Abstract
A rodent model of diet-induced obesity revealed that obesity significantly altered hematopoietic and lymphopoietic functions in the bone marrow and thymus. C57BL/6 mice were fed a mixed high-fat diet (HFD) of 45% fat or 10% fat diet (lean controls) for 180 d. A sustained increase in the numbers of cells found in bone marrow and thymus of HFD mice occurred from day 90 to day 180. However, with the exception of a 10–18% increase in the proportion of lymphocytes, the composition of monocytes, granulocytes, erythrocytes, and mixed progenitor lineages remained normal in the marrow. Likewise, thymuses of HFD mice increased 30–50% in size compared with controls, with analogous increases in thymocyte numbers. The overall thymus cellular composition remained normal. Although increased blood and lymphatic volume in obese mice would play a role in increased hematopoiesis, there were large and disproportionate increases in blood leukocytes of HFD mice, indicating that homeostasis was not maintained. Leptin, which promotes lymphopoiesis and myelopoiesis, reached 100 ng/mL in sera from HFD mice. Moreover, a three- to sixfold increase in adipocytes in marrow resulted in spiked leptin mRNA expression in bones of HFD mice compared with lean controls. Other cytokines and growth factors did not show any increases in obese marrow. The substantial increase in lymphopoietic and hematopoietic processes in HFD mice indicates that the primary tissues are another facet of the immune system dysregulated by obesity, which was perhaps fostered by higher amounts of leptin in marrow and serum.
The bone marrow, which is located throughout the skeletal mass, is one of the largest and most active tissues of the body. Through complex differentiation pathways originating with hematopoietic stem cells, the marrow produces billions of new leukocytes and erythrocytes each day (1–4). Osteoblasts, chondrocytes, myocytes, adipocytes, etc., also arise daily from marrow mesenchymal stem cells. Because it is a highly vascular tissue, the marrow provides a highway for the constant transport of newly produced cells to the systemic circulation. Thus, the breadth of divergent events occurring daily in the marrow is remarkable.
Given the complex operations occurring in the bone marrow each day, it would seem logical to assume that it would be altered by disease states, changes in metabolism and metabolic diseases. However, the roles that the marrow might play in the etiology of disease have been largely overlooked. It is known, however, that increased proliferation of granulocyte percursors is observed in mice in response to inflammation and infection (5, 6). Malnutrition, including zinc deficiency and protein calorie deficiencies, profoundly alters the primary immune tissues, especially the bone marrow (7, 8). Stress, which may create elevated levels of endogenous glucocorticoids, initiates marked apoptosis among pre-B and pre-T cells while enhancing myelopoiesis in the marrow (9, 10). In dextran sodium sulfate-induced colitis, the marrow exhibits marked declines in erythropoiesis and accelerated production of monocytes and granulocytes, thereby exacerbating the anemia and inflammation of the gut that accompanies this disease. These examples make clear that hematopoietic processes in the marrow are actively responding to new and diverse physiological processes and not simply maintaining homeostasis.
In addition to metabolic changes, obesity is also associated with chronic low-grade inflammation that leads to the increased production of adipokines, proinflammatory cytokines, leptin, etc., in blood (11). These factors play a role in the development of several comorbidities associated with obesity, such as cardiovascular disease, diabetes, hypertension, arthritis, and stroke (12, 13). Inflammation, along with documented changes in T-cell and macrophage function, indicates that immune dysregulation has occurred in the obese (14, 15). Considering the system-wide changes occurring during obesity, it is possible that immune cell development in the primary immune tissues would be affected by obesity-associated inflammation, increased adiposity, and/or hormonal changes. Indeed, leptin-deficient obese ob/ob mice have a 40% reduction in the number of bone marrow cells and smaller thymuses compared with lean mice (16). These same mice also have reduced lymphocyte numbers in peripheral blood (17). Similarly, obese db/db mice, which have an inactive leptin receptor, have a deficit in lymphocyte progenitors as well as reduced B- and CD4+ T-cell numbers in peripheral blood (18). Provision of recombinant leptin to ob/ob mice promoted both lymphopoiesis and myelopoiesis (16). Thus, it seemed that leptin, which is substantially elevated in the obese, might alter hematopoiesis. Comparatively little was known about the effects of diet-induced obesity (DIO) on hematopoiesis. It is known that adipose tissue in DIO mice contains increased numbers of proinflammatory macrophages (adipose tissue macrophages) that are bone marrow-derived as well as a large number of stem cells (19–22). Similarly, obesity in humans has been associated with increased white blood cell counts (23–25), but the role of the marrow in these changes was unclear.
Considering all of the aforementioned issues, it seemed plausible to assume that other obesity-related changes would occur in hematopoietic and lymphopoietic processes in bone marrow and the thymus. In this study, we investigated the effects of diet-induced obesity in young mice on hematopoietic and lymphopoietic function. Profound changes in the primary immune tissues were noted in the obese mice that persisted throughout 180 d of this study.
Results
Weight Gain and Leptin.
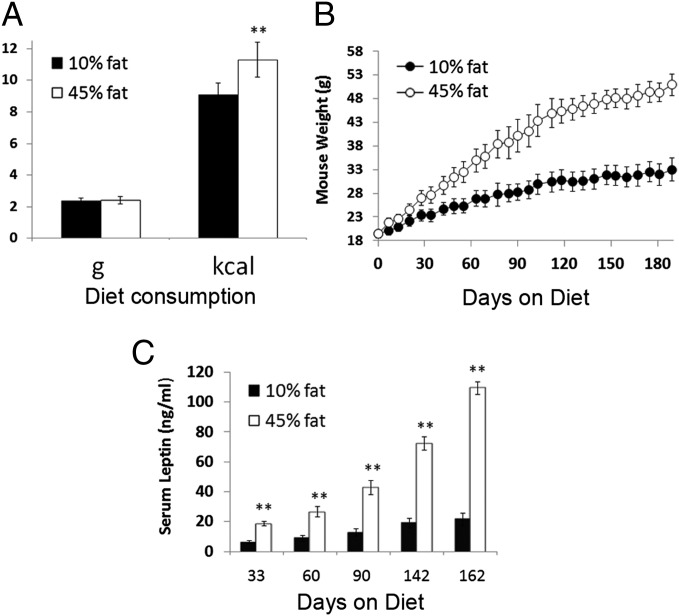
Mice were fed either a 10% (control) or 45 Kcal percent fat (mixed fat; HFD) diet starting at 6–7 wk of age. Diet consumption, body weight, and serum leptin were monitored from day 7 to approximately day 180. HFD mice consumed the same amount of food in grams as mice fed control diet, indicating a lack of hyperphagia (Fig. 1A). Because of the higher caloric density of HFD (4.73 vs. 3.85 kcal/g for HFD vs. control, respectively), HFD mice consumed ∼25% more calories than control mice (Fig. 1A). Over time, this increased calorie consumption resulted in significantly elevated body weights in HFD mice throughout the experiment starting as early as day 7 (Fig. 1B). HFD mice had body weights that were increased 10–15% by day 30, 20–30% by day 60, 40% by day 90, and 50–60% by later time points compared with controls. As expected, this weight gain was accompanied by significant increases in the amount of epididymal fat. Fig. S1 shows epididymal fat weight increased 1.8- to 3.0-fold in HFD mice. HFD mice also had significantly elevated serum leptin throughout the experiment (Fig. 1C), ranging from a threefold increase at day 33 to a fivefold increase by day 162. Given the substantial increase in epididymal fat, this finding would be expected, because adipocytes are the primary producers of leptin. This significant and early rise in leptin would be important, because leptin is known to promote lymphopoiesis and granulopoiesis (16), which will be shown to be enhanced in HFD mice. More modest increases in weight, fat, and serum leptin with age were noted in mice on control diet.
Fig. 1.
Characteristics of mice fed HFD. Mice fed a 45% mixed fat diet (white bars) consumed similar amounts of food in grams as mice fed a 10% fat diet (black bars) but ingested significantly more calories (A). Data shown are average food consumption per day for the entire 180-d experiment. (B) Mice fed a 45% fat diet (○) gained significantly more weight than mice fed a 10% fat diet (●). (C) Serum leptin in mice fed a 45% fat diet (white bars) was significantly elevated compared with mice fed a 10% fat diet (black bars; n = 7–8 mice per group). **P < 0.01.
Effect of HFD on Bone Marrow Classes.
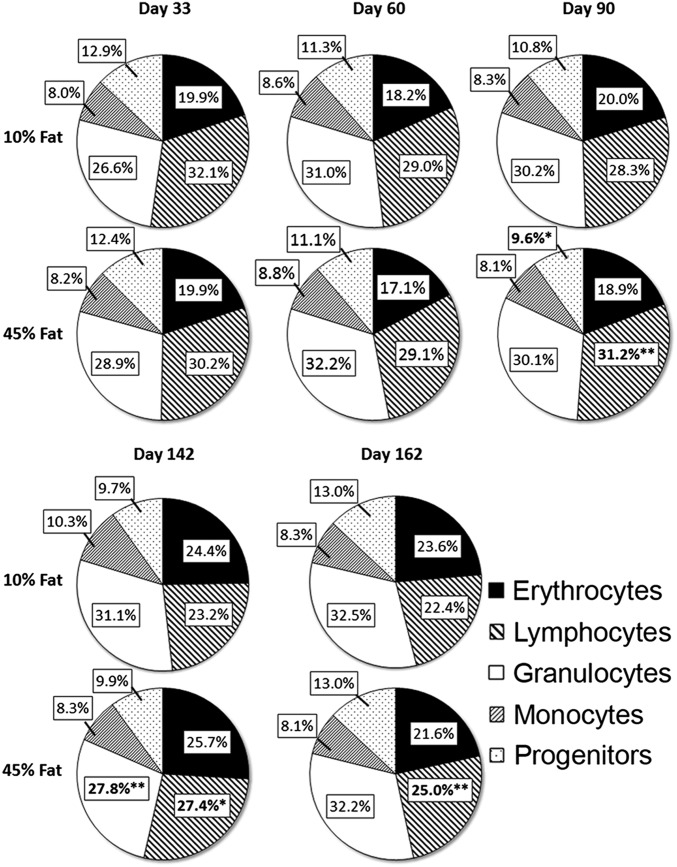
To assess the effects of HFD on bone marrow cellularity and its hematopoietic composition, flow cytometry was used to analyze the proportion and total number of bone marrow cells in both control and HFD mice. A system that delineates the major subclasses of marrow cells of the hematopoietic system (e.g., erythrocytes, lymphocytes, granulocytes, monocytes, and progenitors) was used. Flow cytometric profiles and secondary antibodies used to validate these major hematopoietic populations (Fig. S2) have been used many times by our laboratory and others (8, 9, 26). Examination of early, intermediate, and late time points after initiation of diet revealed that HFD mice showed little or no significant changes in cellular composition compared with control mice (Fig. 2). Only starting at day 90 and continuing thereafter were modest increases in the proportion of marrow lymphocytes observed in HFD mice. The lymphocyte precursors increased at days 90, 142, and 162, ranging from 10% to 18% (Fig. 2). Overall, HFD produced only modest changes in the composition of marrow, with the exception noted for the increase in the proportion of lymphocytes starting at day 90.
Fig. 2.
Distribution of bone marrow hematopoietic cell subpopulations in mice fed 10% or 45% fat diet. Bone marrow cells were labeled with anti-CD31 (clone ER-MP12) and anti–Ly-6C (clone ER-MP20), with cell subpopulations determined as described in Fig. S2, including specific markers to confirm the identity and size of each marrow subpopulation. Data show the percent of total nucleated marrow cells for each cell type at various times after the start of diet. n = 7–8 mice per group. *P < 0.05, **P < 0.01.
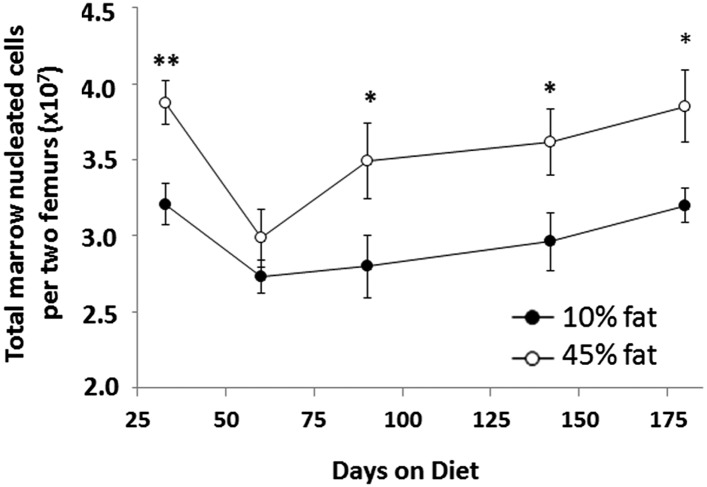
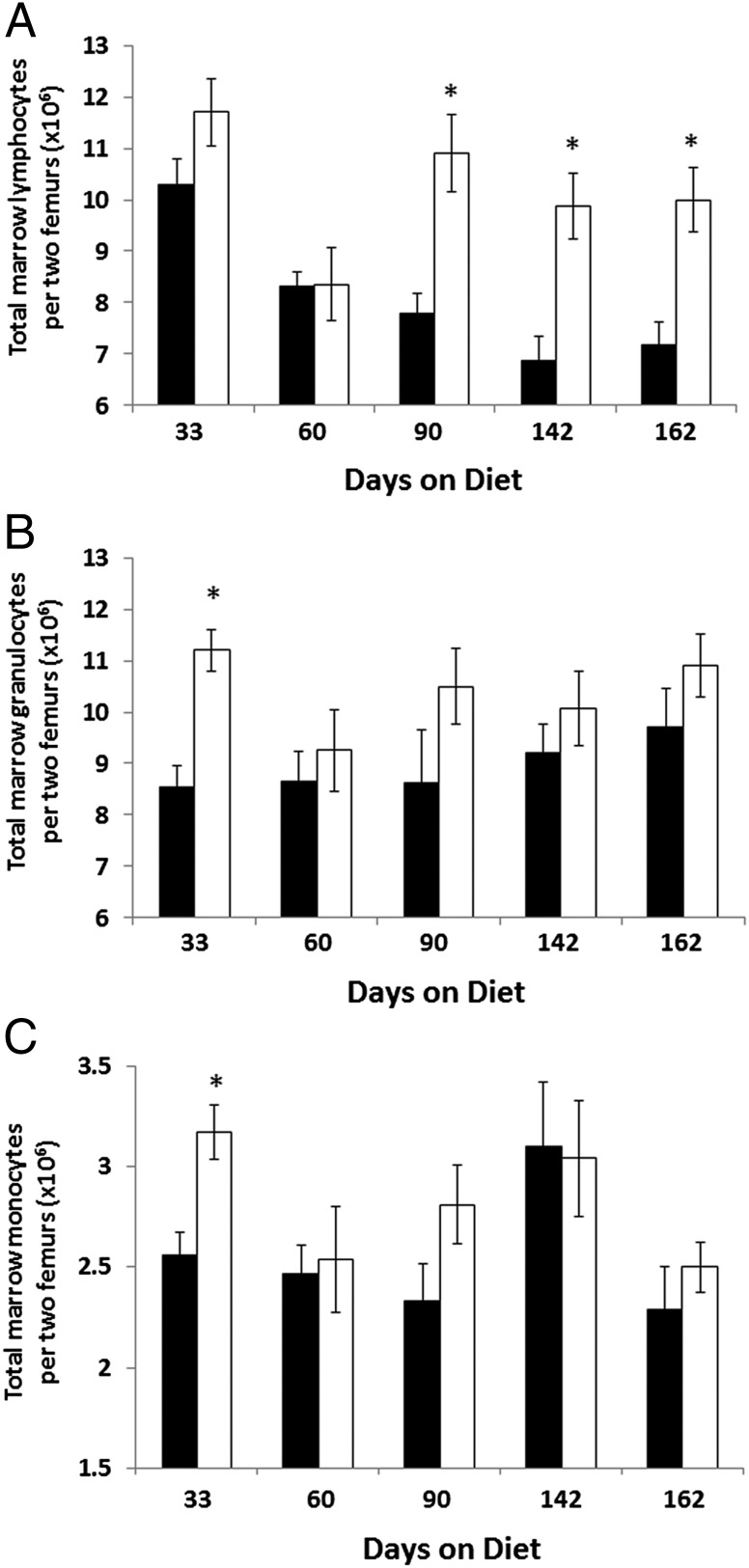
Although the relative proportion of marrow cell subclasses in HFD mice remained mostly unchanged, total marrow cell numbers tell a different story. Indeed, HFD mice had a significantly increased number of nucleated cells in the bone marrow starting at day 90 compared with lean controls. This increase in total marrow nucleated cells continued through day 184 of the study (Fig. 3) and represented a 20–25% increase in total cell numbers in marrow at each time point. This finding translated into increased numbers of cell subclasses in HFD mice compared with controls (Fig. 4). In particular, total marrow lymphocyte number showed the largest increases in total numbers in HFD mice, with increases of ∼40% at day 90 and thereafter (Fig. 4A). Marrow granulocytes and monocytes were elevated in total number in HFD mice but were not significant except for at the earliest time point (day 33) (Fig. 4 B and C). Of note, Fig. 3 shows a drop in marrow cell numbers in lean mice between days 33 and 60. This drop is believed to be an artifact of the experimental model, because modest variations in cell counts are often observed between different groups of mice.
Fig. 3.
Increased numbers of nucleated cells in bone marrow of obese mice. Mice fed the 45% fat diet (○) showed increased bone marrow cell numbers compared with mice fed the 10% fat diet (●). Data shown are the total numbers of nucleated marrow cells per two femurs. n = 7–8 mice per group. *P < 0.05, **P < 0.01.
Fig. 4.
Total numbers of different bone marrow cell subclasses. Mice fed a 45% fat diet (white bars) had increased numbers of lymphocytes (CD31+/Ly-6C−/B220+) in their bone marrow compared with mice fed a 10% fat diet (black bars) starting at day 90 on diet (A). Total numbers of (B) marrow granulocytes (CD31−/Ly-6C+/Ly-6G+/Gr-1+) and (C) monocytes (CD31+/Ly-6Chi/Ly-6G−/Gr-1+) were only modestly affected. Data represent total numbers of each marrow cell type in two femurs. n = 7–8 mice per group. *P < 0.05.
The observed increase in total numbers of bone marrow cells represents a very large overall increase given the large size of the skeletal mass in the body. Similar results were observed when the B-cell subpopulations of marrow were analyzed. Increased total marrow cell number translated to significantly increased total numbers of large and small pre-B cells, immature B cells, and mature B cells (Table S1).
Effect of HFD on Thymus and Thymocytes.
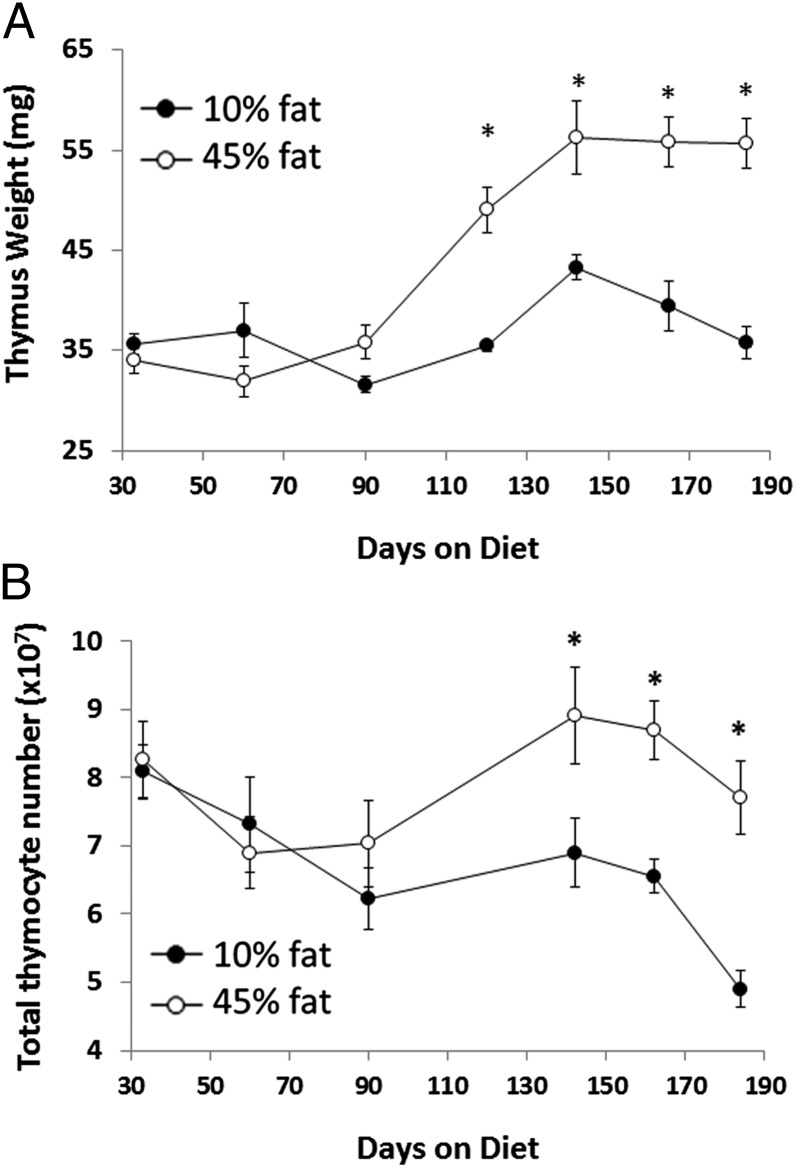
The thymus is often a bellwether of immunological changes, altering its size in response to various stimuli, including stress, infection, nutritional status, etc. (27–29), along with progressive atrophy with aging in both humans and mice (30, 31). Because lymphocyte numbers were significantly elevated in the bone marrow of HFD mice, the state of the thymus was also analyzed. As can be seen in Fig. 5A, thymuses in HFD mice were 30–50% larger in size (wet weight) than thymuses from controls starting at 120 d on diet, and they remained significantly larger for more than 180 d. This size difference was caused by an actual increase in the overall size of thymuses in HFD mice rather than any significant decrease in the size of thymus because of aging among control mice.
Fig. 5.
Thymus size and cell number were elevated in HFD mice. Mice fed a 45% fat diet (○) had increased thymus size (A) and total thymocyte number (B) beyond 120 d on diet compared with mice fed a 10% fat diet (●). n = 7–8 mice per group. *P < 0.05.
Representative time points were chosen for analysis of the thymus for total numbers of cells. As expected based on the lack of change in thymus size up to day 90, no difference was observed in total thymocyte number between HFD mice and controls. However, by days 142, 162, and 184, when thymuses were significantly larger in HFD mice, thymic total cell counts were also 30%, 42%, and 55% higher, respectively (Fig. 5B). Thus, increased thymus size in HFD mice resulted in increased total thymocyte numbers. This finding was also a rather remarkable increase from normal, and it indicates that there is active promotion of lymphopoiesis in both primary tissues.
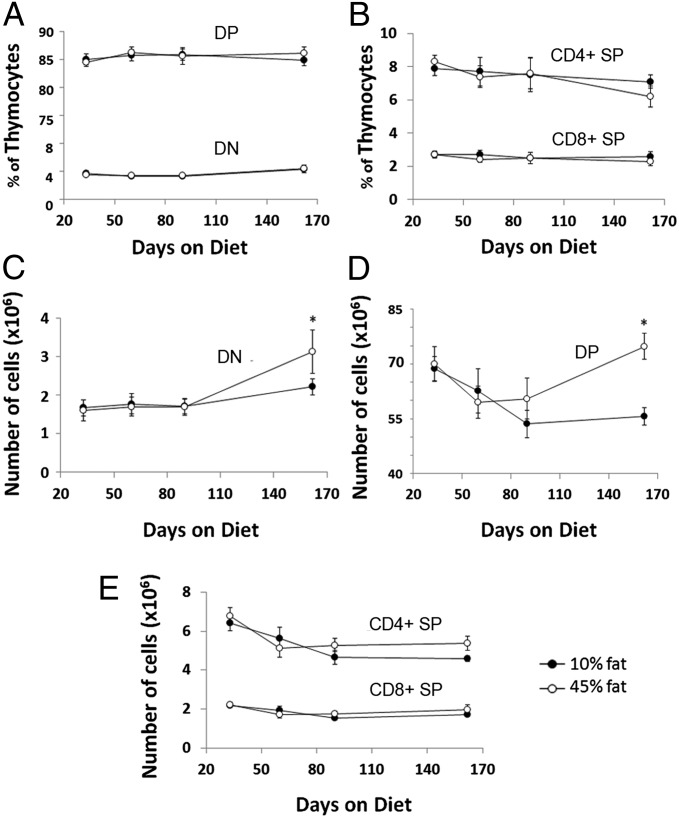
Flow cytometry was then used to determine cellular composition in HFD and control mice. Representative time points were used, namely days 33, 60, and 90 for early time points and day 162 for times when thymus size was larger and bone marrow cell counts were increased in HFD mice compared with controls. The distribution of cells in thymuses from control and HFD mice was not significantly different when analyzing CD4/CD8-labeled subpopulations at any time point tested, including when the thymuses from HFD mice were substantially increased in size (Fig. 6 A and B). Thus, HFD did not significantly alter the distribution of CD4/CD8 cells in mice to include CD4+, CD8+, and CD4+CD8+ subsets.
Fig. 6.
Thymus cellular composition and total numbers in HFD mice. Thymuses from mice fed a 45% fat diet (○) were analyzed by flow cytometry for thymus cell subpopulations and compared with mice fed a 10% fat diet (●). The composition of thymocyte subclasses as defined by CD4 and CD8 expression was not significantly altered by obesity (A and B). When total numbers of thymocytes were assessed, significant increases in (C) DN (CD4−/CD8−) and (D) DP (CD4+/CD8+) subtypes were observed at day 162 on diet, when thymus size was significantly larger in obese mice compared with controls. No significant alterations were seen in total numbers of mature single positive CD4+/CD8− and CD4−/CD8+ thymocytes (E). n = 7–8 mice per group. *P < 0.05.
Double negative (CD4−/CD8−) thymocytes can be divided into four distinct subsets based on CD44 and CD25 expression (named DN1–4, respectively), which comprise sequential early T-cell precursors that make up a minority of the total thymocyte population. The proportion of double negative subpopulations of thymocytes was unchanged in HFD mice throughout the experiment, with the exception of modest changes at day 162 when the proportion of DN3 cells was reduced ∼15%, whereas the proportion of DN4 cells was increased by ∼14% (Fig. S3A). The proportion of DN1 and DN2 subpopulations was not altered by HFD at any time point. (Fig. S3A). These results indicate that only very modest alterations in the proportion of thymocyte subclasses were caused by HFD.
When total numbers of these thymocyte subpopulations were analyzed, no significant differences were observed between HFD and control mice up to day 90 on diet. On day 162, when thymus size was larger in HFD mice, double positive (CD4+/CD8+) and double negative (CD4−/CD8−) thymocytes both exhibited an ∼35% increase in total numbers in HFD mice compared with controls (Fig. 6 C and D). Total numbers of mature CD4+ and CD8+ T cells were not elevated in HFD mice compared with controls at any time point (Fig. 6E).
The total cell counts of double negative thymocyte subpopulations DN1, DN2, and DN4 were also significantly increased in HFD mice by 24%, 31%, and 53%, respectively, at this time point (day 162) (Fig. S3B), whereas the numbers of DN3 thymocytes remained unchanged. These results indicate increases in early stages of T-lymphopoiesis and also fit with the increased total numbers of B cells and their progenitors in marrow. Together, these results show a general increase in lymphopoiesis in primary immune tissues in diet-induced obese mice.
Changes in Leukocytes in Blood Because of HFD.
To determine if increased cell numbers in bone marrow and thymus of HFD mice affected peripheral sites, blood was collected from mice at two different time points (day 142 and day 171); at this time, thymuses were enlarged, and bone marrow cell counts were elevated in obese mice. Analysis of complete blood counts revealed 70–125% increases in total white blood cell counts at both time points as well as 75–120% increases in total blood lymphocyte number (Table 1). In addition, on day 171 when WBC numbers more than doubled, a threefold increase in blood neutrophil numbers was also observed. These results indicate that the changes occurring in the bone marrow and thymus of HFD mice are also manifest in blood, particularly with the observed increases in lymphopoiesis in HFD mice.
Table 1.
Blood differential counts in mice fed HFD
| Diet | Days on diet | WBC (number of cells)* | Neutrophils | Lymphocytes | Monocytes |
| 10% fat | 142 | 4.7 ± 1.2 | 0.70 ± 0.23 | 3.8 ± 0.94 | 0.15 ± 0.05 |
| 45% fat | 142 | 7.9 ± 1.3† | 0.86 ± 0.28 | 6.7 ± 1.2† | 0.26 ± 0.15 |
| 10% fat | 171 | 6.1 ± 2.2 | 0.90 ± 0.39 | 4.8 ± 1.7 | 0.45 ± 0.28 |
| 45% fat | 171 | 13.7 ± 1.5‡ | 2.7 ± 0.63‡ | 10.5 ± 1.8† | 0.45 ± 0.26 |
n = 5 at each time point and diet group.
*Data shown are number of cells (×103) per microliter blood ± SD.
†P < 0.01.
‡P < 0.001.
Expression of Growth Factors and Inflammatory Molecules in Bone Marrow.
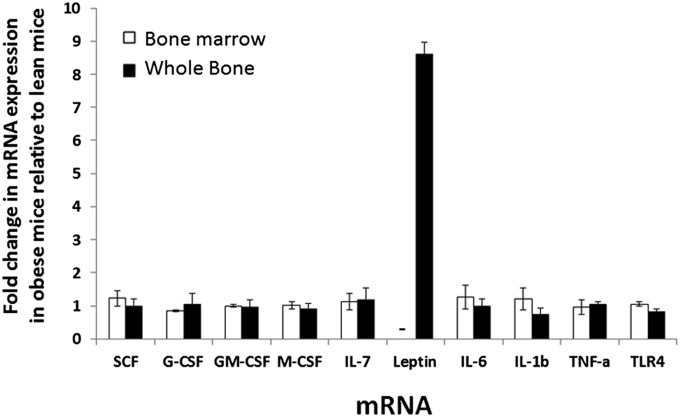
The reason for increased cell counts in bone marrow of HFD mice is not known, but it may have involved increased production of growth factors, cytokines, and proinflammatory molecules that have been shown to have a role in modulating hematopoiesis. To assess this possibility, quantitative RT-PCR was performed on RNA of bone marrow cells from lean and obese mice to determine relative mRNA expression levels of a variety of growth factors and proinflammatory molecules as well as the hormone leptin. Stem cell factor was investigated, because it plays a key role in maintenance of hematopoiesis and expansion of a variety of hematopoietic progenitors (32). Specific growth factors tested included the colony stimulating factors granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), and granulocyte macrophage colony-stimulating factor (GM-CSF), which are known to play a role in the differentiation and proliferation of granulocyte progenitors, monocyte progenitors, and granulocyte–monocyte progenitors, respectively (33, 34). The expression of IL-7 mRNA was investigated because of its role in the development and proliferation of marrow lymphocytes and their progenitors, especially precursor B and T cells (35). Leptin was also a focus of this gene expression analysis because of its known role in hematopoiesis, especially lymphopoiesis and myelopoiesis (16, 36). In addition, the expression of inflammatory factors IL-6, IL-1β, and TNF-α was investigated because chronic inflammation is a characteristic of obesity, and some inflammatory molecules have been implicated in increased granulopoiesis and monopoiesis (6, 28). The expression of pattern recognition receptor Toll-like receptor 4 (TLR4), which plays a role in bacterial endotoxin signaling, was investigated, because TLR signaling has been shown to play a role in myeloid cell differentiation (37). When isolated bone marrow cells were used as source for total RNA, no significant increases were observed in the expression of mRNA of any of the factors tested in HFD mice compared with controls (Fig. 7). Leptin mRNA was not detected in isolated marrow cells. Because isolation of bone marrow cells from bone requires removal of red blood cells and other processing, adipocytes within the bone marrow do not survive the harvesting process. To assess the expression of mRNA from bone marrow containing adipocytes, whole bones (femurs) from control and HFD mice were processed for isolation of RNA, and RT-PCR was performed. Similar to what was observed in isolated bone marrow cells, growth factor and proinflammatory mRNA expression were not changed in whole-bone RNA from HFD mice compared with control mice. In contrast, a more than eightfold increase in leptin mRNA expression was observed in HFD mice compared with controls. These results suggest that leptin plays a role in promoting hematopoiesis in the absence of elevated expression of other growth factors and cytokines.
Fig. 7.
Analysis of expression of various genes in isolated bone marrow cells that would not contain adipocytes because of processing method and whole bone that would contain adipocytes from HFD mice. Total RNA was isolated bone marrow cells (white bars) and whole bone (black bars) and subjected to RT-PCR for relative quantitation of mRNA expression. Data shown represent expression of each mRNA in obese mice relative to expression in control lean mice. Data represent the average of two experiments for bone marrow and whole bone. For marrow, cells from days 142 and 165 on diet were used. For whole bone, femurs from days 157 and 165 were used. n = 8 per group at each time point. –, not detected.
Analysis of Bone Marrow Adipocyte Number.
Bone marrow is also known to contain adipocytes that are the primary producers of leptin. Total RNA from whole bone of HFD mice showed an eightfold increase in leptin mRNA expression compared with whole bone from control mice, whereas isolated marrow cells showed no detectable leptin mRNA expression; therefore, it was hypothesized that an increase in the number of marrow adipocytes might be the cause of the increase in leptin mRNA expression observed in bones of HFD mice. To assess the numbers of adipocytes in bone marrow, femurs were removed and processed for sectioning and immunohistochemical staining, which allows visual identification of adipocyte outlines or ghosts. The adipocytes in marrow were quantified by visual counting. Fig. 8 shows the average number of adipocytes counted per femur section from HFD and control mice. As can be seen in Fig. 8, a significant increase in adipocyte number is seen in HFD mice compared with controls—sixfold increase at day 162 and threefold increase at day 176, indicating that increased leptin mRNA expression in marrow of HFD mice probably resulted from increased adipocyte numbers.
Fig. 8.
Quantification of adipocytes in bone marrow of HFD mice. Femurs from mice fed 10% or 45% fat diet were sectioned and stained with H&E for visualization of adipocytes. Adipocyte number was determined by counting the number of adipocytes per femur section using a light microscope (two sections per animal). Each circle represents one mouse, and black bars indicate mean for that group. n = 7–8 mice per group. *P < 0.05.
Discussion
The collective data herein clearly show that diet-induced obesity in mice substantially increased the number of nucleated cells in the marrow and thymus for the 180-d period studied. This finding alone is in marked contrast to the effects of aging, inflammatory bowel disease, stress, etc., that cause depletions in the primary immune tissues (3, 38, 39). It suggests a complex interplay among the metabolic factors, cytokines, and adipokines that accompany obesity, and therefore, the end result is a promotion of lymphopoiesis and myelopoiesis. Because the marrow is located throughout the skeletal mass of the body, the observed 20–25% increase in marrow nucleated cells in HFD mice by 90 d is a noteworthy rise in cellular mass (Fig. 3). Within the marrow, total cell numbers of the erythrocytic, monocytic, and granulocytic lineages increased, but proportions were unchanged (Figs. 2 and 3). Only cells of the lymphocytic lineages increased 10–18% above normal (Fig. 2). This trend was mimicked by the greatly enlarged weight of the thymus of HFD mice (30–50% increase), which included increases in the number of CD4+CD8+ thymocytes and mature single positive (SP) cells compared with controls (Figs. 5 and 6). In sum, these substantial changes in the primary immune tissues add to the growing list of immune dysregulation known to be associated with obesity.
Thus, the question arises as to why obesity, with its numerous metabolic changes and presumed state of inflammation, would promote hematopoiesis and enlarge the marrow and thymus. A mundane explanation would be that a 40% increase in body weight in HFD mice by day 90, rising to a 50–60% increase beyond day 120, requires a larger blood and lymphatic volume to support this extra weight (Fig. 1). This result, no doubt, is part of the needed change, but a substantial amount of the excess weight found in obese mice includes epididymal fat, which was from two- to fourfold greater than the amount found over time in the lean controls (Fig. S1). In Fig. S1 as well as Figs. 1 and 4, one can see that control mice also underwent modest increases over time in body weight and adipose tissue weight as well. However, these increases in lean mice did not translate to increases in the total number of lymphocyte, monocyte, or granulocyte precursors in their marrow. Moreover, over time, there was an actual decline in the lymphocyte lineages in the marrow and thymus of lean mice—the very lineages that increased the most in HFD mice (Figs. 4 and 5). Finally, the distribution of WBC was changed in HFD mice (Table 1), being almost double the normal number in mice on day 171 with double the proportion of lymphocytes. These increases are out of proportion, being beyond what might be needed for an expanding blood volume, and they correlate with observed increases in WBC in obese human subjects, including the morbidly obese (23, 24).
Another explanation for the cellular increase in the primary tissues may be the two- and fourfold increases in adipose tissue that include substantial increases in adipocytes, which are the primary producers of leptin. Fig. 1C shows a fivefold increase in serum leptin in HFD mice over controls, with up to 100 ng/mL being similar to the increase noted in obese humans. Moreover, there were also three- and sixfold increases in the adipocytes found in the marrow itself by day 162 (Fig. 8). This finding translated into a more than eightfold increase in the expression of leptin mRNA in the bones of HFD mice by day 162 compared with control mice (Fig. 7). Whereas leptin was initially known for its role in controlling food intake, it has been shown to have many immunological properties, with many cells of the immune system bearing leptin receptors.
Recent studies have shown that, in the obese ob/ob mouse, leptin promoted hematopoietic processes to include lymphopoiesis and myelopoiesis when recombinant exogenous leptin was used to rejuvenate the depleted primary tissues of the leptin-deficient ob/ob mice (16). The ob/ob mice produce a nonfunctioning leptin and despite their large size, had atrophied thymuses and depleted hematopoietic compartments (16, 28). Compared with same-aged lean controls, the lymphocytic lineages were depleted by 60%, with 40% and 25% depletions noted in the granulocytic and monocytic compartments, respectively (16, 28); 7 d of provision of recombinant leptin nearly restored the monocytic and granulocytic marrow compartments, whereas 12 d of supplement were required to rejuvenate lymphopoiesis. Others have noted accelerated thymopoiesis in ob/ob mice provided leptin (28). Moreover, the provision of exogenous leptin protected mice from thymic atrophy when subjected to starvation or glucocorticoid treatment (40, 41).
Although many mature cells of the immune system are known to have leptin receptors, there is disagreement over whether thymocytes have significant numbers of them (42, 43). Studies of whole marrow indicate the presence of leptin receptors, but reliable quantitative information on the expression of these receptors on early lineages of cells is not yet available. Even less information is available on whether early cell lineages become leptin-resistant. Interestingly, neutrophils from morbidly obese subjects adequately performed key functions, such as phagocytosis, chemotaxis, and production of superoxide anion, despite being in an environment with high levels of leptin and inflammatory cytokines (25). How high levels of leptin impact immune defense in the obese may well vary among classes of cells.
Interestingly, the enhanced number of WBC precursors in marrow and mature versions circulating in the blood does not translate into better immune defense. Whereas it was recently found that neutrophils even in morbidly obese patients could readily carry out phagocytosis, chemotaxis, and production of superoxide (25), this finding is not normal for obese subjects. More infections, poorer responses to influenza vaccination, and increased production of inflammatory cytokines by T cells and macrophages have been noted in obese humans and mouse models (14, 44). Thus, enhanced numbers of hematopoietic and thymic cells could be benign, or they could contribute to increased malfunction and inflammation. How these observations fit into the big picture of immune status in obesity awaits additional understanding of the status of the immune system as a whole.
One emerging problem is the variance in outcomes from laboratories essentially testing the same immune functions, which is clearly noted in a recent review (14). Within the human population, not enough consideration is given to how variance in body mass index, diabetic status, age, and sex may impact outcomes. With regard to mouse models, there is growing evidence that the feeding of 45% fat vs. 60% fat generates variances in outcomes. It is well-known that a 60% fat diet creates an intensified state of hyperlipidemia, causing a much higher degree of fatty liver compared with a 45% fat diet (45). In addition, discrepancies between 45% and 60% fat diets have been observed in mice when it comes to weight gain, insulin resistance, and fat mass in various mouse models (21, 46, 47). There is evidence that 60% fat diets may also alter observed immune outcomes as well. In experiments where control C57BL/6 mice were fed chow (soy- or alfalfa-based) with 4.5% fat and others were fed a casein-based dietary formulation containing 60% fat, obese mice after 13 mo (390 d) on the diet showed an increase in perithymic fat, with reduced numbers of thymocyte subsets and reduced numbers of peripheral naïve T cells (48). These changes were not noted at 3 mo (90 d) like in our study (Fig. 5). Whether the subsequent decline in thymic size and cell numbers was a product of aging, the HFD, or both is not clear (48). It, however, might be of benefit to the field if the 45% fat diet was used with the complementary low-fat control diet (both with casein as protein source) to provide more unified data to generate a model more analogous to high-fat human nutrition.
In sum, obesity based on the DIO mouse model used herein seems to be among the growing list of diseases and conditions that alter marrow and thymic function. Given the level of proinflammatory cytokines and metabolic changes that accompany obesity, it was somewhat surprising to find enhanced numbers of WBC precursors in marrow and thymus and WBC in blood of HFD mice. Although not the only factor, the large increases in leptin in the blood and bone have a high probability of contributing to the observed results. It is acknowledged that leptin may not have been the only contributing factor and may simply stand in for other factors that accelerated lymphopoiesis and myelopoiesis. Additional experiments are required to address this matter and determine if there are any deleterious effects on the host created by the increased output of the marrow and thymus.
Materials and Methods
Mice and Diet.
Male C57BL/6 mice (4 wk) were purchased from Jackson Labs and maintained according to guidelines approved by the University Laboratory Animal Research Committee at Michigan State University in a facility maintained at 25 °C with 12-h light and dark cycles. Starting at ages 6–7 wk, weight-matched mice were fed either a low-fat diet (control; 10% mixed fats) or HFD (45% mixed fats) ad libitum. Diets were purchased from Research Diets (10% mixed fats Diet D12450B and 45% mixed fats Diet D12451). Food was weighed, and diet was replaced two times per week. Mice were weighed one time per week.
Harvesting and Processing of Tissues.
Mice were anesthetized with isoflurane, blood was collected by cardiac puncture, and serum was collected for additional analysis. In some experiments, blood was transferred to EDTA microtainers (BD Biosciences) for analysis of total blood cell counts. Bone marrow was harvested from both femurs of each mouse into Harvest Buffer (HBSS; Invitrogen), 10 mM Hepes, 4 mM sodium bicarbonate, and 4% heat-inactivated FBS (pH 7.2; Atlanta Biologicals) as described previously (42). After red blood cell lysis, bone marrow cells were resuspended in Label Buffer (HBSS, 10 mM Hepes, 4 mM sodium bicarbonate, 2% heat-inactivated FBS, 0.15% sodium azide, pH 7.2) for counting and flow cytometry analysis. Thymuses were removed, weighed, and processed by mincing and passing through a 100-gauge stainless steel screen. Thymocytes were washed and resuspended in Label Buffer for counting and flow cytometry analysis as previously described (10). Epididymal fat was removed and weighed at each time point.
Determination of Serum Leptin.
Serum leptin was determined using the mouse Leptin Quantikine ELISA kit (R&D Systems).
Flow Cytometry.
All antibodies were from eBiosciences except where indicated. For analysis of major classes of cells within the bone marrow, the antibodies biotinylated rat anti-CD31 (clone ER-MP12; AbD Serotec) and FITC-conjugated rat anti–Ly-6C (clone ER-MP20; AbD Serotec) were used. Five bone marrow subpopulations were identified: erythrocytes (CD31−/Ly-6C−), lymphocytes (CD31+/Ly-6C−), granulocytes (CD31−/Ly-6Cmed), monocytes (CD31+/−/Ly-6Chi), and mixed progenitors (CD31+/Ly-6Cmed). To verify bone marrow subpopulations, antibodies allophycocyanin (APC)-conjugated Ter119, R-phycoerythrin (PE)-conjugated rat anti-CD45R/B220, PE-conjugated rat anti–Ly-6G, and PE-conjugated rat anti–Ly-6G and Ly-6C (Gr-1) were used to identify erythrocytes, lymphocytes, and myeloid cells, respectively, as described previously (8, 26) (Fig. S2).
Composition of B-cell lineages in the marrow was analyzed using CD43-FITC, CD45R-PerCP-Cy5.5, IgM-PE, and biotinylated IgD detected with streptavidin-PECy5 as previously described (8, 49). Gr-1–APC was used to detect and eliminate myeloid cells from analysis. Single-color positive and three-color isotype samples were used for fluorochrome spectral compensation and correction for false-positive staining. All B220+, Gr-1− cells were gated as B lineage-restricted cells. Pro-B cells were defined as CD43highIgM−, Pre-B cells were defined as CD43neg-lowIgM−, and immature–mature B cells were defined as CD43neg-lowIgM+. CD19 was used to distinguish between Pre–Pro-B (CD19−) and late Pro-B cells (CD19+). Forward scatter (FSC) was used to distinguish between early and late Pre-B cells. IgD was used to distinguish between immature (IgD−) and mature B cells (IgD+) (8, 49).
For labeling, bone marrow cells (1 × 106) in Label Buffer were incubated with antibody for 25 min on ice. Biotinylated antibodies were detected by the addition of streptavidin–PE-Cy5 conjugate in a second incubation step.
Thymocytes (1 × 106) in Label Buffer were labeled with anti-CD4 (PE-Cy5–conjugated) and anti-CD8 (FITC-conjugated) to determine major subpopulations of thymocytes. To determine subsets of double negative (DN; CD4−/CD8−) thymocytes, cells were labeled with anti-CD25 (PE-Cy5–conjugated), anti-CD44 (FITC-conjugated), c-kit (PE-conjugated), anti-CD4 (PE-Cy7–conjugated), and anti-CD8–conjugated (PE-Cy7) antibodies. Cells were gated on CD4−/CD8− cells and delineated as follows: DN1, CD44+/CD25−/c-kit+; DN2, CD44+/CD25+/c-kit+; DN3, CD44−/CD25−/c-kit−; DN4, CD44−/CD25+/c-kit−.
Isotype control antibodies used were PE-conjugated rat IgG2a, biotinylated rat IgG2a, FITC-conjugated rat IgG2a, biotinylated rat IgG2b, PE-conjugated rat IgG2b, and APC-conjugated rat IgG2b.
After labeling, cells were kept in 1.5% formaldehyde in PBS (pH 7.2–7.4) until ready for analysis. Flow cytometry was performed on either an FACS Vantage flow cytometer or an LSRII flow cytometer (BD) using a 488-nm laser. Data were analyzed using WinList 6 software (Verity Software House).
Isolation of Whole-Bone RNA, Bone Marrow RNA, and Quantitative RT-PCR.
Isolation of bone marrow cells results in the total loss of marrow adipocytes. Because adipocytes in marrow express certain genes that may play a role in hematopoiesis, total RNA was isolated from both whole bone (with adipocytes) and marrow cells (without adipocytes). Mouse femurs were snap-frozen and crushed, and cells were lysed in TRIzol Reagent (Invitrogen). Separately isolated bone marrow cells were lysed in TRIzol Reagent, with total RNA isolated according to the manufacturer’s recommendations, and they were further purified using the RNeasy Mini Kit (Qiagen) contaminating genomic DNA removed by RNase-free DNase set (Qiagen) for on-column DNase digestion according to the manufacturer’s recommendations.
Reverse transcription of total bone marrow RNA was performed using the AffinityScript QPCR cDNA Synthesis kit (Agilent), and quantitative PCR was performed using the Brilliant II QPCR Master Mix (Agilent) according to the manufacturer’s recommendations. Primers are described in Table S2 and were verified to have efficiency of >90% as estimated by a standard curve of purified amplicon. Analysis of quantitative PCR data was done using the −ΔΔCT method of comparison.
Immunohistochemical Staining of Bone.
Femurs were fixed in 10% formalin for 24 h and then transferred to 70% ethanol. Fixed samples were decalcified in EDTA, embedded in paraffin, and sectioned at 5 μm. Slides were stained with H&E, and adipocytes were enumerated by visual inspection.
Statistics.
Data were analyzed using the Student t test. Statistical significance was set at P < 0.05. Unless otherwise stated, means ± SEM are reported. The number of mice per treatment group was n = 7–8 unless otherwise stated. All experiments were performed two or more times unless noted.
Supplementary Material
Acknowledgments
We thank Gerald Dekker, Brent Campbell, and Zachary Pena for assistance with mouse feeding and weighing. This work was supported by the College of Natural Science and AgBioResearch at Michigan State University.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205129109/-/DCSupplemental.
References
- 1.Hardy RR, et al. B-cell commitment, development and selection. Immunol Rev. 2000;175:23–32. [PubMed] [Google Scholar]
- 2.Rolink AG, Schaniel C, Andersson J, Melchers F. Selection events operating at various stages in B cell development. Curr Opin Immunol. 2001;13:202–207. doi: 10.1016/s0952-7915(00)00205-3. [DOI] [PubMed] [Google Scholar]
- 3.Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- 4.Lodish H, Flygare J, Chou S. From stem cell to erythroblast: Regulation of red cell production at multiple levels by multiple hormones. IUBMB Life. 2010;62:492–496. doi: 10.1002/iub.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueda Y, Cain DW, Kuraoka M, Kondo M, Kelsoe G. IL-1R type I-dependent hemopoietic stem cell proliferation is necessary for inflammatory granulopoiesis and reactive neutrophilia. J Immunol. 2009;182:6477–6484. doi: 10.4049/jimmunol.0803961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraker P, King L. Changes in regulation of lymphopoiesis and myelopoiesis in the zinc-deficient mouse. Nutr Rev. 1998;56:S65–S69. doi: 10.1111/j.1753-4887.1998.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 8.King LE, Fraker PJ. Zinc deficiency in mice alters myelopoiesis and hematopoiesis. J Nutr. 2002;132:3301–3307. doi: 10.1093/jn/132.11.3301. [DOI] [PubMed] [Google Scholar]
- 9.Trottier MD, Newsted MM, King LE, Fraker PJ. Natural glucocorticoids induce expansion of all developmental stages of murine bone marrow granulocytes without inhibiting function. Proc Natl Acad Sci USA. 2008;105:2028–2033. doi: 10.1073/pnas.0712003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King LE, Osati-Ashtiani F, Fraker PJ. Apoptosis plays a distinct role in the loss of precursor lymphocytes during zinc deficiency in mice. J Nutr. 2002;132:974–979. doi: 10.1093/jn/132.5.974. [DOI] [PubMed] [Google Scholar]
- 11.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 12.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 13.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 14.Karlsson EA, Beck MA. The burden of obesity on infectious disease. Exp Biol Med (Maywood) 2010;235:1412–1424. doi: 10.1258/ebm.2010.010227. [DOI] [PubMed] [Google Scholar]
- 15.Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137:1236–1243. doi: 10.1093/jn/137.5.1236. [DOI] [PubMed] [Google Scholar]
- 16.Claycombe K, King LE, Fraker PJ. A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc Natl Acad Sci USA. 2008;105:2017–2021. doi: 10.1073/pnas.0712053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faggioni R, et al. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: Role of tumor necrosis factor alpha and IL-18. Proc Natl Acad Sci USA. 2000;97:2367–2372. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett BD, et al. A role for leptin and its cognate receptor in hematopoiesis. Curr Biol. 1996;6:1170–1180. doi: 10.1016/s0960-9822(02)70684-2. [DOI] [PubMed] [Google Scholar]
- 19.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 22.Ye J, Gimble JM. Regulation of stem cell differentiation in adipose tissue by chronic inflammation. Clin Exp Pharmacol Physiol. 2011;38:872–878. doi: 10.1111/j.1440-1681.2011.05596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nanji AA, Freeman JB. Relationship between body weight and total leukocyte count in morbid obesity. Am J Clin Pathol. 1985;84:346–347. doi: 10.1093/ajcp/84.3.346. [DOI] [PubMed] [Google Scholar]
- 24.Pratley RE, Wilson C, Bogardus C. Relation of the white blood cell count to obesity and insulin resistance: Effect of race and gender. Obes Res. 1995;3:563–571. doi: 10.1002/j.1550-8528.1995.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 25.Trottier MD, Naaz A, Kacynski K, Yenumula PR, Fraker PJ. Functional capacity of neutrophils from class III obese patients. Obesity. 2011 doi: 10.1038/oby.2011.354. [DOI] [PubMed] [Google Scholar]
- 26.de Bruijn MF, et al. Distinct mouse bone marrow macrophage precursors identified by differential expression of ER-MP12 and ER-MP20 antigens. Eur J Immunol. 1994;24:2279–2284. doi: 10.1002/eji.1830241003. [DOI] [PubMed] [Google Scholar]
- 27.Fraker PJ, King LE. A distinct role for apoptosis in the changes in lymphopoiesis and myelopoiesis created by deficiencies in zinc. FASEB J. 2001;15:2572–2578. doi: 10.1096/fj.01-0430rev. [DOI] [PubMed] [Google Scholar]
- 28.Hick RW, Gruver AL, Ventevogel MS, Haynes BF, Sempowski GD. Leptin selectively augments thymopoiesis in leptin deficiency and lipopolysaccharide-induced thymic atrophy. J Immunol. 2006;177:169–176. doi: 10.4049/jimmunol.177.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang SD, Huang KJ, Lin YS, Lei HY. Sepsis-induced apoptosis of the thymocytes in mice. J Immunol. 1994;152:5014–5021. [PubMed] [Google Scholar]
- 30.Dorshkind K, Swain S. Age-associated declines in immune system development and function: Causes, consequences, and reversal. Curr Opin Immunol. 2009;21:404–407. doi: 10.1016/j.coi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]
- 33.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 34.Burgess AW, Metcalf D. The nature and action of granulocyte-macrophage colony stimulating factors. Blood. 1980;56:947–958. [PubMed] [Google Scholar]
- 35.von Freeden-Jeffry U, et al. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–446. [PubMed] [Google Scholar]
- 37.Nagai Y, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aspinall R, Andrew D. Thymic involution in aging. J Clin Immunol. 2000;20:250–256. doi: 10.1023/a:1006611518223. [DOI] [PubMed] [Google Scholar]
- 39.Scollay RG, Butcher EC, Weissman IL. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 40.Howard JK, et al. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trotter-Mayo RN, Roberts MR. Leptin acts in the periphery to protect thymocytes from glucocorticoid-mediated apoptosis in the absence of weight loss. Endocrinology. 2008;149:5209–5218. doi: 10.1210/en.2008-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gruver AL, Ventevogel MS, Sempowski GD. Leptin receptor is expressed in thymus medulla and leptin protects against thymic remodeling during endotoxemia-induced thymus involution. J Endocrinol. 2009;203:75–85. doi: 10.1677/JOE-09-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velloso LA, Savino W, Mansour E. Leptin action in the thymus. Ann N Y Acad Sci. 2009;1153:29–34. doi: 10.1111/j.1749-6632.2008.03973.x. [DOI] [PubMed] [Google Scholar]
- 44.Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol. 2010;184:3127–3133. doi: 10.4049/jimmunol.0903220. [DOI] [PubMed] [Google Scholar]
- 45.Ha S-K, Chae C. Inducible nitric oxide distribution in the fatty liver of a mouse with high fat diet-induced obesity. Exp Anim. 2010;59:595–604. doi: 10.1538/expanim.59.595. [DOI] [PubMed] [Google Scholar]
- 46.Weisberg SP, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang L, et al. Leptin contributes to the adaptive responses of mice to high-fat diet intake through suppressing the lipogenic pathway. PLoS One. 2009;4:e6884. doi: 10.1371/journal.pone.0006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang H, et al. Obesity accelerates thymic aging. Blood. 2009;114:3803–3812. doi: 10.1182/blood-2009-03-213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.