Abstract
Sialyl Lewis x (sLex) and sialyl Lewis a (sLea) glycans are expressed on highly metastatic colon cancer cells. They promote extravasation of cancer cells and tumor angiogenesis via interacting with E-selectin on endothelial cells. Recently, epithelial–mesenchymal transition (EMT) has been noted as a critical phenotypic alteration in metastatic cancer cells. To address the association between sLex/a expression and EMT, we assessed whether sLex/a are highly expressed on colon cancer cells undergoing EMT. Treatment of HT29 and DLD-1 cells with EGF and/or basic FGF (bFGF) induced EMT and significantly increased sLex/a expression resulting in enhanced E-selectin binding activity. The transcript levels of the glycosyltransferase genes ST3GAL1/3/4 and FUT3 were significantly elevated and that of FUT2 was significantly suppressed by the treatment. We provide evidence that ST3GAL1/3/4 and FUT3 are transcriptionally up-regulated by c-Myc with probable involvement of Ser62 phosphorylation, and that FUT2 is transcriptionally down-regulated through the attenuation of CDX2. The contribution of c-Myc and CDX2 to the sLex/a induction was proved to be significant by knockdown or forced expression experiments. Interestingly, the cells undergoing EMT exhibited significantly increased VEGF secretion, which can promote tumor angiogenesis in cooperation with sLex/a. Finally, immunohistological study indicated high E-selectin ligand expression on cancer cells undergoing EMT in vivo, supporting their coexistence observed in vitro. These results suggest a significant link between sLex/a expression and EMT in colon cancer cells and a pivotal role of c-Myc and CDX2 in regulating sLex/a expression during EMT.
Colon cancer is one of the most prevalent cancers worldwide, with more than 1,200,000 new cases and over 600,000 deaths estimated to have occurred in 2008 (1). Although early detection, increased awareness, and developments in treatment have increased complete cure rates especially in some advanced countries, distant metastasis is still a critical event that makes colon cancer a lethal disease. Therefore, novel therapeutic approaches to inhibit metastasis are required.
Sialyl Lewis x (sLex) and sialyl Lewis a (sLea) are E-selectin ligand glycans expressed on the surface of many types of cancer cells, including colorectal, pancreatic, gastric, breast, prostate, and lung cancer (2, 3). These glycans play crucial roles in hematogenous metastasis through interaction with endothelial cells. The most established role is promoting extravasation of cancer cells: circulating cancer cells in blood flow arrest at distant sites by adhering to endothelial cells, which enables their movement out of the vasculature (2, 3). Importantly, the interaction between sLex/a and E-selectin exclusively mediates the adhesion of most epithelial cancer cells to endothelial cells, whereas sLex/a-independent interaction with endothelial ICAM-1 and VCAM-1 mediates the adhesion of nonepithelial malignant cells, such as leukemia and some sarcoma cells, to endothelial cells (4). Another important role of sLex/a in hematogenous metastasis is tumor angiogenesis (3, 5), which can facilitate intravasation and postextravasational proliferation of cancer cells (6–8). In line with these observations, high sLex/a expression levels in colon cancer patients are correlated with poor prognosis (2). Therefore, these glycans are frequently evaluated as tumor markers. Whereas the diagnostic utility of sLex/a has been well established, therapeutic approaches targeting these glycans are not well developed, partly because molecular mechanisms of their expression have been only partially elucidated (9–11).
Recently, epithelial–mesenchymal transition (EMT) has been noted as a critical event in the early step of cancer metastasis (12, 13). It is also notable that EMT is known to be associated with cancer stem cells (14, 15). EMT is defined as a transitional process from epithelial to mesenchymal phenotype, including fibroblast-like morphology, down-regulation of E-cadherin by transcriptional repressors such as SNAIL1, ZEB1, and TWIST, mesenchymal marker expression such as Vimentin, Fibronectin, and N-cadherin, and enhanced cell motility. A variety of EMT inducers have been reported, including TGF-β and receptor tyrosine kinase (RTK) growth factors such as hepatocyte growth factor (HGF), EGF, and basic FGF (bFGF). Although many studies have focused on TGF-β (16), the TGF-β signaling pathway is frequently inactivated in colon cancer due to loss-of-function mutations in TGFBR2 and SMAD genes (17). Therefore, RTK growth factors are likely to figure more heavily than TGF-β in EMT of colon cancer cells. Several clinical studies have suggested the correlation between RTK signaling and metastasis. EGFR was expressed in ∼85% of patients with metastatic colon cancer (18) and its expression level and function in colon cancer cells were correlated with metastatic potential (19, 20). Plasma bFGF levels were significantly higher in patients with metastatic colon cancer than in normal controls, whereas those levels were comparable between patients with nonmetastatic colon cancer and normal controls (21). Sato et al. demonstrated by quantitative RT-PCR that the transcript levels of FGFR1 in colon cancer tissues were significantly higher in patients with liver metastasis than in those without liver metastasis (22).
Despite the significant roles of sLex/a and EMT in cancer metastasis, their association remains unknown. To address this issue, we assessed whether sLex/a is highly expressed on cancer cells undergoing EMT.
Results
Induction of EMT in Colon Cancer Cells by EGF or bFGF.
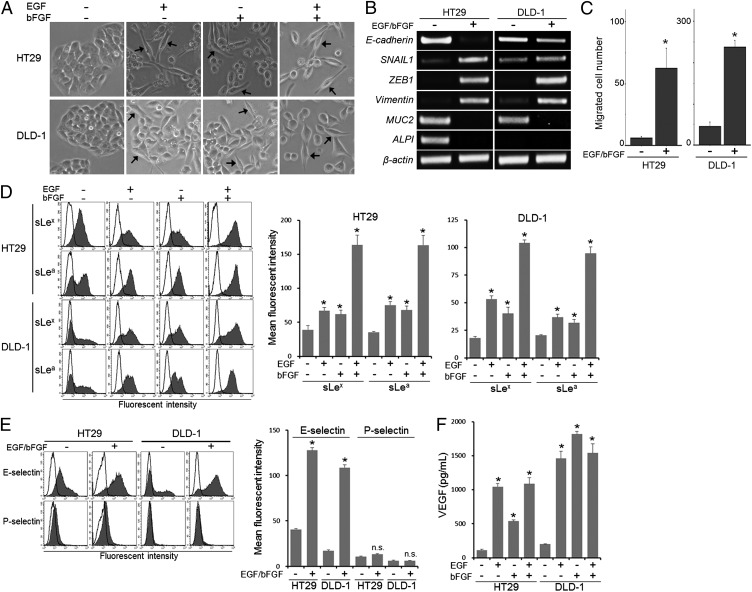
To prepare colon cancer cells undergoing EMT, we treated HT29 and DLD-1 cells with EGF (20 ng/mL) and/or bFGF (10 ng/mL) in serum-deprived medium. Treatment with either EGF or bFGF alone transiently induced a fibroblast-like appearance (Fig. 1A); however, it was very difficult to maintain the cells for further experiments. Treatment with both EGF and bFGF (hereafter EGF/bFGF treatment) permitted better cell survival and induced a fibroblast-like appearance (Fig. 1A). The EGF/bFGF treatment increased the levels of the mesenchymal marker genes SNAIL1, ZEB1, and Vimentin, whereas it reduced the level of E-cadherin and the colon-epithelial differentiation marker genes MUC2 (mucin 2) and ALPI (intestinal alkaline phosphatase) (Fig. 1B). Functionally, the treated cells showed significantly enhanced migration activity (P < 0.05; Fig. 1C). These results indicated that the EGF/bFGF treatment induced EMT in HT29 and DLD-1 cells.
Fig. 1.
Induction of EMT and sLex/a expression in colon cancer cells by EGF or bFGF. (A) HT29 and DLD-1 cells were maintained in culture medium with 10% FBS or in serum-free medium supplemented with EGF (20 ng/mL) and/or bFGF (10 ng/mL). After culture for 7 d, cells were observed under a phase-contrast microscope. (Arrows) Cells exhibiting fibroblastic morphology. (B) Expression of marker genes for EMT and colon-epithelial differentiation was examined by conventional RT-PCR. (C) Cell migration activity was determined with Biocoat Matrigel invasion chambers. (D) Expression levels of sLex/a were determined by flow cytometry. Bold lines, staining control. (E) Selectin-binding activity was determined by flow cytometry. Bold lines, staining control. (F) Culture supernatant VEGF levels were measured by ELISA. (C–F) Statistic analysis was performed in three independent experiments by t test. Error bars, SD; asterisks, P < 0.05 (C), P < 0.01 (D), P < 0.000005 (E), and P < 0.0001 (F) compared with the untreated cells; NS, not significant (P > 0.05).
Induction of sLex/a Expression and E-Selectin Binding Activity in Colon Cancer Cells by EGF or bFGF.
We then evaluated sLex/a expression levels on the cells undergoing EMT by flow cytometry. The results indicated significantly increased sLex/a expression on the EGF/bFGF-treated cells as well as on the cells treated with either factor alone compared with the untreated cells (P < 0.01; Fig. 1D). To test whether the increased sLex/a expression could contribute to the interaction with E-selectin, we examined binding activity of the treated cells to recombinant E-selectin. The cells exhibited significantly enhanced E-selectin binding activity (P < 0.000005), whereas no significant binding activity was detected for P-selectin (P > 0.05; Fig. 1E), which selectively binds to sLex determinants carried on P-selectin glycoprotein ligand 1 (PSGL-1), generally expressed on leukocytes (23). Furthermore, the E-selectin binding activity was significantly inhibited by anti-sLex antibody, anti-sLea antibody, or EDTA (P < 0.000005; Fig. S1), indicating that recombinant E-selectin did bind to the cells through the interaction with sLex/a.
We previously demonstrated that sLex/a expressed on cancer cells promote tumor angiogenesis through interacting with E-selectin on endothelial cells (5). Because E-selectin is known to be induced by VEGF (24), we examined whether VEGF was secreted into the supernatant of the cells treated with EGF and/or bFGF. Results of ELISA indicated that the VEGF level was significantly increased by the treatment (P < 0.0001; Fig. 1F). These results suggest that EGF and/or bFGF can strongly promote angiogenesis synergistically by inducing sLex/a and E-selectin expression on colon cancer cells and endothelial cells, respectively.
Altered Expression of ST3GAL1/3/4, FUT3, and FUT2 Induced by EGF or bFGF.
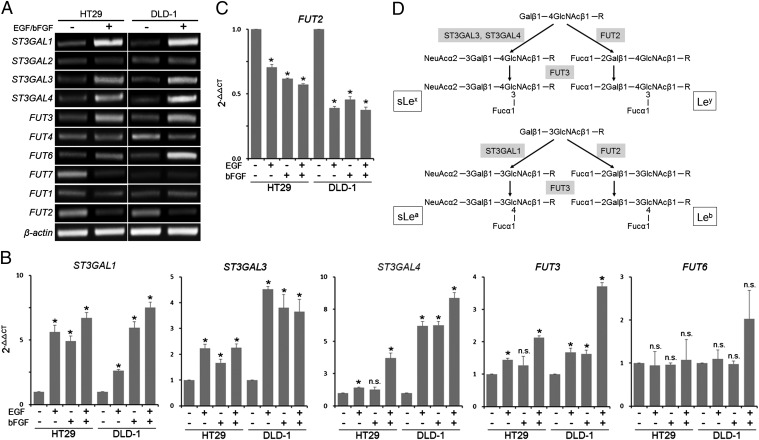
To address the molecular mechanism underlying the EMT-associated sLex/a expression, we focused on the glycosyltransferase genes. Sialyltransferases and fucosyltransferases are essential enzymes for the synthesis of sialic acid and fucose residues of sLex/a, respectively. Screening of the genes involved in the sLex/a synthesis by conventional RT-PCR using primers listed in Table S1 revealed that the levels of ST3GAL1/3/4 and FUT3/6 were increased, whereas that of FUT2 was decreased by the EGF/bFGF treatment of HT29 and DLD-1 cells (Fig. 2A). Quantitation of the expression levels of these genes by real-time RT-PCR using the assays listed in Table S2 indicated that the EGF/bFGF treatment induced significant increases in the ST3GAL1/3/4 and FUT3 levels (P < 0.005; Fig. 2B) and a significant decrease in the FUT2 level (P < 0.00005; Fig. 2C). In addition, these alterations were also induced by treatment with EGF or bFGF alone (Fig. 2 B and C). However, the FUT6 level was not significantly changed by any of the treatment (P > 0.05; Fig. 2B). Therefore, we focused on ST3GAL1/3/4, FUT3, and FUT2 for further experiments. ST3GAL1/3/4 catalyze the addition of N-acetylneuraminic acid (NeuAc) to the nonreducing terminal galactose (Gal) residue of glycans, and FUT3 catalyzes addition of fucose (Fuc) to the N-acetylglucosamine (GlcNAc) residue (Fig. 2D). Therefore, up-regulation of ST3GAL1/3/4 and FUT3 results in increased sLex/a expression. In contrast, FUT2 catalyzes addition of Fuc to the nonreducing terminal Gal, competing with NeuAc addition by sialyltransferases (Fig. 2D). Down-regulation of FUT2 thus contributes to increased sLex/a expression. As expected, quantitative analysis by flow cytometry indicated that EGF/bFGF treatment induced significantly higher increase in the levels of sLex and sLea compared with those of Ley and Leb, respectively (P < 0.01; Fig. S2).
Fig. 2.
Altered glycogene expression induced by EGF or bFGF. (A) Expression of the sialyltransferase and fucosyltransferase genes involved in the sLex/a synthesis was screened by conventional RT-PCR. (B and C) Expression levels of ST3GAL1/3/4, FUT3/6 (B), and FUT2 (C) were determined by quantitative RT-PCR. The mean 2-ΔΔCT values ± SD from three independent experiments are shown. Statistic analysis was performed by t test. Asterisks, P < 0.005 (B) and P < 0.00005 (C) compared with the untreated cells; NS, not significant (P > 0.05). (D) Scheme of the sLex, Ley, sLea, and Leb synthetic pathways showing the roles of ST3GAL1/3/4, FUT3, and FUT2. Note that FUT2 competes with ST3GAL3/4 for sLex synthesis and with ST3GAL1 for sLea synthesis, respectively.
Involvement of c-Myc in the Induction of ST3GAL1/3/4 and FUT3 Expression by EGF/bFGF.
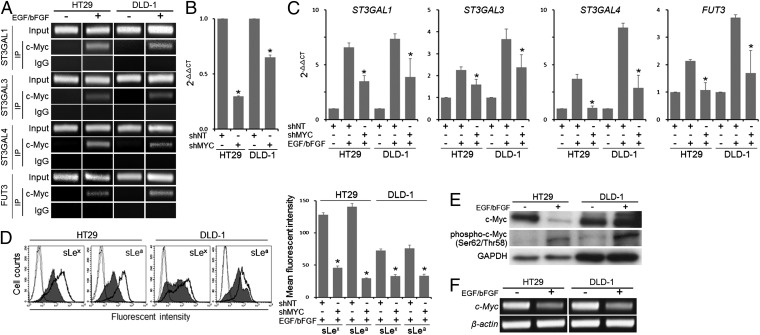
To explore the mechanism of the EGF/bFGF-induced alteration in the glycogene transcription, we next searched for potential transcription factor binding sites in the 5′-regulatory regions of the glycogenes identified above and noticed potential c-Myc binding sites in the promoters of ST3GAL1/3/4 and FUT3 (Fig. S3 A–D). ChIP assays using primers listed in Table S3 indicated increased recruitment of c-Myc to their promoters in the EGF/bFGF-treated cells (Fig. 3A). To determine the role of c-Myc in the sLex/a induction, we performed c-Myc knockdown experiments. Namely, we introduced a c-Myc shRNA-expressing vector into HT29 and DLD-1 cells (Fig. 3B) and treated the cells with EGF/bFGF. Knockdown of c-Myc significantly inhibited the maximal induction of ST3GAL1/3/4 and FUT3 expression (P < 0.05; Fig. 3C). Consequently, the maximal sLex/a induction was also inhibited (P < 0.0005; Fig. 3D). These results suggested a pivotal role of c-Myc in the sLex/a induction through the transcriptional regulation of ST3GAL1/3/4 and FUT3 under the EGF/bFGF treatment. To gain insights into the molecular mechanism by which the EGF/bFGF treatment induced the glycogenes through c-Myc, we performed Western blot analysis. Unexpectedly, the level of total c-Myc was reduced by the treatment in HT29 cells (Fig. 3E) likely caused by a decrease in the transcript level (Fig. 3F). However, the level of phospho–c-MycSer62/Thr58 was strongly enhanced by the treatment both in HT29 and DLD-1 cells (Fig. 3E). It is known that a priming phosphorylation of c-Myc at Ser62 can be followed by phosphorylation at Thr58 by GSK3β (25). Because Western blotting revealed a decrease in the level of total GSK3β and an increase in that of phospho-GSK3βSer9, the inactivated form of GSK3β (Fig. S4), the increase in the phospho–c-MycSer62/Thr58 level most likely reflects the hyperphosphorylation of the Ser62 site, which has been implicated in the enhanced recruitment of c-Myc to the promoter of its target genes as well as in the enhanced transcriptional activity of c-Myc (26, 27).
Fig. 3.
Involvement of c-Myc in the transcriptional regulation of ST3Gal1/3/4 and FUT3. (A) ChIP assays were performed to examine the binding of c-Myc to the 5′-regulatory regions of ST3Gal1/3/4 and FUT3. (B) Effect of c-Myc shRNA (shMYC) on the transcript level of c-Myc was evaluated by quantitative RT-PCR. (C) Effects of shMYC on the expression levels of ST3Gal1/3/4 and FUT3 were examined by quantitative RT-PCR. (D) Expression levels of sLex/a were examined by flow cytometry. Dotted lines, staining control; bold lines, nontarget shRNA (shNT)-introduced cells; filled histogram, shMYC-introduced cells. (E) Levels of c-Myc and phospho–c-MycSer62/Thr58 were determined by Western blotting. (F) Expression of c-Myc was examined by conventional RT-PCR. (B–D) Statistic analysis was performed in three independent experiments by t test. Error bars, SD; asterisks, P < 0.00001 (B), P < 0.05 (C), and P < 0.0005 (D) compared with the shNT-transfected cells (B) or to the shNT-introduced cells treated with EGF/bFGF (C and D).
Involvement of CDX2 in the EGF/bFGF-Induced Transcriptional Suppression of FUT2.
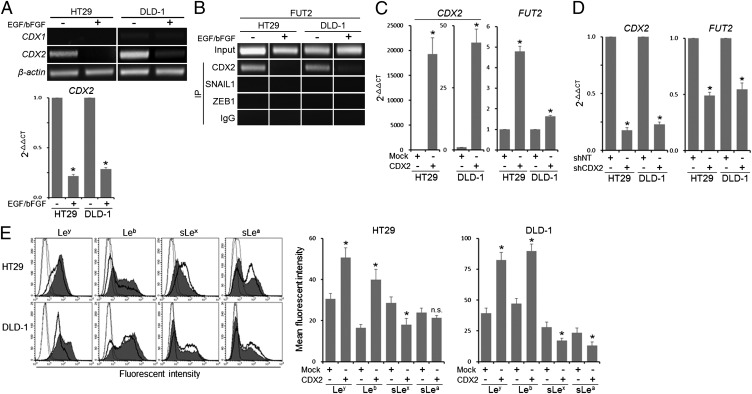
We next examined the mechanism of the EGF/bFGF-induced transcriptional suppression of FUT2. We noticed potential binding sites for CDX1 and CDX2, transcription factors known to regulate several colon-specific genes (28–31), in the 5′-regulatory region of FUT2 (Fig. S3E). Both HT29 and DLD-1 cells showed good levels of CDX2 expression, which were significantly reduced by EGF/bFGF treatment (P < 0.000001; Fig. 4A). ChIP assays revealed that binding of CDX2 to the FUT2 promoter was abolished by the treatment (Fig. 4B). On the other hand, SNAIL1 and ZEB1, EMT-related transcriptional repressors, were not recruited to the promoter (Fig. 4B). To determine the role of CDX2 in the transcriptional regulation of FUT2, we introduced a CDX2 expression vector into HT29 and DLD-1 cells. The cells exhibited significantly elevated FUT2 expression compared with the mock vector-transfected cells (P < 0.0005; Fig. 4C). In contrast, HT29 and DLD-1 cells introduced with CDX2 shRNA showed significantly reduced level of FUT2 (P < 0.0005; Fig. 4D). Furthermore, forced expression of CDX2 elevated Ley/b expression and suppressed sLex/a expression in HT29 and DLD-1 cells (Fig. 4E). These results suggest that the EGF/bFGF-induced down-regulation of CDX2 contributes to the sLex/a induction via suppression of FUT2 transcription.
Fig. 4.
Involvement of CDX2 in the transcriptional regulation of FUT2. (A) Expression of CDX1 and CDX2 was examined by conventional RT-PCR. The transcript level of CDX2 was determined by quantitative RT-PCR. (B) ChIP assays were performed to examine the binding of CDX2, SNAIL1, and ZEB1 to the 5′-regulatory region of FUT2. (C and D) Effect of CDX2 forced expression (C) or CDX2 shRNA (shCDX2; D) on the expression levels of CDX2 and FUT2 were determined by quantitative RT-PCR. (E) Expression levels of Ley/b and sLex/a were examined by flow cytometry. Dotted lines, staining control; bold lines, mock vector-introduced cells; filled histogram, CDX2 expression vector-introduced cells. (A and C–E) Statistic analysis was performed in three independent experiments by t test. Error bars, SD; asterisks, P < 0.000001 (A), P < 0.0005 (C and D), and P < 0.05 (E) compared with the untreated cells (A), to the mock vector-transfected cells (C and E) or to the nontarget shRNA (shNT)-transfected cells (D); NS, not significant (P > 0.05).
E-Selectin Ligand Glycan Expression on Colon Cancer Cells Undergoing EMT in Vivo.
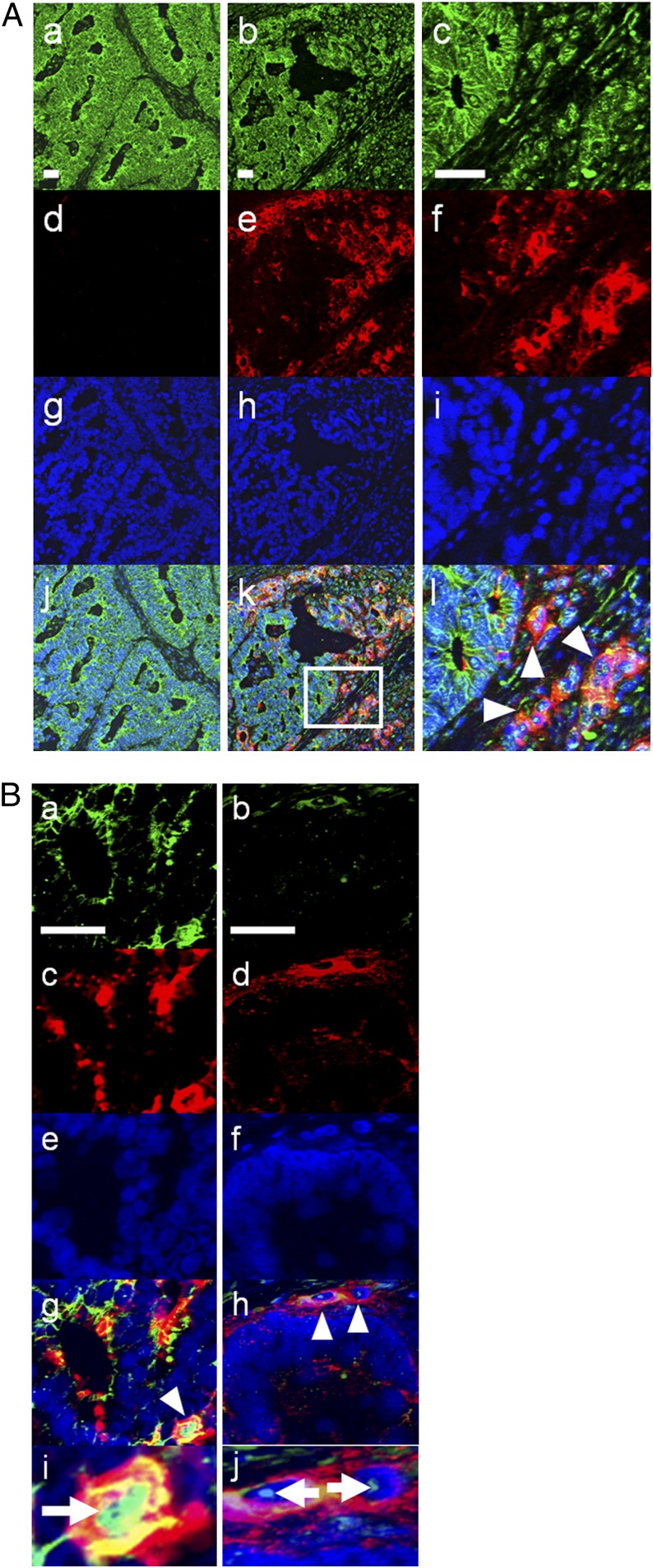
Finally, we examined association between E-selectin ligand glycan expression and EMT in clinial samples by immunohistochemical analysis. We focused on sLea in this experiment, because this glycan is preferentially expressed on cancer cells, whereas sLex is broadly expressed on various normal cells including leukocytes and might complicate the results. We performed double staining with antibodies against sLea and E-cadherin on sections from five colorectal cancer patients. In one section from a 70-y-old male patient with colon cancer, we identified a small area of cancer cells that lacked cell-surface E-cadherin expression at the invasion front (Fig. 5A). Most interestingly, these cancer cells exhibited high sLea expression, whereas cancer cells with cell-surface E-cadherin exhibited no sLea expression (Fig. 5A). Furthermore, double staining with antibodies against SNAIL1 and sLea identified a subset of cancer cells that coexpressed nuclear SNAIL1 and a high level of sLea (Fig. 5B). Similar results were obtained by double staining with antibodies against ZEB1 and sLea (Fig. 5B). These results supported the coincidence of E-selectin ligand expression and EMT observed in vitro.
Fig. 5.
E-selectin ligand glycan expression on colon cancer cells undergoing EMT in vivo. (A) Expression of E-cadherin (green) and sLea (red) was examined on human colon cancer sections by immunohistochemistry. (Left) Region with cancer cells exhibiting high cell-surface E-cadherin expression. (Center) Another region in the same section containing cancer cells without cell-surface E-cadherin expression, a part of which are surrounded by a square (k) and magnified in the Right column. Arrowheads indicate cells with decreased E-cadherin and increased sLea expression on the cell surface. (Blue) Hoechst 33342. (B) Expression of SNAIL1 (a, green), ZEB1 (b, green), and sLea (c and d, red) was examined on sections from the same patient. (e and f) Hoechst 33342. Cells with arrowheads (g and h) are magnified (i and j), with arrows showing nuclear SNAIL1 and ZEB1, respectively. (Scale bars, 50 μm.)
Discussion
The major findings of this study are as follows: (i) sLex/a expression is strongly induced during EMT of colon cancer cells triggered by EGF or bFGF, and (ii) c-Myc and CDX2 play key roles in the sLex/a induction by EGF or bFGF.
Our present results demonstrate that c-Myc contributes to sLex/a expression by transcriptional induction of ST3GAL1/3/4 and FUT3 in the EGF/bFGF-treated cells. Although the detailed mechanism of this glycogene induction by c-Myc remains unclear, we speculate the possible involvement of Ser62 phosphorylation of c-Myc as described above (Fig. 3E). Ser62 of c-Myc is known to be phosphorylated by ERK or cyclin-dependent kinase (CDK) 2 (25–27). The kinase that contributed to this phosphorylation in the EGF/bFGF-treated cells remains to be identified.
In this study, we demonstrated that the transcription of CDX2 was down-regulated by the EGF/bFGF treatment, which resulted in a decrease in the transcript level of FUT2. Although the mechanism underlying the down-regulation of CDX2 by the treatment remains unknown, SNAIL1 may be involved because transcription of CDX2 is known to be repressed by SNAIL1 (32), and SNAIL1 expression was increased by EGF/bFGF (Fig. 1B). Although several lines of evidence indicate that CDX2 is a tumor suppressor (33), the association between CDX2 and metastasis has been unclear. Clinically, Baba et al. reported that the loss of CDX2 expression in colon cancer tissues was significantly correlated with stage IV disease (34). Our present findings may explain at least a part of the mechanisms by which the loss of CDX2 contributes to metastasis.
We previously reported that hypoxia induced sLex/a expression in colon cancer cells (9). In that report, we documented that the transcription of ST3GAL1, FUT7, and UGT1 (UDP-galactose transporter 1), which are all involved in the E-selectin ligand glycan synthesis, was elevated under a hypoxic condition. Hypoxia-inducible factor-1α (HIF-1α) was involved in the induction of these glycogenes. The present study provides additional information on the transcriptional regulation of the sLex/a synthesis-related glycogenes.
Recently, Guan et al. reported a significant association between glycans and EMT, demonstrating that the expression levels of GM2 and Gg4 glycosphingolipids were significantly decreased during TGF-β–induced EMT and that the glucosylceramide synthase inhibitor EtDO-P4 induced EMT (35). From their subsequent observations demonstrating that exogenous addition of Gg4 abrogated the EMT process and that Gg4 was closely associated with E-cadherin and β-catenin, they proposed that Gg4 may be important in maintaining epithelial cell membrane organization (36). Together with these reports, our present study demonstrates a drastic alteration in the glycan expression during the EMT process. It remains an interesting issue whether the alteration in sLex/a expression further promotes the EMT process as the alteration in the Gg4 expression did.
We demonstrated that sLea was preferentially expressed on the cancer cells with low expression of membranous E-cadherin, nuclear SNAIL1, and nuclear ZEB1 in a clinical sample of colon cancer. These results are consistent with the coincidence of sLex/a expression and EMT observed in vitro and suggest that these glycans may serve as a good marker of EMT in cancer patients. Our results indicate that RTK signaling activation confers both EMT and sLex/a expression on cancer cells. As RTK signaling pathways provide effective therapeutic targets, these glycans may serve as surrogate markers for evaluating therapeutic effects of such modalities.
Materials and Methods
Additional information can be found in SI Materials and Methods.
Human colon cancer cell lines, HT29 and DLD-1, were maintained in DMEM and RPMI1640 medium (Invitrogen), respectively, supplemented with 10% (vol/vol) FBS. For treatment with EGF and/or bFGF, recombinant human EGF (Sigma; 20 ng/mL) and/or recombinant human bFGF (Sigma; 10 ng/mL) were added to the serum-free medium with recombinant human insulin (Sigma; 25 μg/mL), human holo-transferrin (Sigma; 100 μg/mL), putrescine dihydrochloride (Sigma; 10 μg/mL), and sodium selenite (Sigma; 5 ng/mL).
Supplementary Material
Acknowledgments
This work was supported in part by Grants-in-Aid for Young Scientists (B) 20790583 and 22790774 from the Japan Society for the Promotion of Science, Grants-in-Aid 24590364 and (on priority areas) 23112520 from the Ministry of Education, Culture, Sports, Science and Technology, Grants-in-Aid for the Third-Term Comprehensive Ten-Year Strategy for Cancer Control from the Ministry of Health and Welfare, and a grant from Uehara Memorial Foundation, Japan.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111135109/-/DCSupplemental.
References
- 1.Jemal A, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Kannagi R. Carbohydrate-mediated cell adhesion involved in hematogenous metastasis of cancer. Glycoconj J. 1997;14:577–584. doi: 10.1023/a:1018532409041. [DOI] [PubMed] [Google Scholar]
- 3.Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 2004;95:377–384. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takada A, et al. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993;53:354–361. [PubMed] [Google Scholar]
- 5.Tei K, et al. Roles of cell adhesion molecules in tumor angiogenesis induced by cotransplantation of cancer and endothelial cells to nude rats. Cancer Res. 2002;62:6289–6296. [PubMed] [Google Scholar]
- 6.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 7.Tien YW, et al. Tumor angiogenesis and its possible role in intravasation of colorectal epithelial cells. Clin Cancer Res. 2001;7:1627–1632. [PubMed] [Google Scholar]
- 8.Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- 9.Koike T, et al. Hypoxia induces adhesion molecules on cancer cells: A missing link between Warburg effect and induction of selectin-ligand carbohydrates. Proc Natl Acad Sci USA. 2004;101:8132–8137. doi: 10.1073/pnas.0402088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyazaki K, et al. Loss of disialyl Lewis(a), the ligand for lymphocyte inhibitory receptor sialic acid-binding immunoglobulin-like lectin-7 (Siglec-7) associated with increased sialyl Lewis(a) expression on human colon cancers. Cancer Res. 2004;64:4498–4505. doi: 10.1158/0008-5472.CAN-03-3614. [DOI] [PubMed] [Google Scholar]
- 11.Yusa A, Miyazaki K, Kimura N, Izawa M, Kannagi R. Epigenetic silencing of the sulfate transporter gene DTDST induces sialyl Lewisx expression and accelerates proliferation of colon cancer cells. Cancer Res. 2010;70:4064–4073. doi: 10.1158/0008-5472.CAN-09-2383. [DOI] [PubMed] [Google Scholar]
- 12.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radisky DC, LaBarge MA. Epithelial-mesenchymal transition and the stem cell phenotype. Cell Stem Cell. 2008;2:511–512. doi: 10.1016/j.stem.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Ikushima H, Miyazono K. TGFbeta signalling: A complex web in cancer progression. Nat Rev Cancer. 2010;10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Pasche B. TGF-beta signaling alterations and susceptibility to colorectal cancer. Hum Mol Genet. 2007;16(Spec No 1):R14–R20. doi: 10.1093/hmg/ddl486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Normanno N, et al. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009;6:519–527. doi: 10.1038/nrclinonc.2009.111. [DOI] [PubMed] [Google Scholar]
- 19.Radinsky R, et al. Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells. Clin Cancer Res. 1995;1:19–31. [PubMed] [Google Scholar]
- 20.Goldstein NS, Armin M. Epidermal growth factor receptor immunohistochemical reactivity in patients with American Joint Committee on Cancer Stage IV colon adenocarcinoma: Implications for a standardized scoring system. Cancer. 2001;92:1331–1346. doi: 10.1002/1097-0142(20010901)92:5<1331::aid-cncr1455>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.George ML, Tutton MG, Abulafi AM, Eccles SA, Swift RI. Plasma basic fibroblast growth factor levels in colorectal cancer: A clinically useful assay? Clin Exp Metastasis. 2002;19:735–738. doi: 10.1023/a:1021322201816. [DOI] [PubMed] [Google Scholar]
- 22.Sato T, et al. Overexpression of the fibroblast growth factor receptor-1 gene correlates with liver metastasis in colorectal cancer. Oncol Rep. 2009;21:211–216. [PubMed] [Google Scholar]
- 23.McEver RP, Cummings RD. Perspectives series: Cell adhesion in vascular biology. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100:485–491. doi: 10.1172/JCI119556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim I, et al. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276:7614–7620. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 25.Sears R, et al. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000;14:2501–2514. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benassi B, et al. c-Myc phosphorylation is required for cellular response to oxidative stress. Mol Cell. 2006;21:509–519. doi: 10.1016/j.molcel.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Hydbring P, et al. Phosphorylation by Cdk2 is required for Myc to repress Ras-induced senescence in cotransformation. Proc Natl Acad Sci USA. 2010;107:58–63. doi: 10.1073/pnas.0900121106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dang DT, Mahatan CS, Dang LH, Agboola IA, Yang VW. Expression of the gut-enriched Krüppel-like factor (Krüppel-like factor 4) gene in the human colon cancer cell line RKO is dependent on CDX2. Oncogene. 2001;20:4884–4890. doi: 10.1038/sj.onc.1204645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto H, Bai YQ, Yuasa Y. Homeodomain protein CDX2 regulates goblet-specific MUC2 gene expression. Biochem Biophys Res Commun. 2003;300:813–818. doi: 10.1016/s0006-291x(02)02935-2. [DOI] [PubMed] [Google Scholar]
- 30.Chan CW, et al. Gastrointestinal differentiation marker Cytokeratin 20 is regulated by homeobox gene CDX1. Proc Natl Acad Sci USA. 2009;106:1936–1941. doi: 10.1073/pnas.0812904106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kakizaki F, et al. CDX transcription factors positively regulate expression of solute carrier family 5, member 8 in the colonic epithelium. Gastroenterology. 2010;138:627–635. doi: 10.1053/j.gastro.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 32.Gross I, et al. The intestine-specific homeobox gene Cdx2 decreases mobility and antagonizes dissemination of colon cancer cells. Oncogene. 2008;27:107–115. doi: 10.1038/sj.onc.1210601. [DOI] [PubMed] [Google Scholar]
- 33.Guo RJ, Suh ER, Lynch JP. The role of Cdx proteins in intestinal development and cancer. Cancer Biol Ther. 2004;3:593–601. doi: 10.4161/cbt.3.7.913. [DOI] [PubMed] [Google Scholar]
- 34.Baba Y, et al. Relationship of CDX2 loss with molecular features and prognosis in colorectal cancer. Clin Cancer Res. 2009;15:4665–4673. doi: 10.1158/1078-0432.CCR-09-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan F, Handa K, Hakomori SI. Specific glycosphingolipids mediate epithelial-to-mesenchymal transition of human and mouse epithelial cell lines. Proc Natl Acad Sci USA. 2009;106:7461–7466. doi: 10.1073/pnas.0902368106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guan F, Schaffer L, Handa K, Hakomori SI. Functional role of gangliotetraosylceramide in epithelial-to-mesenchymal transition process induced by hypoxia and by TGF-β. FASEB J. 2010;24(12):4889–4903. doi: 10.1096/fj.10-162107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.