Abstract
Background and Purpose
Patients with intracerebral hemorrhage (ICH) and intraventricular hemorrhage (IVH) have a reported mortality of 50–80%. We evaluated a clot lytic treatment strategy for these patients in terms of mortality, ventricular infection, and bleeding safety events and for its effect on the rate of intraventricular clot lysis.
Methods
48 Patients were enrolled at 14 centers and randomized to treatment with 3mg recombinant tissue plasminogen activator (rt-PA) or placebo. Demographic characteristics, severity factors, safety outcomes (mortality, infection, bleeding), and clot resolution rates were compared in the two groups.
Results
Severity factors, including admission GCS, ICH volume, IVH volume and blood pressure, were evenly distributed, as were adverse events except for an increased frequency of respiratory system events in the placebo-treated group. Neither ICP nor Cerebral Perfusion pressure (CPP) differed substantially between treatment groups on presentation, with EVD closure, or during the active treatment phase. Frequency of death and ventriculitis was substantially lower than expected and bleeding events remained below the pre-specified threshold: mortality (18%, rt-PA; 23%, placebo); ventriculitis (8%, rt-PA; 9%, placebo); symptomatic bleeding (23%, rt-PA; 5% placebo, which approached statistical significance (p=0.1)). The median duration of dosing was 7.5 days for rt-PA and 12 days for placebo. There was a significant beneficial effect of rt-PA on rate of clot resolution
Conclusions
Low-dose rt-PA for the treatment of ICH with IVH has an acceptable safety profile compared to placebo and prior historical controls. Data from a well-designed Phase III clinical trial, such as CLEAR III, will be needed to fully evaluate this treatment.
Clinical Trial Registration Information
Participant enrollment began prior to July 1, 2005.
Keywords: intracerebral hemorrhage, intraventricular hemorrhage, tissue plasminogen activator, thrombolysis
Introduction
Among the different stroke subtypes, brain hemorrhage has a disproportionately high mortality rate. Mortality rates for patients with intracerebral hemorrhage (ICH) with an associated intraventricular hemorrhage (IVH) range from 50–80%.1, 2 Animal models demonstrate substantial physiologic and functional benefits associated with the early removal of blood clots from either the ventricle or the intraparenchymal spaces.3–5 Small trials have demonstrated the feasibility of a minimally invasive technique using intraventricular catheters and low dose thrombolytics and suggest clinically significant benefits in terms of reduced mortality.6–9 However, little attention has been given to measuring the efficacy of clot removal.10–12
This study was designed primarily to assess the safety of low dose rt-PA administered via extraventricular drainage catheter in the treatment of ICH with massive IVH, in terms of mortality, ventricular infection, and bleeding events. In addition, we tested the secondary hypothesis that administration of 3 mg of rt-PA, via external ventricular device (EVD) every 12 hours, increases the rate of intraventricular clot lysis compared to placebo-irrigated (normal saline) catheters.
Methods
Patient Selection
The study was conducted at 14 neurocritical care centers using a uniform protocol approved by the institutional review boards at each participating center (FDA IND 8523). Inclusion criteria were patients 18 to 75 years of age who experienced a small supratentorial ICH (≤ 30 cc) with massive IVH with an EVD already placed for treatment of obstructive hydrocephalus, per standard of care, and could be randomized within 24 hours of diagnostic CT showing IVH. Thus no patient was exposed to the risk of an EVD insertion that would not otherwise have one. A computerized tomography (CT) scan performed after EVD placement to demonstrate clot stability and proper EVD placement. Exclusion criteria included presence of infra- or sub--tentorial parenchymal bleeding, pregnancy, radiological evidence of arteriovenous malformation, aneurysm, or tumor as a source for the ICH, and evidence of coagulopathy (INR >1.7; platelet count < 100,000).
Clinical Protocol
Subjects were randomized to receive either 3 mg/3 ml of rt-PA or 3 ml of normal saline injected via the EVD into the ventricular space(s). CT scans were performed daily to monitor for asymptomatic bleeding and measure clot resolution while the subject received rt-PA/Placebo administrations and once between days 28 and 32 post enrollment. rt-PA/Placebo administration was continued every 12 hours until CT evidence of clot resolution was sufficient to remove the catheter (at a minimum the opening of the 3rd and 4th ventricles) or until a safety endpoint (symptomatic bleeding, infection, or death) occurred, whichever came first. Data on well-established ICH severity factors were collected at time of presentation.2 Daily monitoring for serum coagulation factors and infection markers in the CSF was undertaken. Subjects were monitored for blood pressure, temperature, and cerebral perfusion pressure (CCP) fluctuations every 8 hours and for ICP elevation every 4 hours while receiving rt-PA/placebo. Functional outcome assessment was performed by a blinded observer.
Test Article Administration
Activase® (Alteplase, recombinant) (rt-PA) is a sterile, lyophilized preparation intended for intravascular infusion. Genentech, Inc. provided the Activase® for the trial in the form of 50-mg vials labeled for investigational use. Drug was reconstituted with sterile water to yield a solution that contained 1 mg of Activase® per mL. The Activase® and a 4 mL normal saline flush were prepared in sterile syringes and delivered to the intensive care unit (ICU). At each site, pharmacists used a strict sterile reconstitution and preparation protocol. An isovolumetric administration technique was used with CSF aspirated prior to dosing. Compliance with dosing and administration mechanics was high for the entire cohort, with 553 of 575 planned doses (96%) administered. Those administering study medication and nursing personnel were blinded to whether the patient received rt-PA or placebo.
Adverse Event Monitoring
All adverse and endpoint events were determined by the site investigator and validated by site visit. Adverse events (AEs) were then reviewed by the coordinating center and the PI using standard Good Clinical Practice definitions (21 CFR, Part 312, Investigational New Drug Application). Endpoint events were reviewed by the PI and then reviewed in a blinded manner by an endpoint committee consisting of the study QA monitor, an intensivist-neurologist, a neurosurgeon and a coordinator.
Safety Monitoring
All AEs and endpoints were reviewed in an unblinded manner by an independent Data Safety and Monitoring Board (DSMB) consisting of two neurologists, a neurosurgeon not associated with the study, and the study statistical consultant. The following were pre-specified to trigger early DSMB analysis and possible study suspension: 30-day survival less than 25%, a symptomatic rebleeding (clot enlargement with concurrent drop in GCS motor of > 2) rate greater than 35%,13 and an infection (fever and positive CSF culture) rate greater than 30% (Fig. 1).14
Fig. 1.
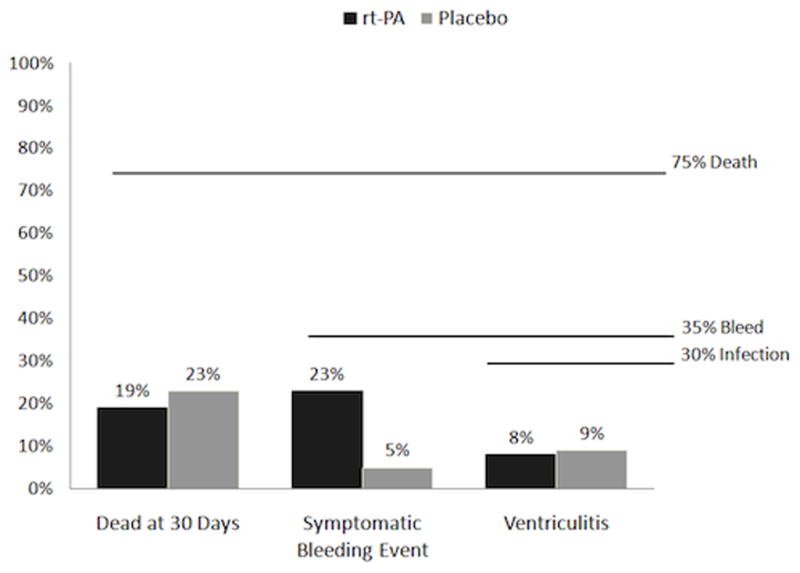
Pre-specified safety triggers and events. None were significant (death at 30 days p=1.00, symptomatic bleeding event p=0.106, ventriculitis p=1.00).
CT Hemorrhage Volume Analysis
On completion of the study, CT scan copies were sent to a central location for analysis by a neuroradiologist blinded to treatment assignment. The CT reader demarcated all areas of ICH and IVH clot on each slice. IVH and ICH volumes were determined using a modification of Steiner et al’s axial CT volume analysis method.15 Within each CT slice custom software was used to determine the pixel count within the marked areas while outlined against a backlit digitizing tablet (Numonics, Montgomeryville PA, model A56BL with Macintosh Accessory Kit). This pixel count was multiplied by area per pixel to obtain the cross-sectional area within the marked portion of that slice. Volume was calculated as the product of this area and the collimation width of the slice. The total volume of interest was calculated as the sum of volumes within all slices. The intra-observer variability in volume determinations with this method has been less than 1.5%.16
Clot Resolution Rate
Random-effects linear regression was carried out to examine whether receiving rt-PA resulted in a different rate of clot resolution than placebo. IVH volumes from all head CTs taken during the 4 days immediately following the stability CT were used with clot volumes standardized as a percent of the stability CT IVH volume. Interaction terms between receiving active drug and time since stability scan were created.
Glasgow Coma Scale Score and Clot Resolution Rate
Random-effects linear regression was also carried out to examine whether a patient’s rate of clot resolution was associated with short term (96-hour) change in level of consciousness, based on change in GCS from the GCS recorded closest in time to the start of treatment. Preference was given to scores from before the start of treatment if taken within two hours of treatment. Individual rates of clot resolution were estimated for each patient using CT scans taken during the first 5 days following the stability CT scan. For this analysis we used CT scans from the first five days, rather than the shorter time periods used in the analyses described above to enable us to better capture the considerable variation in individual rates of clot resolution. All patients, both rt-PA and placebo treated, who survived without symptomatic rebleed were included. Factors independently associated with change in level of consciousness, such as initial GCS score, were also considered. Since change in GCS over time was not expected to be uniform, higher order terms for time were examined and included where significant.
Deaths and symptomatic rebleeding events, which typically result in cessation of treatment, might have biased the result due to “informative” rather than “at random” censoring of both GCS and clot resolution data. To avoid this problem, the analysis was limited to patients who were successfully treated, defined as those surviving and not experiencing a symptomatic rebleed event (n=36). One additional patient who did not have GCS readings recorded after admission was excluded from this analysis. On average, 11.6 GCS readings (range: 1–14) were available for each patient.
Statistical Methods
Categorical baseline characteristics and frequencies of AEs were compared by treatment using Fisher’s exact test. Ordinal variables and non normally distibuted continuous variables were compared using the Wilcoxon rank-sum test and normally distributed continuous baseline characteristics were compared using Student’s t-test. The outcome measure, percent clot resolution rate, was estimated and compared by treatment assignment using random effects generalized least squares regression with the consistency of the estimates evaluated using the Hausman specification test.
All statistical analyses were performed using STATA statistical software (STATA Corporation, College Station, TX) with tests being two-tailed. P≤0.05 was considered to indicate statistical significance.
Results
Patient Characteristics
Forty-eight patients were randomized to twice daily (Q12h) isovolumetric injections of either 3 mg intraventricular rt-PA (n=26) or vehicle (n=22). Demographic characteristics of study subjects are presented in Table 1. Only gender was not distributed evenly across treatment groups, (rt-PA group, 73% vs. placebo, 32% male subjects). Presenting clinical and disease severity characteristics are displayed in Table 2. Severe hypertension and decreased levels of consciousness characterized the entire population. Severity factors, including admission GCS, ICH volume, IVH volume and admission blood pressure, were evenly distributed across the two groups. The location of primary ICH favored deep paramedian regions such as caudate, globus pallidus, putamen, and thalamus.
Table 1.
Patient Characteristics
| Characteristic | Placebo (N=22) | rt-PA (N=26) | Total (N=48) | P-value* |
|---|---|---|---|---|
| Mean Age (Std dev) | 56.5 (1.6) | 54.1 (2.4) | 55.2 (1.5) | 0.45† |
| Male (%) | 31.8 % | 73.1 % | 54.2 % | 0.008 |
| AA, not Hispanic | 45.5 % | 65.4 % | 56.2 % | |
| Asian/Pacific Islander | 4.6 % | 11.6 % | 8.3 % | |
| Caucasian, not Hispanic | 31.8 % | 11.6 % | 20.8 % | |
| Hispanic | 18.2 % | 11.6 % | 14.6 % | 0.26 |
| Hx of HTN | 86.4 % | 76.9 % | 81.2 % | 0.48 |
| Hx of DM | 13.6 % | 26.9 % | 20.8 % | 0.31 |
| Hx Seizures | 0.0 % | 0.0 % | 0.0 % | --- |
| Hx Migraine | 0.0 % | 3.8 % | 2.1 % | 1.00 |
| Hx ETOH | 18.2 % | 15.4 % | 16.7 % | 1.00 |
| Hx Tobacco | 18.2 % | 11.6 % | 14.6 % | 0.69 |
| Hx of Illicit Drug (Cocaine) | 9.1 % | 11.6 % | 10.4 % | 1.00 |
Fisher’s exact test for all variables except age
t-test
Table 2.
Initial patient severity
| Placebo (N=22) | rt-PA (N=26) | Total (N=48) | P-value* | |
|---|---|---|---|---|
| SBP | 189.0 (7.3) | 191.0 (7.4) | 190.1 (5.2) | 0.85 |
| DBP | 101.2 (5.7) | 105.6 (5.6) | 103.6 (4.0) | 0.58 |
| MAP | 130.5 (5.9) | 134.1 (5.9) | 132.4 (4.2) | 0.67 |
| PP - calculated (SBP - DBP) | 87.9 (4.5) | 85.4 (4.6) | 86.5 (3.2) | 0.71 |
| ICP | 9.0 (1.6) | 11.8 (1.0) | 10.5 (0.9) | 0.14 |
| CPP | 86.7 (3.4) | 90.7 (4.3) | 88.8 (2.7) | 0.47 |
| GCS (median;IQR) | 7 (4.8–9.0) | 8 (5.0–10.3) | 7 (5–9.8) | 0.44† |
| Admit NIH Stroke Scale (median:IQR) | 25 (13.0–32.0) | 24 (17.0–37.0) | 24.5 (15.3– 32.8) | 0.46† |
| ICH volume (median:IQR) | 7.9 (0–21.4) | 7.2 (0.7–12.7) | 7.5 (0–16.7) | 0.54† |
| IVH volume | 50.1 (6.7) | 54.8 (5.8) | 52.7 (4.4) | 0.60 |
| IVH Grade (median) | 15.5 (12.5–17.3) | 15 (12.0–17.0) | 15(12.3–17.0) | 0.69† |
| Subarachnoid hemorrhage | 0 % | 0 % | 0 % | --- |
| Location of ICH: | ||||
| No ICH | 6 (27.3 %) | 4 (15.4 %) | 10 (20.8 %) | |
| Caudate | 2 (9.1 %) | 7 (26.9 %) | 9 (18.8 %) | |
| Globus Pallidus | 0 (0.0 %) | 2 (7.7 %) | 2 (4.2 %) | |
| Putamen | 3 (13.6 %) | 3 (11.5 %) | 6 (12.5 %) | |
| Thalamus | 11 (50.0 %) | 10 (38.5 %) | 21 (43.8 %) | 0.34 |
| Mean Predicted Mortality‡ (actual mortality) | 74.52% (23%) | 74.49% (19%) | 74.5% (21%) | <0.00001§ |
t-test for normally distributed continuous variables, Wilcoxon rank-sum test for ordinal and all non-normally distributed continuous variables, and Fisher’s exact test for categorical variables. Data shown are mean (SD) or median (Inter quartile range)
Wilcoxon rank-sum test
Tuhrim S, Dambrosia JM, Price TR: Intracerebral hemorrhage: external validation and extension of a model for prediction of 30-day survival. Ann Neurol 29:658–663, 1991
Represents the binomial probability of actual death rate occurring given the predicted injury severity.
The treatment goal of early initiation, i.e., no sooner than 12 hours and no later than 24 hours from the CT scan diagnosing the IVH, was achieved in 47 of 48 subjects.
Initial Emergency Care
Emergency care did not differ between treatment groups. On average, time from symptom onset to emergency department arrival was 4 hours, and initial diagnostic CT was performed within 50 minutes of arrival. Time from diagnostic CT to complete a ventricular catheter insertion was 6.1 ± 5.8 hrs; from EVD insertion to post-insertion stability CT scan, 6.8 ± 7.0 hrs; and from stability CT scan to first dose of rt-PA/Placebo, 7.8 ± 6.8 hrs. ICP was generally well controlled throughout the entire treatment period. Neither ICP nor CPP differed substantially between treatment groups on presentation, with EVD closure, or during the active treatment phase.
Test Article Administration
The average duration of dosing did not differ significantly: 10.2 ± 8 days, rt-PA; 12.7 ± 8.4 days, placebo. However, a trend toward a shorter dosing period can be seen with the median duration of dosing being 7.5 days for rt-PA and 12 days for placebo. Dosing with test article required closure of the catheter for 1 hour to test the clot lysing abilities of rt-PA. Catheter closure was well tolerated with ICP rising from 12.8 to 17.8 mm Hg for rt-PA patients and 11.5 to 16.7 mm Hg for placebo patients. The median number of test article injections was 11 injections, the mean was 12.0. On 15 of 575 occasions the catheter was opened prior to 1 hour to control ICP. Elevated ICP with IVC closure occurred rarely, compromise of CPP was even less frequent with IVC closure. The percentage of closure-related elevations greater than 30 mm Hg was 8% (46/575): 10.3% (28/272) in the rt-PA group and 5.9% (18/303) in the placebo group. Decreases of CPP lower than 60 mm Hg showed a similar pattern: 3% (18/575); 1.8% (5/272) in the rt-PA group and 4.3% (13/303) in the placebo group.
Four patients underwent craniotomy for uncontrolled intracranial hypertension not responding to drainage and medical management; each case was associated with an episode of intracranial bleeding, as determined by central analysis of serial CT scans (3 rt-PA, 1 placebo). In three cases, surgery was successful providing long-term control of ICP.
Safety Hypothesis
Mortality, bleeding events during the treatment period, and ventriculitis represented the pre-specified safety outcomes against which treatment was judged as our primary goal. Frequency of events was substantially lower than expected for death and ventriculitis and remained below the pre-specified threshold for bleeding, (See Figure 1.) Predicted (30-d) mortality, using a well-validated severity algorithm,17, 18 was 75% for both treatment groups (Table 2). Actual mortality was 19% in the rt-PA treated group and 23% in the placebo group. Ventriculitis occurred among 8% and 9% respectively of those groups and symptomatic bleeding was reported for 23% of the rt-PA treated group and 5% of the placebo group. Asymptomatic bleeding demonstrated a similar trend with five events in the rt-PA and two in the placebo group. Adverse events were frequent in both study groups (Table 3). They were generally similar between groups except for an increased frequency of respiratory system events in the placebo-treated group. None of these differences reached statistical significance. However, differences in the symptomatic bleeding event rate approached statistical significance (p=0.1).
Table 3.
Adverse events
| Number of patients with: | rt-PA | Placebo | p-value |
|---|---|---|---|
| Any adverse event | 18 (69.2) | 20 (90.9) | 0.084 |
| Any serious adverse event | 16 (61.54) | 8 (36.36) | 0.147 |
| Number of patients with events by Body System | |||
| Nervous system | 14 (53.9) | 9 (40.9) | 0.401 |
| Nervous system hemorrhage | 10 (38.5) | 3 (13.6) | 0.101 |
| General body system/misc. | 10 (38.5) | 12 (54.6) | 0.384 |
| Digestive system | 1 (3.9) | 1 (4.6) | 1.000 |
| Cardiovascular system | 6 (23.1) | 6 (27.3) | 0.751 |
| Respiratory system | 10 (38.5) | 15 (68.2) | 0.049 |
| Endocrine/metabolic system | 1 (3.9) | 4 (18.2) | 0.165 |
| Heme/lymphatic system | 0 (0.0) | 1 (4.6) | 0.458 |
| Urogenital | 2 (7.7) | 3 (13.6) | 0.649 |
Death was attributed directly to initial hemorrhage in 9 of the 10 reported deaths. The remaining death was attributed to a delayed mass effect and herniation event. Primary and secondary causes of death include: delayed mass effect (5), withdrawal of care attributed directly to initial hemorrhage (5), rebleeding (2), renal failure (1), ventriculitis (1), hypertension (1), increased ICP (1), ischemia (1), vasospasm (1), and brain death (1).
Clot Size Reduction Hypothesis
The rate of blood clot resolution was significantly greater in the rt-PA-treated patient group (18% per day vs. 8% per day for placebo-treated patients) (p< 0.001, Fig. 2). This was associated with a higher rate of successful removal of catheter at the end of rt-PA/Placebo administration (50% vs. 20%), less reliance on three or more EVDs (4% vs. 32%), and a shorter length of treatment.
Fig. 2.
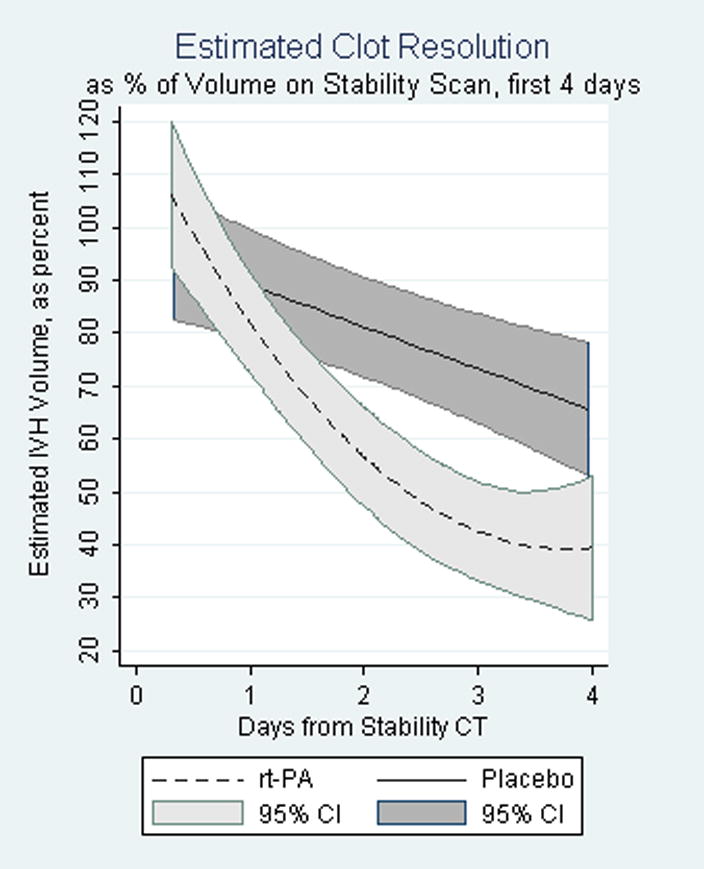
Rates of clot reduction t-PA vs. control, shown with 95% confidence intervals. Note the rate for reduction with t-PA diverges from placebo within two days (p<.001).
Improved 30-day outcomes for rt-PA treated patients were observed for all pre-specified functional outcome measures. GOS ≤ 2: (57% rt-PA vs. 64% placebo); modified Rankin ≤ 4: (52% rt-PA vs. 27% placebo); NIH Stroke Scale ≤ 10: (54% rt-PA vs. 29% placebo); and Barthel Index ≥ 80: (19% rt-PA vs. 18% placebo). None of these differences reached statistical significance.
Effect of Rt-PA and Time on Clot Resolution
Estimates of the rate of clot resolution over the 4 days following the stability CT were based on 147 CT scans. The estimated IVH volume as a % of IVH volume on stability CT was as follows:
where t= time in days since stability CT, up to 4 days, and rt-PA = 1 if treated with rt-PA, = 0 if treated with placebo. The terms are all statistically significant, and composite testing of the 3 rt-PA terms with a Wald test had a Chi2 (3 df) = 29.39, (p-value < 0.001). Estimated volumes for each group over time based on this analysis are shown in Figure 2 with 95% confidence intervals. As seen in Figure 2, much of the lysing activity in the rt-PA group occurs during the first three days. Since time to remove blood clot may be an important treatment variable, and furthermore since the rt-PA effect on clot resolution is nearly constant over time for the first three days, we ran a further analysis limited to the first three days. Estimated resolution for rt-PA treated patients over this period was 22.3%/day (95 % CI, 16.7% – 28.0%) and for placebo-treated patients was 9.9%/day (95 % CI, 3.5% – 16.2 %).
The rate of IVH resolution for placebo-treated patients was estimated to be a constant 7.93% a day, while the rate of IVH resolution for rt-PA treated patients was not constant, being more rapid during the first 2 1/2 days and then leveling off. Forty-eight, 72, and 96 hours after the stability scan, the estimated percent IVH volume remaining for rt-PA treated versus placebo treated patients was 56.6% vs. 81.1%, 42.4% vs. 73.2%, and 39.4% vs. 65.3%.
Model of Relation between Clot Resolution and Change in Consciousness
Initial GCS and a patient’s rate of clot resolution were independently associated with change in GCS during the 96 hours following the start of treatment. Baseline ICH volume was not independently associated with change in GCS once initial GCS was taken into account. The composite testing of all terms in the model with a Wald test had a Chi2 (5 df) = 50.68 (p-value < 0.001). There was a family of curves for the predicted short term GCS over time depending on initial GCS, but the relationship of rate of clot resolution to change in GCS remained the same in each. Figure 3 shows the predicted short term GCS for patients with a GCS of 8 at the start of treatment, the median for these patients, for four rates of clot resolution: 0%/day, 10%/day, 20%/day and 30%/day. In this example, as well as for other initial GCS values, each 10% per day increase in the rate of clot resolution was associated with a 1.1-point improvement in GCS 96 hours after the initial GCS (95% CI, 0.49 – 1.63 point improvement) (p<0.001). For patients with an initial GCS of 8 and a 0%/day short term rate of clot resolution, predicted GCS by 96 hours was about 7. At the other extreme, patients with an initial GCS of 8 and a 30%/day short term rate of clot resolution, had a predicted GCS by 96 hours of above 10.
Fig 3.
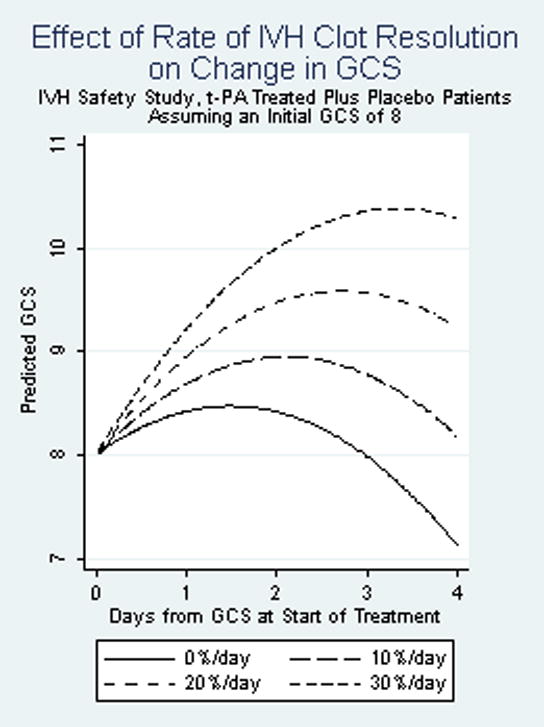
Relation of rate of IVH clot resolution to change in GCS. P-value for rate of IVH clot resolution, as a continuous variable <0.001. Included patients who survived without symptomatic rebleed (N=35)
Discussion
The volume of blood in both the intraparenchymal and intraventricular spaces is a potent factor in determining mortality.2, 19 This relationship has been consistent in multiple cohorts of patients.6, 12, 20 The safety data presented here represent the first prospective effort to define the safety of a minimally invasive approach to removing clot using intraventricular low dose thrombolytics. This trial provides additional data regarding the amount and timing of blood clot removal produced by low dose rt-PA or EVD alone.
The mortality rate in both treatment groups was substantially lower than prior reports,1, 21, 22 despite the selection of a severely-impaired group of patients with a high likelihood of mortality based on their presenting GCS, ICH size, IVH size, and blood pressure.2 These severity factors were equally distributed across treatment groups and a mortality difference between the groups was not demonstrated. Case series data have suggested that extraventricular drainage controls ICP elevation, but does not alter mortality.21 This study confirms prospectively Adams’ finding that extraventricular drainage can produce controlled ICP.
Several factors could account for the substantial difference between our mortality and earlier reports.1, 21 Possible factors include good ICU care and regular patient monitoring (the Hawthorne effect). Furthermore, all patients selected for the study had an EVD in place and one goal of the study was to maintain catheter patency and to continue extraventricular drainage until acute obstructive hydrocephalus was relieved and normal CSF circulation reestablished. Possibly this practice alone is associated with improved mortality. Specifically, these clinical goals were achieved in all study survivors. Both groups also achieved marked reduction of clot size over the initial treatment phase and initial 30 days, with rt-PA treated group achieving a radiologic reduction of approximately 60% over 4 days and the placebo group the same reduction over 8 days as demonstrated by the clot reduction model. An association of improved mortality with clot reduction is consistent with animal models23–25 as well as prior observations in which attempts at blood clot removal were either not made21 or ineffective.8 Differences in patient severity between the Adams and Coplin series and ours do not appear to explain the enhanced survival we demonstrated. However, withdrawal of life sustaining therapies was more frequent in the those cohorts.26 Other cohorts have larger ICHs and smaller IVHs, thus they represent different subgroups of the overall ICH population.12, 27
Lower absolute mortality and the absence of any unfavorable comparison to either the concurrent placebo group or historical controls suggest that low dose thrombolytic therapy can be performed safely in a severely impaired group of deep ICH patients with massive intraventricular hemorrhage. Careful attention to ICP control and IVC catheter antisepsis was associated with a low frequency of ICP elevation and catheter related infections. However, a consistent trend toward increased frequency of bleeding events was noted in the rt-PA treated group. Secondary bleeding events over the 30-day study period included rebleeding at the primary site, rebleeding at secondary sites (predominantly in the catheter tract), and several instances of IVH extension. Because clinical clot stability was required prior to administration of the thrombolytic, these findings could represent evidence of ongoing drug-related susceptibility to bleeding.
Given the finding of significantly enhanced clot lysis and a strong trend towards increased secondary bleeding events, caution with respect to the overall safety of low dose rt-PA in the treatment of ICH needs to be expressed. Multiple factors, including blood pressure, coagulation state, ethnicity, diabetes mellitus, and concurrent medications such as aspirin, BP-elevating drugs and/or illicit drugs, have all been implicated as risk factors for bleeding. Data on the management of these factors and their interactions with low dose rt-PA is absent; our study is not informative as the bleeding event rate is too low to draw conclusions about these factors. Additionally, data demonstrating a dose response relationship between rt-PA and bleeding events or clot lysis rate is absent. A dose finding study is necessary to investigate whether lower doses of rt-PA may be associated with a high degree of clot lysis but provide a substantially better safety margin with respect to rebleeding.
Two methodologically robust studies of clot removal have demonstrated baseline clot lysis rates from 6 to 12% per day.6, 16 The 8 ± 2% per day intraventricular clot lysis rate in our placebo group is consistent with these prior observations and the latest estimate from this study is strengthened by the greater frequency of clot volume measurements used. Thus, we conclude that the enhanced clot lysis rate observed for the rt-PA treated patients is a robust effect, however, it is not as great or as effective as the effects demonstrated in animal models where clot removal produced decreased edema and prevented subependymal inflammation.4, 5, 28 Despite this, several other findings from the animal models were demonstrated in this human study: decreased mortality from herniation and ICP events24 and an enhanced or more rapid recovery of impaired consciousness.3 More rapid removal of clot could be translated into a shorter ICU stay, if the EVDs are removed earlier.
This study was neither designed nor powered to assess functional outcome. Others have suggested that ICH patient functional outcome is best assessed at longer time intervals after the initial event.29, 30 This suggestion is consistent with our data which demonstrated high degrees of impaired consciousness at presentation and initial therapeutic periods, with recovery of consciousness occurring slowly over the 30 day study period. The ability of patients to return to prior independent lifestyles could not be properly assessed over this time frame. However, the study demonstrates that some individuals (3 subjects) were capable of returning to their premorbid functional status within 30 days after severe IVH. Therefore, it seems likely that future studies, including the current CLEAR III (Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage Phase III) Trial, will need to assess functional performance in the 90 to 180-day time frame or as far out as 365 days. The location of ICH as well as extent of ICH should significantly influence functional outcome. The selection criteria for this study controlled for lesion location and ICH volume. Therefore, these factors will require further study and evaluation, if patient selection is to be optimized. The presence of increased brain tissue injury with prolonged blood clot exposure in animal models and humans31, 32 suggests a strong logical rationale for improving the timing and efficiency of blood clot removal in these patient groups. To date, a treatment modality to do this does not exist in the clinical arena. Multiple daily doses of rt-PA could provide the desired intervention capable of rapidly removing blood from tissue contact.
This study demonstrates that low dose rt-PA has an acceptable safety profile compared to placebo and prior historical controls of the natural history of ICH with IVH. While 3 mg of rt-PA irrigation every 12 hours clearly accelerates clot removal from the ventricular system neither dose safety nor dose efficacy was fully explored for this approach. Data from a well-designed Phase III clinical trial, such as CLEAR III, will be needed if this treatment is to be fully evaluated.
Acknowledgments
We sincerely thank the patients, families, and hospital colleagues who participated in the trial and the following Data Safety Monitoring Board: Joseph P. Broderick, MD, Department of Neurology, University of Cincinnati College of Medicine; Robert J. Wityk, MD, Department of Neurology, Johns Hopkins School of Medicine. We gratefully acknowledge the invaluable contributions of Kerri McGovern, Timothy Morgan, and Natalie Ullman to manuscript preparation.
Sources of Funding
The Intraventricular Hemorrhage Thrombolysis Trial was supported by a grants (FD-R-001693 to Drs Naff and Hanley) and (FD-R 002018 to Drs Hanley and Rhoney) from the Office of Orphan Products Development, Food and Drug Administration. Dr. Naff received funding from the American Heart Association and funding from a research grant (FD-R-001693) from the Office of Orphan Products Development. Penny Keyl received funding from a research grant (FD-R-001693) from the Office of Orphan Products Development. Karen Lane and Nichol McBee received funding from a research grant (FD-R-001693) from the Office of Orphan Products Development. Stanley Tuhrim received funding from a research grant (FD-R-001693) from the Office of Orphan Products Development. Anthony Marmarou received funding from a research grant (FD-R-001693) from the Office of Orphan Products Development. Daniel F. Hanley is supported by grants U01NS062851 and RO1NS046309 from the National Institutes of Health/NINDS, grant 272–2007 from the Eleanor Naylor Dana Charitable Trust, the Jeffry and Harriet Legum Endowment, and materials grants from Genentech, Inc.
Footnotes
Disclosures
No party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated. We certify that all financial and material support for this research has been clearly identified in the Sources of Funding.
References
- 1.Coplin WM, Vinas FC, Agris JM, Buciuc R, Michael DB, Diaz FG, et al. A cohort study of the safety and feasibility of intraventricular urokinase for nonaneurysmal spontaneous intraventricular hemorrhage. Stroke. 1998;29:1573–1579. doi: 10.1161/01.str.29.8.1573. [DOI] [PubMed] [Google Scholar]
- 2.Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 1999;27:617–621. doi: 10.1097/00003246-199903000-00045. [DOI] [PubMed] [Google Scholar]
- 3.Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 2: In vivo safety study of intraventricular urokinase. Neurosurgery. 1986;19:547–552. doi: 10.1227/00006123-198610000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Wagner KR, Xi G, Hua Y, Zuccarello M, de Courten-Myers GM, Broderick JP, et al. Ultra-early clot aspiration after lysis with tissue plasminogen activator in a porcine model of intracerebral hemorrhage: Edema reduction and blood-brain barrier protection. J Neurosurg. 1999;90:491–498. doi: 10.3171/jns.1999.90.3.0491. [DOI] [PubMed] [Google Scholar]
- 5.Mayfrank L, Kissler J, Raoofi R. Ventricular dilatation in experimental intraventricular hemorrhage in pigs. Characterization of cerebrospinal fluid dynamics and the effects of fibrinolytic treatment. Stroke. 1997;28:141–148. doi: 10.1161/01.str.28.1.141. [DOI] [PubMed] [Google Scholar]
- 6.Naff NJ, Keyl PM, Tuhrim S, Bederson J, Bullock R, Mayer SA, et al. Intraventricular thrombolysis speeds blood clot resolution: Results of a randomized, double-blinded controlled clinical trial. Neurosurgery. 2004;54:577–583. doi: 10.1227/01.neu.0000108422.10842.60. discussion 583–574. [DOI] [PubMed] [Google Scholar]
- 7.Naff NJ, Carhuapoma JR, Williams MA, Bhardwaj A, Ulatowski JA, Bederson J, et al. Treatment of intraventricular hemorrhage with urokinase: Effect on 30-day survival. Stroke. 2000;31:841–847. doi: 10.1161/01.str.31.4.841. [DOI] [PubMed] [Google Scholar]
- 8.Teernstra OP, Evers SM, Lodder J, Leffers P, Franke CL, Blaauw G. Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: A multicenter randomized controlled trial (sichpa) Stroke. 2003;34:968–974. doi: 10.1161/01.STR.0000063367.52044.40. [DOI] [PubMed] [Google Scholar]
- 9.Shen PH, Matsuoka Y, Kawajiri K. Treatment of intraventricular hemorrhage using urokinase. Neurol Med Chir (Tokyo) 1990;30:329–333. doi: 10.2176/nmc.30.329. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi AI, Tuhrim S, Broderick J, Batjer HH, Hondo H, Hanley D. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 11.Hankey GJ, Hon C. Surgery for primary intracerebral hemorrhage: Is it safe and effective? A systematic review of case series and randomized trials. Stroke. 1997;28:2126–2132. doi: 10.1161/01.str.28.11.2126. [DOI] [PubMed] [Google Scholar]
- 12.Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (stich): A randomised trial. Lancet. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 13.Brott T, Broderick J, Kothari R. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Lozier AP, Sciacca RR, Romagnoli MF, Connolly S. Ventriculostomy-related infections: A critical review of the literature. Neurosurgery. 2002;51:170–182. doi: 10.1097/00006123-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Steiner L, Bergvall U, Zwetnow N. Quantitative estimation of intracerebral and intraventricular hematoma by computer tomography. Acta Radiol Suppl. 1975;346:143–154. doi: 10.1177/0284185175016s34615. [DOI] [PubMed] [Google Scholar]
- 16.Naff NJ, Williams MA, Rigamonti DR, Keyl PM, Hanley DF. Blood clot resolution in human cerebrospinal fluid: Evidence of first-order kinetics. Neurosurgery. 2001;49:614–619. doi: 10.1097/00006123-200109000-00015. discussion 619–621. [DOI] [PubMed] [Google Scholar]
- 17.Ariesen MJ, Algra A, van der Worp HB, Rinkel GJ. Applicability and relevance of models that predict short term outcome after intracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 2005;76:839–844. doi: 10.1136/jnnp.2004.048223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuhrim S, Dambrosia JM, Price TR. Intracerebral hemorrhage: External validation and extension of a model for prediction of 30-day survival. Ann Neurol. 1991;29:658–663. doi: 10.1002/ana.410290614. [DOI] [PubMed] [Google Scholar]
- 19.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage: A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 20.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Recombinant activated factor vii for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–785. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- 21.Adams RE, Diringer MN. Response to external ventricular drainage in spontaneous intracerebral hemorrhage with hydrocephalus. Neurology. 1998;50:519–523. doi: 10.1212/wnl.50.2.519. [DOI] [PubMed] [Google Scholar]
- 22.Lapointe M, Haines S. Fibrinolytic therapy for intraventricular hemorrhage in adults (cochrane review) Cochrane Database Syst Rev. 2002;3:CD003692. doi: 10.1002/14651858.CD003692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qureshi AI, Wilson DA, Hanley DF, Traystman RJ. Pharmacological reduction of mean arterial pressure does not adversely effect regional cerebral blood flow and intracranial pressure in experimental intracerebral hemorrhage. Crit Care Med. 1999;27:965–971. doi: 10.1097/00003246-199905000-00036. [DOI] [PubMed] [Google Scholar]
- 24.Qureshi AI, Wilson DA, Hanley DF, Traystman RJ. No evidence for an ischemic penumbra in massive experimental intracerebral hemorrhage. Neurology. 1999;52:266–272. doi: 10.1212/wnl.52.2.266. [DOI] [PubMed] [Google Scholar]
- 25.Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 1: Canine intraventricular blood cast model. Neurosurgery. 1986;19:540–546. doi: 10.1227/00006123-198610000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Becker KJ, Baxter AB, Cohen WA, Bybee HM, Tirschwell DL, Newell DW, et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology. 2001;56:766–772. doi: 10.1212/wnl.56.6.766. [DOI] [PubMed] [Google Scholar]
- 27.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, Skolnick BE, et al. Efficacy and safety of recombinant activated factor vii for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 28.Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 3: Effects of intraventricular urokinase on clot lysis and posthemorrhagic hydrocephalus. Neurosurgery. 1986;19:553–572. doi: 10.1227/00006123-198610000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Morgenstern LB, Frankowski RF, Sheddon P. Surgical treatment for intracerebral hemorrhage (stich): A single-center, randomized clinical trial. Neurology. 1998;51:1359–1363. doi: 10.1212/wnl.51.5.1359. [DOI] [PubMed] [Google Scholar]
- 30.Zuccarello M, Brott T, Derex L. Early surgical treatment for supratentorial intracerebral hemorrhage: A randomized feasibility study. Stroke. 1999;30:1833–1839. doi: 10.1161/01.str.30.9.1833. [DOI] [PubMed] [Google Scholar]
- 31.Carhuapoma JR, Hanley DF, Banerjee M, Beauchamp NJ. Brain edema after human cerebral hemorrhage: A magnetic resonance imaging volumetric analysis. J Neurosurg Anesthesiol. 2003;15:230–233. doi: 10.1097/00008506-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Carhuapoma JR, Beauchamp NJ. Brain edema after intracerebral hemorrhage: A magnetic resonance imaging volumetric analysis. Neurology. 2000;54:A375–A376. doi: 10.1097/00008506-200307000-00010. [DOI] [PubMed] [Google Scholar]


