Abstract
Research linking post-traumatic stress disorder (PTSD) to hypercortisolism in laboratory experiments was extended to a natural clinical setting. Mothers of children diagnosed with a life-threatening illness (N = 92) completed standardized measures of PTSD and provided a salivary cortisol sample during their child’s medical check-up (Time 1) and again 24 h later, after the threat of possible negative medical reports was removed (Time 2). Women who met diagnostic criteria for PTSD exhibited significantly higher cortisol levels at Time 1 compared to women who did not meet criteria for a diagnosis. No significant differences were observed for cortisol levels at Time 2 between the women with and without PTSD. These findings extend current laboratory findings linking hypercortisolism and PTSD to a natural, stressful situation. Implications for understanding the etiology of PTSD as well as for possible prevention and intervention options are discussed.
Keywords: Chronic illness, Cortisol, Hypothalamic–pituitary–adrenal axis, PTSD, Trauma, Women
1. Introduction
Approximately 13 million people nationwide are diagnosed with post-traumatic stress disorder (PTSD), with women being diagnosed nearly twice as often as men (Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995; Olff, Langeland, Draijer, & Gersons, 2007). One group of women that may be particularly vulnerable is mothers of children diagnosed with a chronic life-threatening illness. Rates for PTSD reportedly range from 7% to 14% for mothers of children diagnosed with cancer, and are equally prevalent among mothers of children diagnosed with other chronic illnesses such as type I diabetes (Kazak et al., 2004; Landolt et al., 2002; Stoppelbein & Greening, 2007). In addition to causing serious personal distress, individuals diagnosed with PTSD are at an increased risk for such comorbid conditions as depression, suicidality, and anxiety-related disorders (Brady, Killeen, Brewerton, & Lucerini, 2000). Manne, Du Hamel, Galleli, Sorgen, and Redd (1998), for example, found that approximately 25% of mothers of pediatric cancer patients also exhibited major depression and or generalized anxiety disorder. Psychosocial consequences for society including absenteeism from work, unemployment, and the high utilization of health-care services further underscore the magnitude of the sequelae as well as the merit of research investigating the underlying etiological mechanisms for PTSD.
There has been considerable research focusing specifically on the neurobiological underpinnings of PTSD, which has revealed differences in the basal functioning and reactivity of the hypothalamic–pituitary–adrenal (HPA) axis in patients with PTSD compared to control groups (Yehuda, 2002). The HPA axis mediates the human stress response through the release of corticotropin releasing factor (CRF) from the hypothalamus, which in turn stimulates the secretion of adrenocorticotropin releasing hormone (ACTH) from the pituitary gland. ACTH then stimulates the release of cortisol from the adrenal glands which acts as part of a feedback loop to regulate HPA-axis activity. Evidence has emerged over the last decade linking PTSD to aberrant HPA-axis functioning. For example, studies examining central nervous system markers of HPA-axis functioning including CRF levels in the cerebrospinal fluid (CSF) reveal elevated levels of CRF among individuals diagnosed with PTSD when compared to a control group (Baker et al., 1999; Bremner et al., 1997; Sautter et al., 2003).
There is also support for aberrant peripheral basal cortisol concentrations but these findings are equivocal. A number of studies, for example, have documented significantly lower levels of urinary and plasma cortisol among individuals with PTSD following different types of traumatic events including motor vehicle accidents (Delahanty, Raimonde, & Spoonster, 2000; Delahanty, Raimonde, Spoonster, & Cullado, 2003), having a child diagnosed with a chronic illness (Glover & Poland, 2002), combat (Yehuda et al., 1990), and breast cancer (Luecken, Dausch, Gulla, Hong, & Compas, 2004). Interestingly, police officers with post-traumatic stress show lower levels of salivary cortisol (Neylan et al., 2005) as well as a significantly reduced increase in cortisol upon awakening (Wessa, Rohleder, Kirschbaum, & Flor, 2006). Compared to individuals with other psychiatric diagnoses, trauma survivors with PTSD have been found to have lower 24-h urinary cortisol levels (Mason, Giller, Kosten, Ostroff, & Podd, 1986). However, the research linking hypocortisolism to PTSD is not unequivocal. Others have reported increased urinary, plasma, and salivary cortisol secretion among PTSD patients (Delahanty, Nugent, Christopher, & Walsh, 2005; Hawk, Dougall, Ursano, & Baum, 2000; Inslicht et al., 2006; Resnick, Yehuda, Pitman, & Foy, 1995) and still others find no relation (Lindley, Carlson, & Benoit, 2004; Young & Breslau, 2004; Young, Tolman, Witkowski, & Kaplan, 2004).
Much of the current research on cortisol and PTSD has focused on differences in basal cortisol level and has neglected to compare HPA-axis responses during stressful versus non-stressful events. Examining differences under these two conditions might help to explain some of the equivocal findings that have been reported to date. More recent research has revealed that individuals with PTSD have higher cortisol levels just prior to, during, and immediately after a cognitive challenge task (Bremner et al., 2003). Similarly, Elzinga, Schmahl, Vermetten, van Dyck, and Bremner (2003) found that abused women with PTSD had higher cortisol levels than controls during and shortly after exposure to a trauma script. Theorists suggest that patients with PTSD show increased cortisol responsivity during stressful events and an ongoing suppression of basal cortisol levels when they are not under stress to compensate for this rise (Bremner et al., 2003). That is, when people diagnosed with PTSD are allowed to engage in avoidant behaviors or when they do not perceive any threat from a stressful stimulus, they are expected to show normal or lower cortisol levels. However, when faced with a potentially threatening or uncontrollable stressor, they would be expected to exhibit hypercortisolism.
Interestingly, Simeon et al. (2007) did not find a difference between individuals with and without PTSD in their basal cortisol or their cortisol response to a social stressor task. Similarly, Brady et al. (2006) found no difference between individuals diagnosed with PTSD and a control group on their peak cortisol response to a cold pressor task. The lack of differences may be attributed to using a non-trauma-related stimulus that did not elicit feelings of threat in participants rather than to the lack of a relation. Hence, the purpose of the present study was to test the hypothesis that individuals with PTSD show greater cortisol responsivity when confronted with a real-world and more trauma-specific stressor compared to individuals without PTSD. This hypothesis was tested in a non-contrived clinical setting with mothers of children diagnosed with a chronic, potentially life-threatening illness (i.e., cancer or type 1 diabetes). Cortisol samples were collected at the time of their child’s routine medical follow-up appointment with their respective physicians (Time 1) and again 24 h after their medical appointment (Time 2). It was hypothesized that (a) the mothers with PTSD would have higher cortisol levels compared to mothers without PTSD at Time 1 because of the threat of possible negative medical reports, and that (b) the mothers with PTSD would exhibit similar or lower levels of cortisol than the mothers without PTSD at Time 2 because the threat of negative medical feedback is removed. The uncontrollability and unpredictability of a traumatic event (Foa, Steketee, & Rothbaum, 1989; Greening & Stoppelbein, 2007), such as a recurrence of cancer as well as the impact of daily and developmental stressors on blood glucose, can heighten the risk of negative medical feedback for these populations. The present findings might help extend previous research examining cortisol responsivity among individuals with PTSD beyond controlled experimental situations to naturalistic settings.
2. Method
2.1. Participants
Mothers of children diagnosed with a life-threatening disease (i.e., cancer or type I diabetes; N = 118) were recruited from a southeastern children’s hospital. Exclusion criteria included a history of previous psychiatric diagnoses1 or substance abuse, history of endocrine disorders or use of synthetic glucocorticoid or exogenous steroid treatment within the past month, and receiving negative medical feedback about their child’s health status during their medical visit. Nineteen participants were subsequently excluded (9 received negative feedback, 4 had endocrine disorders or were on hormonal treatments, 6 reported history of psychiatric diagnoses or substance use) and an additional 7 mothers declined to participate due to lack of time/interest. Of the remaining 92, 62% were from the pediatric oncology clinic (n = 57) and 38% were from the pediatric endocrinology clinic (n = 35). Unequal n analyses of variance (ANOVA) and chi-square tests revealed that there were no significant differences between the two illness groups on demographic variables or on a measure of PTSD.
Demographic and disease-related data are presented in Table 1. The mothers ranged from 20 to 44 years of age; 57% were African-American and 43% were Caucasian. Over half (57%) were married to the child’s father; the remaining were either divorced (13%), separated (4%), widowed (4%), living with a partner (8%), or never married (14%). Most of the children with cancer had been treated with chemotherapy (87%); 43% had received radiation therapy and 6% had received a bone marrow transplant (BMT). Most of the children diagnosed with diabetes were taking daily insulin injections (77%), with an average of 2 shots per day (M = 2.03, SD = 1.38); the remaining were using a subcutaneous insulin pump. The children in the sample were on average 10 years of age (M = 10.70, SD = 5.22) and were diagnosed with their respective diseases approximately 2 years prior to assessment (M = 2.21 years, SD = 2.29). None of the mothers were currently on any medication.
Table 1.
Sample characteristics (N = 92).
| Variable | Total group | PTSD | Non-PTSD |
|---|---|---|---|
| Age in years, mean (SD) | 36.48 (8.47) | 36.45 | 37.00 |
| Ethnicity/race % | |||
| African American (Caucasian) | 57 (43) | 60 (40) | 55 (45) |
| SESa, mean (SD) | 3.13 (1.17) | 2.94 (0.98) | 3.32 (1.36) |
| Hollingshead class I % | 9% | 0% | 9% |
| Hollingshead class II % | 27% | 20% | 28% |
| Hollingshead class III % | 11% | 20% | 11% |
| Hollingshead class IV % | 47% | 60% | 46% |
| Hollingshead class V % | 6% | 0% | 6% |
| Marital status % | |||
| Unmarried | 14% | 19% | 8% |
| Married | 57% | 69% | 56% |
| Divorced | 13% | 11% | 20% |
| Separated | 4% | 1% | 4% |
| Living with partner | 8% | 0% | 8% |
| Widowed | 4% | 0% | 4% |
| Years since child’s diagnosis, mean (SD) | 2.21 (2.29) | 2.67 (2.44) | 1.75 (2.14) |
| SCID PTSDb diagnosis % | 11% | 100% | 0% |
| BDI-IIc score, mean (SD) | 13.24 (10.21) | 15.34 (8.08) | 11.14 (12.34) |
| STAI-Td T-score, mean (SD) | 57.16 (12.64) | 61.16 (13.82) | 53.00 (11.46) |
| Stressful life events, mean (SD) | 3.94 (2.56) | 3.90 (2.55) | 3.98 (2.57) |
Socioeconomic status.
Structured Clinical Interview for the DSM-IV, PTSD module.
Beck Depression Inventory—2nd edition.
State Trait Anxiety Inventory—Trait Scale.
2.2. Measures
2.2.1. Demographic and health history
Participants were interviewed about disease-related, demographic, maternal health-related information (e.g., medications, history of endocrine-related disorders, etc.) and personal history of a psychiatric diagnosis or substance use/abuse. Demographic data were used to compute a Hollingshead Index (Hollingshead, 1975) as a measure of socioeconomic status (SES).
2.2.2. Structured clinical interview for the DSM-IV (SCID), PTSD module
The SCID-PTSD module is a structured clinical interview designed to assess for PTSD using DSM-IV criteria (First, Gibbon, Spitzer, & Williams, 2002). A dichotomous score indicating whether the interviewee currently meets or does not meet criteria for PTSD is derived from responses. Participants were asked to respond to the occurrence of symptoms as they related to their child’s diagnosis. The interviewers were trained to 90% inter-rater reliability. Reliability ratings for the SCID-PTSD range from 0.78 to 1.0 (Zanarini & Frankenburg, 2001). The module also has good sensitivity and specificity, >0.80 (Solomon, Keane, Newman, & Kaloupek, 1996).
2.2.3. State-trait Anxiety Inventory-Trait Scale (STAI-T)
The STAI-T is a 20-item, self-report measure of trait anxiety (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). Respondents indicated on a 4-point scale how much they agreed with statements assessing anxiety. Internal consistency is high (αs = 0.90 for STAI-T) and was equally high for the present sample, α = 0.93. Test–retest reliability for the measure is acceptable, with a median coefficient of 0.76 (Spielberger et al., 1983).
2.2.4. Beck Depression Inventory-2nd edition (BDI-II)
The BDI-II is a 21-item, self-report measure of depressive symptoms. Respondents rated along a 4-point scale ranging from 0 to 3 the severity of symptoms described for each item. Scores for clinically significant symptoms are <14 = minimal, 14–19 = mild, 20–28 = moderate, and >28 = severe. The scale has high internal consistency, moderate concurrent validity, and discriminates between depressive and anxiety symptoms (Beck, Steer, & Brown, 1996). Internal consistency was high in the present study, α = 0.93.
2.2.5. Life events checklist
A checklist similar to a measure used by Breslau (2001) was used to assess for previous trauma exposure. Respondents indicated if they had experienced events from a list of 16 qualifying events for PTSD [e.g., physical assault, tornado/earthquake, serious car accident; American Psychiatric Association (APA), 2000]. Endorsed events were summed for a total score.
2.2.6. Cortisol
Salivary cortisol provides a reliable and accurate index of free plasma cortisol and avoids confounds that could arise from the stress of drawing blood intravenously (Bhagwagar, Hafizi, & Cowen, 2002; Smyth et al., 1997). Approximately 2 ml of saliva were collected from each participant using a pre-weighed Salivette kit. The collected samples were frozen and stored in a subzero freezer at −80 °C until data collection was completed. Cortisol samples were analyzed as a group in a single assay after completing data collection to prevent possible confounds with interassay variability. Samples were assayed in duplicate and the average for the duplicate was used for analyses. Samples were analyzed with the Salimetrics HS Salivary Cortisol EIA kit for unbound cortisol using kit instructions. The Salivary Cortisol EIA kit has intra-assay coefficients of variation ranging from 3.35% to 3.65% and the intra-assay variability of 3.41% for the current study is similar to that reported by the kit manufacturer. Salivary cortisol as analyzed using this kit is highly correlated with serum cortisol (r = 0.91, p < .0001).
2.3. Procedure
After obtaining approval from the Institutional Review Board, mothers were invited to participate while they waited for their child’s quarterly or annual follow-up medical oncology or endocrinology appointment. After providing written consent, the mothers completed the study protocol prior to seeing their child’s physician but following any medical procedures (e.g., MRI, blood work). Recent research suggests that differences in HPA-axis functioning among individuals with and without PTSD, particularly women, is prominent in the afternoon and evening (Freidenberg et al., 2010; Yehuda, Golier, & Kafman, 2005). In an effort to control for circadian variations in cortisol, mothers were recruited for participation between 3:00 p.m. and 5:00 p.m. Participants first completed the demographic questionnaire. Then a clinical researcher collected a salivary cortisol sample (Time 1). The researcher confirmed that the participant had not consumed any food, liquid, or chewed gum at least 60 min before the salivary sample was obtained. None of the participants were tobacco users. The researcher then completed the clinical interview with mothers, followed by the mothers completing the self-report measures. Upon completion of data collection, the participants were instructed to collect a second saliva sample between 3:00 p.m. and 5:00 p.m. the following day (Time 2), and not to eat, drink, chew gum, or brush one’s teeth for at least 60 min before collecting the sample. The mothers received a phone call the following day as a reminder to collect their saliva sample and to answer any questions they had regarding the collection procedure. The mothers were instructed to store the sample in the freezer immediately upon collection. Salivary cortisol is stable at room temperature or colder for 2 to 3 weeks (Groschel, Wagner, Rauh, & Dorr, 2001). The participants were provided with a self-addressed envelope in which the sample was shipped overnight to the researcher. This procedure has been used in similar studies examining the role of cortisol in PTSD (Glover & Poland, 2002; Hawk et al., 2000; Heinrichs et al., 2005; Lindley et al., 2004; Neylan et al., 2005). All samples were received 2 days after the initial assessment suggesting that the samples were provided on the day following their child’s medical appointment. Mailed samples were stored in a subzero freezer until they were analyzed as a group. Participants were compensated $30 for their participation, which was mailed to the participant 1 to 2 weeks after participation.
2.4. Data analytic plan
Descriptive statistics were calculated and correlational analyses were performed to investigate the correlations between demographic variables (e.g., SES), PTSD status, cortisol level, depression, anxiety, and negative life events. These variables were included in correlational analyses because they have been linked to either post-traumatic stress or cortisol level in the literature (Brown, Madan-Swain, & Lambert, 2003; Goldberg-Libov, Nevid, Pelcovitz, & Carmony, 2002; Heinrichs et al., 2005; Stoppelbein & Greening, 2007). Significant covariates of cortisol level were included as control variables in analyses that were performed to test the study’s hypotheses. Tests of differences between the PTSD and non-PTSD groups were also performed. Given the unequal sample sizes for the PTSD and non-PTSD groups, a non-parametric test – the Kruskal–Wallis test – was performed to compare the groups on age, length of time since diagnosis, BDI-II score, STAI-T score, and negative life events. Fisher’s exact test was used to compare the groups on categorical variables including SES, race/ethnicity, and marital status because of the small sample of women with PTSD. The Bonferroni correction was applied (.05/8) to account for the number of group comparisons.
A series of random effects regression models using maximum likelihood estimation, adjusting for any covariates, was estimated to test the primary hypotheses that mothers who met criteria for PTSD would have higher cortisol levels at the time of their child’s medical appointment (Time 1) compared to mothers who did not meet criteria for PTSD; that the mothers with PTSD would have similar or lower levels of cortisol compared to mothers without PTSD 24 h after their child’s medical appointment and after the threat of negative medical feedback was removed (Time 2). Random effects regression analyses have a number of advantages when used with repeated measures analyses. First, they use all available data and use missing data appropriately, they account for within subject correlations between repeated measurements, and they do not assume homogeneity of variance across groups and time points (Gueorgieva & Krystal, 2004; Raudenbush & Bryk, 2002). Cortisol was entered as the dependent variable. Time was the within-group variable and PTSD diagnostic status was the between-group variable. Finally, the Time × PTSD diagnostic status interaction was examined. Significant interactions were examined using contrast analyses. Covariates of the dependent variable that were identified in preliminary correlational analyses were planned to be included as control variables.
3. Results
Eleven percent of the participants (n = 10) met criteria for PTSD on the SCID. Of those who met criteria, 60% of them were mothers of children with cancer and 40% were mothers of children with diabetes. ANOVAs and chi square tests did not reveal significant differences between the two illness groups on demographic variables for the rate of PTSD (p > .01). The Fisher’s exact test and the Kruskal–Wallis test also did not reveal significant differences between the women who did and who did not meet criteria for PTSD on SES, race/ethnicity, marital status, age, length of time since diagnosis, BDI-II score, STAI-T score, and negative life events (p > .01). Mean BDI-II (M = 13.24, SD = 10.21) and STAI-T (M T-Score = 57.16, SD = 12.64) scores for the total sample fell in the non-clinical range; the participants reported experiencing an average of 4 negative life events in their lifetime (M = 3.94, SD = 2.56).
3.1. Correlational analyses and group comparisons
Correlational analyses revealed that mothers with higher Time 1 cortisol levels were observed to be significantly more likely to meet diagnostic criteria for PTSD on the SCID, r = 0.21, p < .05 (see Table 2). However, there were no significant relations observed between Time 1 cortisol and the BDI-II, STAI-T, or negative life events. Mothers from lower SES households tended to exhibit higher Time 2 cortisol levels, r = 0.24, p < .05; mothers who met diagnostic criteria for PTSD tended to report more depressive and anxiety symptoms, rs = 0.22 and 0.24, respectively, p < .05. Mothers who reported more depressive symptoms also tended to report more anxiety symptoms, r = 0.83, p < .001. Finally, the mothers of children with diabetes were older, r = 0.32, p < .01. Illness group was not related to cortisol, the SCID, BDI-II, STAI-T or to negative life events.
Table 2.
Correlational analyses (N = 92).
| Variable | 1 | 2 | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | – | −0.10 | −0.15 | 0.11 | −0.12 | 0.32** | 0.01 | 0.08 | 0.02 | 0.09 |
| 2. Ethnicitya | – | 0.33** | 0.07 | 0.14 | 0.04 | −0.18 | −0.16 | −0.16 | 0.01 | |
| 3. SESb | – | 0.13 | 0.24* | 0.11 | 0.06 | 0.19 | 0.17 | −0.02 | ||
| 4. Time 1 cortisol | – | 0.16 | 0.17 | 0.21* | 0.01 | 0.10 | −0.06 | |||
| 5. Time 2 cortisol | – | 0.02 | −0.08 | 0.02 | −0.05 | −0.15 | ||||
| 6. Illness groupc | – | 0.11 | 0.13 | 0.08 | 0.06 | |||||
| 7. SCIDd | – | 0.22* | 0.24* | −0.07 | ||||||
| 8. BDI-IIe | – | 0.83*** | 0.19 | |||||||
| 9. STAI-Tf | – | 0.18 | ||||||||
| 10. Negative life events | – |
0 = Caucasian, 1 = African-American.
Socioeconomic status.
0 = cancer, 1 = diabetes.
Structured Clinical Interview for the DSM-IV, PTSD module.
Beck Depression Inventory—2nd edition.
State Trait Anxiety Inventory—Trait Scale.
p < .05.
p < .01.
p < .001.
3.2. Predicting change in cortisol
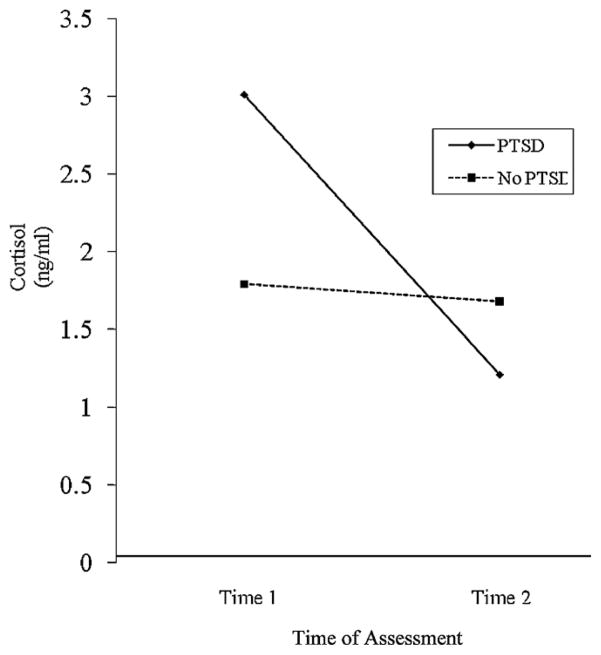
Next, a series of random effects regression analyses were performed to assess the relation between cortisol and PTSD status. SES was included as a covariate because it was significantly related to cortisol at Time 2. The main effect of time, F(1, 90) = 0.11, p = .74, was not statistically significant; however, the main effect of diagnostic group status, F(1, 90) = 3.20, p = .08, was marginally significant while controlling for SES. The interaction variable –Time × Diagnostic Group Status – was observed to be significant, F(1, 90) = 3.76, p = .05. Post hoc contrast analyses revealed that mothers with PTSD had higher cortisol levels at Time 1 (M = 3.01 ng/ml) than mothers without PTSD (M = 1.65 ng/ml), and that there was no significant difference in cortisol level between mothers with PTSD (M = 1.22 ng/ml) and mothers without PTSD (M = 1.56 ng/ml) at Time 2 (see Fig. 1).
Fig. 1.
Change in cortisol from Time 1 to Time 2 for mothers who do and do not meet diagnostic criteria for PTSD.
4. Discussion
The hypothesis that mothers of chronically ill children with PTSD would exhibit significantly higher salivary cortisol levels than mothers without PTSD while waiting for their child’s medical appointment was supported. These findings are consistent with previous reports of higher cortisol levels during laboratory-induced, event-related stressors (e.g., trauma script; Elzinga et al., 2003) among those with PTSD, and suggest that applying a repeated measures model to understand HPA-axis functioning in stressful and non-stressful conditions might offer further insight into the equivocal findings reported in the literature. Unlike past laboratory studies that failed to find support for the role of cortisol in PTSD (Brady et al., 2006; Simeon et al., 2007), the participants in the present study were evaluated exposed to a trauma-related stressor. According to behavior theorists, patients with PTSD are conditioned to react to potential trauma-related stimuli with heightened autonomic nervous system (ANS) arousal and cortisol secretion as a result of their initial trauma exposure and ANS arousal (Keane, Zimering, & Caddell, 1985; Kilpatrick, Veronen, & Best, 1985). Hence, such reactions would not be expected when exposed to non-trauma-related stimuli necessarily. Explanations for the mothers’ heightened arousal could be biological. For example, having a hyper-reactive HPA axis, might account for their hypercortisolism. That is, the mothers with PTSD might be more vulnerable to exhibiting hypercortisolism in response to trauma-related stimuli because they possess a more sensitive HPA axis. While not tested directly, the mothers’ significantly higher cortisol secretion while waiting for their child’s medical appointment lends some correlational support for this hypothesis. The present study, however, precludes inferring a cause-and-effect relation. Hence, research is warranted with additional cortisol assessments across time including prior to trauma exposure, in cases where feasible, before any cause-and-effect conclusions can be drawn.
Some may question if a child’s diagnosis of a life-threatening condition can be conceptualized as a traumatic event that could lead to PTSD. However, according to the American Psychiatric Association (2000) having a child diagnosed with a life-threatening illness is recognized as a traumatic event because of “the threat of death”. The unpredictability and uncontrollability of a life-threatening illness and the risk of future recurring threats of the illness further qualifies this event as a traumatic stressor. Furthermore, epidemiological research reveals a higher prevalence rate of PTSD among mothers of children dignosed with such life-threatening chronic illnesses as cancer and diabetes than in the general population (Kessler et al., 1995; Lefkowitz, Baxt, & Evans, 2010; Libov, Nevid, Pelcovitz, & Carmony, 2002; Stoppelbein & Greening, 2007). The 11% prevalence rate observed in the present study is also higher than the 2% to 7% reported in the literature for the general population (Kessler et al., 1995). Rates for mothers of children diagnosed with life-threatening illnesses tend to range from 15% to 20% in the literature (Lefkowitz, Baxt, & Evans, 2010; Libov, Nevid, Pelcovitz, & Carmony, 2002; Stoppelbein & Greening, 2007). Others (e.g., Brown, Madan-Swain, & Lambert, 2003; Landolt, Vollrath, Ribi, Gnehm, & Sennhauser, 2003; Pelcovitz et al., 1996) have reported higher rates (25% to 28%) for mothers of children with life-threatening illnesses but these studies included larger samples, which may account for the higher rates.
4.1. Limitations
There are methodological limitations that preclude inferring definitive conclusions including the inclusion of only mothers in the present study. Although women are twice as likely as men to be diagnosed with PTSD (Kessler et al., 1995; Olff et al., 2007), research with men and victims of other types of traumatic events is warranted to maximize generalizations. Secondly, although there were no differences observed in the rates of PTSD among mothers of children with cancer or diabetes, other disease-related factors such as diagnostic status and severity of disease should be considered in future research. Third, the relatively small sample of mothers who met diagnostic criteria for PTSD (11%) limits generalizations and further suggests that the present pilot study warrants replications with larger samples.
Other limitations include the collection of only two salivary cortisol samples, not collecting basal cortisol samples, and testing the hypothesized relation in natural non-contrived settings, all of which can increase the risk of potential confounds. We also did not have experimental control over the collection of Time 2 salivary cortisol samples. However, home collections of salivary cortisol samples have been used in other similar studies (Glover & Poland, 2002; Hawk et al., 2000; Heinrichs et al., 2005; Lindley et al., 2004; Neylan et al., 2005). Although previous research comparing cortisol in those with and without PTSD has not found any differences in cortisol in the days/hours prior to exposure to a stressful task (Simeon et al., 2007). Nevertheless, more controlled assessments as well as collecting basal and more frequent cortisol samples over time are recommended for future research.
4.2. Conclusion
Although cause-and-effect conclusions are precluded by the present study’s design, the study attempts to provide some support for the hypothesized relation between hypercortisolism and PTSD. It is the first known study that documents such a relation using a longitudinal design and in the natural environment. Further longitudinal research with a cross-section of trauma survivors and with multiple cortisol assessments are recommended to help ascertain causal relations. Efforts to unveil the underlying neurobiological underpinnings of PTSD could provide possible implications for clinical interventions. For example, empirical support for the role of cortisol in PTSD has been implicated in the treatment efficacy of corticosteroids and beta-adrenergic antagonists on the mitigation of and prevention of PTSD symptoms (Pitman et al., 2002; Schelling et al., 2004; Valva et al., 2003). Further longitudinal research examining how changes in cortisol levels might act as mediating variables for these treatment effects could shed light on the neurobiological underpinnings of the effects and possibly furthering the development of effective treatments.
Acknowledgments
Role of the funding source
This study was funded by the Center for Psychiatric Neuroscience, University of Mississippi Medical Center, NIH Grant Number P20 RR17701, Institutional Development Award Program of the National Center for Research Resources. The Center for Psychiatric Neuroscience was not involved in the design, data collection, data analysis, data interpretation, writing of the manuscript, or decision to submit the paper for publication.
Footnotes
Participants with no previous psychiatric diagnoses were recruited to control for comorbid disorders. While no comorbid diagnoses is uncommon among individuals with PTSD, the mothers in the present sample had not been diagnosed with PTSD prior to their participation in the present study. Hence, they either neglected seeking mental health services for their symptoms or were managing their symptoms and were subsequently not diagnosed with PTSD as well as comorbid disorders.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Press; 2000. DSM-IV. [Google Scholar]
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill K, et al. Serial CSF corticotrophin-releasing hormone levels and adrenocorticol activity in combat veterans with posttraumatic stress disorder. American Journal of Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown BK. Beck depression inventory manual. 2. San Antonio: Psychological Corporation; 1996. [Google Scholar]
- Bhagwagar Z, Hafizi S, Cowen PJ. Acute citalopram administration produces correlated increases in plasma and salivary cortisol. Psychopharmacology. 2002;163:118–120. doi: 10.1007/S00213-002-1149-4. [DOI] [PubMed] [Google Scholar]
- Brady KT, Killeen TK, Brewerton T, Lucerini S. Comorbidity of psychiatric and posttraumatic stress disorder. Journal of Clinical Psychiatry. 2000;61:22–32. [PubMed] [Google Scholar]
- Brady KT, Waldrop AE, McRae AL, Back SE, Saladin ME, Upadhyaya HP, et al. The impact of alcohol dependence and posttraumatic stress disorder on cold pressor task response. Journal of Studies on Alcohol. 2006;67:700–706. doi: 10.15288/jsa.2006.67.700. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, et al. Elevated CSF corticotrophin-releasing factor concentrations in posttraumatic stress disorder. American Journal of Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, et al. Cortisol responses to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28:733–750. doi: 10.1016/S0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- Breslau N. The epidemiology of posttraumatic stress disorder: what is the extent of the problem? Journal of Clinical Psychiatry. 2001;62:16–22. [PubMed] [Google Scholar]
- Brown RT, Madan-Swain A, Lambert R. Posttraumatic stress symptoms in adolescent survivors of childhood cancer and their mothers. Journal of Traumatic Stress. 2003;16:309–318. doi: 10.1023/A:1024465415620. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Nugent NR, Christopher NC, Walsh M. Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Psychoneuroendocrinology. 2005;30:121–128. doi: 10.1016/j.psyneuen.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Raimonde AJ, Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biological Psychiatry. 2000;48:940–947. doi: 10.1016/S0006-3223(00)00896-9. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Raimonde AJ, Spoonster E, Cullado M. Injury severity, prior trauma history, urinary cortisol levels, and acute PTSD in motor vehicle accident victims. Journal of Anxiety Disorders. 2003;17:149–164. doi: 10.1016/S0887-6185(02)00185-8. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Schmahl CG, Vermetten E, van Dyck R, Bremner JD. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28:1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorder: research version. New York: Columbia University; 2002. [Google Scholar]
- Foa E, Steketee G, Rothbaum O. Behavioral/cognitive conceptualizations of post-traumatic stress disorder. Behavior Therapy. 1989;20:155–176. [Google Scholar]
- Freidenberg BM, Gusmano R, Hickling EJ, Blanchard EB, Bremner JD, Frye C. Women with and without PTSD have lower basal salivary cortisol levels later in the day than do men with PTSD: a preliminary study. Physiological Behavior. 2010;9:234–236. doi: 10.1016/j.physbeh.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover DA, Poland RE. Urinary cortisol and catecholamines in mothers of child cancer survivors with and without PTSD. Psychoneuroendocrinology. 2002;27:805–819. doi: 10.1016/S0306-4530(01)00081-6. [DOI] [PubMed] [Google Scholar]
- Goldberg-Libov B, Nevid JS, Pelcovitz D, Carmony TM. Posttraumatic stress symptomatology in mothers of pediatric cancer survivors. Psychology and Health. 2002;17:501–511. doi: 10.1080/0887044022000004975. [DOI] [Google Scholar]
- Greening L, Stoppelbein L. Pediatric cancer, parental coping style, and risk for depressive, posttraumatic stress, and anxiety symptoms. Journal of Pediatric Psychology. 2007;32:1272–1277. doi: 10.1093/jpepsy/jsm057. [DOI] [PubMed] [Google Scholar]
- Groschel M, Wagner R, Rauh M, Dorr HG. Stability of salivary steroids: the influence of storage, food and dental care. Steroids. 2001;66:737–741. doi: 10.1016/S0039-128X(01)00111-8. [DOI] [PubMed] [Google Scholar]
- Gueorgieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated measures data and its reflection in papers published in the Archives of General Psychiatry. Archives of General Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- Hawk LW, Dougall AL, Ursano RJ, Baum A. Urinary catecholamines and cortisol in recent-onset posttraumatic stress disorder after motor vehicle accidents. Psychosomatic Medicine. 2000;62:423–434. doi: 10.1097/00006842-200005000-00016. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Wagner D, Schoch W, Soravia LM, Hellhammer DH, Ehlert U. Predicting posttraumatic stress symptoms from pretraumatic risk factors: a 2-year prospective follow-up study in firefighters. American Journal of Psychiatry. 2005;162:2276–2286. doi: 10.1176/appi.ajp.162.12.2276. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Inslicht SS, Marmar CR, Neylan TC, Metzler TJ, Hart SL, Otte C, et al. Increased cortisol in women with intimate partner violence-related posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:825–838. doi: 10.1016/j.psyneuen.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Kazak AE, Alderfer M, Rourke MT, Simms S, Streisand R, Grossman JR. Posttraumatic stress disorder (PTSD) and posttraumatic stress symptoms (PTSS) in families of adolescent childhood cancer survivors. Journal of Pediatric Psychology. 2004;29:211–219. doi: 10.1093/jpepsy/jsh022. [DOI] [PubMed] [Google Scholar]
- Keane TM, Zimering RT, Caddell JM. A behavioral formulation of posttraumatic stress disorder. Behavior Therapist. 1985;8:9–12. [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Veronen LJ, Best CL. Factors predicting psychological distress among rape victims. In: Figley CR, editor. Trauma and its wake. New York: Brunner/Mazel; 1985. pp. 113–141. [Google Scholar]
- Landolt MA, Ribi K, Laimbacher J, Vollrath M, Gnehm HE, Sennhauser FH. Posttraumatic stress disorder in parents of children with newly diagnosed type I diabetes. Journal of Pediatric Psychology. 2002;27:647–652. doi: 10.1093/jpepsy/27.7.647. [DOI] [PubMed] [Google Scholar]
- Landolt MA, Vollrath M, Ribi K, Gnehm HE, Sennhauser FH. Incidence and associations of parental and child posttraumatic stress symptoms in pediatric patients. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2003;44:1199–1207. doi: 10.1111/1469-7610.00201. doi:10:1111/1469-7610.00201. [DOI] [PubMed] [Google Scholar]
- Lefkowitz DS, Baxt C, Evans JR. Prevalence and correlates of post-traumatic stress and postpartum depression in parents of infants in neonatal care units (NICU) Journal of Clinical Psychology in Medical Settings. 2010;17:230–237. doi: 10.1007/s10880-010-9202-7. doi:10:1007/s10880-010-9202-7. [DOI] [PubMed] [Google Scholar]
- Libov BB, Nevid JS, Pelcovitz D, Carmony TM. Posttraumatic stress symptomatology in mothers of pediatric cancer survivors. Psychology and Health. 2002;17:501–511. doi:10:1018/0887044022000004975. [Google Scholar]
- Lindley SE, Carlson EB, Benoit M. Basal and dexamethasone suppressed salivary cortisol concentrations in a community sample of patients with posttraumatic stress disorder. Biological Psychiatry. 2004;55:940–945. doi: 10.1016/j.biopsych.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Dausch B, Gulla V, Hong R, Compas B. Alterations in morning cortisol associated with PTSD in women with breast cancer. Journal of Psychosomatic Research. 2004;56:13–15. doi: 10.1016/S0022-3999(03)00561-0. [DOI] [PubMed] [Google Scholar]
- Manne SL, Du Hamel K, Gallelli K, Sorgen K, Redd WH. Posttraumatic stress disorder among mothers of cancer survivors: diagnosis, comorbidity, and utility of the PTSD checklist as a screening instrument. Journal of Pediatric Psychology. 1998;23:357–366. doi: 10.1093/jpepsy/23.6.357. [DOI] [PubMed] [Google Scholar]
- Mason JW, Giller EL, Kosten TR, Ostroff RB, Podd L. Urinary-free cortisol levels in posttraumatic stress disorder patients. Journal of Nervous and Mental Disease. 1986;174:145–149. doi: 10.1097/00005053-198603000-00003. [DOI] [PubMed] [Google Scholar]
- Neylan TC, Brunet A, Pole N, Best SR, Metzler TJ, Yehuda R, et al. PTSD symptoms predict waking salivary cortisol levels in police officers. Psychoneuroendocrinology. 2005;30:373–381. doi: 10.1016/j.psyneuen.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Draijer N, Gersons BPR. Gender differences in posttraumatic stress disorder. Psychological Bulletin. 2007;133:183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- Pelcovitz D, Goldenberg B, Kaplan S, Weinblatt M, Madnel F, Vineiguerra V. Posttraumatic stress disorder in mothers of pediatric cancer survivors. Psychosomatics. 1996;37:116–126. doi: 10.1016/S0033-3182(96)71577-3. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propanolol. Biological Psychiatry. 2002;51:189–192. doi: 10.1016/S0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: applications and data analysis methods. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Resnick H, Yehuda R, Pitman RK, Foy DW. Effect of previous trauma on acute plasma cortisol level following rape. American Journal of Psychiatry. 1995;152:1675–1677. doi: 10.1176/ajp.152.11.1675. [DOI] [PubMed] [Google Scholar]
- Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, et al. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biological Psychiatry. 2003;54:1382–1388. doi: 10.1016/S0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- Schelling G, Kilger E, Roozendaal B, de Quervain D, Briegel J, Dagge A, et al. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: a randomized study. Biological Psychiatry. 2004;55:627–633. doi: 10.1016/j.biopsych.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Simeon D, Knutelska M, Yehuda R, Putnam F, Schmeidler J, Smith LM. Hypothalamic–pituitary–adrenal axis function in dissociative disorders, posttraumatic stress disorder, and healthy volunteers. Biological Psychiatry. 2007;61:966–973. doi: 10.1016/j.biopsych.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth A, Ockenfels JM, Gorin MC, Catley AA, Porter D, Kirschbaum LS, et al. Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology. 1997;22:89–105. doi: 10.1016/S0306-4530(96)00039-X. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Keane TM, Newman E, Kaloupek DG. Choosing self-report measures and structured interview. In: Carlson EB, editor. Trauma research methodology. Baltimore: Sidran Press; 1996. pp. 56–81. [Google Scholar]
- Spielberger CD, Gorsuch RI, Lushene R, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory, STAI. Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- Stoppelbein L, Greening L. The risk of posttraumatic stress disorder in mothers of children diagnosed with pediatric cancer and type I diabetes. Journal of Pediatric Psychology. 2007;32:223–229. doi: 10.1093/jpepsy/jsm057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valva G, Ducrocq F, Jezequel K, Averland B, Lestavel P, Brunet A, et al. Immediate treatment with propanolol decreases posttraumatic stress disorder two months after trauma. Biological Psychiatry. 2003;54:947–949. doi: 10.1016/S0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- Wessa M, Rohleder N, Kirschbaum C, Flor H. Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:209–215. doi: 10.1016/j.psyneuen.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Current status of cortisol of findings in post-traumatic stress disorder. Psychiatric Clinics of North America. 2002;25:341–368. doi: 10.1016/S0193-953X(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Kafman S. Circadian rhythm of salivary cortisol in Holocaust survivors with and without PTSD. American Journal of Psychiatry. 2005;162:998–1000. doi: 10.1176/appi.ajp.162.5.998. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Nussbaum G, Wahby V, Giller EL, Mason JW. Low urinary cortisol excretion in patients with posttraumatic stress disorder. Journal of Nervous and Mental Disease. 1990;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Young EA, Breslau N. Cortisol and catecholamines in post-traumatic stress disorder. Archives of General Psychiatry. 2004;61:394–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- Young EA, Tolman R, Witkowski K, Kaplan G. Salivary cortisol and posttraumatic stress disorder in a low-income community sample of women. Biological Psychiatry. 2004;55:621–626. doi: 10.1016/j.biopsych.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR. Attainment and maintenance of reliability of axis I and axis II disorders over the course of a longitudinal study. Comprehensive Psychiatry. 2001;42:369–374. doi: 10.1053/comp.2001.24556. [DOI] [PubMed] [Google Scholar]