Abstract
Vascular endothelial cells (ECs) are constantly exposed to blood flow-induced shear stress, but the mechanism of force-specific activation of their signaling to modulate cellular function remains unclear. We have demonstrated that bone morphogenetic protein receptor (BMPR)-specific Smad1/5 can be force-specifically activated by oscillatory shear stress (OSS) in ECs to cause cell cycle progression. Smad1/5 is highly activated in ECs of atherosclerotic lesions in diseased human coronary arteries from patients with end-stage heart failure undergoing heart transplantation and from apolipoprotein E-deficient mice. Application of OSS (0.5 ± 4 dyn/cm2) causes the sustained activation of Smad1/5 in ECs through activations of mammalian target of rapamycin and p70S6 kinase, leading to up-regulation of cyclin A and down-regulations of p21CIP1 and p27KIP1 and, hence, EC cycle progression. En face examination of rat aortas reveals high levels of phospho-Smad1/5 in ECs of the straight segment of thoracic aorta and the inner, but not the outer, curvature of aortic arch. Immunohistochemical and en face examinations of the experimentally stenosed abdominal aorta in rats show high levels of phospho-Smad1/5 in ECs at poststenotic sites, where OSS occurs. These OSS activations of EC Smad1/5 in vitro and in vivo are not inhibited by the BMP-specific antagonist Noggin and, hence, are independent of BMP ligand. Transfecting ECs with Smad1/5-specific small interfering RNAs inhibits the OSS-induced EC cycle progression. Our findings demonstrate the force-specificity of the activation of Smad1/5 and its contribution to cell cycle progression in ECs induced by disturbed flow.
Keywords: atherosclerosis, stenosis
Vascular endothelial cells (ECs), which provide an interface between the blood and the vessel wall, are constantly exposed to blood flow-induced shear stress (1). Increasing evidence suggests that laminar flow with physiological levels of laminar shear stress (LSS) in the straight parts of the arterial tree is atheroprotective, whereas disturbed flow with low and oscillatory shear stress (OSS) at the curvatures and bifurcations is proatherogenic, attributable, in part, to the distinct roles of this shear flow pattern in enhancing EC cycle progression and proliferation (1–5). Previous studies have demonstrated that ECs exposed to OSS turn over more rapidly and have a higher DNA synthesis rate than the cells exposed to LSS or under static condition (6, 7). This enhancement of EC proliferation by OSS is accompanied by the promotion of cell cycle progression to allow G0/G1-synthetic phase transition (8). These in vitro results are consistent with the ex vivo microscopic examination of the luminal surface of the entire rabbit thoracic aorta showing that ECs in regions of disturbed flow exhibit higher mitotic rates (9). In addition, rapid EC turnover were observed frequently at sites prone to lesion development in apolipoprotein E-deficient (ApoE−/−) mice, indicating a correlation between disturbed blood flow and atherosclerosis (10). A recent study by Guo et al. (11) demonstrated that OSS and LSS regulate EC cycle by distinct modes of interactions between AMP-activated protein kinase (AMPK) and Akt and, hence, differential time courses in the activation of downstream mammalian target of rapamycin (mTOR) and p70S6 kinase (p70S6K). However, the molecular signaling that can be specifically activated by OSS per se to modulate EC cycle progression in vitro and in vivo has not been identified.
Bone morphogenetic proteins (BMPs) transduce signals by forming heteromeric complex cognate with type I and II BMP receptors (BMPRs) to phosphorylate BMPR-specific Smads (i.e., Smad 1/5/8) that may play important roles in the pathogenesis of several diseases (12). Application of OSS to ECs has been shown to induce their production of BMP-4, which plays proinflammatory roles in vascular biology (13). A recent study by Ankeny et al. (14) demonstrated that BMPR-specific Smads are highly activated in the calcified fibrosa endothelium of human aortic valve, which experiences disturbed flow with OSS (1). However, whether OSS per se can activate BMPR-specific Smads in vascular ECs without requiring the BMP ligands to modulate the mechanical responses of ECs remains unclear. Smad proteins can modulate cell cycle, which is controlled by cyclin-dependent protein kinases (Cdks) and their regulatory subunits cyclins and inhibitors p21CIP1 and p27 KIP1 (15). We postulated that OSS per se may activate BMPR-specific Smads in vascular ECs, which would lead to activation of downstream signaling and changes in expression of cell cycle regulatory proteins to induce EC cycle progression.
In the present study, which ranges from in vitro cell culture studies on effects of OSS on molecular signaling to in vivo investigations on the experimentally stenosed rat abdominal aorta and ApoE−/− mice and clinical specimens from patients with coronary artery disease, we have demonstrated that BMPR-specific Smad1/5 can undergo sustained activation in ECs without requiring the BMP ligands. The OSS-induced sustained activation of Smad1/5 led to activations of mTOR and p70S6K, with consequential up-regulation of cyclin A and down-regulations of p21CIP1 and p27KIP1 and, hence, cell cycle progression in ECs. Our findings indicate the force-specificity of EC Smad1/5 activated by disturbed flow in vitro and in vivo and implicate these molecules as hemodynamic-based targets for intervention against vascular disorders associated with OSS-induced EC cycle progression and proliferation.
Results
Smad1/5 Is Highly Activated in the Luminal EC Layer of Atherosclerotic Lesions in Diseased Human Coronary Arteries and ApoE−/− Mice.
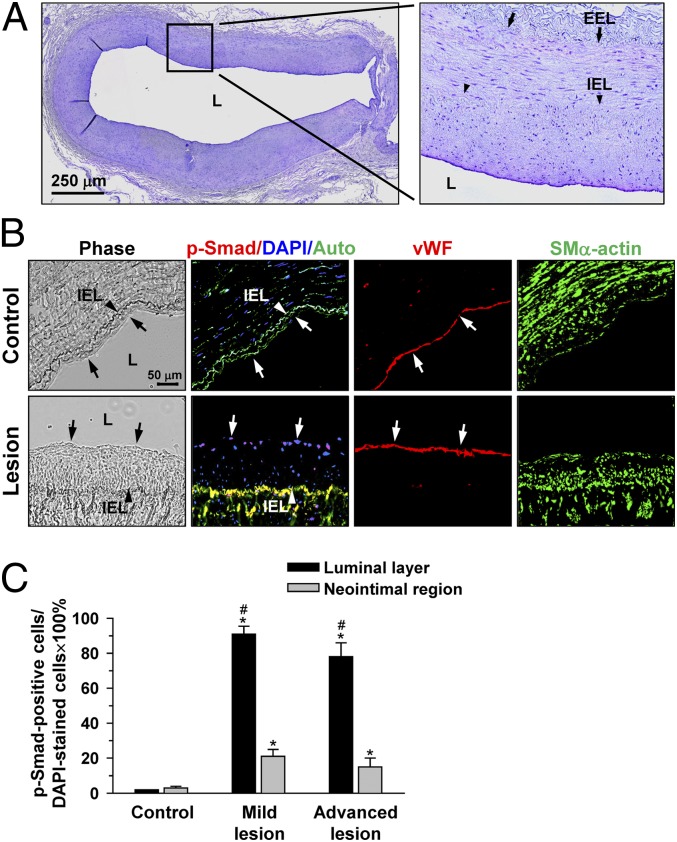
To assess the activation of Smad1/5 in atherosclerotic lesions, immunohistochemical examinations were made on the diseased human coronary arteries, with the use of internal thoracic arteries from the same patients as controls. Fig. 1A shows a representative cross-section [hematoxylin/eosin (H&E) staining] of a diseased human coronary artery containing mild lesions with intimal thickening. Fig. 1B shows a pronounced staining of phospho-Smad1/5 in the cell nuclei in the EC layer (indicated by arrows), but not the neointima, in the lesion region (Fig. 1B, bottom row). In contrast, the control thoracic aorta shows virtually no detectable staining of phospho-Smad1/5 in the EC layer (Fig. 1B, top row). A positive staining of phospho-Smad1/5 is also apparent in ECs of advanced lesions in human coronary artery (Fig. S1A) and atherosclerotic aortas of ApoE−/− mice fed an atherogenic diet for 1 y, as confirmed by immunohistochemical examination (Fig. S1B). Quantitative analysis of the percentage of phospho-Smad1/5-positive cells relative to 4′,6-diamidino-2-phenylindole (DAPI)-stained cells in different regions of human atherosclerotic lesions confirmed the high prevalence of phospho-Smad1/5 in the luminal EC layer, rather than the neointimal region (Fig. 1C).
Fig. 1.
Phospho-Smad1/5 is strongly stained in the EC layer, rather than neointimal region, of human coronary atherosclerotic lesions. (A) Representative H&E staining of cross sections of diseased human coronary arteries showing prominent intimal thickening of the vessel wall. (B) Serial cross-sections of diseased human coronary arteries were stained for human phospho-Smad1/5, vWF, and SMα-actin and counterstained with DAPI. Arrow, phospho-Smad1/5-positive cells. Auto, autofluorescence of the samples; EEL, external elastic lamina; IEL, internal elastic lamina; L, lumen. (C) Quantitative analysis of the percentage of numbers of phospho-Smad1/5-positive cells relative to DAPI-stained cells. Data are means ± SEM of 100–120 cells for EC layer and 180–200 cells for neointimal region from three to five independent experiments. *P < 0.05 vs. control vessels; #P < 0.05 vs. neointimal region.
OSS Induces Sustained Phosphorylation of Smad1/5 and, Hence, Cell Cycle Progression in ECs, Without Requiring the BMP Ligands.
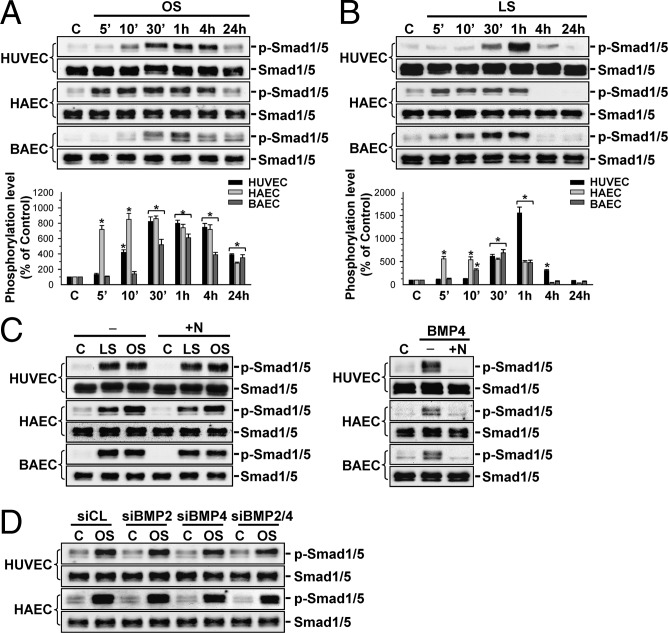
To investigate whether the pattern of shear stress plays a role in modulating EC Smad1/5 activation, OSS at 0.5 ± 4 dyn/cm2 or LSS at 12 dyn/cm2 were applied over a time course of 24 h to human umbilical vein ECs (HUVECs), human aortic ECs (HAECs), and bovine aortic ECs (BAECs). The phosphorylation of Smad1/5 in these ECs was induced rapidly (within 5–30 min) by OSS and remained elevated after 24 h of shearing (although less than the peak value), compared with static control cells (Fig. 2A). In contrast, the increases in Smad1/5 phosphorylation in these ECs induced by LSS were transient and returned to near or even below the basal levels after 4 h of shearing (Fig. 2B). Pretreating these ECs with the BMP specific antagonist Noggin did not inhibit the shear-induced Smad1/5 phosphorylation in all three types of ECs; hence, the shear activation of EC Smad1/5 was not mediated by BMPs (Fig. 2C). This BMP ligand independence of OSS-activation of Smad1/5 was confirmed by the lack of inhibition in OSS-induced Smad1/5 phosphorylation in ECs by transfections with BMP-2- and BMP-4-specific small interfering RNAs (siRNAs) and their combination (40 nM for each; Fig. 2D), which reduced the respective BMP mRNA expression by 70–80% compared with control siRNA (Fig. S2A). As positive controls, Noggin treatment did strongly inhibit the BMP-4-induced Smad1/5 phosphorylation in these ECs (Fig. 2C). Application of OSS to HUVECs and HAECs caused increases in cell percentages in synthetic and G2/M phases and decreases in the G0/G1 phase, compared with static control cells (Table S1). These changes in cell cycle distributions were inhibited by transfecting the ECs with either Smad1- or Smad5-specific siRNA (compared with control siRNA; all at 40 nM), which caused 80–90% reductions in the corresponding Smad protein expression (Fig. S2B), indicating the involvement of Smad1/5 in OSS-induced cell cycle progression in ECs.
Fig. 2.
OSS and LSS induce, respectively, sustained and transient increases in Smad1/5 phosphorylation in ECs, without requiring the BMP ligands. HUVECs, HAECs, and BAECs were kept under static condition (C) or exposed to OSS (OS) (0.5 ± 4 dyn/cm2) (A) or LSS (LS) (12 dyn/cm2) (B) for 5 min (5′), 10 min (10′), 30 min (30′), 1 h, 4 h, and 24 h. (C) ECs were kept as controls (−) or pretreated with Noggin (100 ng/mL) for 1 h (+N) before flow or BMP-4 (100 ng/mL) treatment for 30 min. (D) ECs were transfected with BMP-2-specific (siBMP2) or BMP-4-specific (siBMP4) siRNA (40 nM each) or their combination for 48 h and then subjected to OSS for 30 min. Data in A and B are means ± SEM from three independent experiments and presented as percentage changes in band density from control cells normalized to Smad1/5 protein levels. Results in C and D are representative of triplicate experiments with similar results. *P < 0.05 vs. static control cells.
High Levels of Phospho-Smad1/5 Are Present in ECs in the Inner Curvature of Aortic Arch, but Not the Outer Curvature and the Straight Segment of Thoracic Aorta in Rats.
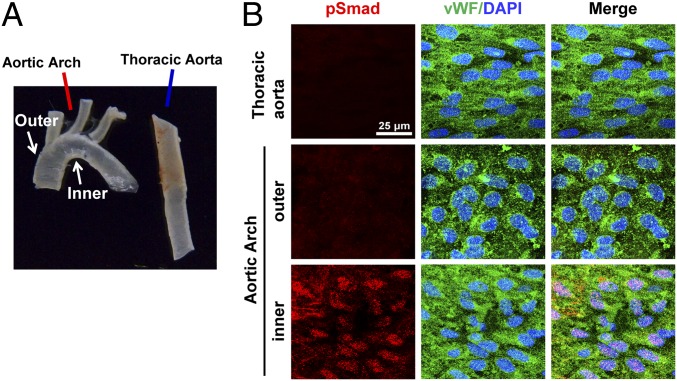
To investigate whether the flow pattern-specific regulation of Smad1/5 activation found in cultured ECs in vitro also exists in the native circulation in vivo, we examined the aortic arch and the straight segment of thoracic aorta of normal rats (Fig. 3A) by en face coimmunostaining for phospho-Smad1/5 and von Willebrand factor (vWF), with DAPI nuclear counterstains. High levels of phospho-Smad1/5 are present in EC nuclei in the inner curvature of aortic arch, where disturbed flow occurs with relatively low and oscillating shear stress (16), but not the outer curvature and the straight segment of thoracic aorta (Fig. 3B), where the shear stress is high and more laminar. These results demonstrate the disturbed flow-specific activation of Smad1/5 in ECs in the native circulation in vivo.
Fig. 3.
In vivo activation of EC Smad1/5 in the native disturbed flow-prone areas. The explants (A) of aortic arch and straight segment of thoracic aorta of normal rats were examined by en face coimmunostaining for rat-specific phospho-Smad1/5 and vWF, with DAPI nuclear counterstains (B). Results are representative of five independent experiments with similar results.
Smad1/5 Is Highly Activated in ECs and Smooth Muscle Cells (SMCs) at Poststenotic Sites in the Stenosed Rat Abdominal Aorta, but only the Activation in SMCs Is Mediated by BMPs.
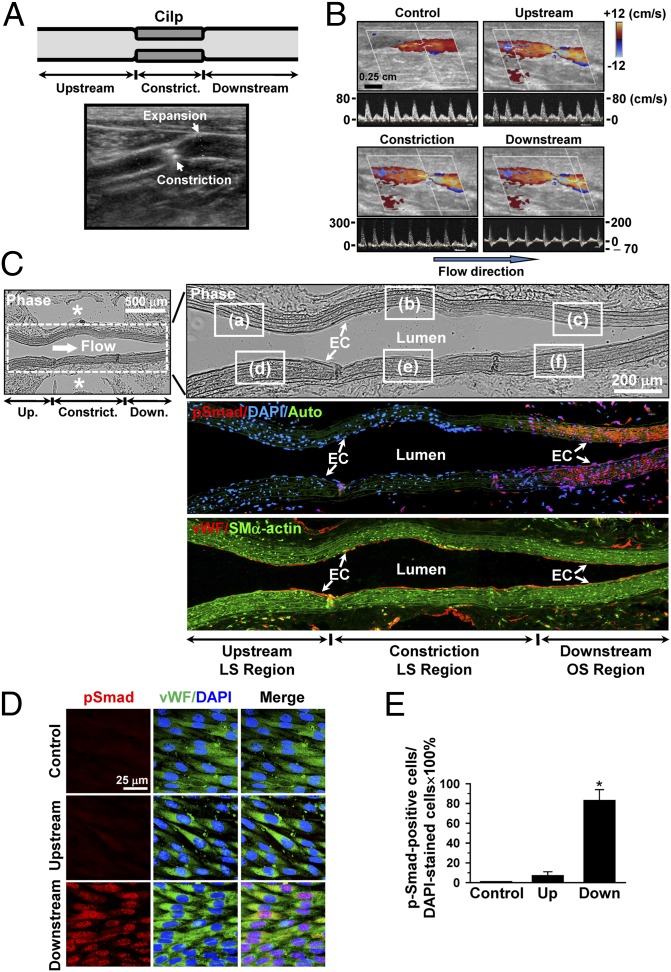
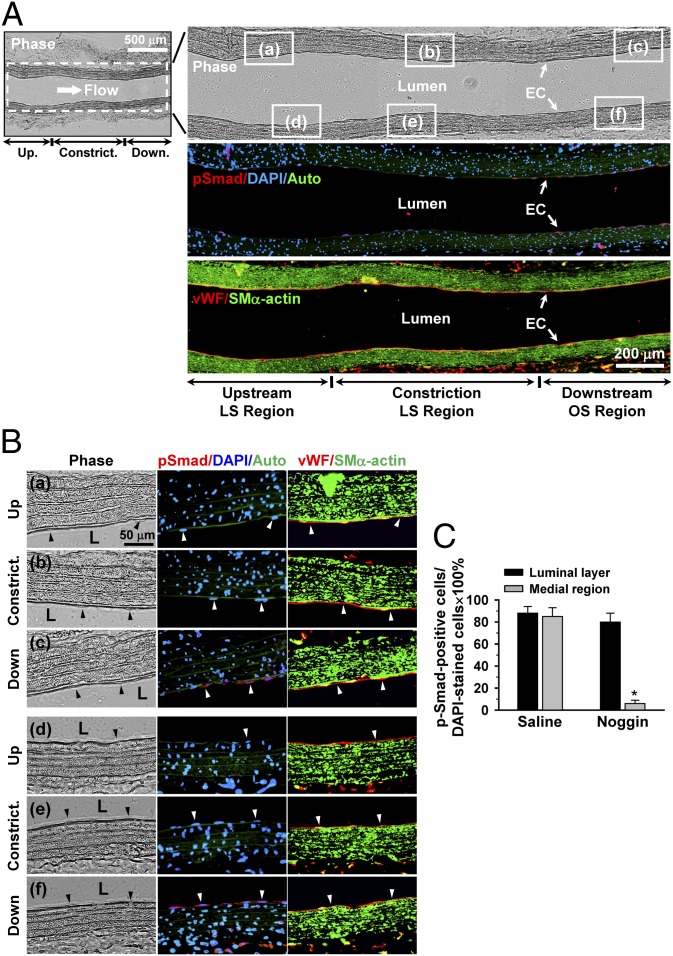
To further assess the effect of an experimentally created disturbed flow pattern in vivo on Smad1/5 activation, we used a stenosis model in which the rat abdominal aorta was subjected to partial constriction by using a U-clip (Fig. 4A). Ultrasonography indicated that placement of the U-clip resulted in a 65% constriction of the aorta diameter, which induced an accelerated forward laminar flow in the constricted region, followed by a pronounced oscillating flow with the existence of retrograde velocities downstream in the region of poststenotic dilatation (Figs. 4 A and B). This poststenotic dilatation was confirmed by examining the explants of the aorta 2 wk after surgery (Fig. S3). The flow patterns and wall shear stress distributions in the constricted rat abdominal aorta were further characterized by computational fluid dynamic modeling using the Comsol Multiphysics software, which confirmed the existence of recirculation eddies with retrograde velocities downstream to the constricted sites (Fig. S4, SI Results, and Table S2). Immunohistochemical examinations of serial sections of the constricted aorta along the longitudinal axis 2 wk later (n = 5) show that the poststenotic sites have high levels of rat-specific phospho-Smad1/5 in both the luminal EC layer and the medial SMC region, which are marked with positive-staining of vWF and smooth muscle-α-actin (SMα-actin), respectively (Fig. 4C and Fig. S5 C and F). In contrast, virtually no detectable levels of phospho-Smad1/5 are seen upstream to (Fig. S5 A and D) and within (Fig. S5 B and E) the constriction (Fig. 4C). These differential levels of phospho-Smad1/5 in the EC layer on the downstream and upstream sides of the constriction were confirmed by the en face staining of the luminal surfaces of the aorta (n = 5), which also shows a high degree of colocalization of phospho-Smad1/5 with DAPI in the cell nucleus (Fig. 4D), and this was further substantiated by the quantitative analysis of the number of cells being positive for both phospho-Smad1/5 and DAPI staining (Fig. 4E). As controls, the straight part of the rat abdominal aorta (n = 5) shows minimal activation of endogenous Smad1/5 proteins in either the EC layer (Fig. 4D) or the medial SMC region.
Fig. 4.
Smad1/5 is highly phosphorylated at the poststenotic site with disturbed flow in the stenosed rat abdominal aorta. (A) Schematic diagram (Upper) and ultrasound illustration (Lower) of the stenosis in rat abdominal aorta produced by using a U-shaped clip. (B) Ultrasound measurements on the rat abdominal aorta subjected to stenosis. Upper images in B show vessels illustrated by using 2D and color Doppler mode, and lower images show Doppler spectral display of velocity profiles for several heartbeats measured by pulse wave Doppler mode. (C) Panoramic examinations on Smad1/5 phosphorylation levels in the stenosed rat abdominal aorta from the upstream through midpoint to downstream of constriction. Asterisk indicates clip-induced injury holes. Auto, autofluorescence of the vessel wall. The magnified views of the indicated areas (white line box) in the phase image (Right) in C are shown in Fig. S5. (D) En face immunohistochemical detections of EC Smad1/5 phosphorylation levels in the upstream and downstream sides of constriction. Flow direction is from left to right. Control is straight part of aorta away from stenosis. (E) Quantitative analysis of the en face staining results. Data are means ± SEM of 80–100 cells from five independent experiments for each area. *P < 0.05 vs. control vessels or upstream area. Images shown in each picture are representative of five rats with similar results.
To test the role of BMPs in Smad 1/5 activation in poststenotic sites, Noggin was infused intraarterially into the constricted aorta (n = 5) by osmotic pump-based controlled release system (90 μg/kg per day). Noggin treatment resulted in an inhibition of Smad1/5 activation in the medial SMC region, but not the luminal EC layer, at the poststenotic sites (Fig. 5 and Fig. S6), compared with control animals receiving saline injection (Fig. 4C and Fig. S5). These results indicate that: (i) Smad1/5 activation can be modulated by different flow patterns in vivo in a manner similar to that observed in vitro; and (ii) Smad1/5 activation at the poststenotic site in vivo involves both ECs and SMCs, but with different mechanisms: the OSS-induced Smad1/5 activation is mediated by BMPs in SMCs, but not in the EC layer.
Fig. 5.
Smad1/5 activation in the endothelium at the poststenotic sites with disturbed flow is not mediated by BMPs. Noggin (90 μg/kg per day) was infused into the stenosed abdominal aorta in rats (n = 5) by osmotic pump. Control rats (n = 5) received saline. Animals were killed 2 wk after surgery, and serial sections of affected aortas were subjected to immunohistochemical examinations of rat-specific phospho-Smad1/5, vWF, and SMα-actin. (A) Panoramic view of immunohistochemical examinations on Smad1/5 phosphorylation levels in the stenosed rat abdominal aorta. Auto, autofluorescence of the vessel wall. Photomicrographs in B are the magnified views of the indicated areas (white line box) in the phase image of A (Right). L, lumen. Arrowhead, ECs. (C) Quantitative analysis of the percentage of numbers of phospho-Smad1/5-positive cells relative to DAPI-stained cells in different cell regions downstream to the constriction for animals injected with or without Noggin. Images shown in each examination are representative of five rats with similar results. Data in C are means ± SEM of 150 cells for the EC layer and 200 cells for the medial SMC region from five independent experiments. *P < 0.05 vs. saline group.
OSS Induces Sustained Activations of mTOR/p70S6K and, Hence, Up-Regulation of Cyclin A and Down-Regulations of p21CIP1 and p27KIP1 in ECs, Which Are Mediated by Smad1/5.
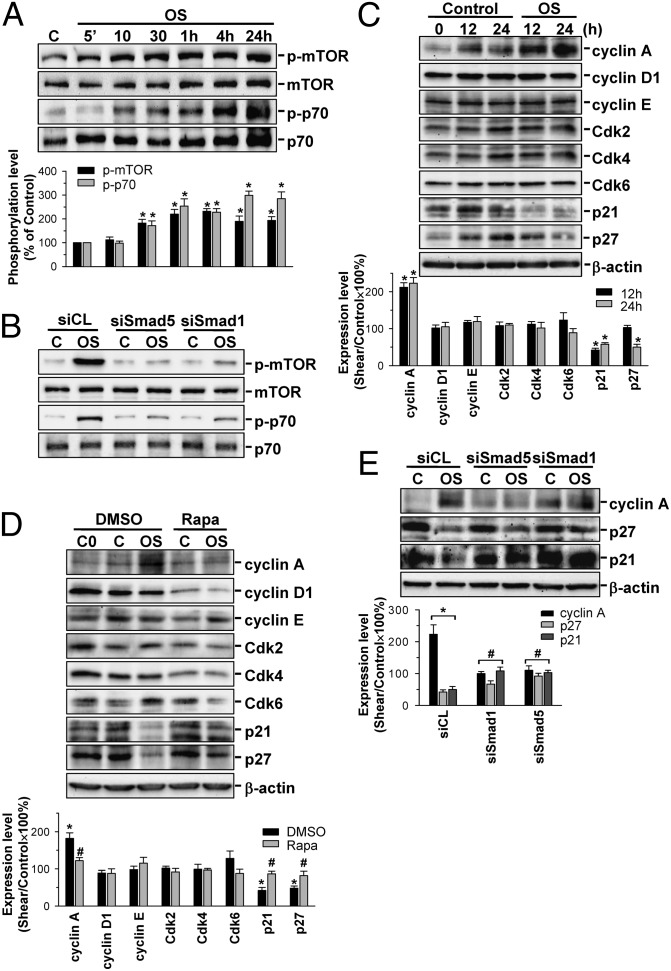
Application of OSS at 0.5 ± 4 dyn/cm2 to HUVECs induced rapid (significant within 10 min) and sustained (24 h) increases in mTOR and p70S6K phosphorylations (Fig. 6A). In contrast, LSS at 0.5 dyn/cm2 had only minor effects on these signaling events (Fig. S7). These OSS-induced responses were inhibited by transfecting ECs with Smad1- and Smad5-specific siRNAs (Fig. 6B). OSS applied to HUVECs for 12 or 24 h resulted in an increase in cyclin A expression and decreases in p21CIP1 and p27KIP1 expressions in these ECs (Fig. 6C). These OSS-induced responses were rescued by pretreating the cells with specific mTOR inhibitor rapamycin (10 nM) (Fig. 6D) or transfecting with Smad1- or Smad5-specific siRNAs (compared with control siRNA; 40 nM for each) (Fig. 6E). These results indicate that the OSS-induced EC cycle progression is mediated by Smad1/5 through the mTOR/p70S6K signaling cascade.
Fig. 6.
Smad1/5 regulates OSS-mediated mTOR/p70S6K activation and cyclin A, p21CIP1, and p27KIP1 expressions in ECs. HUVECs were kept under static condition or exposed to OSS for the indicated times (A and C), 10 min (B) or 24 h (D and E). Cells were transfected with control siRNA or specific siRNA (40 nM each) of Smad1 and Smad5 (B and E) for 48 h, or pretreated with rapamycin (Rapa) (10 nM) for 1 h (D). Data in A–E are means ± SEM from three independent experiments. Results in B are representative of triplicate experiments with similar results. *P < 0.05 vs. static control cells; #P < 0.05 vs. siCL- or DMSO-treated cells.
Discussion
The present systematic in vitro and in vivo investigations have characterized BMPR-specific Smad1/5 as important mechanosensitive molecules critical for vascular EC cycle progression in response to disturbed flow with OSS. The OSS-activation of Smad1/5 is force-specific and contributes to the development of atherosclerotic lesions. In recent years, >30 signaling molecules and gene products in ECs, including those involved in modulating EC inflammation [e.g., intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule-1] (16, 17), survival (e.g., histone deacetylase 3) (18), and permeability (e.g., connexin 43) (19, 20), have been identified in vitro and in vivo to be differentially regulated by different flow patterns (disturbed vs. laminar flows) and the associated shear stresses (OSS vs. LSS) [reviewed elsewhere (1)]. However, it remains unclear whether these molecules are regulated by flow pattern and shear stress per se or by the autocrine or paracrine effect of chemical mediators released from the sheared ECs. The in vivo results in our current study showed that EC Smad1/5 are activated in the rat aortic arch and experimentally stenosed abdominal aorta, where disturbed flow occurs. Ankeny et al. (14) showed that Smads1/5 are highly activated in the calcified fibrosa endothelium of human aortic valve, which experiences disturbed flow. However, the mechanisms underlying the flow activation of these Smads in vascular and valvular ECs are different. BMP ligands probably play a key role in modulating the side-dependent activation of Smad1/5 in valvular ECs, as shown by the effects of antagonists (14). In contrast, the activation of Smad1/5 specifically by OSS in vascular ECs does not require the BMP ligands, because pretreating these ECs in vitro with Noggin and transfecting with BMP-2- and BMP-4-specific siRNAs cannot inhibit the OSS-activation of these Smads. This BMP ligand independence of OSS-activation of EC Smad1/5 was substantiated by our in vivo findings that intraarterial injection of Noggin into the stenosed rat abdominal aorta cannot inhibit the Smad1/5 activation in the EC layer at poststenotic sites. The difference in the role of BMPs in Smad1/5 activation between vascular and valvular ECs in response to disturbed flow may be attributable to their endothelial heterogeneity (1). Our present study demonstrates the force-specificity of molecular signaling induced by disturbed flow in ECs by in vivo longitudinal-sectional observations on the stenosed aortas spanning from the upstream laminar flow area through the constricted throat to the downstream dilated area with disturbed flow.
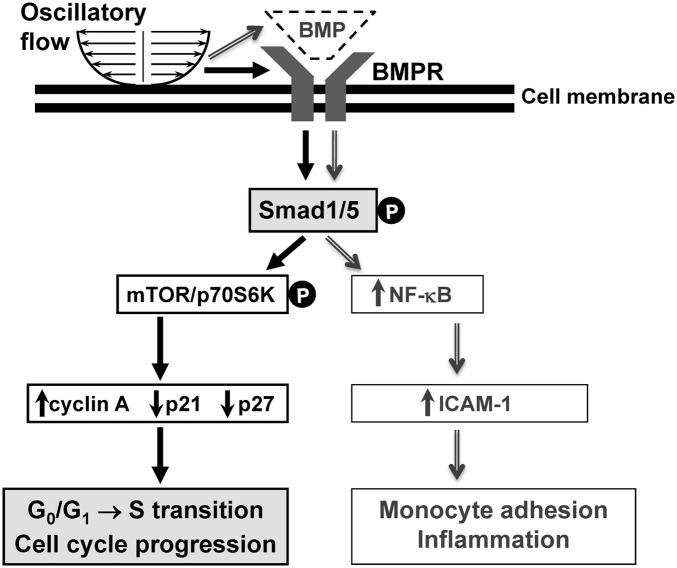
Disturbed flow is atherogenic through its induction of EC proliferation and inflammation. The present study has shown that OSS enhances EC cycle progression through the activation of BMPR-specific Smad1/5 independent of BMP ligand. There is evidence, however, that the mediation of proinflammatory responses [nuclear factor-κB (NF-κB) activation, ICAM-1 expression, and monocyte adhesion] of ECs by OSS in vitro is dependent on BMP ligand, because these responses are inhibited by pretreating ECs with Noggin (13). We have performed in vivo studies to show that disturbed flow induces a high expression of BMP-4 in ECs at poststenotic sites of the stenosed rat abdominal aorta, but not upstream to, nor within, the constriction (Fig. S8). Our in vitro studies using HUVECs have further demonstrated that pretreating ECs with Noggin or transfecting with BMP-4-specific siRNA inhibits the OSS-induction of ICAM-1 (Fig. S9 A and B) and that transfecting ECs with Smad1- and Smad5-specific siRNAs inhibits OSS- and BMP-4-inductons of ICAM-1 (Fig. S9 C and D). The present in vivo and in vitro results are in concert with the in vitro findings of Sorescu et al. (13) that the OSS-induced EC proinflammatory response is mediated by BMP-4 through Smad1/5. Our present study advances the notion that disturbed flow with OSS stimulates EC inflammation and cycle progression through the activation of Smad1/5 by different mechanisms involving BMP/BMPR: the Smad1/5-induction of inflammation is BMP-dependent, but the Smad1/5-induction of cell cycle progression is BMP-independent; the OSS-induced EC cycle progression is mediated by the force-activation of Smad1/5 via the BMRP (Fig. 7).
Fig. 7.
OSS induces EC cycle progression and inflammation through the activation of Smad1/5 by different mechanisms. The black solid line arrows show the findings of this study that OSS induces EC cycle progression by activating Smad1/5 through BMPR without requiring the BMP ligands. The gray double-line arrows show the findings in ref. 13 and this study that OSS induces EC inflammation via the activation of Smad1/5 by the EC-released BMPs.
Our study also demonstrates that the OSS-induced BMP-4 expression contributes to the Smad1/5 activation in SMCs (Fig. 4C and Fig. S5 C and F), since this can be abolished by local Noggin injection (Fig. 5 A and B, c and f), in contrast to the lack of Noggin effect on EC Smad1/5. Thus, Smad1/5 are highly activated in both ECs and SMCs at poststenotic sites with disturbed flow, but only the activation in SMCs is mediated by BMPs.
The importance of EC Smad1/5 activation in atherogenesis is shown by our immunohistochemical examinations on the diseased human coronary arteries. Approximately 90% of ECs in endothelial patches on the atherosclerotic lesions have positive staining for phospho-Smad1/5, but such staining is undetectable in ECs on the normal (minimally diseased) human artery. This high level of phospho-Smad1/5 in the EC layer of human atherosclerotic coronary arteries may be attributed, at least in part, to their complex flow environment arising from the irregular geometry (i.e., branches, curvatures, and tortuosity) (1), rather than the autocrine or paracrine stimuli by BMPs from ECs or SMCs in the lesions (21). This notion is supported by the recent findings by Chang et al. (22) that the BMP antagonists Noggin, follistatin, and matrix Gla proteins are highly coexpressed with BMP-4 in ECs in human atherosclerotic coronary arteries, which may provide a negative feedback mechanism in an attempt to minimize the BMP effects in lesions.
Our present findings are in agreement with the results of Guo et al. (11) that disturbed flow with OSS induces sustained activation of mTOR/p70S6K mitogenic signaling cascade in ECs. The present study further demonstrated that this OSS-activation of mTOR/p70S6K cascade is mediated by Smad1/5 and subsequently regulates the OSS-induced changes in downstream cyclin A, p21CIP1, and p27KIP1 protein expressions. OSS-activation of mTOR/p70S6K has been shown to be modulated by the interplay between AMPK and Akt in response to OSS, with AMPK serving as a negative regulator and Akt as a positive activator for OSS-induced p70S6K activity and EC cycle progression (11). AMPK activation counteracts the effect of Akt to attenuate EC cycle progression in response to OSS (11). Whether Smad1/5 interferes with the interplay between AMPK and Akt to modulate mTOR/p70S6K activations and EC cycle progression in response to OSS warrants further investigations.
The present study has the following physiological and pathophysiological significance. We have characterized BMPR-specific Smad1/5 as a connecting link for the chain of events of disturbed flow, mechanical sensing, EC cycle progression, and atherogenic responses in the arterial wall. We also present evidence that the role of Smad1/5 in modulating OSS-induced EC cycle progression is mediated through its regulation in mTOR/p70S6K activations and downstream cyclin A, p21CIP1, and p27KIP1 expressions. Our findings that Smad1/5 activation by disturbed flow enhances EC cycle progression without requiring the BMP ligands, in contrast to the requirement of BMP for inflammation, indicate that the Smad1/5 serves as a convergent molecular signaling site for chemical (e.g., BMPs) and mechanical (e.g., OSS) stimulations. Thus, BMPR-specific Smad1/5 may serve as a promising hemodynamic-based target for the development of anti-atherogenic strategies for interventions against vascular disorders associated with EC dysfunction.
In summary, the present study used a combination of cell culture, experimental animals, and clinical specimens to demonstrate that BMPR-specific Smad1/5 is highly activated in ECs by disturbed flow with OSS both in vitro and in vivo (including the endothelial layers of mouse aorta subjected to different flow patterns and diseased human coronary arterial wall), with consequent promotion of EC cycle progression. This OSS-activation of BMPR-specific Smad1/5 is force-specific, without requiring the BMP ligands. Our findings that endothelial Smad1/5 can be activated by disturbed flow with OSS per se indicate the convergence and divergence of molecular signaling in response to chemical and mechanical stimuli and provide insights to therapeutic intervention against disturbed flow-associated vascular disorders, e.g., atherosclerosis.
Methods
Materials.
A detailed list of materials used in this study is provided in SI Methods.
Cell Cultures.
HUVECs were isolated and cultured in M199 (Gibco) supplemented with 20% (vol/vol) FBS (Gibco), as described (7). HAECs were purchased from Cell Applications and cultured in Endothelial Cell Growth Medium (Cell Applications). BAECs were obtained from Clonetics and cultured in DMEM with 10% (vol/vol) FBS. Cells were incubated in phenol red-free medium supplemented with 10% (vol/vol) FBS (pretreated with charcoal to remove endogenous BMPs in the serum) for 24 h before the BMP-knockdown experiments.
Flow Apparatus.
Cultured ECs were subjected to different patterns of flow in a parallel-plate flow chamber that can produces OSS at 0.5 ± 4 dyn/cm2 and LSS at 12 dyn/cm2. Details of the system are provided in SI Methods.
Human Coronary Arteries, ApoE−/− Mice, Aortic Stenosis Animal Model, and Noggin Delivery.
Seven diseased human coronary arteries and four control internal thoracic arteries were harvested from five patients with end-stage heart failure undergoing heart transplantation at Tri-Service General Hospital in Taipei, as approved by the Hospital Human Subjects Review Committee, and were fixed and embedded in paraffin blocks. These diseased arteries contain various stages of atherosclerosis from mild lesions with fatty streaks to advanced lesions, according to American Heart Association guidelines for histological classification of atherosclerotic lesions (23). Control C57BL/6J and ApoE−/− mice were purchased from Jackson Laboratory and were fed normal chow diet and atherogenic diet containing 15% (wt/vol) cocoa butter and 0.25% (wt/vol) cholesterol, respectively. For aortic stenosis experiments, a U-shaped titanium clip (Ethicon Endo-Surgery) was surgically applied to the abdominal aorta of 10 rats to create local stenosis, after which 5 sham control rats received normal saline and another 5 rats were intraarterially injected with a specific BMP antagonist Noggin (BD Biosciences) by osmotic pump (Alzet) at a controlled rate (90 μg/kg per day; Fig. S10). All rats were provided with normal diet. Animal experiments were performed in accordance with National Institutes of Health guidelines and with the approval of the Animal Research Committee of National Health Research Institutes.
Ultrasound Measurements.
All ultrasound measurements were taken using the iE33 ultrasound imaging system (Philips Medical Systems) equipped with a 7-to 15-MHz linear array transducer (L15-7io).
An expanded SI Methods section is provided in SI Text.
Supplementary Material
Acknowledgments
This work was supported by National Science Council Grant NSC-99-2321-B-400-002/NSC-100-2325-B-400-011 and National Health Research Institutes Grant NHRI-ME-100-PP06 (to J.-J.-C.), National Institutes of Health Grant HL-106579/HL-104402 (to S.C.), and National Science Council Grant NSC-99-2911-I-009-101 (to S.C. and J.-J.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205476109/-/DCSupplemental.
References
- 1.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Resnick N, Gimbrone MA., Jr Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J. 1995;9:874–882. doi: 10.1096/fasebj.9.10.7615157. [DOI] [PubMed] [Google Scholar]
- 3.Traub O, Berk BC. Laminar shear stress: Mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 4.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadohama T, Nishimura K, Hoshino Y, Sasajima T, Sumpio BE. Effects of different types of fluid shear stress on endothelial cell proliferation and survival. J Cell Physiol. 2007;212:244–251. doi: 10.1002/jcp.21024. [DOI] [PubMed] [Google Scholar]
- 6.DePaola N, Gimbrone MA, Jr, Davies PF, Dewey CF., Jr Vascular endothelium responds to fluid shear stress gradients. Arterioscler Thromb. 1992;12:1254–1257. doi: 10.1161/01.atv.12.11.1254. [DOI] [PubMed] [Google Scholar]
- 7.Chiu JJ, Wang DL, Chien S, Skalak R, Usami S. Effects of disturbed flow on endothelial cells. J Biomech Eng. 1998;120:2–8. doi: 10.1115/1.2834303. [DOI] [PubMed] [Google Scholar]
- 8.Davies PF. Spatial hemodynamics, the endothelium, and focal atherogenesis: A cell cycle link? Circ Res. 2000;86:114–116. doi: 10.1161/01.res.86.2.114. [DOI] [PubMed] [Google Scholar]
- 9.Chien S. Molecular and mechanical bases of focal lipid accumulation in arterial wall. Prog Biophys Mol Biol. 2003;83:131–151. doi: 10.1016/s0079-6107(03)00053-1. [DOI] [PubMed] [Google Scholar]
- 10.Foteinos G, Hu Y, Xiao Q, Metzler B, Xu Q. Rapid endothelial turnover in atherosclerosis-prone areas coincides with stem cell repair in apolipoprotein E-deficient mice. Circulation. 2008;117:1856–1863. doi: 10.1161/CIRCULATIONAHA.107.746008. [DOI] [PubMed] [Google Scholar]
- 11.Guo D, Chien S, Shyy JY. Regulation of endothelial cell cycle by laminar versus oscillatory flow: Distinct modes of interactions of AMP-activated protein kinase and Akt pathways. Circ Res. 2007;100:564–571. doi: 10.1161/01.RES.0000259561.23876.c5. [DOI] [PubMed] [Google Scholar]
- 12.Bobik A. Transforming growth factor-betas and vascular disorders. Arterioscler Thromb Vasc Biol. 2006;26:1712–1720. doi: 10.1161/01.ATV.0000225287.20034.2c. [DOI] [PubMed] [Google Scholar]
- 13.Sorescu GP, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem. 2003;278:31128–31135. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- 14.Ankeny RF, et al. Preferential activation of SMAD1/5/8 on the fibrosa endothelium in calcified human aortic valves—association with low BMP antagonists and SMAD6. PLoS ONE. 2011;6:e20969–e20977. doi: 10.1371/journal.pone.0020969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang SF, et al. BMP-4 induction of arrest and differentiation of osteoblast-like cells via p21 CIP1 and p27 KIP1 regulation. Mol Endocrinol. 2009;23:1827–1838. doi: 10.1210/me.2009-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suo J, et al. Hemodynamic shear stresses in mouse aortas: Implications for atherogenesis. Arterioscler Thromb Vasc Biol. 2007;27:346–351. doi: 10.1161/01.ATV.0000253492.45717.46. [DOI] [PubMed] [Google Scholar]
- 17.Chappell DC, Varner SE, Nerem RM, Medford RM, Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res. 1998;82:532–539. doi: 10.1161/01.res.82.5.532. [DOI] [PubMed] [Google Scholar]
- 18.Zampetaki A, et al. Histone deacetylase 3 is critical in endothelial survival and atherosclerosis development in response to disturbed flow. Circulation. 2010;121:132–142. doi: 10.1161/CIRCULATIONAHA.109.890491. [DOI] [PubMed] [Google Scholar]
- 19.Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ Res. 1998;83:636–643. doi: 10.1161/01.res.83.6.636. [DOI] [PubMed] [Google Scholar]
- 20.DePaola N, et al. Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro. Proc Natl Acad Sci USA. 1999;96:3154–3159. doi: 10.1073/pnas.96.6.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boström K, et al. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang K, et al. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: Role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation. 2007;116:1258–1266. doi: 10.1161/CIRCULATIONAHA.106.683227. [DOI] [PubMed] [Google Scholar]
- 23.Stary HC, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.