Abstract
Zinc-supplementation (20 μM) effects on growth, photosynthesis, antioxidant enzyme activities (superoxide dismutase, ascorbate peroxidase, catalase), and the expression of phytochelatin synthase gene were investigated in four marine diatoms (Amphora acutiuscula, Nitzschia palea, Amphora coffeaeformis and Entomoneis paludosa). Zn-supplementation reduced the maximum cell density. A linear relationship was found between the evolution of gross photosynthesis and total chlorophyll content. The Zn treatment decreased the electron transport rate except in A. coffeaeformis and in E. paludosa at high irradiance. A linear relationship was found between the efficiency of light to evolve oxygen and the size of the light-harvesting antenna. The external carbonic anhydrase activity was stimulated in Zn-supplemented E. paludosa but was not correlated with an increase of photosynthesis. The total activity of the antioxidant enzymes did not display any clear increase except in ascorbate peroxidase activity in N. palea. The phytochelatin synthase gene was identified in the four diatoms, but its expression was only revealed in N. palea, without a clear difference between control and Zn-supplemented cells. Among the four species, A. paludosa was the most sensitive and A. coffeaeformis, the most tolerant. A. acutiuscula seemed to be under metal starvation, whereas, to survive, only N. palea developed several stress responses.
1. Introduction
Marine diatoms fulfill important roles in the biosphere. Among these, diatoms are responsible for about 25% of annual inorganic carbon fixation in oceans [1]. This CO2 is fixed through the photosynthetic process into energy-rich molecules that ultimately serve to feed the other levels of the trophic networks. To fulfill this role, diatoms as other living organisms must find in their environment good conditions, including the right range of macro- and microelements. Among the mandatory microelements required for cell functioning, zinc (Zn) occupies a particular place because it acts as a structural component [2] and as functional component of numerous enzymes, in some gene transcription regulators [3] and as a cofactor in zinc-finger protein involved in mitosis regulation [4] (for review, see [5]). As for other nutrient, Zn should be present within a definite range to allow optimum cell functioning and growth. In Zn-deficient conditions, diatoms cannot develop whereas when Zn is present in excess, crucial processes are inhibited partially or totally (growth: [6–8], photosynthesis: [9, 10]) while the oxidative stress develops [11–13]. Because the optimal range of Zn concentrations depends on diatom species, this type of algae is used as bioindicators [14].
Physiological and biochemical studies have demonstrated that the capacity to tolerate Zn is linked to the ability to establish defense mechanisms (for reviews see [5, 15]). Among these mechanisms, Zn chelation seems to be major. Zn ions can be chelated by exopolysaccharides as in the diatom Skeletonema costatum [16] or in the cytoplasm by phytochelatins, which are cysteine-rich pseudopeptides. Phytochelatins are synthesized by addition of glutathione units (γ-Glu-Cys-Gly) through the catalytic action of phytochelatin synthase (PCS), a γ-glutamyl cysteine transpeptidase [17].
The aim of this study was to compare the effect of an increase of Zn ion concentration on the growth, photosynthetic process, and responses to metal stress of four diatom species. Amphora acutiuscula and Nitzschia palea were harvested and isolated from the South-East Vietnamese coast, at the Can Gio site, which is confronted by pollution from the Mekong River, and two other diatom species (A. coffeaeformis and Entomoneis paludosa) isolated from the French Atlantic coast. N. palea often develops in polluted waters [18], and A. coffeaeformis has been shown to be a tolerant species to UV [19, 20] and Cu [10] but sensitive to Cd [14].
2. Materials and Methods
2.1. Culture Conditions
Amphora acutiuscula Kützing and Nitzschia palea (Kützing) Smith were collected at the Can Gio coastal site in South East Vietnam (latitude: 10°40′09′′; longitude: 107°00′59′′), whereas A. coffeaeformis (Agardh) Kützing and Entomoneis paludosa (W. Smith) Reimer were collected on the French Atlantic coast and were obtained from the Nantes Culture Collection (strains UTC58 and NCC18.2, resp.). Each taxon was axenically cultured in artificial seawater (ASW) prepared from Millipore ultrapure water according to Harrison et al. [21]. Diatoms originating from the Vietnamese coast and from the French coast were maintained at 23°C and 16°C, respectively. The cultures were illuminated using cool-white fluorescent tubes (at a photon flux density of 300 μmol photons PAR m−2 s−1, Philips TLD, 18 W) under a light-dark (14/10 h) cycles. The photon flux density was measured using a 4π waterproof light probe (Walz, Germany) connected to a Li-Cor 189 quantum meter. The growth temperatures were maintained for measurements. For experiments, exponentially growing cells were harvested from precultures, centrifuged gently (900 ×g, 10 min, 4°C) and inoculated sterilely into fresh ASW supplemented or not with a sterile ZnCl2 stock solution. The final Zn concentration was 20 μM. The Zn concentration of fresh ASW was 0.25 μM. The cultures were performed in Erlenmeyer flasks of 250 mL capacity that were inoculated at a cell density of 104 cells mL−1. This concentration was chosen after preliminary trials showing that this Zn concentration was the highest Zn concentration tolerated by all four diatoms for at least 10 days (results not shown). All the measurements were performed with cells from cultures at the exponential growth phase that is 5 days from inoculation (data not shown).
2.2. Algal Growth and Chlorophyll a and c Contents
Growth in the cultures was monitored by daily cell counts using a Neubauer type hemacytometer. The growth rate was calculated during the exponential phase, and the maximum cell density was determined from the stationary phase of the growth curves. Chlorophyll (Chl) a and Chl c were measured spectrophotometrically according to Speziale et al. [22].
2.3. Oxygen Evolution and Chlorophyll Fluorescence Measurements
Oxygen evolution was determined using a thermostated chamber equipped with a Clark-type oxygen electrode (DW2, Hansatech Instruments Ltd., UK). The oxygen evolution was measured under actinic irradiance ranging from 0 to 1200 μmol photons PAR m−2 s−1. The gross photosynthesis was calculated as the net photosynthesis plus respiration, assuming that the respiration rate was constant in light and in darkness. The gross photosynthesis versus irradiance curves (P versus E curves) were fitted according to the model of Eilers and Peeters [23] using the Sigma-plot software.
Chl fluorescence was measured using a FMS1 modulated fluorometer (Hansatech Ltd., UK) modified to make it suitable for use at low Chl a concentrations [24]. To obtain the relative electron transport rate versus irradiance (rETR versus E) curves, algae were submitted to 11 levels of actinic light progressing from 0 to 1200 μmol photons PAR m−2 s−1. The fitting of experimental data to rETR versus E curves were calculated as indicated by Eilers and Peeters [23] and Mouget et al. [25].
2.4. Carbonic Anhydrase Activity
The carbonic anhydrase (CA) activity was measured according to Dionisio-Sese and Miyachi [26] and Morant-Manceau et al. [27]. Intact cells were used to quantify the extracellular CA activity (CAext), while the total CA activity was quantified using cells homogenized in liquid nitrogen (CAtot). The internal CA activity (CAint) was calculated as CAtot activity minus CAext activity.
2.5. Antioxidant Enzymatic Activities
The algae were harvested by centrifugation (900 ×g, 4°C) and ground in a liquid nitrogen frozen potassium phosphate buffer (K2HPO4 50 mM, EDTA Na2 1 mM, pH 7) using a mortar and a pestle. The homogenate was centrifuged (10,000 ×g, 15 min, 4°C), and the supernatant was used for spectrophotometric determination of enzymatic activity. Catalase (CAT) activity was estimated by tracking the reduction of H2O2 at 240 nm and 20°C [28]. The reaction mixture contained 200 μM H2O2 in 50 mM of pH 7.5 potassium phosphate buffer. Ascorbate peroxidase (APX) activity was evaluated by tracking the changes in absorbance at 290 nm of the ascorbate substrate in a reaction mixture composed of ascorbate 10 mM and H2O2 10 mM in 50 mM of pH 7.0 potassium phosphate buffer. Ascorbate oxidation was measured at 25°C [29]. One unit of enzymatic activity (CAT and APX) was defined as the amount of enzymes that catalyses the conversion of one μmole of substrate per min [30]. Superoxide dismutase (SOD) activity was determined by measuring the inhibition of photochemical reduction of nitroblue tetrazolium (NBT), which absorbs at 560 nm. One unit of SOD activity was calculated as the amount required to cause 50% inhibition of the photoreduction of NBT [31]. Protein concentration of diatom extracts was determined by standardizing versus bovine serum albumin, according to Hartree [32].
2.6. Extraction of Nucleic Acids, PCR Amplification, and Bacterial Transformation
DNA was extracted from about 1 g of fresh tissues as described by J. J. Doyle and J. L. Doyle [33] after grinding in liquid nitrogen. The samples were dissolved in 80 μL water. Partial genomic DNA sequences of phytochelatin synthase were obtained by the following PCR procedure. Primer sequences FPCdia/RPCdia (5′-ATGGAARGGACCATGGAGRTG-3′ and 5′-ATRGGWGAAAAATGYCCMGTTCC-3′) and nested primer sequences NFPCdia/NRPCdia (5′-ACCATGGAGRTGGTAYGARGA-3′ and 5′-TTCCAGTTTGMCC-3′) corresponding to conserved sequences were designated from the alignment of PCS nucleic acid sequences of both model diatoms: Thalassiosira pseudonana and P. tricornutum (http://genome.jgi-psf.org/). Thirty cycles consisting of denaturing for 30 s at 94°C, annealing for 1 min at 57.2°C, and extension for 2 min at 72°C were performed. The reaction was completed by an extension step at 72°C. The first PCR was performed with 0.2 μM of FPCdia, 0.2 μM of RPCdia, and 2.5 units of Thermus aquaticus (Taq) DNA polymerase (Promega). Amplified DNA products were subjected to a second PCR with nested primers using the same conditions, apart from a slightly higher annealing temperature (57.5°C). PCR products were cloned into pGEMT-Easy vector (Promega) containing a cassette conferring the resistance to ampicillin. The ligation productions were transformed into Escherichia coli DH5α. Recombinant bacteria were selected and sequenced on both strands (Operon, Deutschland). Total RNAs (control and sample with Zn 20 μM) were extracted using the RNeasy Plant Mini Kit (Qiagen, MD, USA), and stored at −80°C before northern blot analysis.
2.7. Sequence Analysis
The sequences obtained after PCR were subjected to a homology search through the BLAST program available at the NCBI GenBank biocomputing site (http://blast.ncbi.nlm.nih.gov/Blast.cgi) [34]. The deduced amino acid sequences were obtained using the translate software available at the server: (http://www.bioinformatics.org/sms/index.html). The multiple alignments of the sequenced fragments were carried out using the ClustalW EBI program and visualized using Genedoc, version 2.6 [35].
2.8. Northern Blot Analysis
Equal amounts (7.5 μg) of total RNA samples were denatured and fractionated by electrophoresis in 1.2% agarose denaturing gel [36]. Total RNA quality was confirmed by ribosomal RNA integrity observed after agarose gel ethidium bromide treatment [36]. Gels were blotted by a capillary procedure [36] on NY Plus membrane (Porablot, Macherey-Nagel, Düren, Germany). Fractionated RNAs were crosslinked at 80°C. The membrane was stained with methylene blue to check the ribosomal RNA quality. The radiolabeled PCS probe was obtained by using the Prime a Gene Labeling System kit (Promega, Madisson, WI, USA) with the cloned cDNA adding 50 μCi (330 nM) of [α 32P]dCTP. The probe was purified on G50 microcolumns (Amersham-Pharmacia, Orsay, France). Membranes were prehybridized in a hybridization buffer [36] for 2 h, and then [α 32P]dCTP radiolabeled probes 1 × 1010 cpm μg−1 were added. Membranes were exposed to X-ray film (Kodak) for 12 h at –70°C. These experiments were duplicated.
2.9. Statistical Analysis
We used a one-way analysis of variance (ANOVA) to determine the statistical significance of differences in all experiments. To be statistically significant, a difference had to display a level of significance of at least 5% (P ≤ 0.05) using the Tukey test run on SigmaStat version 3.1 software compatible with SigmaPlot 9.0. All measurements were made on 3–5 replicates (from different cultures), and the results were expressed as means and standard errors.
3. Results and Discussion
3.1. Effects of Zinc on Growth
In the absence of Zn-supplementation, the highest cell density was reached with N. palea, which was also the taxon dividing with the slowest rate (Table 1). The two Amphora taxons behaved similarly, reaching medium cell densities but the highest dividing rate. E. paludosa reached the lowest cell density and the division rate was intermediate between those measured for Amphora sp. and N. palea (Table 1). These data agree with those published previously on the same Amphora species but not for N. palea and E. paludosa, for which higher values were found by Nguyen-Deroche et al. [10]. The supplementation of the growth medium with Zn affected differentially the growth of the different taxons. For the four taxons, the maximum cell density decreased, while the growth rate remained constant in the Amphora species, increased in N. palea, and dramatically decreased in E. paludosa (Table 1). Altogether, the data suggests that in N. palea, Zn stimulated mitosis for a short period before to inhibit this process, leading to a reduced maximum cell density. In the other taxons, Zn ions have only negative effects on culture growth. This negative effect has been already observed for lower Zn concentrations in different species such as Nitzschia closterium (0–1.52 μM: [6]), S. costatum (24 pM: [37]), and P. tricornutum (0.05–10 μM: [38]).
Table 1.
Growth rate, maximum cell density, chlorophyll a and c contents, total Chl, Chl a/Chl c ratio in Amphora acutiuscula, Amphora coffeaeformis, Nitzschia palea, and Entomoneis paludosa grown in ASW (control) or in the presence of Zn 20 μM added to ASW.
| Species | A. acutiuscula | A. coffeaeformis | N. palea | E. paludosa | ||||
|---|---|---|---|---|---|---|---|---|
| Control | Zn 20 μM | Control | Zn 20 μM | Control | Zn 20 μM | Control | Zn 20 μM | |
| Growth rate (day−1) | 1.210 ± 0.074a | 1.281 ± 0.014a | 1.198 ± 0.192a | 0.957 ± 0.108a | 0.258 ± 0.028a | 0.405 ± 0.069b | 0.630 ± 0.430a | 0.020 ± 0.017b |
| Maximum cell density (103 cells mL−1) | 591± 8a | 498 ± 5b | 617 ± 4a | 480 ± 4b | 1308 ± 133a | 627 ± 8b | 298 ± 14a | 48 ± 1b |
| Chl a (μg 10−6 cells) | 3.22 ± 0.20a | 2.72 ± 0.20b | 1.10 ± 0.03a | 0.87 ± 0.03b | 1.03 ± 0.05a | 1.42 ± 0.01b | 2.79 ± 0.36a | 2.27 ± 0.10a |
| Chl c (μg 10−6 cells) | 0.32 ± 0.01a | 0.37 ± 0.03b | 0.13 ± 0.01a | 0.11 ± 0.01a | 0.21 ± 0.02a | 0.29 ± 0.01b | 0.34 ± 0.06a | 0.37 ± 0.03a |
| Total Chl (μg 10−6 cells) | 3.54 ± 0.21a | 3.09 ± 0.09a | 1.24 ± 0.04a | 0.97 ± 0.03b | 1.23 ± 0.07a | 1.71 ± 0.22a | 3.13 ± 0.42a | 2.64 ± 0.13a |
| Chl a/Chl c | 10.09 ± 0.40a | 7.52 ± 0.75b | 8.30 ± 0.14a | 8.07 ± 0.09a | 4.96 ± 0.12a | 4.89 ± 0.22a | 8.2 ± 0.40a | 6.13 ± 0.15a |
Mean values ± SE (n = 3–5). Significant different data are indicated with different superscripted letters (Tukey Test, P ≤ 0.05).
The results of this experiment allowed us to range both Amphora species as Zn-tolerant taxons and both P. paludosa and N. palea as Zn-sensitive taxons. This conclusion fits with the results already published on Zn sensitivity of Nitzschia [6]. Interestingly, these taxons reacted differently when facing to an increase of Cu [10]. Despite the fact that Zn can be important for mitosis regulation [4], algal growth depends primarily on photosynthesis. Therefore, this process was characterized at the biochemical and physiological level in the four diatom species grown in the presence or in the absence of Zn.
3.2. Effects of Zinc on Chlorophyll Contents
Chl quantifications in the four taxons grown in the absence of the Zn-supplementation revealed that A. acutiuscula and E. paludosa contained three times more total Chl than the two other taxons. Chl a was always the major pigment (Table 1). This difference was not reflected in the Chl a/Chl c ratio, always higher than 8, except for N. palea for which the ratio was close to 5. Because the Chl a/Chl c ratio constitutes a rough measure of the size of the light-harvesting antenna [39], this result suggests that the antenna of N. palea is larger than in the other species. The addition of Zn did not significantly impact the total Chl amount in A. acutiuscula, whereas it triggered an increase in N. palea and a decrease in A. coffeaeformis and E. paludosa. The Chl a/Chl c ratio was not affected in A. acutiuscula and N. palea, whereas it was decreased by at least two units in E. paludosa and A. coffeaeformis (Table 1). Although the different culture protocols used in the literature make difficult the comparison of Zn effects on Chl contents, it is generally found that metals in excess, including Zn, reduce the Chl a amount (Zn-Chlorella vulgaris: [40]; Zn-Pavlova viridis: [11]; Cd, Cu-multispecies: [41]) with a notable exception in the diatom Asterionella japonica for which an increase was reported [42]. The way used by Zn to impact the Chl amount is not clear and no reasonable hypothesis can be proposed at the present state of our knowledge. Regardless of this reason, it is worse to mention that the Chl a/Chl c ratio remained stable while in green algae, the ratio Chl a/Chl b decreases due to the inhibition of Chl b formation from Chl a [43, 44]. Because Chl c derived from the Chl precursor protochlorophyllide (reviewed in [45]), any block or stimulation of the biochemical steps prior protochlorophyllide formation would affect similarly the amount of both pigment types leading the ratio to remain unchanged. Altogether, the results suggest that the Zn excess does not modify the size of the light-harvesting complexes except in E. paludosa and in A. acutiuscula. In the two other species, the increase in total Chl content would speak in favor of a Zn-induced increase of the number of photosynthetic chains. In order to test this hypothesis, we measured the variation of the gross photosynthesis and of the relative electron transfer rate versus the irradiance level.
3.3. Effects of Zinc on Photosynthesis
In the absence of Zn-supplementation, the curves P/E presented the same trends. Both increased and saturated between 600–800 μmol photons PAR m−2 s−1 (Figure 1). However, the maximum amplitude reached was different for the different species (Table 2). The Zn-supplementation affected negatively the gross photosynthesis in E. paludosa and N. palea but positively that of both Amphora species (Figure 1), confirming that these species are better in managing the excess of zinc. A decrease in photosynthetic activity has also been observed in other microalgae at various Zn concentrations (S. costatum- >24 pM: [37]; Chlamydomonas reinhardtii-30.8 μM: [46]; Pseudokirchneriella subcapitata-14 μM: [47]).
Figure 1.
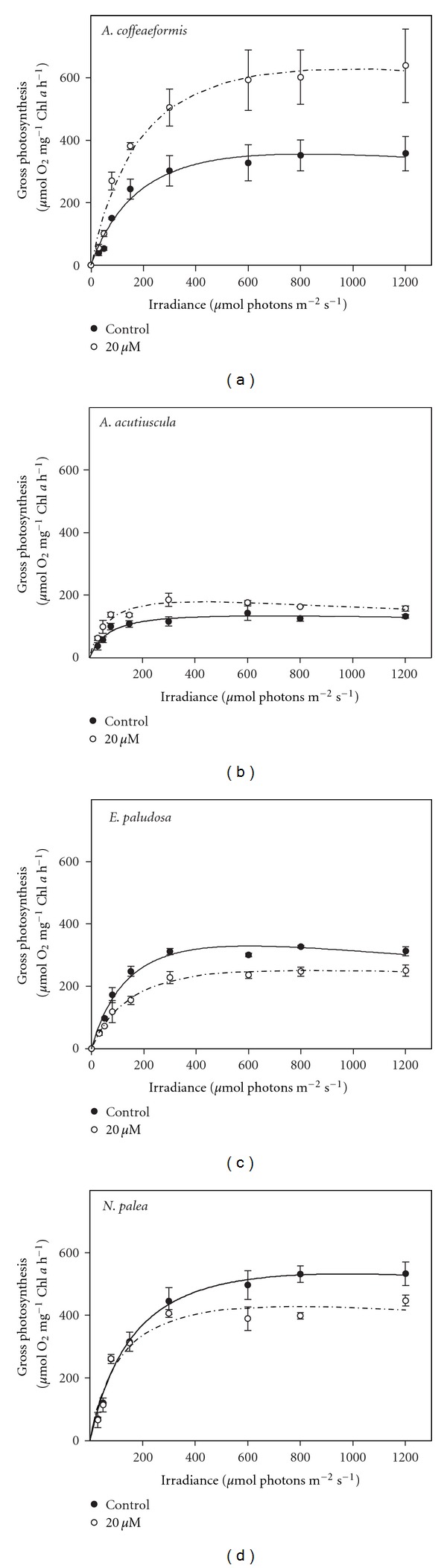
Gross photosynthesis versus irradiance curves in Amphora coffeaeformis, Amphora acutiuscula, Entomoneis paludosa, and Nitzschia palea grown in ASW (control) or in the presence of 20 μM Zn added to ASW. Mean values ± SE (n = 3–5).
Table 2.
Parameters (α B, light utilization coefficient; P max B, maximum gross photosynthesis; E k, irradiance for the light saturation of photosynthesis) of gross photosynthesis versus irradiance curves. α B: μmol O 2 mg−1 Chl a h −1 (μmol photons m−2 s−1)−1; P max B: μmol O2 mg−1 Chl a h −1; E k: μmol photons m−2 s−1. Parameters (α rETR, light utilization coefficient; rETR max, maximum relative electron transport rate; E krETR, irradiance for the light saturation of photosynthesis) of relative electron transport rate versus irradiance curves. α rETR/rETR (μmol photons m−2 s−1)−1; rETR max: relative units; E krETR: μmol photons m−2 s−1.
| Species | A. acutiuscula | A. coffeaeformis | N. palea | E. paludosa | ||||
|---|---|---|---|---|---|---|---|---|
| Control | Zn 20 μM | Control | Zn 20 μM | Control | Zn 20 μM | Control | Zn 20 μM | |
| α B | 2.23 ± 0.68a | 3.27 ± 0.31b | 2.31 ± 0.05a | 3.87 ± 0.20b | 3.71 ± 0.36a | 4.64 ± 0.75a | 3.01 ± 0.14a | 2.24 ± 0.13b |
| P max B | 146 ± 11a | 177 ± 3b | 407 ± 44a | 580 ± 33b | 545 ± 27a | 438 ± 5b | 335 ± 2a | 259 ± 6b |
| E k | 88 ± 29a | 55 ± 4a | 175 ± 15a | 178 ± 18a | 153 ± 21a | 102 ± 16a | 110 ± 5a | 116 ± 4a |
| α rETR | 0.21 ± 0.02a | 0.41 ± 0.03b | 0.36 ± 0.03a | 0.36 ± 0.01a | 0.42 ± 0.02a | 0.39 ± 0.03a | 0.48 ± 0.05a | 0.29 ± 0.01b |
| rETRmax | 37 ± 1a | 24 ± 1b | 36 ± 1a | 49 ± 2b | 95 ± 7a | 62 ± 3b | 43 ± 3a | 53 ± 3b |
| E krETR | 187 ± 28a | 62 ± 7b | 112 ± 9a | 139 ± 8a | 224 ± 7a | 166 ± 19b | 90 ± 6a | 181 ± 8c |
Mean values ± SE (n = 3–5). Significant different data are indicated with different superscripted letters (Tukey Test, P ≤ 0.05).
The impairment of photosynthesis is reflected in the values of the parameters characterizing P/E curves (Table 2).
The α B Parameter —
It reflects the affinity of the algae for light. In the absence of Zn-supplementation, α B ranged between 2.2-2.3 for the Amphora species to 3.0–3.7 for the two other species. These values were higher in the presence of the Zn-supplementation except in E. paludosa for which it decreased. For the same photon flux density, the speed at which O2 is evolved is primarily dependent on the size of the light harvesting complex, which is reflected in the Chl a/Chl c ratio. Therefore, a linear relationship between the two parameters should be observed. To test this hypothesis, the α B values were plotted against the Chla/Chlc values obtained with diatoms grown in the presence of an excess of Zn (Figure 2(a)). The linear relationship obtained suggests the validity of the hypothesis.
Figure 2.
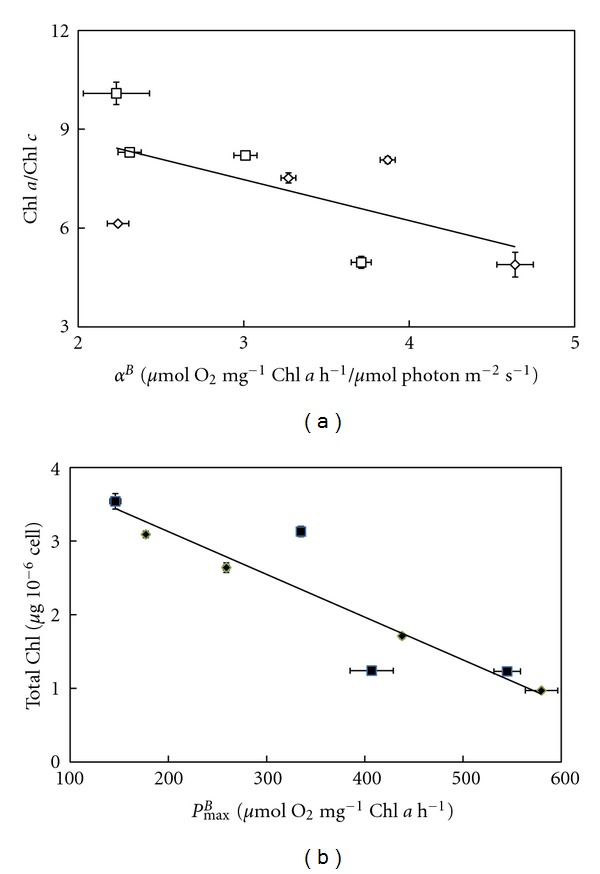
(a) Relationship between α B calculated from gross photosynthesis versus light intensity curves (P/E in Figure 1) and Chl a/Chl c ratio (Table 1) in Amphora coffeaeformis, Amphora acutiuscula, Entomoneis paludosa, and Nitzschia palea grown in ASW in the absence (□) or the presence (◊) of Zn supplementation. (b) Relationship between the total Chl content (Table 1) and the maximum gross photosynthesis (P max B) (Figure 1) in Amphora coffeaeformis, Amphora acutiuscula, Entomoneis paludosa, and Nitzschia palea grown in ASW in the absence (■) or the presence (◆) of Zn supplementation.
The P max B Parameter —
This factor reflects the photosynthetic activity when the light is saturating. In the absence of Zn-supplementation, the values of P max B were high except for A. acutiuscula for which the value was reduced by 50 to 75% (Table 2). P max B was increased in both Amphora species, but was lower in the other two diatoms in comparison to controls. The maximum oxygen evolved is primarily related to the total Chl present and therefore a linear relationship should be found when the P max B values are plotted against the total Chl amount. This linear relationship was indeed found (Figure 2(b)).
The Parameter E k —
It reflects the photon flux density from which the photosynthetic activity does not increase proportionally to the light intensity. In the absence of Zn supplementation, the values of E k for A. acutiuscula and E. paludosa were lower than those obtained for the two other species suggesting that the two former species are more sensitive to high-light than the others. This is also reflected by the lower value of P max B for these two species. The Zn supplementation did not change significantly the E k levels (Table 2).
The P/E curves give information on how Zn affects the PSII functioning. In order to enlarge our picture on the impact of Zn on the photosynthetic process, we followed the response of the relative electron transport rate (rETR) to increasing photon flux density. In the absence of Zn-supplementation, the curves rETR/E presented the same trends as the P/E curves except that they never completely saturated. rETRmax reached were similar among the different species (around 40) except for N. palea, which reached 80 (Figure 3). The Zn-supplementation affected negatively the rETR in E. paludosa and N. palea, suggesting that Zn might have several targets. To get more information from the curves, the characteristic parameters were calculated (Table 2).
Figure 3.
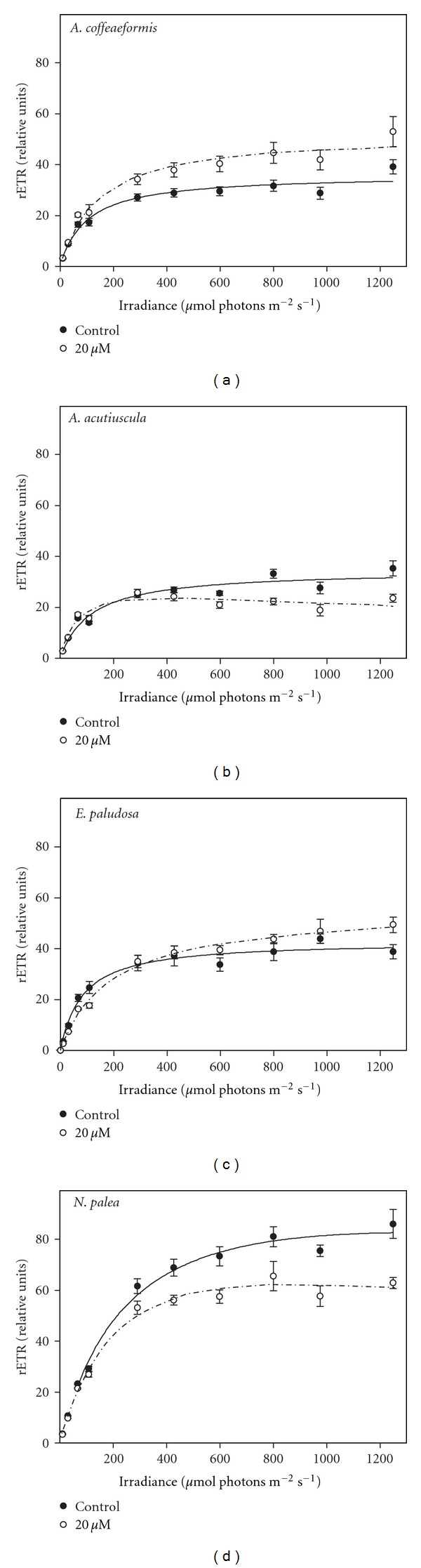
Relative electron transport rate (rETR) versus irradiance curves in Amphora coffeaeformis, Amphora acutiuscula, Entomoneis paludosa, and Nitzschia palea grown in ASW (control) or in the presence of 20 μM Zn added to ASW. Mean values ± SE (n = 3–5).
The α rETR Parameter —
It reflects the efficiency of the algae to use the incoming light to drive the electron transport. In the absence of Zn-supplementation, the taxons were equally performant in using the incoming light except A. acutiuscula, which was the less efficient. In N. palea and A. coffeaeformis, α rETR was not modified, whereas it was considerably higher in A. acutiuscula (+95%) and lower in E. paludosa (−40%).
The rETRmax Parameter —
This factor reflects the maximum ETR when the light is saturating. In the absence of Zn-supplementation, the taxons reached the same value for this parameter except N. palea, which exhibited a much higher level at saturation. In the presence of Zn excess, the intensity of this parameter significantly increased in A. coffeaeformis and E. paludosa, whereas it significantly decreased in the two other species.
The E krETR parameter —
It reflects the photon flux density from which the ETR does not increase proportionally to the light intensity. E. paludosa and A. coffeaeformis presented lower values than for the two other species. The values of this parameter were reduced in N. palea and A. acutiuscula, but considerably increased in E. paludosa (+107%) (Table 2).
P/E and rETR/E are two ways to measure the photosynthetic activity [48]. Therefore, from the theoretical point of view, both parameters vary in the same way [49] as shown in the case of A. coffeaeformis in the absence or in the presence of Zn supplementation (Figure 4). However, a stress may affect differentially the PSII and the electron transport chain and disrupts the linear relationship between these two parameters. This is obviously the case in A. acutiuscula (Figure 4), in which the absence of Zn made the electron rate slower than the oxygen evolution rate. The Zn supplementation restored the proportionality between the two activities. This result suggests that in the ASW used here, A. acutiuscula underwent a slight Zn deprivation. This slight Zn deprivation would also affect A. coffeaeformis because both parameters were most intense in the presence of Zn (Figures 1 and 3).
Figure 4.
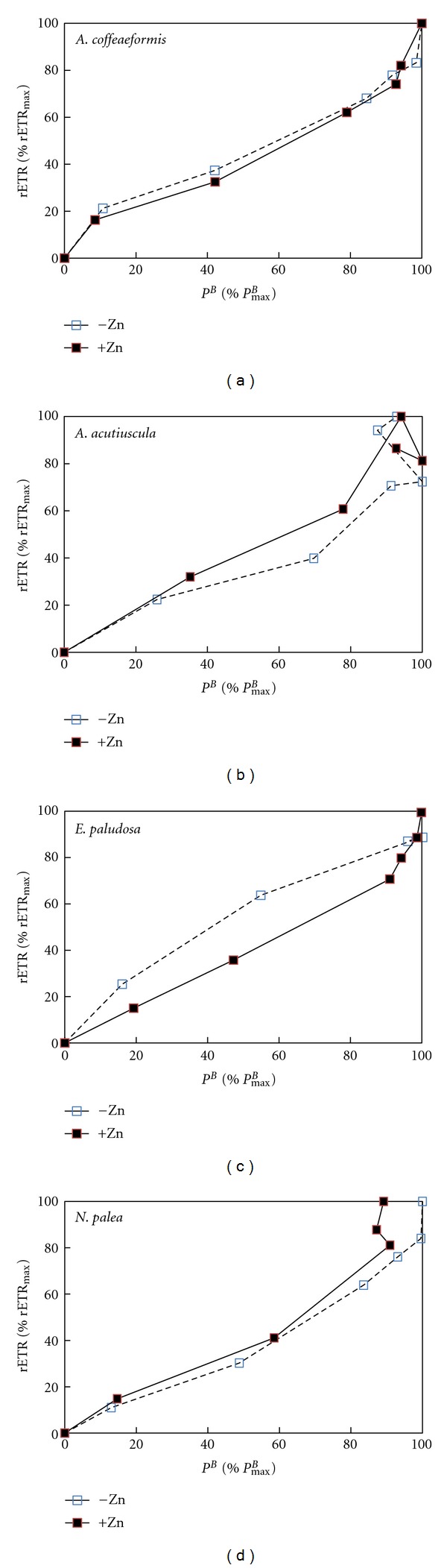
Relationship between the relative intensity of rETR and the relative intensity of P B in Amphora coffeaeformis, Amphora acutiuscula, Entomoneis paludosa, and Nitzschia palea grown in the absence (□) or the presence (■) of a Zn supplementation.
In the absence of Zn, the electron transport rate was faster than the oxygen evolution rate in E. paludosa. Such a behavior could be explained by the involvement of other mechanisms such as Mehler reaction, cyclic electron transport around PSII and/or PSI, photorespiration, and light-dependent mitochondrial respiration. The intensity of these mechanisms depends on the experimental conditions [50]. We observed that the Zn supplementation restored the proportionality between the two parameters, suggesting that Zn may target some component(s) of the electron transfer chain (Figure 4). The cytochrome of the electron transport chain can be proposed as a putative target of Zn ions in excess. Actually, it has been shown that Zn ions interact with the Q0 pocket of cytochrome b6/f complex [51]. These ions have also been shown to impair the proton transport function of cytochromes in bacteria and mitochondria [52]. Because the structure of cytochrome has been highly conserved during evolution [53, 54], this possibility is also likely.
In N. palea, Zn slowed down both oxygen evolution and the electron transport rates, with the rETR being more impacted at the highest photon flux densities (>800 μmol photon PAR m−2 s−1) than the oxygen evolution rate. Several nonexclusive causes can be involved in this inhibition: (i) photoinhibition due to a reduced activity of the xanthophyll cycle: the cycle consists in the reversible conversion of diadinoxanthin to diatoxanthin. It is activated by the acidification of the thylakoid. It is used as a photoprotection mechanism allowing the dissipation of the excess of energy absorbed by PSII. When this capacity is over, the photoinhibition starts [55, 56]. In our conditions, an impairment of the xanthophyll cycle is unlikely as no photoinhibition was observed (Figures 1 and 3). If this phenomenon would occur, both P/E and rETR/E curves would have presented a strong decreasing phase at high photon flux densities. So far the only metal known to inhibit the xanthophyll cycle activity in diatoms is cadmium [57]. (ii) PSII inhibition: it can be due to the impairment of the water oxidizing enzymes itself or/and by the destabilization of the binding cofactors in the oxygen evolving polypeptides associated with PSII [58]. For instance, Vaillant et al. [59] established that the replacement of Mn2+ in the water oxidizing complex by Zn2+ leads to a reduction of oxygen emission. Altogether these data indicate that in N. palea, the reduction of photosynthetic activity triggered by the excess of Zn explains the lower maximum cell density presented in Table 1, with the cell becoming at this Zn concentration unable to cope with its toxicity. (iii) A shortage of carbon supply: Subrahmanyam and Rathore [60] found that a reduced demand for ATP and NADPH in the Calvin cycle causes a downregulation of PSII photochemistry. On the other hand, Sunda and Huntsman [61] have identified a relationship between the addition of Zn and the C fixating rate at saturating light intensity in the diatom Thalassiosira pseudonana and in higher plants, Zn can inhibit the carboxylase activity of RuBisCO, leading intact the oxygenase capacity [62].
In diatoms, carbonic anhydrase, a Zn-dependent enzyme catalyses the reversible interconversion of HCO3 − and CO2 and is an important component of the inorganic carbon concentration mechanism [63–65]. This enzyme supplies RubisCO with CO2 [27, 66]. The positive effects of Zn on the photosynthetic activity of A. coffeaeformis suggest that the amount of Zn in the ASW constitutes a limiting factor (Figures 1 and 3) that could limit the CA activity. In order to test this hypothesis, the effect of Zn-supplementation on the CA activity was measured. These data are presented in the next section.
3.4. Effects of Zinc on Carbonic Anhydrase Activity
In the absence of the Zn-supplementation, the carbonic anhydrase activity was detected at the cell surface (external CA) and in the cytosol (internal CA) in all four diatoms, with the highest total activity being found in A. coffeaeformis and N. palea. The addition of Zn did not stimulate CA activity except the CAext activity in E. paludosa (Figure 5). It can be noticed that the weak increase of CAext activity in A. acutiuscula could be reflected in the higher photosynthetic activity (Figures 1 and 3).
Figure 5.
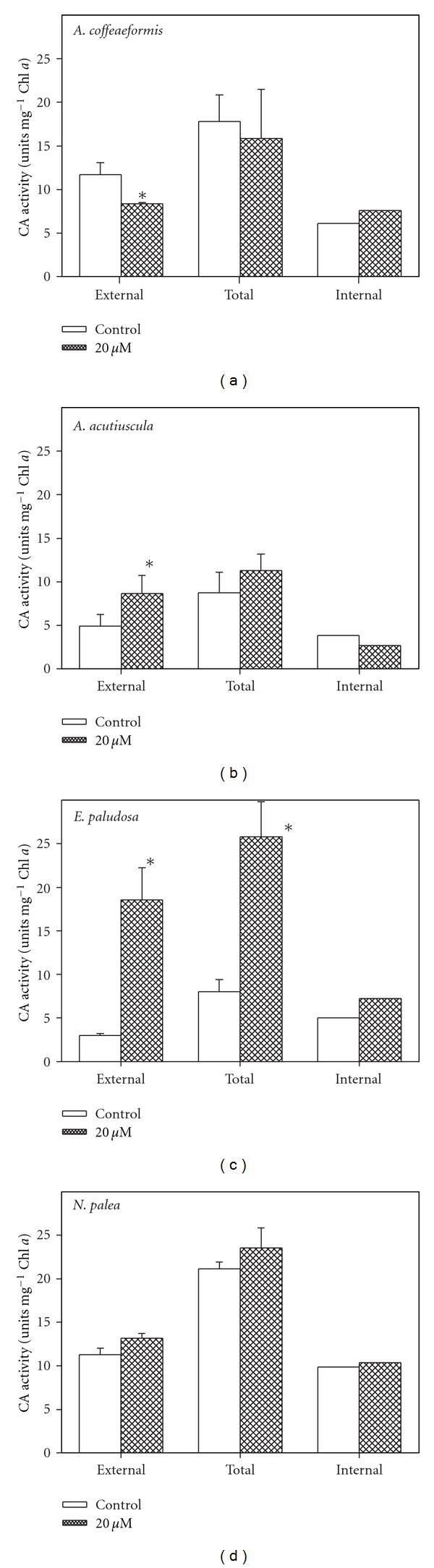
External, internal, and total carbonic anhydrase (CA) activities in Amphora coffeaeformis, Amphora acutiuscula, Entomoneis paludosa, and Nitzschia palea grown in ASW (control) or in the presence of 20 μM Zn added to ASW. Mean values ± SE (n = 3–5). Significant differences are indicated by an asterisk (P ≤ 0.05).
It is well established that metal stresses induce the production of ROS that disturbs the functioning of the different cell compartments [15]. To test this possibility in our growth conditions, the total activity of the main antioxidant enzymes that is, SOD, APX, and CAT were measured after 5 days of growth in the presence or the absence of Zn-supplementation.
3.5. Antioxidant Enzymatic Activities
Each taxon presented an activity APX, CAT, and SOD in the absence of Zn-supplementation but with different relative intensities (Figure 6). In the four species, the SOD activity represented about 70% the total antioxidant activity measured, the remaining activities being shared unequally between APX and CAT activities. For instance, in E. paludosa, the CAT activity was 12 times higher than the APX one (Figure 6).
Figure 6.
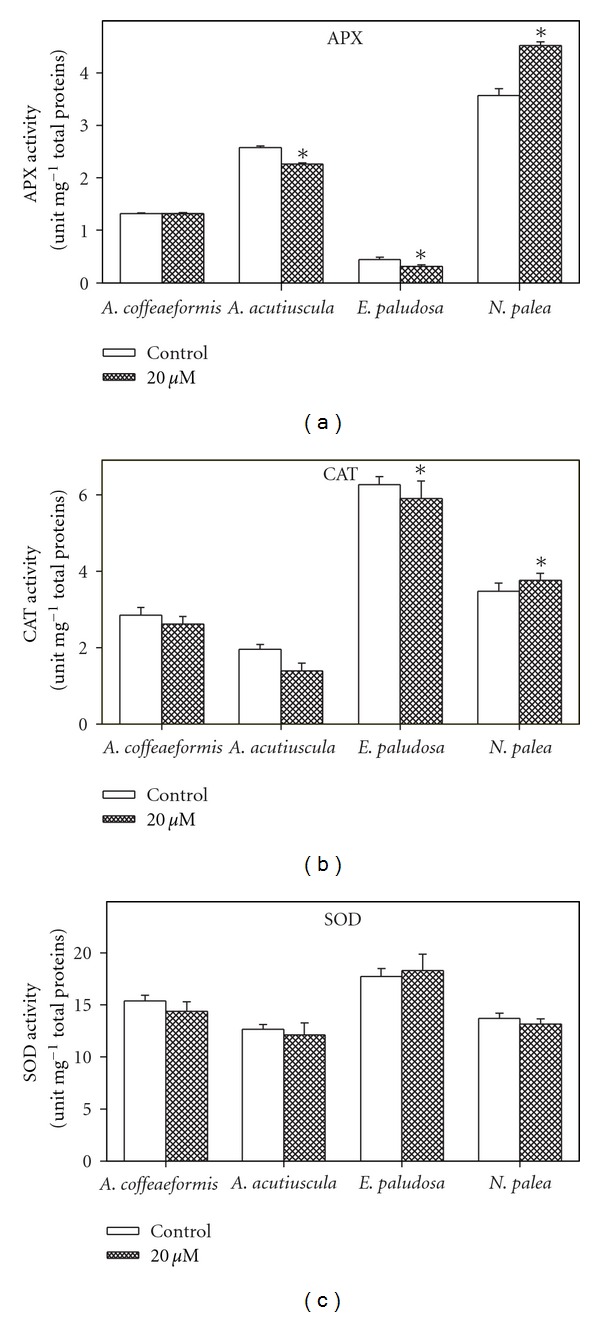
Antioxidant enzymes activities (superoxide dismutase, SOD; catalase, CAT and ascorbate peroxidase, APX) in Amphora coffeaeformis, Amphora acutiuscula, Entomoneis paludosa, and Nitzschia palea grown in ASW (control) or in the presence of 20 μM Zn added to ASW. Significant differences are indicated by an asterisk (P ≤ 0.05). Mean values ± SE (n = 3–5).
In the Zn-supplemented growth medium, the activity of the three antioxidant enzymes did not display any clear increase, except the APX activity in N. palea that increased by 22%. However, we could not exclude the possibility that the activity of the enzymes is modified in individual cell compartments, such as the chloroplasts, in which the ROS production can elevate in case of photosynthetic impairment (reviewed in [5]), these results presented here suggest that in our conditions, the excess of Zn did not triggered an intensive oxidative stress requiring additional antioxidative enzymes to cope with Pinto et al. [67] have shown that in Pavlova viridis an excess of Zn (c.a. 50 μM) enhanced lipid peroxidation, which can be considered as an indication of the oxidation damages. Alternatively, we can suggest that a part of the ions in excess is quenched, with the remaining part being unable to trigger an intense oxidative stress. So far, two main mechanisms of ion quenching have been found to be active in algae, including diatoms (reviewed in [5, 15]). The first mechanism occurs outside the cells and involved the binding of the metal ions to exopolysaccharides (Zn-S. costatum: [16]; Cu-Amphora sp.: [10]). Although these exopolysaccharides were not quantified in this study, we observed that the four diatoms tended to agglutinate when placed in the Zn-supplemented medium (data not shown), suggesting the production of these compounds as reported in A. coffeaeformis [68]. However, the binding capacity of the exopolysaccharides seems not intense enough to avoid Zn penetrating into the cells. The second mechanism occurs mostly in the cytoplasm and consists in the phytochelatins (Cu, Zn-Scenedesmus sp.: [31]; Zn-Nitzschia closterium: [6]) (reviewed in [67]). In order to test the second possibility, the genes corresponding to phytochelatin synthase were searched and their expression was measured in the different taxons grown in the presence or in the absence of Zn-supplementation.
3.6. Partial Phytochelatin Synthase Sequences
The use of the designed primers allowed the recovery of the partial DNA sequences in each taxon studied. The DNA sequence analysis showed open reading frames ranging from 279 to 321 bp (data not shown) coding for 92 to 106 amino acids residues, respectively, for the four taxons investigated in this study (Figure 7). Blast searches using the nucleotide sequences against those of higher plants as well as the sequenced genomes of the diatoms P. tricornutum and T. pseudonana revealed identities up to 100% with phytochelatin synthase gene (98%: E. paludosa and both A. coffeaeformis; 100%: A. acutiuscula). The in silico translation of the open-reading frames revealed the presence of four conserved cysteine residues belonging to the catalytic domain located at the N-terminal region of the enzyme [69, 70] (Figure 7). Both these DNA sequences and the corresponding deduced amino acid sequences have been deposited to the EML-EBI database (N. palea no. FN995985; A. coffeaeformis, no. FN995986; E. paludosa, no. FN995987; A. acutiuscula, no. FN995989). To evaluate the expression level of the phytochelatin synthase gene in the absence and in the presence of Zn-supplementation, total RNA were extracted after 5 days of growth in the absence or the presence of Zn-supplementation and quantified by northern blotting. The good quality of the total RNA extracted was revealed by two clearly defined electrophoretic bands corresponding to 18S and 28S ribosomal RNA (data not shown). Despite the fact that Zn is the second best inducer of phytochelatin synthesis [71], the mRNAs corresponding to phytochelatin synthase were only detected in equal amount in N. palea in the presence or in the absence of Zn-supplementation (data not shown), and using this method, no change in the expression level was suspected due to the presence of the Zn supplementation. Interestingly, the diatom P. tricornutum did not express phytochelatin synthase for a Zn concentration one order lower than our (2.2 μM: [72]). On the other hand, Zn has been reported to trigger phytochelatin synthesis in the green alga Dunaliella tertiolecta for a Zn concentration one order higher than the one used in this study (200 μM: [73]). This suggests that each taxon would sense the Zn internal concentration and would express the phytochelatin synthase gene according to a threshold, with this level being one ecological characteristic of this taxon. Our data suggest that this minimum level was crossed only in the case of N. palea. The synthesis of phytochelatins would then contribute to the resistance of N. palea in Zn-supplemented growth medium. This result also confirms that this taxon is especially sensitive to Zn elevation. More investigations are needed to find out whether the phytochelatin synthase genes are completely repressed or slightly expressed in the other three species.
Figure 7.
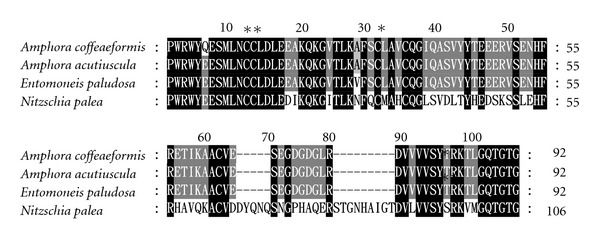
Alignment of the amino acid sequences of phytochelatin synthase fragments isolated from Amphora acutiuscula (FN995989), Amphora coffeaeformis (FN995985), Entomoneis paludosa (FN995987) and Nitzschia palea (FN995985) grown in ASW. Black boxes indicated 100% identity, dark grey 80%, and light grey 60%. The cysteine residues are indicated by asterisks.
4. Conclusions
A Zn supplementation to the growth medium has different effects on the metabolism of diatoms. Of the four diatoms tested, E. paludosa was found to be the most sensitive taxon to Zn supplementation since its growth is drastically decreased. This study also showed that Zn in ASW is a limiting factor for both Amphora species. A. coffeaeformis is the most tolerant species in our culture condition. In N. palea, a higher antioxidant enzyme activity and the expression of phytochelatin gene are mechanisms providing cellular tools to cope with the excess of Zn and allowing the cells to develop equally to the tolerant species A. coffeaeformis.
Acknowledgments
The authors thank Dr. Y. Rincé for identifying the diatoms. The Embassy of French Republic at Hanoï is thanked for the fellowship to T. L. N. Nguyen-Deroche. The authors are grateful to P. Gaudin (Nantes Culture Collection) for the supply of the diatoms.
References
- 1.Granum E, Raven JA, Leegood RC. How do marine diatoms fix 10 billion tonnes of inorganic carbon per year? Canadian Journal of Botany. 2005;83(7):898–908. [Google Scholar]
- 2.Atkins CA, Patterson BD, Graham D. Plant carbonic anhydrases. II preparation and some properties of monocotyledon and dicotyledon enzyme types. Plant Physiology. 1972;50(2):218–223. doi: 10.1104/pp.50.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riechmann JL, Ratcliffe OJ. A genomic perspective on plant transcription factors. Current Opinion in Plant Biology. 2000;3(5):423–434. doi: 10.1016/s1369-5266(00)00107-2. [DOI] [PubMed] [Google Scholar]
- 4.Flor-Parra I, Vranes M, Kämper J, Pérez-Martín J. Biz1, a zinc finger protein required for plant invasion by Ustilago maydis, regulates the levels of a mitotic cyclin. Plant Cell. 2006;18(9):2369–2387. doi: 10.1105/tpc.106.042754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertrand M, Poirier I. Photosynthetic organisms and excess of metals. Photosynthetica. 2005;43(3):345–353. [Google Scholar]
- 6.Stauber JL, Florence TM. Mechanism of toxicity of zinc to the marine diatom Nitzschia closterium . Marine Biology. 1990;105(3):519–524. [Google Scholar]
- 7.Cid A, Herrero C, Torres E, Abalde J. Copper toxicity on the marine microalga Phaeodactylum tricornutum: effects on photosynthesis and related parameters. Aquatic Toxicology. 1995;31(2):165–174. [Google Scholar]
- 8.Gagneux-Moreaux S. Les métaux (Cd, Cu, Pb et Zn) dans la production des microalgues sur différents milieux de culture: biodisponibilité, bioaccumulation et impact physiologique. Nantes university; 2006. Ph.D. thesis. [Google Scholar]
- 9.El-Sarraf WM, Taha OE. Effets of copper on photosynthetic activity and chlorophyll-a content of Chaetoceros radicans Schütt. Bulletin of the High Institute of Public Health. 1995;25(2):439–446. [Google Scholar]
- 10.Nguyen-Deroche TLN, Le TT, Bui TV, Rincé Y, Tremblin G, Morant-Manceau A. Effects of copper on growth and photosynthesis in marine diatoms: a comparison between species from two different geographical areas. Cryptogamie, Algologie. 2009;30(2):97–109. [Google Scholar]
- 11.Li M, Hu C, Zhu Q, Chen L, Kong Z, Liu Z. Copper and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in the microalga Pavlova viridis (Prymnesiophyceae) Chemosphere. 2006;62(4):565–572. doi: 10.1016/j.chemosphere.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Rijstenbil JW, Derksen JWM, Gerringa LJA, et al. Oxidative stress induced by copper: defense and damage in the marine planktonic diatom Ditylum brightwellii, grown in continuous cultures with high and low zinc levels. Marine Biology. 1994;119(4):583–590. [Google Scholar]
- 13.Rijstenbil JW. Effects of UVB radiation and salt stress on growth, pigments and antioxidative defence of the marine diatom Cylindrotheca closterium . Marine Ecology Progress Series. 2003;254:37–48. [Google Scholar]
- 14.Branco D, Lima A, Almeida SFP, Figueira E. Sensitivity of biochemical markers to evaluate cadmium stress in the freshwater diatom Nitzschia palea (Kützing) W. Smith. Aquatic Toxicology. 2010;99(2):109–117. doi: 10.1016/j.aquatox.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Solymosi K, Bertrand M. Soil metals, chloroplasts, and secure crop production: a review. Agronomy for Sustainable Environment. 2010;32(1):245–272. [Google Scholar]
- 16.Imber BE, Robinson MG, Ortega AM, Burton JD. Complexation of zinc by exudates from Skeletonema costatum grown in culture. Marine Chemistry. 1985;16(2):131–139. [Google Scholar]
- 17.Grill E, Loffler S, Winnacker EL, Zenk MH. Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase) Proceedings of the National Academy of Sciences of the United States of America. 1989;86(18):6838–6842. doi: 10.1073/pnas.86.18.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sylvestre F, Beck-Eichler B, Duleba W, Debenay JP. Modern benthic diatom distribution in a hypersaline coastal lagoon: the lagoa de araruama (R.J.), Brazil. Hydrobiologia. 2001;443:213–231. [Google Scholar]
- 19.Rech M, Mouget J-L, Morant-Manceau A, Rosa P, Tremblin G. Long-term acclimation to UV radiation: effects on growth, photosynthesis and carbonic anhydrase activity in marine diatoms. Botanica Marina. 2005;48(5-6):407–420. [Google Scholar]
- 20.Fouqueray M, Mouget JL, Morant-Manceau A, Tremblin G. Dynamics of short-term acclimation to UV radiation in marine diatoms. Journal of Photochemistry and Photobiology B. 2007;89(1):1–8. doi: 10.1016/j.jphotobiol.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Harrison PJ, Waters RE, Taylor FJR. A broad spectrum artificial seawater medium for coastal and open ocean phytoplankton. Journal of Phycology. 1980;16(1):28–35. [Google Scholar]
- 22.Speziale BJ, Schreiner SP, Giammatteo PA, Schindler JE. Comparison of N,N- dimethylformamide, dimethyl sulfoxide, and acetone for extraction of phytoplankton chlorophyll. Canadian Journal of Fisheries and Aquatic Sciences. 1984;41(10):1519–1522. [Google Scholar]
- 23.Eilers PHC, Peeters JCH. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecological Modelling. 1988;42(3-4):199–215. [Google Scholar]
- 24.Rech M, Mouget J-L, Tremblin G. Modification of the Hansatech FMS fluorometer to facilitate measurements with microalgal cultures. Aquatic Botany. 2003;77(1):71–80. [Google Scholar]
- 25.Mouget J-L, Tremblin G, Morant-Manceau A, Morançais M, Robert JM. Long-term photoacclimation of Haslea ostrearia (Bacillariophyta): effect of irradiance on growth rates, pigment content and photosynthesis. European Journal of Phycology. 1999;34(2):109–115. [Google Scholar]
- 26.Dionisio-Sese M, Miyachi S. The effect of sodium chloride on carbonic anhydrase activity in marine microalgae. Journal of Phycology. 1992;28(5):619–624. [Google Scholar]
- 27.Morant-Manceau A, Nguyen TLN, Pradier E, Tremblin G. Carbonic anhydrase activity and photosynthesis in marine diatoms. European Journal of Phycology. 2007;42(3):263–270. [Google Scholar]
- 28.Aebi H. Catalase in vitro . Methods in Enzymology. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 29.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology. 1981;22(5):867–880. [Google Scholar]
- 30.Morelli E, Scarano G. Copper-induced changes of non-protein thiols and antioxidant enzymes in the marine microalga Phaeodactylum tricornutum . Plant Science. 2004;167(2):289–296. [Google Scholar]
- 31.Tripathi BN, Mehta SK, Amar A, Gaur JP. Oxidative stress in Scenedesmus sp. during short- and long-term exposure to Cu2+ and Zn2+ . Chemosphere. 2006;62(4):538–544. doi: 10.1016/j.chemosphere.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 32.Hartree EF. Determination of protein: a modification of the lowry method that gives a linear photometric response. Analytical Biochemistry. 1972;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- 33.Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- 34.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 35.Nicholas KB, Nicholas HB. GENEDOC a Tool for Editing and Annoting Multiple Sequence. Alignments, 1997, http://www.psc.edu/biomed/genedoc.
- 36.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A laboratory Manual. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 37.Hu H, Shi Y, Cong W, Cai Z. Growth and photosynthesis limitation of marine red tide alga Skeletonema costatum by low concentrations of Zn2+ . Biotechnology Letters. 2003;25(22):1881–1885. doi: 10.1023/b:bile.0000003976.18970.cd. [DOI] [PubMed] [Google Scholar]
- 38.Morelli E, Scarano G. Synthesis and stability of phytochelatins induced by cadmium and lead in the marine diatom Phaeodactylum tricornutum . Marine Environmental Research. 2001;52(4):383–395. doi: 10.1016/s0141-1136(01)00093-9. [DOI] [PubMed] [Google Scholar]
- 39.Lamote M, Darko E, Schoefs B, Lemoine Y. Assembly of the photosynthetic apparatus in embryos from Fucus serratus L. Photosynthesis Research. 2003;77(1):45–52. doi: 10.1023/A:1024999024157. [DOI] [PubMed] [Google Scholar]
- 40.Kralova K, Masarovicova E, Gyoryova K. The physiological response of green algae (Chlorella vulgaris) to pH-dependent inhibitory activity of some zinc(II) compounds: carboxylato- and halogenocarboxylatozinc(II) complexes. Chemical Papers. 2004;58(5):353–356. [Google Scholar]
- 41.Shehata SA, Lasheen MR, Ali GH, Kobbia IA. Toxic effect of certain metals mixture on some physiological and morphological characteristics of freshwater algae. Water, Air, and Soil Pollution. 1999;110(1-2):119–135. [Google Scholar]
- 42.Fisher NS, Jones GJ, Nelson DM. Effects of copper and zinc on growth, morphology, and metabolism of Asterionella japonica (Cleve) Journal of Experimental Marine Biology and Ecology. 1981;51(1):37–56. [Google Scholar]
- 43.Omar HH. Bioremoval of zinc ions by Scenedesmus obliquus and Scenedesmus quadricauda and its effect on growth and metabolism. International Biodeterioration and Biodegradation. 2002;50(2):95–100. [Google Scholar]
- 44.Rai L, Sing A, Mallick N. Studies on photosynthesis, the associated electron transport system and some physiological variables of Chlorella vulgaris under heavy metal stress. Journal of Plant Physiology. 1991;137(4):419–424. [Google Scholar]
- 45.Beale SI. Enzymes of chlorophyll biosynthesis. Photosynthesis Research. 1999;60(1):43–73. [Google Scholar]
- 46.Danilov RA, Ekelund NGA. Effects of Cu2+, Ni2+, Pb2+, Zn2+ and pentachlorophenol on photosynthesis and motility in Chlamydomonas reinhardtii in short-term exposure experiments. The BMC Ecology. 2001;1, article 1 doi: 10.1186/1472-6785-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koukal B, Guéguen C, Pardos M, Dominik J. Influence of humic substances on the toxic effects of cadmium and zinc to the green alga Pseudokirchneriella subcapitata . Chemosphere. 2003;53(8):953–961. doi: 10.1016/S0045-6535(03)00720-3. [DOI] [PubMed] [Google Scholar]
- 48.Lefebvre S, Mouget J-L, Loret P, Rosa P, Tremblin G. Comparison between fluorimetry and oximetry techniques to measure photosynthesis in the diatom Skeletonema costatum cultivated under simulated seasonal conditions. Journal of Photochemistry and Photobiology B. 2007;86(2):131–139. doi: 10.1016/j.jphotobiol.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 49.Kühl M, Glud RN, Borum J, Roberts R, Rysgaard S. Photosynthetic performance of surface-associated algae below sea ice as measured with a pulse-amplitude-modulated (PAM) fluorometer and O2 microsensors. Marine Ecology Progress Series. 2001;223:1–14. [Google Scholar]
- 50.Flameling IA, Krompkamp J. Light dependence of quantum yields for PSII charge separation and oxygen evolution in eukaryotic algae. Limnology and oceanography. 1998;43(2):284–287. [Google Scholar]
- 51.Roberts AG, Bowman MK, Kramer DM. Certain metal ions are inhibitors of cytochrome b 6 f complex “Rieske” iron-sulfur protein domain movements. Biochemistry. 2002;41(12):4070–4079. doi: 10.1021/bi015996k. [DOI] [PubMed] [Google Scholar]
- 52.Giachini L, Francia F, Veronesi G, et al. X-ray absorption studies of Zn2+ binding sites in bacterial, avian, and bovine cytochrome bc1 complexes. Biophysical Journal. 2007;93(8):2934–2951. doi: 10.1529/biophysj.107.110957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kügler M, Kruft V, Schmitz UK, Braun H-P. Characterization of the PetM subunit of the b 6 f complex from higher plants. Journal of Plant Physiology. 1998;153(5-6):581–586. [Google Scholar]
- 54.Allen JF. Cytochrome b 6 f: structure for signalling and vectorial metabolism. Trends in Plant Science. 2004;9(3):130–137. doi: 10.1016/j.tplants.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 55.Bertrand M. Carotenoid biosynthesis in diatoms. Photosynthesis Research. 2010;106(1-2):89–102. doi: 10.1007/s11120-010-9589-x. [DOI] [PubMed] [Google Scholar]
- 56.Lemoine Y, Schoefs B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: a multifunctional response to stress. Photosynthesis Research. 2010;106(1-2):155–177. doi: 10.1007/s11120-010-9583-3. [DOI] [PubMed] [Google Scholar]
- 57.Bertrand M, Schoefs B, Siffel P, Rohacek K, Molnar I. Cadmium inhibits epoxidation of diatoxanthin to diadinoxanthin in the xanthophyll cycle of the marine diatom Phaeodactylum tricornutum . The FEBS Letters. 2001;508(1):153–156. doi: 10.1016/s0014-5793(01)03050-2. [DOI] [PubMed] [Google Scholar]
- 58.Rashid A, Camm EL, Ekramoddoullah AKM. Molecular mechanism of action of Pb2+ and Zn2+ on water oxidizing complex of photosystem II. The FEBS Letters. 1994;350(2-3):296–298. doi: 10.1016/0014-5793(94)00789-6. [DOI] [PubMed] [Google Scholar]
- 59.Vaillant N, Monnet F, Hitmi A, Sallanon H, Coudret A. Comparative study of responses in four Datura species to a zinc stress. Chemosphere. 2005;59(7):1005–1013. doi: 10.1016/j.chemosphere.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 60.Subrahmanyam D, Rathore VS. Influence of manganese toxicity on photosynthesis in ricebean (Vigna umbellata) seedlings. Photosynthetica. 2000;38(3):449–453. [Google Scholar]
- 61.Sunda WG, Huntsman SA. Effect of CO2 supply and demand on zinc uptake and growth limitation in a coastal diatom. Limnology and Oceanography. 2005;50(4):1181–1192. [Google Scholar]
- 62.Monnet F, Vaillant N, Vernay P, Coudret A, Sallanon H, Hitmi A. Relationship between PSII activity, CO2 fixation, and Zn, Mn and Mg contents of Lolium perenne under zinc stress. Journal of Plant Physiology. 2001;158(9):1137–1144. [Google Scholar]
- 63.Aizawa K, Miyachi S. Carbonic anhydrase and CO2 concentrating mechanisms in microalgae and cyanobacteria. FEMS Microbiology Letters. 1986;39(3):215–233. [Google Scholar]
- 64.Sültemeyer D, Schmidt C, Fock HP. Carbonic anhydrases in higher plants and aquatic microorganisms. Physiologia Plantarum. 1993;88(1):179–190. [Google Scholar]
- 65.Moroney JV, Bartlett SG, Samuelsson G. Carbonic anhydrases in plants and algae. Plant, Cell and Environment. 2001;24(2):141–153. [Google Scholar]
- 66.Lane TW, Morel FMM. Regulation of carbonic anhydrase expression by zinc, cobalt, and carbon dioxide in the marine diatom Thalassiosira weissflogii . Plant Physiology. 2000;123(1):345–352. doi: 10.1104/pp.123.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinto E, Sigaud-Kutner TCS, Leitao MAS, Okamoto OK, Morse D, Colepicolo P. Heavy metal-induced oxidative stress in algae. Journal of Phycology. 2003;39(6):1008–1018. [Google Scholar]
- 68.French MS, Evans LV. The effects of copper and zinc on growth of the fouling diatoms Amphora and Amphiprora . Biofouling. 1988;1:3–18. [Google Scholar]
- 69.Cobbett C, Goldsbrough P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annual Review of Plant Biology. 2002;53:159–182. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- 70.Tsuji N, Hirayanagi N, Iwabe O, et al. Regulation of phytochelatin synthesis by zinc and cadmium in marine green alga, Dunaliella tertiolecta . Phytochemistry. 2003;62(3):453–459. doi: 10.1016/s0031-9422(02)00559-9. [DOI] [PubMed] [Google Scholar]
- 71.Souza JF, Rauser WE. Maize and radish sequester excess cadmium and zinc in different ways. Plant Science. 2003;165(5):1009–1022. [Google Scholar]
- 72.Kawakami SK, Gledhill M, Achterberg EP. Production of phytochelatins and glutathione by marine phytoplankton in response to metal stress. Journal of Phycology. 2006;42(5):975–989. [Google Scholar]
- 73.Hirata K, Tsujimoto Y, Namba T, et al. Strong induction of phytochelatin synthesis by zinc in marine green alga, Dunaliella tertiolecta . Journal of Bioscience and Bioengineering. 2001;92(1):24–29. doi: 10.1263/jbb.92.24. [DOI] [PubMed] [Google Scholar]


