Abstract
Trophic transfer of Hg across lakes within a region has been related to multiple environmental factors, but the nature of these relationships across distinct basins within individual large lakes is unknown. We investigated Hg bioaccumulation in zooplankton in basins of differing trophic status in Lake Champlain (Vermont, USA) to determine the strongest predictors of Hg bioaccumulation. Zooplankton were sampled in Malletts Bay (oligotrophic) and Missisquoi Bay (eutrophic) in 2005–2008. Zooplankton in the eutrophic basin had lower concentrations of total Hg and MeHg than those in the oligotrophic basin in all years but 2007, when no bloom occurred in Missisquoi. In addition, Hg concentrations in seston and small zooplankton, sampled during 2009 at 12 sites spanning the lake, decreased with increasing phytoplankton and zooplankton biomass. Thus, Hg bioaccumulation in zooplankton across basins in Lake Champlain is related to trophic status, as observed previously in multiple lake studies.
Keywords: Methylmercury, Biomass dilution, Zooplankton, Trophic, Lake Champlain
1. Introduction
Large lakes such as Lake Champlain (1127 km2) and the Great Lakes are physically complex and contain basins with distinct trophic characteristics that can span the range of conditions found across multiple lakes in a broad region. In numerous multi-lake studies conducted throughout the US, mercury (Hg) bio-accumulation and trophic transfer in lakes was related to physical, chemical, and ecological factors, including positive relationships to lake and watershed area, and negative relationships to human land use, nutrient concentrations, pH, alkalinity, and abundances of phytoplankton and zooplankton (Watras et al., 1998; Kamman et al., 2004; Chen et al., 2005; Driscoll et al., 2007; Yu et al., 2011). In general, when compared to lakes of other trophic status, eutrophic lakes have lower Hg concentrations in top trophic level fish compared to lakes with lower productivity, in part due to higher zooplankton biomass and lower Hg concentrations in zooplankton (Chen and Folt, 2005; Chen et al., 2005).
Although these spatial patterns in Hg bioaccumulation and trophic transfer have been documented for different lakes within a region, studies have not been conducted within larger lakes, where trophic conditions can vary within their basins. Large lakes such as the Laurentian Great Lakes in the Upper Midwest and Lake Champlain in Vermont and New York (USA) have extensive and varying watersheds surrounding basins that are often hydrologically distinct. These separate basins can vary in trophic status ranging from highly eutrophic to oligotrophic, the same range of conditions captured in past studies of multiple lakes. Although the differences in trophic status among basins have been documented in these large lake ecosystems (McCarty et al., 2004), the influence of such differences on Hg bioaccumulation and transfer in the food web has not been examined.
We hypothesize that the mechanisms influencing between-lake differences in Hg bioaccumulation would also cause differences among basins within large lakes.
Eutrophic lakes have chemical and ecological attributes that may result in reduced Hg bioaccumulation and food web transfer relative to oligotrophic lakes. Increases in lake water pH and alkalinity may reduce the MeHg available for bioaccumulation in the food web (Driscoll et al., 2007). In addition, fish in eutrophic lakes may exhibit growth dilution of mercury when food is abundant, reducing concentrations in tissues relative to those in fish inhabiting oligotrophic lakes (Essington and Houser, 2003; Ward et al., 2009, in review). Nutrient conditions in eutrophic lakes stimulate algal blooms that spread the pool of dissolved inorganic and MeHg over a greater biomass of algal cells (Pickhardt et al., 2002; Luengen and Flegal, 2009), thereby reducing Hg and MeHg concentrations in algae and their zooplankton grazers (Chen and Folt, 2005; Chen et al., 2005). The increase in algal biomass may also alter other factors (e.g., biogeochemical) that could change MeHg production or the degree of Hg uptake via absorption or ingestion of non-living particulate organic matter.
Studies of temporal variability of Hg bioaccumulation in lakes have most often focused on intra-annual changes demonstrating that variability in Hg bioaccumulation through the growing season differs greatly between lakes (Herrin et al., 1998; Monson and Brezonik, 1998; McCarty et al., 2004; Ward et al., in review). However, little is known about inter-annual variation within a single lake or a single basin within a lake. Algal blooms can vary from year to year in lakes, with major nuisance algal blooms in some years and none in others. These temporal changes within lake systems could greatly influence the bioaccumulation of Hg by lower trophic levels and transfer of Hg to higher trophic levels.
Lake Champlain is a spatially complex large lake that experiences elevated Hg levels in fish, leading to fish consumption advisories for top trophic level species such as walleye, Sander vitreus (Vermont Department of Health, 2007). This is despite the low water column concentrations of total mercury (THg) and MeHg as compared to smaller lakes in the region (Shanley et al., 1999; Kamman et al., 2004; Gao et al., 2006). Trophic status and Hg inputs vary greatly across different basins in Lake Champlain (Gao et al., 2006; Miller et al., in review). The most oligotrophic basin in the lake is Malletts Bay in the mid-reach of the lake and the most eutrophic is Missisquoi Bay at the northernmost end bordering Canada. Missisquoi Bay has received substantial nutrient inputs from the surrounding agricultural watershed that have stimulated annual algal blooms. Most of the remaining basins in the lake are mesotrophic, surrounded by watersheds with mixed land uses dominated by forest and agriculture (LCBP, 2010).
In this study, we address the overarching questions of whether basins within a large lake ecosystem like Lake Champlain differ significantly in the degree to which Hg enters the base of the food web. We investigated factors controlling Hg bioaccumulation in zooplankton across contrasting basins in Lake Champlain to identify the strongest chemical and ecological predictors of Hg bio-accumulation in zooplankton, which are important in the trophic transfer of MeHg to fish. To do this, we used two different approaches. In the first, we compared two basins within the lake, one eutrophic and the other oligotrophic, over 4 consecutive years to determine if bioaccumulation of THg and MeHg in large zooplankton differed between basins and whether differences changed through time. In the second, we conducted a broader spatial analysis of 12 sites within Lake Champlain during one season and identified chemical and ecological factors that predict elevated Hg concentrations in particulate and zooplankton size fractions.
2. Methods
2.1. Two-basin comparison
2.1.1. Sampling
In 2005, 2006, 2007 and 2008, we compared Hg bioaccumulation in zooplankton from Malletts Bay and Missisquoi Bay in mid-summer. Two size fractions of zooplankton, small (45–202 μm) and large (>202 μm), were sampled each year. The smaller size fraction incorporates taxa that are primarily herbivores and the larger size fraction incorporates large omnivores. Past studies indicate that the size fractions represent 2 different trophic levels with higher MeHg concentrations in the larger fraction (Chen et al., 2000; Ward et al., in review). Zooplankton were collected with multiple tows in the deepest portion of each site in the lake from 0.5 m above the bottom to the surface with a cone net (202-μm nylon mesh) for large zooplankton, and a Wisconsin net (45-μm nylon mesh) for small zooplankton until enough sample was obtained for each size fraction (>50 mg DW). The contents of the 45-μm net were filtered through a 202-μm filter (with >202 μm material discarded) to generate a sample representing the 45–202-μm size range. Zooplankton samples were taken from one location in each basin on one date in late August of each year (except in 2007, when samples were taken in June and July). Multiple plankton tows (3–5) were usually taken for each replicate sample. Triplicate samples for Hg speciation analysis were collected for both zooplankton size fractions. Samples were placed on ice in the dark and transported to the laboratory, where they were transferred to pre-weighed trace-metal clean glass vials, weighed, freeze dried, and reweighed to determine sample dry weight.
To minimize contamination, zooplankton sampling was conducted with great care with previously established protocols (Chen et al., 2000). Zooplankton samples were collected with trace-metal clean technique and non-metallic sampling gear, deployed from an aluminum boat with outboard motor. Prior to field sampling, Teflon™ sample vials and sampling apparatus were acid cleaned in sequential concentrated nitric acid, dilute HCl, and trace-metal grade, dilute nitric acid baths. Plankton nets were rinsed in Citranox and deionized water.
Data for phytoplankton abundance (measured as chlorophyll a, phytoplankton biovolume, and phytoplankton density), zooplankton density, and water quality parameters (total phosphorus (TP), total nitrogen (TN), dissolved organic carbon (DOC)) for 2005–2008 were obtained from the water quality monitoring conducted by the Vermont Department of Environmental Conservation (VTDEC) Lake Champlain Monitoring Program (LCMP, 2010). We used data obtained during LCMP sample dates that were closest to those dates when our sampling was conducted.
2.1.2. Hg and MeHg analyses
All freeze-dried zooplankton samples were analyzed at the Trace Element Analysis (TEA) Facility at Dartmouth College. Hg speciation of small and large zooplankton was conducted with isotope dilution and alkaline digestion followed by purge and trap GC-ICPMS. The zooplankton samples were first spiked with appropriate amounts of enriched Hg199 and MeHg201 and then extracted by standard alkaline extraction procedures. Our previous work (which describes these techniques in detail) has shown that this double-spiking method can produce accurate and precise measurements of both inorganic Hg and MeHg from biological tissues (Taylor et al., 2008; Jackson et al., 2009). Quality control was achieved by analyses of two standard reference materials, TORT-2 (NRC-CNRC Canada n = 1) and Mussel 2976 (NIST, Gaithersburg, MD, n = 11). Recoveries of MeHg and total Hg were 120% and 118% for TORT 2, respectively, and 102% and 101%, respectively, for NIST 2976. Coefficients of variation for MeHg and total Hg for NIST 2976 were 11.4% and 10.9%, respectively. The method detection limits (based on 3 standard deviations of 11 blank extracts) were 18 pg and 0.43 ng and the average blank values were 12 pg and 0.59 ng for MeHg and inorganic Hg, respectively. All sample values for MeHg were above blank + 3 standard deviations. For inorganic Hg, all sample values were above the mean blank value.
2.2. Lake-wide comparison
In 2009, we sampled water, seston, and zooplankton at 12 stations representing the full range of trophic conditions in the lake (Fig. 1). Samples were collected between August 7 and September 11, 2009 during scheduled sampling of designated water quality monitoring stations conducted by the VTANR-LCMP (Vermont Department of Environmental Conservation, 2009). Water quality sampling included measurements of chlorophyll a, secchi depth, temperature, alkalinity, nutrients (TN and TP), chloride, and dissolved oxygen. The methods for this sampling are described elsewhere (Vermont Department of Environmental Conservation, 2009).
Fig. 1.
Map of Lake Champlain with 12 sampling sites, including Malletts Bay (oligotrophic) and Missisquoi Bay (eutrophic). Site codes are as follows: South Lake A (4), Port Henry (7), Otter Creek (9), Shelburne Bay (16), Main Lake (19), Burlington Bay (21), Malletts Bay (25), Northeast Arm (34), Isle LaMotte off Grand Isle (36), St. Albans Bay (40), Isle LaMotte off Rouses Point (46), Missisquoi Bay (51).
Sampling for Hg speciation analysis, seston mass, and DOC was conducted in triplicate. Samples were collected off the windward bow rail of a fiberglass research vessel. Water grab samples for Hg speciation were collected at ~20 cm depth in the epilimnion with the EPA “clean hands/dirty hands” method directly into pristine 2-L PTEG sampling vessels (see Jackson et al., 2009). Samples were triple bagged and placed immediately in the dark on ice in a cooler for shipment to the laboratory. Separate 2-L grab samples were collected in PTEG bottles for gravimetric analysis of seston mass. These samples were also stored in the dark on ice. Filtered samples were collected from the filtrate of the VTDEC chlorophyll a sampling apparatus (0.2-μm Teflon filter; see Vermont Department of Environmental Conservation, 2009) into pre-combusted 60-ml amber glass bottles and stored in the dark on ice for later measurement of DOC.
Field sampling personnel transported samples on ice directly to the Dartmouth College Trace Element Analytical Facility laboratory within 48 h of collection. Previous work (Jackson et al., 2009) identified a 48-h hold time as acceptable to maintain mercury speciation in Lake Champlain waters. The 2-L water samples were immediately filtered with pre-combusted Whatman GF/F filters to operationally separate seston from “dissolved” mercury. Filter blanks were analyzed with each set of samples. Inorganic mercury and MeHg were determined in both the filtrate and particulate (seston) fractions following the ultra low-level methods described in Jackson et al. (2009). The 2-L samples collected for seston mass determinations were filtered in the same manner as the mercury speciation samples with the exception that the filters were weighed pre- and post- filtration (after drying).
Samples collected for measurement of DOC were shipped on ice to the University of New Hampshire Water Resources Research Center. Samples were held in the dark at 4 °C for up to 4 weeks prior to analysis. Samples were analyzed for DOC with a Shimadzu TOCV-CH and EPA Method 415.1.
Zooplankton samples in two size fractions for Hg speciation and biomass analyses were collected as described above with some modifications. Multiple tows with a bongo net frame with 45- and 202-μm nets were conducted until enough sample of each size fraction was obtained for analysis. Vertical tows were conducted from 1 m above the bottom to the surface; length and number of tows was recorded. Due to shallow water depth in Missisquoi Bay, horizontal zooplankton tows were taken, and tow distance was recorded with GPS. Water volumes sampled for zooplankton were calculated from the net opening diameter and tow length (Stemberger and Chen, 1998). Freeze-dried zooplankton samples were analyzed at the Dartmouth TEA Facility for Hg speciation measurements as described above.
2.3. Data analysis
2.3.1. Two-basin comparison
Total Hg in large zooplankton was compared between sites and sample dates for Malletts Bay and Missisquoi Bay but small zooplankton data were not included in the statistical analysis because of missing data due to low biomass samples and algal biomass in the samples. Concentrations of THg and MeHg and percent MeHg in large zooplankton in Malletts and Missisquoi Bays from 2005 to 2007 were compared with two-way ANOVA to test for site and year differences and site × sample date interactions. Data for THg and MeHg were log10 transformed and percent MeHg data were arcsin transformed. Data for 2008 were not included in the analysis because there were only two replicate samples per site. One-way ANOVAs were also calculated to compare sites within each year for 2005, 2006, and 2007.
2.3.2. Lake-wide comparison
For lake-wide patterns of Hg bioaccumulation across 12 sites sampled in 2009, environmental variables (DOC, chlorophyll a, phytoplankton biovolume and density) and concentrations of Hg and MeHg in seston, particulates, and small (48–202 μm) and large (>202 μm) zooplankton were compared with Spearman rank correlation. All data analyses were conducted with JMP 5.0.1a (SAS Institute Inc., 2002).
3. Results
3.1. Two-basin comparison
Environmental measurements in the oligotrophic and eutrophic basins, Malletts and Missisquoi Bay, reflected their long-term, differing trophic status in all years but 2007 (Table 1). In 2005–2008, total P was consistently lower in Malletts Bay than in Missisquoi Bay by a factor of 4.6–8.8 across years. However, differences in chlorophyll a between the two basins were much lower in August 2007 (1.6 times) compared to other years (3.7–7.7 times), and phytoplankton density in 2007 was greater in Malletts Bay in June 2007 and virtually the same (1.07 times) in both basins in August 2007 compared to much greater densities in Missisquoi than Malletts Bay in August 2006 (5.7 times) and 2008 (355 times). Data for zooplankton total densities were only available from VTDEC for 2005 (June) and 2006 and 2007 (June and August, Table 1). In June 2005 and 2006 zooplankton densities were slightly greater in Malletts Bay than Missisquoi Bay. But in August 2006, Malletts Bay zooplankton densities were 3.8 times lower than those in Missisquoi Bay, coincident with an algal bloom in Missisquoi Bay. In contrast, Mallets Bay zooplankton densities were 6 times higher than those in Missisquoi in June 2007 and 3 times higher in August 2007 when there was no algal bloom in Malletts Bay.
Table 1.
Environmental factors and large zooplankton Hg data for Malletts and Missisquoi Bays on sample dates in 2005–2008. Data are from the Lake Champlain Monitoring Program. TP = total phosphorus, TN = total nitrogen, Chl a = Chlorophyll a, na = not available. Mean and standard error (in parentheses) are given for THg, MeHg, and %MeHg in large zooplankton.
| Bay and sampling date
|
TP (mg/L) | TN (mg/L) | Chl a (mg/L) | Phytoplankton density (cells/ml) | Zooplankton density (no./ml) | Mercury in large zooplankton, DW (>202 μm)
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | Month | THg (ng/g) | MeHg (ng/g) | MeHg (%) | N | |||||
| Malletts Bay | ||||||||||
| 2005 | August | 8.4 | 0.23 | 3.76 | na | na | 78 (14) | 18 (0.7) | 25 (3) | 3 |
| 2006 | August | 8.6 | 0.32 | 4.54 | 239,000 | 31,000 | 71 (3) | 28 (0.4) | 39 (1) | 3 |
| 2007 | June | 9.8 | 0.40 | 2.90 | 48,800 | 63,600 | 333 (28) | 25 (4) | 78 (17) | 3 |
| 2007 | August | 7.0 | 0.30 | 2.95 | 166,000 | 27,900 | 620 | na | na | 1 (July) |
| 2008 | June | 17.1 | 0.35 | 2.35 | 42,500 | na | 308 (56) | 91 (18) | 30 (4) | 3 |
| 2008 | August | 13.7 | 0.32 | 3.70 | 62,500 | na | 195 (54) | 98 (4) | 54 (13) | 2 |
| Missisquoi Bay | ||||||||||
| 2005 | August | 74.7 | 0.52 | 29.00 | na | 321,000 | 24 (1) | 3 (0.2) | 14 (1) | 3 |
| 2006 | August | 43.0 | 0.63 | 17.00 | 1,360,000 | 118,000 | 30 (1) | 10 (0.4) | 34 (3) | 3 |
| 2007 | June | 48.0 | 0.70 | 21.30 | 6370 | 10,300 | 65 (42) | 33 (14) | 46 (7) | 3 |
| 2007 | August | 50.8 | 0.35 | 4.84 | 179,000 | 9350 | 440 | na | na | 1 (July) |
| 2008 | June | 41.9 | 1.00 | 14.60 | 150,000 | na | 74 (6) | 31 (5) | 42 (10) | 2 |
| 2008 | August | 63.8 | 0.59 | 26.80 | 22,200,000 | na | 25 (20) | 4 (2) | 27 (12) | 2 |
THg in large zooplankton varied greatly within site: in Malletts Bay, concentration across years ranged from 70 to 330 ng/g DW and in Missisquoi from 24 to 65 ng/g DW. MeHg concentration in large zooplankton also varied within site across years: Malletts Bay, 18–99 ng/g DW and in Missisquoi, 3–33 ng/g DW. Percent MeHg also varied greatly across sample dates within site: Malletts Bay, 7.7–41%, and in Missisquoi, 14–48%. Comparisons of THg, MeHg, and %MeHg in large zooplankton in Malletts and Missisquoi Bay with two-way ANOVA were significant for all three Hg variables. THg in large zooplankton was significantly lower in Missisquoi Bay than Malletts Bay across years (Fig. 2a); however, there was a significant sample date effect due to the differences in THg concentrations in large zooplankton, particularly in Malletts Bay where there was a marked increase in 2007 (and in 2008 which was not included in the analysis). For MeHg, there were also significant site and sample date effects due to changes in the relative differences between sites across years (Tables 1 and 2). MeHg concentrations were significantly greater in Malletts Bay than in Missisquoi Bay in 2005 and 2006 (one-way ANOVA; p = 0.0001 for both years), but the two sites did not differ in 2007 (p = 0.5939). Although the differences in MeHg and %MeHg in 2007 could have been due to the month sampled (June rather than August), temporal data for 2008 showed relative differences between sites for THg and MeHg to be consistent from June through August (Eric Miller, Ecosystems Research Group, Ltd., Norwich, VT, USA, unpublished data), suggesting that the unusual lack of a bloom in 2007 rather than the month sampled was the reason for the differences in MeHg concentrations and %MeHg. Moreover, single data points for THg in July 2007 (Missisquoi 440 ng/g, Malletts 620 ng/g) showed the relative magnitudes to be similar to June 2007. Two-way ANOVA of %MeHg between sites across sample dates showed no significant site or sample date effect, but a significant interaction of the site and sample date (Tables 1 and 2). This was due to the within site variability across sample dates and the switch from greater %MeHg in Malletts Bay in 2005–2006 to greater %MeHg in Missisquoi Bay in 2007. Interestingly, the return of a bloom in 2008 coincided with the %MeHg in Missisquoi Bay once again dropping lower than in Malletts Bay. However, relative values of %MeHg across the season in 2008 did not show consistent differences between the two basins (Table 1, Eric Miller, Ecosystems Research Group, Ltd., Norwich, VT, USA, unpublished data).
Fig. 2.
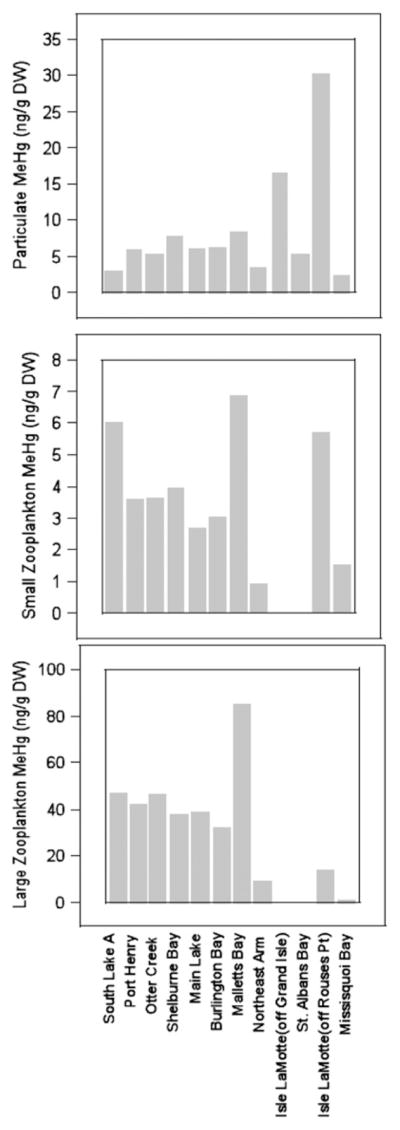
Concentrations of MeHg (ng/g dry weight) in suspended particulate matter and zooplankton across 12 sites sampled in 2009: (a) particulates; (b) 48–202 μm zooplankton; and (c) >202 μm zooplankton.
Table 2.
Results of two-way ANOVA for THg, MeHg, and %MeHg in large zooplankton >202 μm between two embayments (Malletts and Missisquoi) in Lake Champlain across years (2005, 2006, 2007). P-values are shown for the full model, site, year, and site × year interaction (n = 18).
| Factor | Model p-value | Adjusted R2 | Site | Year | Site × Year |
|---|---|---|---|---|---|
| Total Hg (ng/g) | 0.0001 | 0.7349 | 0.0001 | 0.0067 | 0.1293 |
| MeHg (ng/g) | 0.0022 | 0.4716 | 0.0008 | 0.0338 | 0.5581 |
| %MeHg (arcsin transformed) | 0.0040 | 0.5151 | 0.1466 | 0.2110 | 0.0011 |
3.2. Lake-wide comparison
Concentrations of MeHg in seston, particulates, small and large zooplankton varied greatly over the 12 sampling locations (Tables 3 and 4). It is likely that some algae were present in small zooplankton samples from Missisquoi Bay due to the algal bloom in mid-summer. To determine predictors of Hg bioaccumulation in zooplankton across 12 sites in the lake sampled in 2009, Spearman rank correlation analyses of environmental variables related to Hg and MeHg concentrations in seston, particulates, and zooplankton showed that THg and MeHg concentrations in seston decreased with increasing phytoplankton measured as phytoplankton bio-volume (Rho = −0.6606, p = 0.0376 for THg: Rho = −0.8182, p = 0.0038 for MeHg), chlorophyll a (Rho = −0.7818, p = 0.0075 for THg), or phytoplankton density (Rho = −0.6970, p = 0.0251 for MeHg). Similar relationships were found for particulate MeHg concentrations, which decreased with increasing DOC and phytoplankton biovolume. Seston MeHg concentrations were also negatively related to DOC (Rho = −0.7762, p = 0.0030). In addition, THg concentrations in small (48–202 μm) zooplankton decreased with increasing small zooplankton biomass (Rho = −0.6364, p = 0.0479) and MeHg in large (>202 μm) zooplankton increased significantly with increased MeHg in small zooplankton (Rho = 0.7091, p = 0.0217). These latter relationships may have been influenced by algal biomass in the small zooplankton samples. However, none of the relationships between seston or particulate THg or MeHg and small or large zooplankton THg or MeHg were significant.
Table 3.
2009 lake-wide data for nutrients and DOC in water and seston measurements of Hg, phytoplankton abundance, and nutrients (SE = standard error, na = not available).
| Lake basin
|
Seston
|
THg (ng/g)
|
MeHg (ng/g)
|
DOC (mg/L)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Name | Date | N | mean | SE | mean | SE | N | mean | SE |
| 4 | South Lake A | 8/19/09 | 3 | 30.2 | 0.7 | 3.05 | 0.01 | 3 | 4.33 | 0.12 |
| 7 | Port Henry | 8/5/09 | 3 | 64.1 | 7.8 | 4.62 | 0.17 | 3 | 3.27 | 0.02 |
| 7 | Port Henry | 8/24/09 | 3 | 54.2 | 6.7 | 5.92 | 0.29 | 3 | 3.46 | 0.02 |
| 7 | Port Henry | 9/8/09 | 3 | 104.5 | 9.8 | 9.85 | 0.39 | 3 | 3.19 | 0.07 |
| 9 | Otter Creek | 8/5/09 | 3 | 54.4 | 3.5 | 4.36 | 0.22 | 3 | 3.35 | 0.03 |
| 9 | Otter Creek | 8/24/09 | 3 | 48.6 | 1.7 | 5.39 | 0.63 | 3 | 3.19 | 0.04 |
| 16 | Shelburne Bay | 8/12/09 | 3 | 59.9 | 4.0 | 7.87 | 0.63 | 3 | 3.02 | 0.01 |
| 19 | Main Lake | 8/12/09 | 3 | 72.4 | 6.4 | 7.87 | 1.18 | 3 | 2.98 | 0.04 |
| 19 | Main Lake | 8/26/09 | 3 | 56.6 | 0.8 | 6.15 | 0.27 | 3 | 3.32 | 0.09 |
| 21 | Burlington Bay | 8/26/09 | 3 | 61.0 | 1.9 | 6.20 | 0.48 | 3 | 3.26 | 0.05 |
| 25 | Malletts Bay | 8/18/09 | 3 | 103.2 | 4.8 | 8.39 | 0.15 | 3 | 3.55 | 0.23 |
| 25 | Malletts Bay | 9/10/09 | 3 | 45.3 | 1.7 | 4.32 | 0.44 | 3 | 3.27 | 0.04 |
| 33 | Cumberland Bay | 8/10/09 | 3 | 105.2 | 7.2 | 9.01 | 0.32 | 3 | 3.17 | 0.05 |
| 34 | Northeast Arm | 8/6/09 | 3 | 80.3 | 20.4 | 9.56 | 3.44 | 3 | 3.35 | 0.06 |
| 34 | Northeast Arm | 8/25/09 | 3 | 52.9 | 3.4 | 3.41 | 0.53 | 3 | 3.61 | 0.15 |
| 36 | Isle LaMotte (off Grand Isle) | 8/10/09 | 3 | 184.7 | 94.4 | 16.48 | 7.61 | 3 | 2.93 | 0.05 |
| 40 | St. Albans Bay | 8/6/09 | 3 | 79.3 | 6.2 | 5.45 | 0.73 | 3 | 3.53 | 0.04 |
| 46 | Isle LaMotte (off Rouses Pt) | 8/11/09 | 3 | 216.4 | 94.8 | 30.33 | 11.51 | 3 | 2.96 | 0.03 |
| 51 | Missisquoi Bay | 8/11/09 | 3 | 65.8 | 1.0 | 2.34 | 0.21 | 3 | 4.60 | 0.15 |
| Lake basin no. | Chlorophyll-a (mg/L) | Phytoplankton biovolume (μm3/L) | Phytoplankton density (cells/L) | Temperature (°C) | Total nitrogen (mg/L) | Total phosphorus (μg/L) | Dissolved phosphorus (μg/L) |
|---|---|---|---|---|---|---|---|
| 4 | 12.6 | 2,200,000,000 | 851,000 | 25.7 | 0.41 | 41.9 | 14.3 |
| 7 | 6.48 | 180,000,000 | 281,000 | 20.8 | 0.33 | 18.5 | 7.1 |
| 7 | 8.17 | 635,000,000 | 685,000 | 22.7 | 0.32 | 19.8 | 9.6 |
| 7 | 4.14 | 16,900,000 | 68,600 | 20.7 | 0.32 | 16.9 | 7.6 |
| 9 | 7.08 | 173,000,000 | 142,000 | 21.6 | 0.33 | 18.7 | 9 |
| 9 | 9.03 | 185,000,000 | 298,000 | 23.0 | 0.28 | 12.6 | 6.8 |
| 16 | 0.76 | 23,200,000 | 88,000 | 23.2 | 0.31 | 12.7 | 6.8 |
| 19 | 1.23 | 84,500,000 | 157,000 | 22.2 | 0.32 | 10.3 | 6.4 |
| 19 | 3.77 | 117,000,000 | 220,000 | 22.5 | 0.3 | 11.3 | 5 |
| 21 | 5.21 | 56,400,000 | 304,000 | 23.6 | 0.32 | 13.4 | 7.6 |
| 25 | 3.48 | 92,900,000 | 234,000 | 25.2 | 0.27 | 9.8 | 6.7 |
| 25 | 4.37 | 103,000,000 | 199,000 | 21.2 | 0.24 | 8.8 | 5 |
| 33 | na | na | na | na | na | na | na |
| 34 | 1.92 | 415,000,000 | 184,000 | 21.9 | 0.25 | 14.8 | 7.3 |
| 34 | 5 | 113,000,000 | 167,000 | 23.9 | 0.25 | 15.2 | 9.6 |
| 36 | na | na | na | na | na | na | na |
| 40 | na | na | na | na | na | na | na |
| 46 | 2.13 | 6,600,000 | 13,300 | 23.0 | 0.3 | 21.8 | 18.3 |
| 51 | 2.52 | 395,000,000 | 2,880,000 | 22.9 | 0.51 | 62.9 | 37.7 |
Table 4.
Biomass and concentrations of total Hg and MeHg in small zooplankton (45–200 μm) and large zooplankton (>200 μm) in basins of Lake Champlain sampled in 2009 (SE = standard error, na = not available).
| Lake basin | Date | N | Biomass (μg/L)
|
THg (ng/g)
|
MeHg (ng/g)
|
|||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | |||
| Small zooplankton | ||||||||
| South Lake A | 8/19/09 | 3 | 19.4 | 0.3 | 23.0 | 0.7 | 6.03 | 0.07 |
| Port Henry | 8/5/09 | 1 | 2.6 | na | na | na | na | na |
| Port Henry | 8/24/09 | 3 | 7.2 | 2.9 | 32.2 | 7.8 | 3.63 | 0.45 |
| Port Henry | 9/8/09 | 3 | 2.3 | 0.1 | 22.0 | 2.3 | 2.43 | 0.42 |
| Otter Creek | 8/5/09 | 1 | 3.4 | na | na | na | na | na |
| Otter Creek | 8/24/09 | 3 | 3.4 | 0.1 | 31.0 | 0.7 | 3.67 | 0.03 |
| Shelburne Bay | 8/12/09 | 3 | 8.5 | 0.7 | 21.7 | 2.3 | 3.97 | 0.37 |
| Main Lake | 8/12/09 | 3 | 3.3 | 0.2 | 40.6 | 19.1 | 2.77 | 0.12 |
| Main Lake | 8/26/09 | 3 | 1.8 | 0.6 | 46.4 | 9.4 | 2.70 | 0.15 |
| Burlington Bay | 8/26/09 | 3 | 5.5 | 0.7 | 28.9 | 4.0 | 3.07 | 0.09 |
| Malletts Bay | 8/18/09 | 3 | 3.4 | 0.5 | 64.0 | 3.6 | 6.90 | 0.36 |
| Malletts Bay | 9/10/09 | 3 | 4.6 | 0.6 | 23.0 | 3.3 | 1.73 | 0.03 |
| Northeast Arm | 8/6/09 | 1 | 6.1 | na | na | na | na | na |
| Northeast Arm | 8/25/09 | 3 | 2.6 | 0.2 | 27.7 | 2.5 | 0.95 | 0.05 |
| Isle LaMotte (off Rouses Pt) | 8/11/09 | 3 | 1.4 | 0.3 | 34.7 | 6.7 | 5.73 | 0.35 |
| Missisquoi Bay | 8/11/09 | 2 | 52.8 | 8.0 | 18.7 | 0.5 | 4.15 | 2.15 |
| Large zooplankton | ||||||||
| South Lake A | 8/19/09 | 3 | 9.7 | 0.9 | 37.4 | 11.6 | 47.45 | 0.15 |
| Port Henry | 8/5/09 | 1 | 14.8 | na | na | na | na | na |
| Port Henry | 8/24/09 | 3 | 11.7 | 1.6 | 33.3 | 0.7 | 42.15 | 2.35 |
| Port Henry | 9/8/09 | 3 | 6.7 | 0.4 | 18.8 | 3.2 | 28.27 | 1.15 |
| Otter Creek | 8/5/09 | 1 | 13.4 | na | na | na | na | na |
| Otter Creek | 8/24/09 | 3 | 6.9 | 0.3 | 41.0 | 1.1 | 46.77 | 1.05 |
| Shelburne Bay | 8/12/09 | 3 | 12.6 | 0.7 | 68.1 | 7.0 | 38.07 | 1.58 |
| Main Lake | 8/12/09 | 3 | 6.5 | 0.5 | 27.4 | 2.1 | 38.00 | 7.88 |
| Main Lake | 8/26/09 | 3 | 8.9 | 0.7 | 25.4 | 1.3 | 39.03 | 10.99 |
| Burlington Bay | 8/26/09 | 2 | 8.5 | na | 31.4 | na | 31.90 | na |
| Malletts Bay | 8/18/09 | 3 | 5.4 | 0.9 | 52.2 | 4.1 | 84.93 | 3.34 |
| Malletts Bay | 9/10/09 | 3 | 5.1 | 0.5 | 27.5 | 0.9 | 29.43 | 1.79 |
| Northeast Arm | 8/6/09 | 1 | 23.0 | na | na | na | na | na |
| Northeast Arm | 8/25/09 | 3 | 14.3 | 3.0 | 24.4 | 4.6 | 9.30 | 3.49 |
| Isle LaMotte (off Rouses Pt) | 8/11/09 | 3 | 0.9 | 0.5 | 55.1 | 16.1 | 14.37 | 6.17 |
| Missisquoi Bay | 8/11/09 | 3 | 23.0 | 7.3 | 32.0 | 5.8 | 1.07 | 0.26 |
4. Discussion
The patterns of Hg bioaccumulation in contrasting basins of Lake Champlain are consistent with the patterns of Hg bio-accumulation across studies of multiple lakes that capture ranges of environmental conditions and trophic status. Just as individual eutrophic lakes exhibit a biomass dilution of Hg in lower trophic levels, the more eutrophic basins in Lake Champlain also have higher densities and biovolumes of phytoplankton as well as higher mass pools (μg dry mass per liter of lake water) of seston (the fraction containing phytoplankton, cyanobacteria, other microorganisms, and abiotic particles). Eutrophic Missisquoi Bay has lower THg and MeHg concentrations in large zooplankton than in oligotrophic Malletts Bay in most years. This is likely due to the higher algal densities stimulated by nutrient inputs from the largely agricultural land use in the watershed of Missisquoi Bay. However, this biomass dilution of MeHg was not evident in Missisquoi Bay in 2007 when no algal bloom occurred. In early summer, the MeHg concentrations were not different between bays and the %MeHg in zooplankton in Missisquoi Bay was greater than in Malletts Bay. Across multiple basins in Lake Champlain, a similar spatial pattern emerged; lake compartments with higher DOC, particulate densities, and chlorophyll a had lower concentrations of MeHg in particulates and seston, and small zooplankton had lower MeHg concentrations in basins with higher biomass of small zooplankton (Tables 3 and 4). Both these lines of evidence suggest that the trophic status of these contrasting basins influences the degree of bioaccumulation of THg and MeHg by zooplankton. Moreover, the bioaccumulation of Hg by large zooplankton in Lake Champlain largely determines the trophic transfer of Hg to fish (Miller et al., in review).
Trophic status and DOC concentrations have been related to Hg bioaccumulation in phytoplankton, zooplankton, and fish in past lake studies. Sensitivity thresholds have been calculated for Hg in fish based upon total phosphorus and DOC across multiple lakes in the northeast US (Driscoll et al., 2007). Based on total phosphorus sensitivity thresholds, Malletts Bay falls in the range of systems (below 30 ng/g total phosphorus in water) predicted to have high Hg in fish. In contrast, sensitivity thresholds for DOC suggest the opposite: Missisquoi Bay with higher DOC levels (4.3 mg/L) has greater potential to have fish with >300 ng/g MeHg than Malletts Bay with lower DOC (3.4 mg/L) (Driscoll et al., 2007). However in this study, DOC was negatively related to MeHg in seston indicating that DOC may reduce the bioavailability of MeHg to plankton, as suggested in other studies (Watras et al., 1995; Kamman et al., 2004). Alternatively, the different response may be due to differences in the sources of DOC in Lake Champlain vs. the lakes studied in Driscoll et al. (2007), which were generally smaller lakes where DOC is primarily of terrestrial origin including wetlands. In Lake Champlain and other large lakes the DOC is primarily of aquatic origin (secreted by phytoplankton). Thus, large lake DOC maybe somewhat decoupled from watershed transport of Hg, while in small lakes DOC is well correlated with watershed sources of organic matter. Terrestrial sources of DOC may still exert influence on Hg availability in smaller lake segments with restricted circulation.
In a study of 20 lakes of varied trophic status, THg concentrations in particulates decreased with increasing chlorophyll concentrations suggesting, algal biomass dilution (Chen and Folt, 2000). The range of chlorophyll concentrations in that field study (0.7–13.7 μg/L) was exceeded by the range for Lake Champlain (3.0–29.0 μg/L). This suggests that there could be even greater biomass dilution of Hg by algal blooms in Lake Champlain, particularly in Missisquoi Bay. Biomass dilution in experimentally induced algal blooms in mesocosm tanks spanned a range of chlorophyll concentrations up to 30 μg/L, comparable to the highest levels in Lake Champlain (Pickhardt et al., 2002).
The concentrations of THg in zooplankton in Lake Champlain fall within the range of concentrations found in other lake studies and are comparable to the range of concentrations found in smaller lakes in Vermont and New Hampshire (Watras et al., 1998; Chen et al., 2005). The range of concentrations of THg in zooplankton in Lake Champlain are on average similar to ranges found in Lake Michigan (11–376 ng/g) and Lake Superior (20–130 ng/g DW) (Mason and Sullivan, 1997; Back et al., 2003; USEPA, 2004) and lower than concentration ranges in smaller lakes in Wisconsin (33–206 ng/g DW) and the Adirondacks (7–890 ng/g) (Watras et al., 1998; Yu et al., 2011). In 15 small lakes in Wisconsin, Watras et al. (1998) found MeHg concentrations ranging from 6 to 161 ng/g DW in zooplankton, and Yu et al. (2011) found a concentration range of 0.7–250 ng/g DW across 44 Adirondack lakes. This is in contrast to a range of 1–47 ng/g MeHg DW across the 12 basins in Lake Champlain and 15–50 ng/g MeHg DW in Lake Superior, indicating that the range of MeHg concentrations are possibly lower in large lakes than small lakes (Back et al., 2003). Percent MeHg in Malletts and Missisquoi Bay was highly variable between and within basins—ranging from 4 to 53% for large zooplankton—which is slightly lower than values observed across Wisconsin lakes (15–83%) and Adirondack lakes (0–74%) (Watras et al., 1998; Yu et al., 2011). Finally, in Lake Champlain and other lakes in the region, the concentrations of MeHg in smaller zooplankton are correlated with MeHg in large zooplankton and exhibit biomagnification from small to large size classes (Chen et al., 2000; Ward et al., in review).
Other studies have linked MeHg concentrations in zooplankton to MeHg in fish (Westcott and Kalff, 1996). However, in Lake Champlain, fish THg was best related to zooplankton pools of THg, rather than MeHg (Miller et al., in review). Caution should be taken in inferring that generalized fish Hg concentrations among Lake Champlain basins will directly vary with Hg in zooplankton by lake segment. This is because in some instances, fish in Lake Champlain are highly mobile, ranging seasonally among different basins. For example, walleye captured in the Missisquoi River during spawning runs may in fact spend most of their lifecycle in more southern portions of the lake. The same may be said for other long-lived mobile fishes such as lake trout (Salvelinus namaycush) or salmon (Salmo salar). By contrast, smaller or more resident fishes, such as yellow perch (Perca flavescens), white perch (Morone Americana), or smallmouth bass (Micropterus dolomieu), may more directly reflect inter-basin differences in Hg of lower trophic level biota (Miller et al., in review). These important differences constitute important questions for further inquiry and modeling analyses.
The inter-annual differences in zooplankton bioaccumulation of Hg and MeHg in Malletts and Missisquoi Bays highlight the potential impact of seasonal algal blooms on Hg bioaccumulation and trophic transfer in lower levels of the food web. The lack of a bloom in 2007 in Missisquoi Bay was coincident with comparable MeHg concentrations in zooplankton in that year. When algal densities were once again high in Missisquoi relative to Malletts Bay in 2008, the MeHg concentrations in Missisquoi Bay zooplankton were once again lower due to biodilution resulting in reduced trophic transfer to fish.
5. Conclusions
Spatial and temporal patterns in THg and MeHg bio-accumulation in zooplankton in Lake Champlain demonstrate that Hg concentrations vary greatly with trophic status of different basins and that year-to-year variation in algal densities can cause large changes in the bioaccumulation of MeHg in lower levels of the food web. Factors controlling this variation in bioaccumulation are fundamentally linked to productivity and algal biomass. Therefore, land use and management practices that alter the productivity of individual basins in Lake Champlain can be expected to alter the bioaccumulation and ultimate fate of Hg in the lake’s food webs. However, the increase in phytoplankton biomass—while diluting the bioaccumulation of THg and MeHg—does not alter the total amount of Hg in the system since phytoplankton eventually die and sink down to the sediments. Nonetheless, the bioaccumulation of Hg and MeHg at the lower levels of the pelagic food web could influence trophic transfer of Hg and MeHg to planktivorous and piscivorous fish.
Acknowledgments
We thank Pete Stangel and Angela Shambaugh of the Vermont Department of Environmental Conservation for allowing us to coordinate our field work with their field monitoring program. We also thank Elizabeth Traver, Tessa Peart, Curtis Hansen, Eddie Moreno, and Lydia Smith for their assistance in the field and laboratory. This research was supported by NOAA grant number NA06OAR4600222 as part of the Lake Champlain Research Consortium. VTANR and USGS provided data and research support. We also received support from NIH Grant Number P42 ES007373 from the National Institute of Environmental Health Sciences.
Contributor Information
Celia Chen, Email: celia.chen@dartmouth.edu, celia.y.chen@dartmouth.edu.
Neil Kamman, Email: neil.kamman@state.vt.us.
Jason Williams, Email: jason.williams@terragraphics.com.
Deenie Bugge, Email: deenie.bugge@dartmouth.edu.
Vivien Taylor, Email: vivien.taylor@dartmouth.edu.
Brian Jackson, Email: brian.jackson@dartmouth.edu.
Eric Miller, Email: ekmiller@ecosystems.research.com.
References
- Back RC, Gorski PR, Cleckner LB, Hurley JP. Mercury content and speciation in the plankton and benthos of Lake Superior. Science of the Total Environment. 2003;304:349–354. doi: 10.1016/S0048-9697(02)00580-6. [DOI] [PubMed] [Google Scholar]
- Chen CY, Folt CL. Bioaccumulation and diminution of arsenic and lead in a freshwater food web. Environmental Science and Technology. 2000;34:3878–3884. [Google Scholar]
- Chen CY, Folt CL. High plankton biomass reduces mercury bio-magnification. Environmental Science and Technology. 2005;39:115–121. [PubMed] [Google Scholar]
- Chen CY, Folt CL, Stemberger RS, Blum JD, Klaue B, Pickhardt PC. Accumulation of heavy metals in food web components across a gradient of lakes. Limnology and Oceanography. 2000;45:1525–1536. [Google Scholar]
- Chen CY, Stemberger RS, Kamman NC, Mayes BM, Folt CL. Patterns of Hg bioaccumulation and transfer in aquatic food webs across multi-lake studies in the Northeast US. Ecotoxicology. 2005;14:135–147. doi: 10.1007/s10646-004-6265-y. [DOI] [PubMed] [Google Scholar]
- Driscoll CT, Han YJ, Chen CY, Evers DC, Lambert KF, Holsen T, Kamman NC, Munson R. Mercury contamination in remote forest and aquatic ecosystems in the Northeastern U.S.: sources, transformations and management options. Bioscience. 2007;57:17–28. [Google Scholar]
- Essington TE, Houser JN. The effect of whole lake nutrient enrichment on mercury concentration in age-1 yellow perch. Transactions of the American Fisheries Society. 2003;132:57–68. [Google Scholar]
- Gao N, Armatas NG, Shanley JB, Kamman NC, Miller EK, Keeler GJ, Scherbatskoy T, Holsen TM, Young T, McIlroy L, Drake S, Olsen B, Cady C. A mass balance assessment for mercury in Lake Champlain. Environmental Science and Technology. 2006;40:82–89. doi: 10.1021/es050513b. [DOI] [PubMed] [Google Scholar]
- Herrin RT, Lathrop RC, Gorski PR, Andren AW. Hypolimnetic methyl-mercury and its uptake by plankton during fall destratification: a key entry point of mercury into lake food chains? Limnology and Oceanography. 1998;43:1476–1486. [Google Scholar]
- Jackson B, Taylor V, Baker RA, Miller E. Low-level mercury speciation in freshwaters by isotope dilution GC-ICP-MS. Environmental Science and Technology. 2009;43:2463–2469. doi: 10.1021/es802656p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamman NC, Lorey PM, Driscoll CT, Estabrook R, Major A, Pientka B, Glassford E. Assessment of mercury in waters, sediments, and biota of New Hampshire and Vermont lakes, USA, sampled using a geographically randomized design. Environmental Toxicology and Chemistry. 2004;23:194–207. doi: 10.1897/03-170. [DOI] [PubMed] [Google Scholar]
- LCBP. Lake Champlain Basin Program – Opportunities for Action 2010. 2010 http://www.lcbp.org/
- Luengen AC, Flegal AR. Role of phytoplankton in mercury cycling in the San Francisco Bay estuary. Limnology and Oceanography. 2009;54:23–40. [Google Scholar]
- Mason RP, Sullivan KA. Mercury in Lake Michigan. Environmental Science and Technology. 1997;31:942–947. [Google Scholar]
- McCarty HB, Miller K, Brent RN, Schofield J, Rossmann R. Results of the Lake Michigan Mass Balance Study: Mercury Data Report. U.S. Environmental Protection Agency, Great Lakes National Program Office; Chicago, Illinois: 2004. EPA-905/R-01/012. [Google Scholar]
- Miller EK, Chen CY, Kamman N, Shanley J, Chalmers A, Jackson B, Taylor V, Smeltzer E, Stangel P, Shambaugh A. Mercury in the Lake Champlain food web. Ecotoxicology. doi: 10.1007/s10646-011-0829-4. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson BA, Brezonik PL. Seasonal patterns of mercury species in water and plankton from softwater lakes in Northeastern Minnesota. Biogeochemistry. 1998;40:147–162. [Google Scholar]
- Pickhardt PC, Folt CL, Chen CY, Klaue B, Blum JD. Algal blooms reduce the uptake of methylmercury in freshwater food webs. Proceedings of the National Academy of Sciences (USA) 2002;99:4419–4423. doi: 10.1073/pnas.072531099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. JMP™ The Statistical Discovery Software™ 5.0.1a. 2002. [Google Scholar]
- Shanley JB, Donlon AF, Scherbatskoy T, Keeler GJ. Mercury cycling and transport in the Lake Champlain basin. In: Manley TO, Manley PL, editors. Lake Champlain in Transition: From Research Toward Restoration. Water and Science Application 1. American Geophysical Union; Washington, DC: 1999. pp. 277–299. [Google Scholar]
- Stemberger RS, Chen CY. Fish tissue metals and zooplankton assemblages of Northeastern US lakes. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:339–352. [Google Scholar]
- Taylor VF, Jackson BP, Chen CY. Mercury speciation and total trace element determination of low-biomass biological samples. Analytical and Bio-analytical Chemistry. 2008;392:1283–1290. doi: 10.1007/s00216-008-2403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA. Results of the Lake Michigan Mass Balance Study: Mercury Data Report EPA 905 R-01-012. Great Lakes National Program Office; Chicago, IL: 2004. [Google Scholar]
- Vermont Department of Environmental Conservation. Long-Term Water Quality and Biological Monitoring Project for Lake Champlain. 2009 http://www.anr.state.vt.us/dec//waterq/lakes/docs/lcmonitoring/lp_lc-ltmworkplan.pdf.
- Vermont Department of Health. Mercury in Fish Health Alert. 2007 http://healthvermont.gov/enviro/fish_alert/fish_alert.aspx.
- Ward DM, Mayes B, Sturup S, Folt CL, Chen CY. Assessing metal-specific patterns of trace metal bioaccumulation across New England lakes. Science of the Total Environment. doi: 10.1016/j.scitotenv.2012.01.058. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DM, Nislow KH, Chen CY, Folt CL. Rapid, efficient growth reduces mercury concentrations in stream-dwelling Atlantic salmon. Transactions of the American Fisheries Society. 2009;139:1–10. doi: 10.1577/T09-032.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watras CJ, Morrison KA, Host JS, Bloom NS. Concentration of mercury species in relationship to other site-specific factors in the surface waters of northern Wisconsin lakes. Limnology and Oceanography. 1995;40:556–565. [Google Scholar]
- Watras CJ, Back RC, Halvorsen S, Hudson RJM, Morrison KA, Wente SP. Bioaccumulation of mercury in pelagic freshwater food webs. Science of the Total Environment. 1998;219:183–208. doi: 10.1016/s0048-9697(98)00228-9. [DOI] [PubMed] [Google Scholar]
- Westcott K, Kalff J. Environmental factors affecting methyl mercury accumulation in zooplankton. Canadian Journal of Fisheries and Aquatic Sciences. 1996;53:2221–2228. [Google Scholar]
- Yu X, Driscoll CT, Montesdeoca M, Evers D, Duron M, Williams K, Schoch N, Kamman NC. Spatial patterns of mercury in biota of Adirondack, New York lakes. Ecotoxicology. 2011 doi: 10.1007/s10646-011-0717-y. [DOI] [PMC free article] [PubMed] [Google Scholar]