Abstract
The nonselective cation channel transient receptor potential canonical (TRPC)5 is found predominantly in the brain and has been proposed to regulate neuronal processes and growth cones. Here, we demonstrate that semaphorin 3A-mediated growth cone collapse is reduced in hippocampal neurons from TRPC5 null mice. This reduction is reproduced by inhibition of the calcium-sensitive protease calpain in wild-type neurons but not in TRPC5−/− neurons. We show that calpain-1 and calpain-2 cleave and functionally activate TRPC5. Mutation of a critical threonine at position 857 inhibits calpain-2 cleavage of the channel. Finally, we show that the truncated TRPC5 predicted to result from calpain cleavage is functionally active. These results indicate that semaphorin 3A initiates growth cone collapse via activation of calpain that in turn potentiates TRPC5 activity. Thus, TRPC5 acts downstream of semaphorin signaling to cause changes in neuronal growth cone morphology and nervous system development.
Keywords: hippocampus, TRP channel, calcium signaling, neuronal development
A multitude of signals regulate development of the nascent mammalian nervous system. Secreted and contact-mediated extracellular cues may be transduced to the interior of the cell by surface receptors, initiating both short and long-term changes in cellular physiology. Proper formation of the nervous system requires axons, dendrites, and even the soma of neurons to accurately traverse great distances and connect with specific targets. Such movements require spatially and temporally regulated expression of extracellular guidance cue molecules by the surrounding tissues or distant targets that bind to surface receptors and attract or repel the developing neuron (1, 2).
Several members of the semaphorin class of secreted guidance peptides cause intracellular calcium elevations upon binding to their cognate receptors (3, 4), but how they effect this change is unclear. Transient receptor potential (TRP) proteins, a family of calcium-permeable nonselective cation channels, are widespread in the nervous system and affect neuronal development and nervous system function (5). In particular, studies of TRP canonical (TRPC)5 suggest that it influences the growth of neuronal processes (axons or dendrites). TRPC5 mRNA is found at high levels in several brain regions, including the hippocampus, olfactory bulb, amygdala, and cerebellum, and may also be detected diffusely throughout the cortex (6, 7). siRNA-mediated knockdown or pharmacologic inhibition of this channel has been reported to both stimulate and inhibit neurite sprouting and extension, depending upon the cell type and assays used (8–10). In addition, the TRPC5 null mouse displays deficits in dendritic development in the cerebellum (11) and abnormal amygdalar function (12). Little is known about normal modes of TRPC5 activation, although G protein-coupled receptor (GPCR-Gαq) signaling (13) and other molecules (14–19) strongly potentiate TRPC5 current.
Semaphorin (sema)3A is a secreted guidance peptide that binds to the neuropilin-1/plexin A1 receptor complex and may cause either attraction, repulsion, or collapse of neuronal growth cones depending upon the intracellular milieu (20–22). Multiple filopodia on growth cones at the tip of neurites are thought to sense cues that guide the extension of axons and dendrites (23). One current model proposes that sema3A signaling activates the MAPK family of kinases; these kinases directly phosphorylate and activate calpain-2 (24). Calpains have numerous proteolytic targets in the synapse, processes, and soma of neurons, including ion channels and receptors (25). We investigated molecular mechanisms that might link neuropilin–plexin receptor activation to TRPC5 gating. We demonstrate that TRPC5 and calpain influence sema3A signaling in cultured hippocampal neuronal growth cones. We also demonstrate that calpains cleave and activate TRPC5. We propose that calpains activate TRPC5 downstream of sema3A signaling to contribute to growth cone collapse and neuronal development.
Results
TRPC5 and Calpain Are Critical to Sema3A-Induced Growth Cone Collapse.
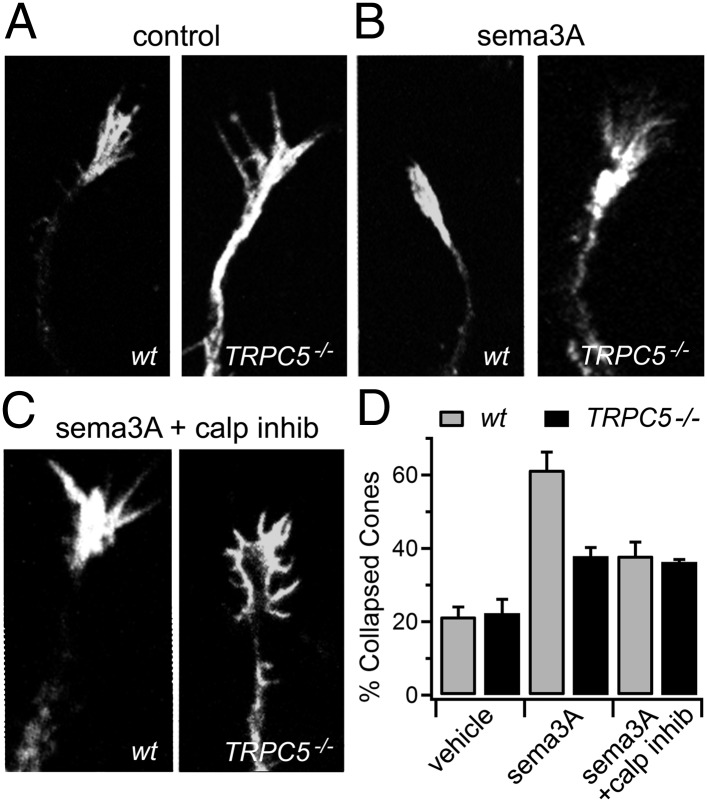
We first compared representative growth cone collapse frequency at the neurite tips of cultured hippocampal neurons from WT and TRPC5 null (Trpc5−/−) mice (Fig. 1 A and D). WT and Trpc5−/− neurons had comparable levels of collapse (21 ± 3% vs. 22 ± 4%). Sema3A treatment (1 nM for 5 min) significantly increased the proportion of collapsed growth cones to 61 ± 5% in WT neurons, but to only 38 ± 2% in Trpc5−/− neurons (Fig. 1 B and D). This represents a significant (P = 0.007) reduction in the ability of sema3A to collapse growth cones in Trpc5−/− neurons.
Fig. 1.
TRPC5 knockout and calpain inhibition reduce sema3A-induced hippocampal growth cone collapse. (A) Untreated, cultured hippocampal neuronal growth cones from WT and TRPC5 null (Trpc5−/−) mice, fixed and stained with the actin-binding peptide phalloidin conjugated to Alexa Fluor 568. (B) Growth cones from neurons treated for 5 min with 1 nM sema3A before fixation. (C) Growth cones from neurons preincubated with the calpain inhibitors calpain inhibitor III (5 μM) and calpeptin (10 μM) for 30 min and then treated with sema3A. (D) Growth cones from the indicated genotypes and treatments were counted and the collapsed fraction quantified (three coverslips from two separate experiments; n = 98–100 cones per condition). Ambiguous growth cones not closely resembling the examples presented were excluded from analysis. P values in text are calculated using Student t test.
The downstream targets of the sema3A receptor complex of neuropilin-1/plexin A1 have not been fully elucidated. Recent reports suggest that sema3A can activate the calcium-sensitive cysteine protease, calpain, via direct phosphorylation by MAPK-family kinases (24). To determine whether the residual sema3A response we observed in Trpc5−/− neurons was attributable to calpain, we also preincubated WT and Trpc5−/− neurons with the cell-permeant calpain inhibitors calpeptin (10 μM) and calpain inhibitor III (5 μM) for 30 min and then treated those neurons with sema3A (Fig. 1 C and D). Preincubation with calpain inhibitors diminished the effect of sema3A on WT neurons, reducing the collapsed fraction from 61% to 38 ± 4% (P = 0.012 compared with sema3A alone). However, calpain inhibition did not further reduce the effect of sema3A on Trpc5−/− neuronal growth cones; the collapsed fraction remained nearly constant at 36 ± 1% (P = 0.98 compared with sema3A alone). These results suggest that calpain and TRPC5 function in the same pathway downstream of sema3A signaling.
Calpains Activate TRPC5 Channels.
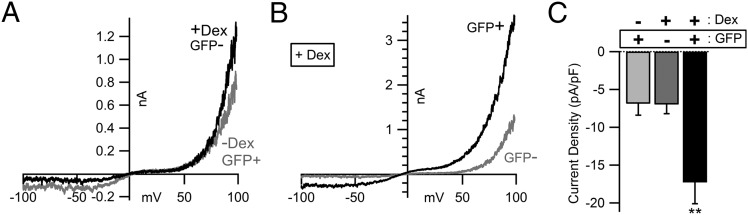
Calpains cleave and alter the activity of several ion channels, receptors, and synaptic proteins (26). The ubiquitous proteases calpain-1 (μ-calpain) and calpain-2 (m-calpain) have the highest expression levels in brain. Calpain-2 may be activated by phosphorylation at serine 50; mutation of this serine to glutamic acid creates a constitutively active protease (27). We tested whether coexpression of constitutively active calpain-2 (S50E) could alter basal TRPC5 channel activity in a heterologous expression system. Calpain-2 S50E (large subunit; under the control of a dexamethasone-inducible promoter), the calpain small subunit (CAPNS1), and eGFP were cotransfected into HEK cells stably expressing mouse TRPC5 (mTrpc5). Dexamethasone-treated GFP-positive cells expressing calpain-2 S50E exhibited a 2.5-fold increase in basal current compared with uninduced, GFP-positive or induced, GFP-negative cells (Fig. 2 A and B); the whole-cell current density at −100 mV shortly after break-in was −17 ± 3 pA/pF from induced, GFP-positive cells, but only −7 ± 2 pA/pF from uninduced, GFP-positive cells (P = 0.004) and −7 ± 1 pA/pF from induced, GFP-negative cells (P = 0.003; Fig. 2C).
Fig. 2.
Coexpression of constitutively active calpain-2 S50E with TRPC5 increases basal channel currents. (A and B) Representative whole-cell current–voltage (I-V) relationships from HEK cells stably expressing TRPC5 cotransfected with calpain-2 S50E under the control of a dexamethasone-inducible promoter, CAPNS1, and eGFP as a marker. −Dex, uninduced; +Dex, dexamethasone-treated cells; GFP−, GFP-negative cells; GFP+, GFP-positive cells. The pipette contained 5 mM HEDTA and 5 μM free Ca2+ to accentuate basal TRPC5 currents. The bath contained the standard 2 mM Ca2+ extracellular solution. I-V relationships were obtained shortly after break-in (<1 min). Both traces in B are from dexamethasone-treated cells. (C) Quantification of whole-cell current density at −100 mV from uninduced, GFP-positive (light gray column; n = 10), induced but GFP-negative (dark gray column; n = 10), and induced, GFP-positive (black column, n = 10) cells. **P < 0.01 (Student t test).
Next, we determined whether purified calpain-1 and calpain-2 could activate heterologously expressed TRPC5 channels. Calpain-1 requires micromolar Ca2+ concentrations for activation in vitro (1–20 μM), whereas calpain-2 requires near millimolar concentrations (0.3–0.8 mM) (25). Thus, we first used pipette solutions with free Ca2+ within or above these ranges to activate purified calpains. Because calpains are large proteins (∼110 kDa) and do not readily diffuse from the pipette into the cell during whole-cell patch clamp, we applied these purified calpains to the intracellular surface of excised inside-out patches from HEK cells stably expressing mTRPC5. Because TRPC5 is sensitive to intracellular calcium (18), we used high calcium solutions (5 μM free Ca2+ buffered with 5 mM (2-Hydroxyethyl)ethylenediaminetriacetic acid (HEDTA) for calpain-1; 2 mM unbuffered Ca2+ for calpain-2) throughout the experiment to first establish a baseline level of channel activity. Using a fast perfusion system, we then rapidly transitioned the patches into a solution stream containing purified calpain-1 or calpain-2.
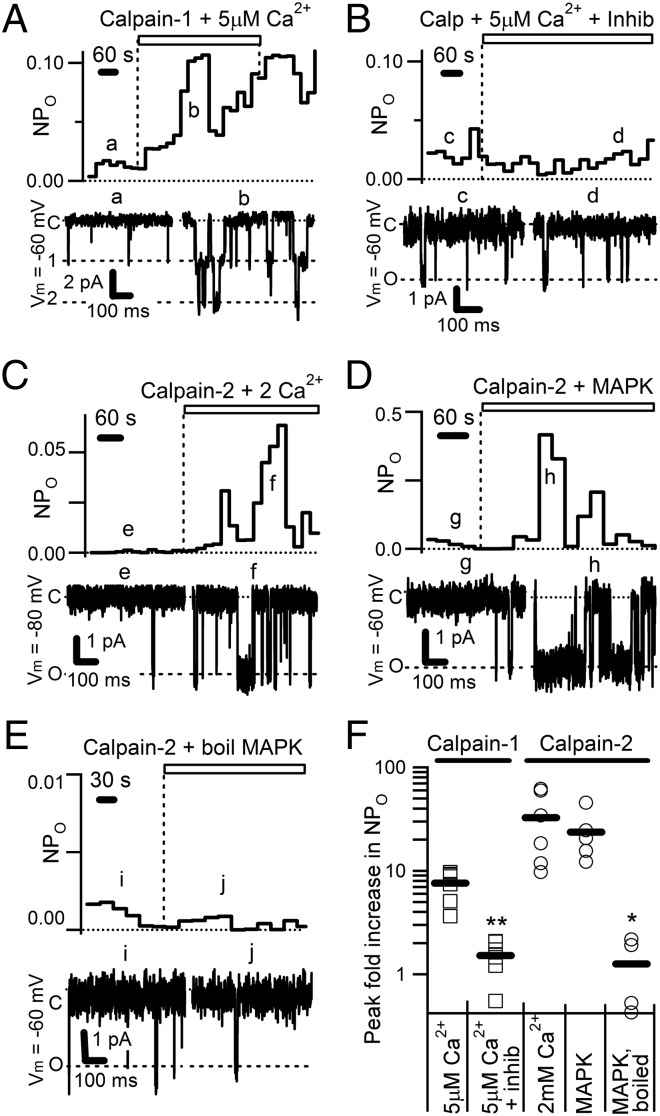
Both calpain-1 and calpain-2 induced a significant increase in TRPC5 single-channel activity, with activity increasing to a peak over several minutes (Fig. 3 A–D). Channel activity, as represented by NPO (number of channels times the open probability of a channel) was collected in 30-s bins for each patch. Calpain-1 increased average peak NPO by 7 ± 1-fold; calpain-2 increased channel activity 33 ± 10-fold (Fig. 3 A and C). Perfusion of a solution containing calpain-1 and a protease inhibitor mixture including the cysteine protease inhibitor E-64 prevented activation (1.5 ± 0.2-fold peak increase; P = 0.0003 compared with calpain-1 or P = 0.009 to calpain-2 alone; Fig. 3 B and F). Average single-channel conductance observed for TRPC5 was 39.5 ± 0.5 pS. Importantly, we observed no activity of channels with a conductance similar to that of TRPC5 in response to calpain-1 or calpain-2 perfusion onto patches from naive HEK cells. The peaks and troughs in NPO (e.g., Fig. 3A, Upper) represent burst-like channel activity, as the channel enters a long series of open or closed states. Because there is significant overlap between the substrates of calpain-1 and calpain-2 (26), it is not surprising that both proteases activate TRPC5.
Fig. 3.
Purified calpains activate TRPC5 channels in excised patches. Average single channel conductance observed for TRPC5 was 39.5 ± 0.5 pS (n = 20). (A, Upper) Activity (NPO) of TRPC5 channels in a representative inside-out excised patch from a HEK cell stably expressing mTRPC5, activated by 15 μg/mL purified calpain-1 (indicated by dashed line and open bar), collected in 30-s bins. (A, Lower) Currents at the time points indicated by the small letters above showing individual TRPC5 channel openings at the indicated voltage. C, closed (no open channels); O or 1, one open channel; 2, two open channels. We used an intracellular solution with 5 mM HEDTA and 5 μM free Ca2+ throughout the experiment. (B) Same as A, except that we perfused purified calpain-1 plus a protease inhibitor mixture including the cysteine protease inhibitor E-64 (14 μM). (C) Same as A, except that we perfused purified calpain-2 onto the patch and used an intracellular solution containing 2 mM Ca2+. (D) Same as A, except that we perfused calpain-2, which had been phosphorylated in vitro with constitutively active MAPK1, onto the patch in an intracellular solution containing 100 nM free Ca2+. (E) Same as D, except that MAPK1 was boiled before incubation with calpain-2 before the experiment. (F) Quantification of the effects of purified calpains on TRPC5. Fold increase is the ratio between the NPO of the peak bin following treatment, divided by the average NPO preceding treatment. Each patch is indicated by its own marker; the solid bar is the mean. *P < 0.05; **P < 0.01 ( Student t test; n = 6–8 for each experiment). Burst activity shown as rising and falling NPO is characteristic of TRPC5.
MAPK1 has been reported to directly phosphorylate and activate calpain-2 (27). Therefore, we incubated purified, constitutively active MAPK1 in the presence of ATP and Mg2+ with calpain-2 in vitro. Perfusion of MAPK1-phosphorylated calpain-2 in 100 nM free Ca2+ [1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)] onto the intracellular surface of TRPC5 channel-containing inside-out excised patches increased channel activity by 24 ± 5-fold (Fig. 3 D and F). Boiling MAPK1 before incubation with calpain-2 increased channel activity by only 1.3 ± 0.4-fold (Fig. 3 E and F; P = 0.012 compared with nonboiled MAPK1-treated calpain-2). Finally, we recorded semaphorin responses from cultured hippocampal neurons using whole-cell patch clamp at the soma but observed no large immediate current changes in response to 1 nM semaphorin application; this may be attributable to space clamp limitations in these cells and the localization of TRPC5 to distal processes.
Calpain-1 and Calpain-2 Cleave TRPC5 in Vitro.
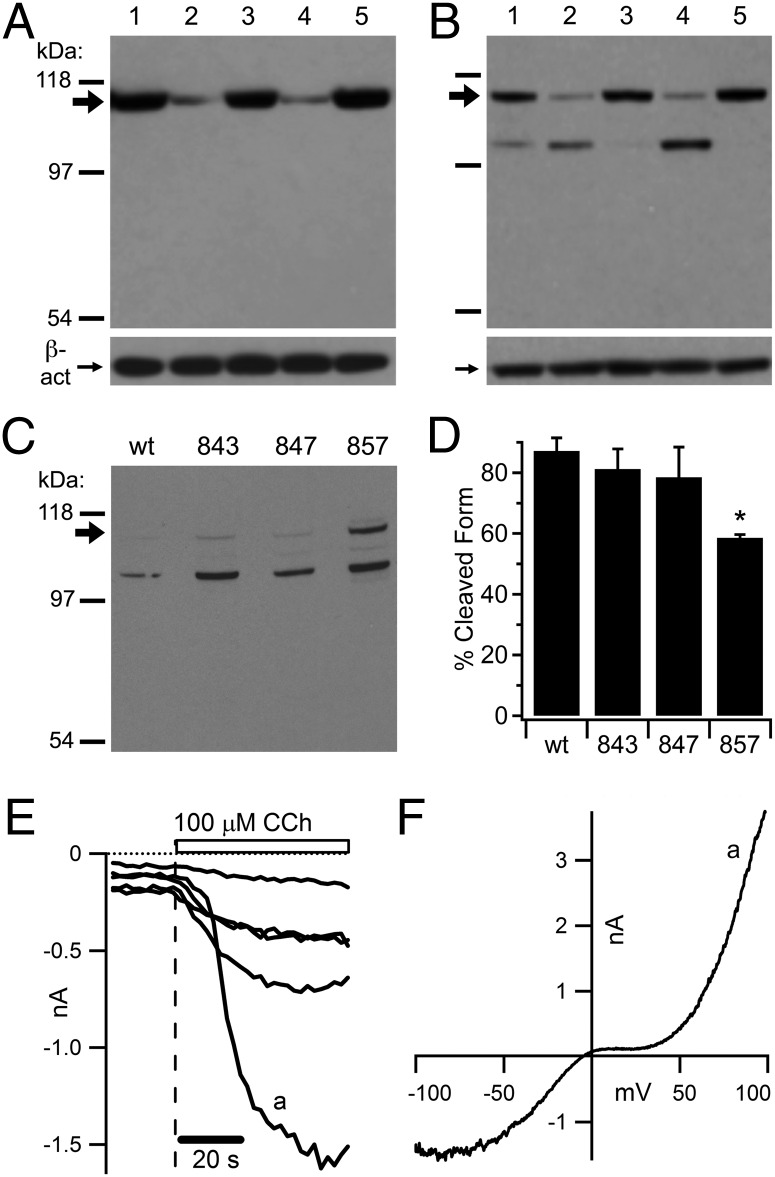
Thus far, the experiments have shown that calpain strongly potentiates TRPC5 current but do not identify the substrate. Next, we subjected TRPC5 to an in vitro calpain cleavage assay, using a HEK cell line stably expressing mouse (m)TRPC5 fused to an N-terminal HA tag. We homogenized cells with a brief sonication and then subjected these homogenates to digestion by calpain-1 or calpain-2. We performed Western blot analysis on the homogenates and probed the blots with an anti-TRPC5 antibody (Fig. 4A; directed against the extreme C terminus) or an anti-HA antibody (Fig. 4B). The TRPC5-directed antibody revealed a substantial reduction in full-length TRPC5 protein in those homogenates digested with calpain-1 and calpain-2; this reduction was prevented by the inclusion of calpain inhibitors. The HA-directed antibody produced the same result but also revealed a cleavage product ∼15 kDa smaller than full-length TRPC5 in those homogenates digested with calpain-1 and calpain-2. We also observed this product, although to a lesser extent, in untreated homogenates, likely reflecting endogenous calpain digestion activated by the high-calcium digestion buffer. These results indicate that calpain cleaves an ∼15-kDa segment of the C terminus of TRPC5, leaving an ∼100-kDa N-terminal fragment that includes the HA tag and the transmembrane regions responsible for ion conduction. We attempted to perform the same analysis on homogenates from cultured hippocampal neurons, but the expression level of TRPC5 proved too low for accurate detection in Western blots.
Fig. 4.
Calpain-1 and calpain-2 cleave TRPC5 at its C terminus, generating a fragment likely to retain ion channel function, and this cleavage is significantly reduced by mutation of threonine 857 to alanine. (A and B) We briefly sonicated HEK cells stably expressing an N-terminally HA-tagged mTRPC5 protein and treated the resulting homogenates with 5 μg/mL purified calpain-1 (lane 2), calpain-1 plus 10 μM calpeptin and 5 μM calpain inhibitor III (lane 3), 5 μg/mL purified calpain-2 (lane 4), or calpain-2 plus the aforementioned inhibitors (lane 5) in a 2 mM Ca2+ buffer (control untreated; lane 1). (A, Upper) Western blot of calpain-treated HA-TRPC5 probed with a TRPC5 C-terminal monoclonal antibody. The large arrow below the 118-kDa marker indicates full-length HA-TRPC5 protein. (B, Upper) Western blot of calpain-treated HA-TRPC5 probed with an HA monoclonal antibody. (A, Lower; and B, Lower) A monoclonal antibody to β-actin shows that equal protein was loaded. (C) Western blot of calpain-2 treated homogenates from HEK cells transfected with an HA-TRPC5 wild-type construct or constructs containing the point mutations F843A, G847A, and T857A probed with anti-HA. The large arrow below the 118-kDa marker indicates full-length HA-TRPC5 protein. (D) Quantification of TRPC5 present in the cleaved form from the blot in C. *P < 0.05 (Student t test; n = 2 blots from independent experiments). (E and F) A modified plasmid containing mTRPC5 truncated at N854 was coexpressed with the muscarinic-type 1 receptor (M1R) and eGFP in HEK cells. (E) Representative whole-cell currents recorded at −100 mV; application of the M1R agonist carbachol at 100 μM (vertical dashed line and open bar) significantly increased current density in five GFP-positive cells. The pipette contained a solution with 5 mM HEDTA and 5 μM free Ca2+ to potentiate any TRPC5-like currents. (F) Current–voltage relationship (I-V) taken at the point indicated by a in E.
A recent study proposed an in silico method for predicting the location of calpain digestion sites within putative substrates (28). Using the full-length mTRPC5 amino acid sequence, this model predicted calpain cleavage of the channel in the C terminus at threonine 857 (T857). Because our results from Fig. 4 A and B indicated that cleavage occurred close to the C terminus, a cleavage site at T857 seemed plausible. Additionally, a molecular mass prediction model (expasy.org/tools/pi_tool.html) suggested that the large fragment remaining after calpain digestion would be ∼13 kDa smaller than full-length, which agrees with our results from Fig. 4 A and B. We tested this prediction by mutating T857 to alanine and quantified the ratio of cleaved to uncleaved HA-TRPC5. After treatment of homogenates with calpain-2 (Fig. 4C), 88% of wild-type TRPC5 was detected in the cleaved state, but only 58% of TRPC5 T857A was cleaved (Fig. 4D; P = 0.045), suggesting that this residue resides within the calpain recognition site. TRPC5 channels with two unrelated mutations (F843A and G847A) were cleaved ∼80% by calpain-2, similar to WT. The amount of cleavage inhibited by mutation of T857A (∼30%) suggests the possibility of either additional calpain cleavage sites or incomplete disruption of the local tertiary structure recognized by the active site of the protease. To confirm that a fragment of TRPC5 generated by cleavage near T857 could function as an ion channel, we generated a modified TRPC5 protein truncated at asparagine 854. Using whole-cell patch clamp, we confirmed that this truncated channel was still functional by coexpressing it with the muscarinic type-1 G protein coupled receptor (M1R) in HEK cells (Fig. 4 E and F). The truncated channel produced constitutive TRPC5-like current in all cells analyzed (−10 ± 1 pA/pF at −100 mV); carbachol significantly increased the average current density to −62 ± 13 pA/pF (P = 0.005).
Discussion
The primary finding of this work is that TRPC5, a calcium-permeant ion channel, and calpain, a calcium-activated protease, contribute to sema3A-mediated growth cone collapse. Taking into account what is known about semaphorin signaling, we propose a model in which sema3A binds to the neuropilin-1/plexin A1 receptor complex, activating MAPK that in turn phosphorylates and activates calpain (29). Calcium flowing into the cell through the cleaved potentiated TRPC5 leads to growth cone collapse.
Calcium influx has been linked to changes in growth cone morphology, collapse, and neurite outgrowth in a number of different pathways (30–32), such as those involving Ca2+/calmodulin-dependent protein kinase (CaMK)II (33), calcineurin, and Rho-Rho kinase (4). Semaphorin signaling has been shown to cause calcium influx in both a neuronal-derived cell line (3) and native neuronal growth cones (4). Other TRP channels have also been implicated in growth cone collapse, such as TRP vanilloid (TRPV)1 (34). Although the connection between in vitro growth cone collapse and in vivo axon path-finding is not entirely clear, the collapse assay nevertheless provides evidence that functionally links semaphorin-dependent signals with calpain and TRPC5. Our data agree with and extend similar studies on sema3A, calpain, calcium, and TRP channel function to define a mechanism for the regulation of growth cones.
Site-directed mutagenesis indicates that threonine 857 of TRPC5 is likely to represent one, but not necessarily the only, cleavage site of the channel. Alternatively, mutagenesis at this position may not fully disrupt the tertiary structure recognized by the protease; such observations are not uncommon (28). There is significant overlap in calpain substrates between family members, so it is possible that other calpains may activate TRPC5 as well (26). The data in Fig. 3 suggest that calpain-2 induces a larger increase in channel activity (NPO) than calpain-1, but this may result from changes in basal channel activity attributable to the differing calcium concentrations used. The data also indicate a much larger relative increase in TRPC5 current following calpain cleavage in excised patches than that observed from cotransfection of calpain-2 S50E with TRPC5. However, this discrepancy is likely attributable to either desensitization (suggested by the data in Fig. 3 C and D) or down-regulation of calpain-2-cleaved TRPC5 channels. Genetic titration of calpain in transgenic mice might shed more light on some of these issues and on the links between semaphorin signaling, calpain, TRPC5, and growth cone morphology. Unfortunately, deletion of the calpain-2 gene from mice is embryonically lethal (25). Intriguingly, genetic deletion of TRPC5 leads to irregularities in dendrite formation and brain function, supporting a role for the channel in neuronal development (12).
A number of groups have published seemingly incongruous reports of TRPC5 exerting both positive and negative effects on neuritogenesis and development. Previous work from our laboratory indicated that TRPC1 was excluded from distal processes but may form heteromeric channels with TRPC5 in and near the soma (8). A recent study suggests that TRPC1 exerts a positive effect, whereas TRPC5 negatively regulates neurites (35). Therefore, we suggest that TRPC1/TRPC5 heteromeric channels, primarily located near the soma and proximal processes, might promote neuritogenesis, whereas TRPC5 homomeric channels present in distal processes respond to inhibitory signals, such as sema3A. Another possibility is that TRPC5 could serve as a common calcium influx pathway downstream of the neuropilin-1/plexin A1 receptor complex and that other changes in the intracellular milieu, such as cyclic nucleotide levels, determine the sign (positive or negative) of sema3A and TRPC5 signaling. Intriguingly, although inhibition of calpain in TRPC5−/− neurons did not change the overall growth cone collapse rate, the appearance of growth cones from this condition was more delicate, with less intense actin staining and many small filopodia (although not always arranged in the bulb-like structure shown in Fig. 1C). This might suggest calpain-independent effects of TRPC5 on growth cones or compensation of calpain and TRPC5 for one another in a common signaling pathway.
Sema3A−/− mice exhibit nerve defasciculation (36), abnormal patterning in the olfactory bulb (37), mistargeting within the hippocampus (38), and several other embryonic abnormalities that resolve by birth (39). TRPC5−/− mice also display abnormalities in nervous system development, but similarly to sema3A−/− mice, these effects are not lethal. It should be noted that TRPC5 is expressed at the highest levels in hippocampus and olfactory bulb (40, 41), two of the regions implicated in sema3A function. Direct and specific block of these two molecules in postnatal mice could reveal more details about their functions. Unfortunately, although inhibitors of sema3A have been reported (42), no specific blocker of TRPC5 is available. In addition its influence on brain development, TRPC5 might also be a target for nerve regeneration therapies; intriguingly, inhibition of sema3A improved regenerative responses and recovery in a model of spinal cord injury (43). Regenerating neurons do establish new growth cones (44), and if TRPC5 is present as a negative regulator of their development, pharmacologic inhibition of TRPC5 may enhance CNS regeneration.
Materials and Methods
Growth Cone Analysis.
Primary hippocampal neurons from P1 TRPC5−/− mice and WT littermates were prepared as previously described (45) and cultured for 40 h. After treatment at 37 °C, neurons on 12-mm coverslips were fixed with 4% (vol/vol) paraformaldehyde (PFA) for 10 min, permeabilized with 0.1% Triton X-100 for 5 min, and stained with phalloidin conjugated to Alexa Fluor 568 (Invitrogen) for 20 min. Coverslips were mounted onto slides with VectaShield medium (Vector Laboratories) containing DAPI, and 15–20 random cell-containing fields were acquired per coverslip using an Olympus FV-1000 confocal microscope with a 60× objective. Neurons were identified based on standard morphological characteristics (8).
Electrophysiology.
We filtered whole-cell currents using an AxoPatch 200A at 5 kHz (four-pole low-pass Bessel), sampled at 20 kHz, and used voltage ramps consisting of a 40 ms step to −100 mV, a 200-ms ramp to +100 mV, and a 40-ms step at +100 mV applied at 0.5 Hz from a holding potential of −40 mV. Series resistance was compensated by 85%. Voltages are corrected for the −11-mV junction potential between internal and external solutions. We filtered single-channel currents at 2 kHz and sampled at 50 kHz. In recordings using calpain-2 (Fig. 3C), the high free [Ca2+] activated two other channels (12 pS and 28 pS); we excluded such openings from analysis. The standard internal solution contained (in mM): 150 Cs-aspartate, 2 MgCl2, 0.3 CaCl2, 1 BAPTA, 4 MgATP, 0.3 NaGTP, 10 Hepes (pH 7.20) with CsOH (100 nM free Ca2+). For the 5 μM free Ca2+ solution, we replaced BAPTA with 5 mM HEDTA and added 0.95 mM CaCl2; when using inhibitors containing 1 mM EDTA, we added an additional 0.8 mM CaCl2. The 2 mM CaCl2 solution contained no chelator. Free [Ca2+] was calculated using WebMaxChelator (http://maxchelator.stanford.edu), assuming an ionic strength of 0.16 M. The standard external solution contained (in mM): 150 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes, 10 d-glucose (pH 7.40) with NaOH. Electrophysiological experiments were performed at room temperature.
In Vitro Cleavage.
We collected HEK cells stably expressing mTRPC5 tagged with an N-terminal HA epitope in 150 mM NaCl with 10 mM Hepes (pH 7.5). We sonicated the cells and added CaCl2 to the homogenates for a final concentration of 2 mM. Purified calpain-1 or calpain-2 (EMD) was added at 5 μg/mL; calpeptin and calpain inhibitor III (EMD/Calbiochem) were added at 10 μM and 5 μM, respectively. After 30 min, a standard lysis buffer containing 1% Triton X-100 and 1× protease inhibitors (Sigma) was added. For blotting, the primary antibody was either mouse monoclonal to TRPC5 (1:500; clone: N67/15; NeuroMab), mouse monoclonal to HA (1:1,000; Cell Signaling), or mouse monoclonal to β-actin (1:2,000; Santa Cruz). The secondary antibody was goat anti-mouse HRP-conjugated (1:5,000; Jackson ImmunoResearch).
All statistical comparisons were calculated with Student's t test.
Acknowledgments
We thank Svetlana Gapon for cell culture and Nathaniel T. Blair for electrophysiology advice and reagents. We also thank Dr. Alan Wells for the constitutively active calpain-2 S50E clone. This work was supported by National Institutes of Health Grant 1 R01 MH090293 (to D.E.C.).
Footnotes
The authors declare no conflict of interest.
References
- 1.O’Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: Receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature. 2000;403:93–98. doi: 10.1038/47507. [DOI] [PubMed] [Google Scholar]
- 3.Sakai T, et al. Mouse semaphorin H induces PC12 cell neurite outgrowth activating Ras-mitogen-activated protein kinase signaling pathway via Ca2+ influx. J Biol Chem. 1999;274:29666–29671. doi: 10.1074/jbc.274.42.29666. [DOI] [PubMed] [Google Scholar]
- 4.To KC, Church J, O’Connor TP. Combined activation of calpain and calcineurin during ligand-induced growth cone collapse. Mol Cell Neurosci. 2007;36:425–434. doi: 10.1016/j.mcn.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Gerhold KA, Bautista DM. Molecular and cellular mechanisms of trigeminal chemosensation. Ann N Y Acad Sci. 2009;1170:184–189. doi: 10.1111/j.1749-6632.2009.03895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 7.Riccio A, et al. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res Mol Brain Res. 2002;109:95–104. doi: 10.1016/s0169-328x(02)00527-2. [DOI] [PubMed] [Google Scholar]
- 8.Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6:837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- 9.Davare MA, et al. Transient receptor potential canonical 5 channels activate Ca2+/calmodulin kinase Igamma to promote axon formation in hippocampal neurons. J Neurosci. 2009;29:9794–9808. doi: 10.1523/JNEUROSCI.1544-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian D, et al. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal. 2010;3:ra77. doi: 10.1126/scisignal.2001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puram SV, et al. A TRPC5-regulated calcium signaling pathway controls dendrite patterning in the mammalian brain. Genes Dev. 2011;25:2659–2673. doi: 10.1101/gad.174060.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riccio A, et al. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell. 2009;137:761–772. doi: 10.1016/j.cell.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaefer M, et al. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- 14.Kim MT, et al. Involvement of calmodulin and myosin light chain kinase in activation of mTRPC5 expressed in HEK cells. Am J Physiol Cell Physiol. 2006;290:C1031–C1040. doi: 10.1152/ajpcell.00602.2004. [DOI] [PubMed] [Google Scholar]
- 15.Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci. 2010;30:1560–1565. doi: 10.1523/JNEUROSCI.4816-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu G, Lu ZH, Obukhov AG, Nowycky MC, Ledeen RW. Induction of calcium influx through TRPC5 channels by cross-linking of GM1 ganglioside associated with alpha5beta1 integrin initiates neurite outgrowth. J Neurosci. 2007;27:7447–7458. doi: 10.1523/JNEUROSCI.4266-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung S, et al. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem. 2003;278:3562–3571. doi: 10.1074/jbc.M211484200. [DOI] [PubMed] [Google Scholar]
- 18.Blair NT, Kaczmarek JS, Clapham DE. Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J Gen Physiol. 2009;133:525–546. doi: 10.1085/jgp.200810153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross SA, et al. TRPC5 is a Ca2+-activated channel functionally coupled to Ca2+-selective ion channels. J Biol Chem. 2009;284:34423–34432. doi: 10.1074/jbc.M109.018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolodkin AL, et al. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T, et al. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama M, et al. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;423:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- 23.Raper J, Mason C. Cellular strategies of axonal pathfinding. Cold Spring Harb Perspect Biol. 2010;2:a001933. doi: 10.1101/cshperspect.a001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin Q, Liao G, Baudry M, Bi X. Role of calpain-mediated p53 truncation in semaphorin 3A-induced axonal growth regulation. Proc Natl Acad Sci USA. 2010;107:13883–13887. doi: 10.1073/pnas.1008652107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zadran S, Bi X, Baudry M. Regulation of calpain-2 in neurons: Implications for synaptic plasticity. Mol Neurobiol. 2010;42:143–150. doi: 10.1007/s12035-010-8145-1. [DOI] [PubMed] [Google Scholar]
- 26.duVerle D, Takigawa I, Ono Y, Sorimachi H, Mamitsuka H. CaMPDB: A resource for calpain and modulatory proteolysis. Genome Inform. 2010;22:202–213. [PubMed] [Google Scholar]
- 27.Glading A, et al. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol Cell Biol. 2004;24:2499–2512. doi: 10.1128/MCB.24.6.2499-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DuVerle DA, Ono Y, Sorimachi H, Mamitsuka H. Calpain cleavage prediction using multiple kernel learning. PLoS ONE. 2011;6:e19035. doi: 10.1371/journal.pone.0019035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zadran S, et al. Brain-derived neurotrophic factor and epidermal growth factor activate neuronal m-calpain via mitogen-activated protein kinase-dependent phosphorylation. J Neurosci. 2010;30:1086–1095. doi: 10.1523/JNEUROSCI.5120-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connor JA, Kater SB, Cohan C, Fink L. Ca2+ dynamics in neuronal growth cones: Regulation and changing patterns of Ca2+ entry. Cell Calcium. 1990;11:233–239. doi: 10.1016/0143-4160(90)90074-5. [DOI] [PubMed] [Google Scholar]
- 31.Lowery LA, Van Vactor D. The trip of the tip: Understanding the growth cone machinery. Nat Rev Mol Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimprich F, Bolsover SR. Calcium channels in neuroblastoma cell growth cones. Eur J Neurosci. 1996;8:467–475. doi: 10.1111/j.1460-9568.1996.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 33.Diefenbach TJ, Guthrie PB, Kater SB. Stimulus history alters behavioral responses of neuronal growth cones. J Neurosci. 2000;20:1484–1494. doi: 10.1523/JNEUROSCI.20-04-01484.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goswami C, Schmidt H, Hucho F. TRPV1 at nerve endings regulates growth cone morphology and movement through cytoskeleton reorganization. FEBS J. 2007;274:760–772. doi: 10.1111/j.1742-4658.2006.05621.x. [DOI] [PubMed] [Google Scholar]
- 35.Heo DK, et al. Opposite regulatory effects of TRPC1 and TRPC5 on neurite outgrowth in PC12 cells. Cell Signal. 2012;24:899–906. doi: 10.1016/j.cellsig.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Gu C, et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taniguchi M, et al. Distorted odor maps in the olfactory bulb of semaphorin 3A-deficient mice. J Neurosci. 2003;23:1390–1397. doi: 10.1523/JNEUROSCI.23-04-01390.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pozas E, et al. Age-dependent effects of secreted Semaphorins 3A, 3F, and 3E on developing hippocampal axons: In vitro effects and phenotype of Semaphorin 3A (-/-) mice. Mol Cell Neurosci. 2001;18:26–43. doi: 10.1006/mcne.2001.0999. [DOI] [PubMed] [Google Scholar]
- 39.White FA, Behar O. The development and subsequent elimination of aberrant peripheral axon projections in Semaphorin3A null mutant mice. Dev Biol. 2000;225:79–86. doi: 10.1006/dbio.2000.9822. [DOI] [PubMed] [Google Scholar]
- 40.Okada T, et al. Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J Biol Chem. 1998;273:10279–10287. doi: 10.1074/jbc.273.17.10279. [DOI] [PubMed] [Google Scholar]
- 41.Philipp S, et al. A novel capacitative calcium entry channel expressed in excitable cells. EMBO J. 1998;17:4274–4282. doi: 10.1093/emboj/17.15.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kikuchi K, et al. In vitro and in vivo characterization of a novel semaphorin 3A inhibitor, SM-216289 or xanthofulvin. J Biol Chem. 2003;278:42985–42991. doi: 10.1074/jbc.M302395200. [DOI] [PubMed] [Google Scholar]
- 43.Kaneko S, et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med. 2006;12:1380–1389. doi: 10.1038/nm1505. [DOI] [PubMed] [Google Scholar]
- 44.Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- 45.Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res. 1995;42:674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]