Abstract
Trafficking of neurotransmitter receptors between intracellular and cell surface compartments is important for regulating neurotransmission. We developed a method for determining if an in vivo treatment has altered receptor distribution in a particular region of rodent brain. After the treatment, brain slices are rapidly prepared from the region of interest. Then cell surface-expressed receptors are covalently crosslinked to nearby proteins using the membrane-impermeable, bifunctional crosslinker bis(sulfosuccinimidyl)suberate (BS3). This increases the apparent molecular weight of surface receptors, while intracellular receptors are not modified. Thus, surface and intracellular receptor pools can be separated and quantified using SDS-PAGE and immunoblotting. This method is particularly useful for analyzing AMPA receptor subunits, offering advantages in accuracy, efficiency and cost compared to biotinylation. A disadvantage is that some antibodies no longer recognize their target protein after crosslinking. We have used this method to quantify changes in receptor distribution after acute and chronic exposure to psychomotor stimulants.
Keywords: BS3, cell surface expression, glutamate receptors, protein crosslinking, receptor trafficking
INTRODUCTION
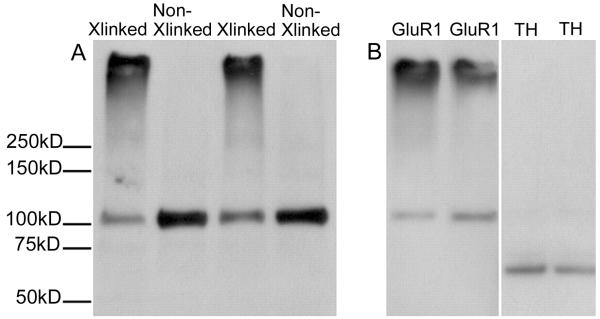
The realization that neurotransmitter receptor trafficking is critical for brain function has made it important to develop methods to monitor changes in receptor distribution. Several methods exist to measure receptor surface expression in cultured cells (e.g., Collingridge et al., 2004). Here we describe an approach that can be used to determine whether an in vivo treatment altered the surface expression of ionotropic glutamate receptors. This is accomplished by administering an experimental treatment to live rodents, quickly preparing brain slices or minces at the appropriate post-treatment time, and then incubating the tissue with BS3 [bis(sulfosuccinimidyl)suberate]. BS3 is a water soluble analog of disuccinimidyl suberate, a bifunctional N-hydroxysuccinimide ester that reacts with primary amines. BS3 does not cross cell membranes so it covalently crosslinks cell surface-expressed receptors while intracellular receptors are not modified. This enables surface and intracellular receptor pools to be distinguished and measured (Fig. 1).
Fig. 1.
BS3 crosslinking enables the measurement of surface and intracellular pools of GluR1. A) The nucleus accumbens was dissected from a naive rat. Tissue from one hemisphere was cross-linked, whereas the other was not, generating paired samples that were then subjected to SDS-PAGE and immunoblotting for GluR1. Both high (surface-expressed) and predicted (intracellular) molecular weight bands are detected in tissue from the crosslinked side (Xlinked), whereas non-crosslinked (Non-Xlinked) tissue yields only a band corresponding to the predicted weight of GluR1. B) In crosslinked nucleus accumbens tissue, only proteins found both on the surface and inside the cell (GluR1, left) result in both high and predicted molecular weight bands. Proteins that are exclusively intracellular [tyrosine hydroxylase (TH); right] result in a predicted molecular weight band only, confirming that the crosslinker does not cross cell membranes. Reprinted from Boudreau and Wolf (2005) with permission from The Journal of Neuroscience.
This article includes a Basic Protocol for BS3 crosslinking in freshly dissected brain tissue, an Alternate Protocol for crosslinking in primary cultures, and a Basic Protocol for quantification of crosslinked and intracellular proteins using SDS-PAGE and immunoblotting. [For an earlier review that focused on combining protein crosslinking and slice electrophysiology, see Grosshans et al. (2002b)]. A Support Protocol is also included for BS3 preparation. We have used these protocols to study the effect of cocaine and amphetamine exposure on surface expression of ionotropic glutamate receptors in the nucleus accumbens and other brain regions (Boudreau & Wolf, 2005; Boudreau et al., 2007; Boudreau et al., 2009; Conrad et al., 2008; Nelson et al., 2009; Ferrario et al., 2010; Li and Wolf, 2011), as well as surface expression of G-protein coupled receptors (Conrad et al., 2010) and an ion channel (Ford et al., 2009). Our protocols have also been used by other laboratories (e.g., Xie et al., 2007; Mickiewicz and Napier, 2011). We hope that this article, which focuses on ionotropic glutamate receptors, will serve as a useful starting point for those who wish to apply the assay to additional proteins of interest, but we note that success will depend on many factors including the properties of the protein, availability of suitable antibodies, and adherence to details of these protocols (see Strategic Planning and Commentary).
STRATEGIC PLANNING
Before beginning an experiment, it is important to verify that the protein of interest has extracellular lysine residues because primary amines in the side chain of lysine residues are the target of the BS3 crosslinking reagent. This can be checked on a variety of websites, including http://www.expasy.org/. The next critical issue is whether an antibody exists that can recognize the protein after it has been crosslinked. This must be determined through trial and error, although antibodies directed against an intracellular epitope are less likely to be affected by crosslinking of extracellular portions of the target protein.
When setting up the BS3 assay, it is best to start with a protein/antibody pair that has been used successfully by others in conjunction with the BS3 assay. Other important points are that freshly dissected brain tissue must be used, there must minimal delay (~3-5 min) between decapitating the animal and placing the tissue in the sample tube, and the timing of all incubations must be identical for samples that are destined to be compared (see Critical Parameters for more discussion). To achieve accurate timing, we recommend a team of at least three people: 1) One person should decapitate the rats, quickly dissect the tissue, and chop or mince the tissue. 2) A second person should add BS3 to the sample tube and, after samples have been crosslinked and quenched, handle subsequent processing. 3) A third person (“runner”) should keep track of start and stop times for crosslinking and quenching, and take samples to and from the cold-room. Proximity of the tissue harvesting room to a cold-room is very helpful. We recommend a dry-run before undertaking an experiment. Finally, after BS3 crosslinking, proteins may become very large, increasing the difficulty of transfer from the gel to the PVDF membrane. Transfer equipment and procedures that are adequate for noncrosslinked proteins may not be adequate to achieve complete transfer of larger crosslinked proteins. Therefore, it may be necessary to purchase the equipment specified in Basic Protocol 2.
BASIC PROTOCOL 1: CROSSLINKING SURFACE-EXPRESSED PROTEINS IN FRESHLY DISSECTED BRAIN SLICES
This protocol describes steps for selective modification of surface-expressed proteins using BS3 so that the effect of an experimental manipulation on receptor surface expression can be determined. Rats or mice are divided into experimental groups that receive different in vivo treatments (e.g., saline or cocaine injections) and are later killed. Brain regions of interest are rapidly dissected on ice and fresh brain slices (400μm) are immediately incubated with BS3 for 30 min at 4°C. Surface-expressed receptors are crosslinked, forming high molecular weight aggregates, while internal receptors are not. This enables surface and internal receptors to be distinguished based on molecular weight by SDS-PAGE and immunoblotting as described in BASIC PROTOCOL 2.
Materials
Materials: Tissue processing
Live rats or mice
Guillotine
Ice buckets
Tips and eppendorf tubes
Pipetman
Dissecting instruments
Glass petri dish
Brain matrix (we recommend a metal brain matrix, so that it can be chilled on ice, from ASI Instruments in Warren, MI, USA)
McIlwain-type Tissue Chopper, set at 400 microns/slice Double edge razor blades for use with brain matrix and tissue chopper Flat plate inverter (Thermo Scientific LABQUAKE Shaker, Cat # C400110) Sonicator [Sonic Dismembrator Model 100, Fisher Scientific; setting of 3-5 (max) output power]
Refrigerated microcentrifuge
Freezer (− 80°C) for storing samples prior to SDS-PAGE
Additional reagents for SDS-PAGE and immunoblotting (Basic Protocol #2)
Preparations on the morning of the experiment
1. Prepare the following solutions on the morning of the experiment (note that the BS3 stock solution should be reconstituted only when everything else is ready and the experiment is about to begin; see Support Protocol for instructions on making the BS3 stock, and Reagents and Solutions for detailed recipes for the other buffers):
Lysis Buffer: prepare the lysis buffer just prior to use. Store on ice. 1M Glycine stock (10X in distilled H20): this is used to quench the crosslinking reaction after the 30 min incubation with BS3. [*Au: pH @ what temp?]
52 mM BS3 stock solution (26X) in 5mM sodium citrate, pH 5.0 (please refer to Support Protocol for detailed instructions for preparation of BS3 stock)
Artificial CSF (aCSF), pH 7.4: We recommend HEPES for making this stock, but alternate buffers may be used for aCSF (e.g. phosphate-based buffers) depending on experimental requirements provided the buffer is devoid of primary and secondary amines (3° amines are acceptable).
2. Prepare samples tubes (1.5 mL or 2 ml eppendorfs) containing 1 ml ice cold aCSF prior to tissue preparation. Keep tubes on ice.
A volume of 1 ml can be used for dissected brain regions of many sizes, since it is advantageous for BS3 to be in excess. Due to the large number of aliquots generated (~20 aliquots per rat for bilateral nucleus accumbens dissection), we also recommend labeling aliquot tubes prior to tissue preparation for use in step 11.
Preparation of fresh brain tissue
3. Prepare an ice bucket containing the brain matrix, dissecting instruments, and an inverted glass petri dish on which to perform the dissection. We recommend placing a piece of slightly wetted filter paper (Whatman #1, Cat. # 1001-070) on top of the inverted petri dish to keep tissue sections in place during the dissection.
4. Bring a rat into the tissue preparation room and decapitate. Remove brain rapidly and place brain in the matrix (in ice bucket). Use two double edge razor blades to obtain a coronal slice that contains the region of interest (e.g., we dissect the nucleus accumbens from a 2 mm thick slice). One side of the double edge blade can be covered with tape for safety. Remove the slice and place on the inverted petri dish. Remove region of interest using chilled tissue punch or perform free hand dissection with chilled scalpel.
Because brain proteins may be altered by acute stress (e.g., a novel environment or the smell of blood), rats are brought to a holding area in their home cages, far from the tissue harvesting room, at least 1 h before tissue collection begins. Once the experiment begins, each rat is brought individually into the tissue harvesting room and immediately decapitated. Before retrieving each rat, make sure that rat-handling glove (if used) and lab coat are free of blood. Rats in all experimental groups to be compared must be killed on the same day and tissue processed in parallel.
5. Quickly transfer the dissected pieces of tissue to the stage of a McIllwain chopper (The Vibratome Company, St. Louis, MO) and prepare 400 μm slices.
Place clean filter paper on the stage of the chopper for each tissue sample. We also recommend wiping the chopper blade with ice-cold aCSF before use to minimize sticking of tissue to the blade. Chopping the tissue in this manner increases the surface area accessible to BS3, enabling reproducible results. However, small brain regions or tissue punches can be difficult to chop using the McIllwain chopper, so we manually cut them into small pieces with a sharp scalpel instead of using the chopper.
Crosslinking of proteins in brain slices and subsequent tissue processing
6. Using a chilled metal spatula (rinsed between uses), transfer the tissue into a sample tube. The sample tube should contain 1 mL of ice-cold artificial cerebral spinal fluid (aCSF) and should be spiked with 40 ul of the 52 mM BS3 stock solution immediately before adding the tissue.
Because of the concerns outlined in the Support Protocol (Preparing the BS3 stock solution), BS3 must be added to the tube just seconds before the tissue is added to ensure consistent crosslinking results between animals. A pipetman should be dedicated solely to this step. Never reintroduce a used pipet tip into BS3 stock solution vial. We recommend that one person spike the sample tube containing aCSF with BS3 at the time that the tissue harvester is finishing the job of chopping the dissected tissue. Once chopped tissue has been added to the sample tube, the third person (“runner”) should immediately begin inverting the tube to separate the pieces of tissue as they walk the sample to the cold room (4°C). Steps 3-6 should be completed as quickly as possible and their timing should be kept consistent between all rats.
The need to minimize the time between decapitation and placement of a tissue sample in BS3 (typically 3-4 min in our hands) places a limit on the number of brain regions that can be harvested from a single rat. If too much time elapses, results can become variable for rats within an experimental group.
7. Incubate sample tube at 4°C for 30 min with gentle mixing by placing on a rocker in a cold room.
Each sample must be crosslinked for EXACTLY the same period of time, so keep a record of start and stop times for each sample, using a laboratory timer. Begin timer when tissue is added to sample tube #1 and record. The timer should not be stopped once started and additional samples should be recorded in series (e.g. Sample #1 - Begin crosslinking at 0:00, quench at 30:00, end quench at 40:00; Sample #2 - Begin crosslinking at 5:00, quench at 35:00, end quench at 45:00).
8. After 30 min, quench the reaction by adding 100 μl of 1M Glycine stock (see recipe; this 1:10 dilution results in a final concentration of 100mM glycine). Incubate at 4°C for 10 min with gentle mixing (on rocker in cold room).
9. After quenching is complete, spin samples at 20 000 x g (centrifuge set at 4°C) for 2 min to recover tissue. Discard supernatant.
10. Resuspend tissue in a brain region-specific amount of ice cold lysis buffer containing protease and phosphatase inhibitors (see recipe). For bilateral rat nucleus accumbens (~15 mg wet weight), we use 400 μl of lysis buffer. Cover tube with parafilm (to prevent any loss of sample) and then insert tip of sonicator just below surface of the liquid. Sonicate for 5 sec, keeping tip in liquid.
We recommend sonication rather than other methods of tissue disruption because it results in a higher yield.
11. Spin samples at 20 000 x g (centrifuge set at 4°C) for 2 min and save the supernatant on ice (discard pellet). Aliquot the supernatant as soon as possible. The volume of the aliquot should be selected such that it is sufficient to run 1-2 gels. We typically generate 20 aliquots (20 μl each) from the bilateral nucleus accumbens samples of a single rat. One aliquot is used for determination of total protein concentration (see Basic Protocol #2).
Although this is a relatively high speed spin, it is very brief. The intention is not to pellet membranes – rather, the spin is intended to pellet materials not solubilized by this lysis buffer. For AMPA receptors, about 75-85% of starting protein is recovered in the supernatant.
12. Store aliquots at −80°C until ready for use. Avoid freeze/thaw.
ALTERNATE PROTOCOL: BS3 CROSSLINKING OF SURFACE-EXPRESSED PROTEINS IN PRIMARY NEURONAL CULTURES
This Alternate Protocol briefly describes adaptation of the BS3 assay to measuring receptor surface expression in primary cultures, as reported previously (e.g., Hall and Soderling, 1997a,b; Hall et al., 1997; Gao and Wolf, 2008; Sun et al., 2008).
Materials
Reagents are the same as described for Basic Protocol 1, except that Hanks’ Balanced salt solution (HBSS, Invitrogen) is also required and primary cultures are used rather than tissue harvested from live rats.
1. After cultures have been treated as desired, they are washed twice with HBBS and then incubated with 2 mM BS3 in this solution for 10 min at 37°C in the incubator.
During the incubation, gently swirl several times by hand (alternatively, place a small rocker in the incubator).
2. Crosslinking is terminated by quenching the reaction with 100 mM glycine (10 min at 4°C in the cold room on a rocker).
To add glycine, spike HBBS+ BS3 already in the well with a high concentration glycine stock (e.g., 100 μl of 10X glycine into 1 ml of HBBS+ BS3).
3. Remove liquid and add ice-cold lysis buffer (from this point forward, cells must be kept on ice).
The volume added will depend on the size of the well and cell density. For a 6 well plate with ~200,000 cells plated per well, we typically add 100-200 μl of lysis buffer per well.
4. Cultures are harvested by scraping in ice-cold lysis buffer (see Materials for Basic Protocol #1).
5. Sonicate cells for 5 sec and then centrifuge at 20 000 x g (centrifuge set at 4°C) for 2 min to remove unsolubilized material (only a very small amount of material will be pelleted).
6. The supernatant is aliquoted and stored at −80°C. Protein concentration was determined by the Bio-Rad assay (Bio-Rad, Hercules, CA, USA).
SUPPORT PROTOCOL: PREPARING THE BS3 STOCK SOLUTION
BS3 (Pierce Biotechnology, Rockford, IL) is a water soluble, bifunctional crosslinking reagent that consists of an 8-carbon spacer arm with an N-hydroxysuccinimide ester (NHS ester) at each end. The NHS esters react with primary amines at pH 7-9 to form stable amide bonds and thus covalently crosslink proteins. BS3 cannot cross cell membranes so it only crosslinks proteins expressed on the cell surface while leaving intracellular proteins intact. The NHS ester moiety also undergoes hydrolysis which increases with increasing pH. If BS3 hydrolyzes, it is not available for the crosslinking reaction. Therefore, the BS3 must be prepared in a low pH solution devoid of amines. We suggest using 5mM Na citrate, pH 5.0, to help prevent hydrolysis and consumption of ester moieties.
Note that preparing BS3 at a low pH does not eliminate hydrolysis, but merely slows it. So it is important that BS3 be prepared fresh for each use. We have found that citrate, which has good buffering capacity at pH 5.0, works best for solubilizing BS3. The working concentration of citrate buffer is 5 mM in order to minimize the ionic strength of the BS3 diluent (since BS3 has limited solubility in aqueous solutions). However, 5mM citrate solution at pH 5.0 does not have a stable shelf life; the pH will rise over time and increase the hydrolysis of BS3. For this reason, the buffer is made from 20X stocks, i.e. 100 mM citric acid and 100 mM sodium citrate. The acid and the base are diluted to 5 mM and combined to achieve pH 5.0 and a 52 mM stock solution of BS3 is then made. By preparing the citrate and the BS3 in this way, the ionic strength of the working solution is minimized and reproducible results can be obtained. The citrate buffer must be made fresh for each batch of tissue processed, to insure pH 5.0, and BS3 should be dissolved immediately before tissue collection begins. Finally, neither the citrate buffer nor the BS3 solution should ever be put on ice as this will cause the BS3 to precipitate.
Materials
Sodium citrate
Citric acid
Bis(sulfosuccinimidyl)suberate (BS3); Pierce cat # 21580 (50 mg tubes; store at 4-8°C in a dessicator prior to use)
1. Prepare Sodium Citrate Buffer, 5mM in ddH2O, pH 5.0
2. Remove BS3 from the refrigerator about 1 h prior to dissolving in citrate buffer.
This improves solubility.
3. When everything else is ready and the first rat is ready to be killed, reconstitute BS3 in its original vial by adding 1.68 ml of sodium citrate buffer. Vortex immediately for ~30 s. The BS3 powder should readily go into solution. This achieves a 52 mM BS3 stock solution (26X).
To prevent difficulties with precipitation of BS3, we recommend hydrating the entire bottle of BS3 in the purchased vial. If, after 30 seconds of vortexing, the solution is cloudy or a suspension is present, assist solubility by placing vial in a hot sonication bath. DO NOT repeatedly vortex the vial as this will only favor particulate formation. If BS3 does not go into solution completely after sonication, start over by using a new vial, because any initial problems with solubility will persist throughout the experiment.
We recommend removing the label from the BS3 bottle before reconstituting in citrate and saving the label with data sheets for the experiment. This serves two purposes. First, it makes it easier to visually check that the BS3 goes into solution and stays in solution throughout the experiment. Second, it helps keep track of the BS3 lot used in a particular experiment.
4. Monitor the solubility of the crosslinker throughout the duration of the experiment. Once reconstituted, BS3 is stable for 2-3 h. If tissue preparation will last longer than 2-3 h, prepare a new vial after this period of time. BS3 does not tolerate freeze-thaw cycles. Utilize the prepared solution only once.
BASIC PROTOCOL 2: SDS-PAGE AND IMMUNOBLOTTING ANALYSIS OF CROSSLINKED GLUTAMATE RECEPTOR SUBUNITS
This protocol describes steps for quantifying surface and intracellular receptor pools after crosslinking in tissue slices (Basic Protocol 1). The same steps can be used for crosslinked proteins from cultured neurons (Alternate Protocol).
Materials
Buffers (see Reagents and Solutions for all buffer recipes, or commercial sources)
Sample Treatment Buffer (STB)
Electrophoresis Buffer
Transblotting Buffer
Western Blotting Buffers
Antibodies
Rabbit anti-GluR1; Thermo Scientific (Pierce), cat # PA1-37776
Rabbit anti-GluR2/3; Chemicon, cat # AB1506
Mouse anti-GluR2; Neuromab (Antibodies Inc), cat # 75-002
Rabbit anti-GluR3; Cell Signaling, cat # 3437
Goat anti-Rabbit IgG-HRP conjugate; Invitrogen, cat # G21234
Goat anti-Mouse IgG-HRP conjugate; Invitrogen, cat # G21040
These are the antibodies that are currently able to recognize AMPA receptor subunits after they have been crosslinked with BS3. However, each new lot of antibody should be tested with control crosslinked tissue prior to use in experiments.
Electrophoresis and Transblotting Materials
Tris-HCl polyacrylamide gradient (4-15%) gels (BioRad)
Precision Plus Prestained SDS-PAGE standards (BioRad) or comparable visible molecular weight markers
Invitrogen MagicMark XP Western Standard (cat # LC5602)
Detergent compatible protein determination system Mini-PROTEAN ® 3 cell (BioRad, cat # 165-3301)
BioRad Trans-blot ® cell (cat # 170-3939)
BioRad power supply Power Pac HC (cat # 164-5052)
Transblot sponges Polyvinylidene Fluoride (PVDF) membrane
Whatman 3MM chromatography paper Plastic covered containers for incubations (PerfectWestern, GenHunter.com)
ECL substrate; Amersham/GE Healthsciences
Film
Plate shaker (low to medium shaking)
Centrifuge
Heat Block
Total Lab® or other comparable data analysis program
Ponceau S (Sigma-Aldrich, cat # P7170-1L; 1X stock)
Samples: Lysates generated according to Basic Protocol 1 and removed from −80°C. (Note: Prior to analyzing samples, one aliquot should have been used for protein concentration determination).
Sample preparation
1. Thaw samples to RT and dilute 1 aliquot in distilled water for protein assay.
2. Determine total protein/sample with Bio-Rad DC Protein Assay (Bio-Rad, Hercules, CA, USA).
3. Dilute the samples 1:1 with 2x STB.
4. Heat samples in heating block at 70°C for 10 min and allow to cool to RT prior to loading on gel. DO NOT PLACE SAMPLES ON ICE FOR COOLING.
5. Briefly centrifuge samples to pellet any debris (10 000 rpm for ~3 min)
6. Load samples in gel wells according to desired total protein/well. This depends on the abundance of the protein in the brain region analyzed. For extremely abundant proteins, we load 5-10μg whereas for less abundant proteins we load as much as 35-50μg of protein.
Electrophoresis
Details in Steps 7-12 apply specifically to the BioRad Mini-PROTEAN ® 3 cell and precast Ready® Gels. The Tris-HCl ion pair within Bio-Rad Ready Gels (cat # 161-1158) combined with the Bio-Rad Tris/Glycine/SDS running buffer (cat # 161-0772) results in the best crosslinked and non-crosslinked bands. Other ion pairs like Bis-Tris along with MOPS running buffer (Invitrogen) yield crosslinked bands that are diffuse and streaky, and thus difficult to analyze.
7. Assemble the electrophoresis cell with 4-15% gradient gel(s) according the BioRad Assembly Guide.
8. Add 1x running buffer to upper chamber and inspect for leaks. If no leaks are present, proceed by pouring running buffer into the lower chamber. Remove any air bubbles from bottom chamber gel contacts with a pipet.
9. Load wells with desired amount of standard and samples.
Two different types of molecular weight standards are recommended (see Materials). Visible standards help you track the quality of gel electrophoresis and the transfer. MagicMark standards are visible on the film or image after ECL development and are used for relative molecular weight determination.
10. Place lid on top of the buffer chamber and apply power: 200 volts, constant voltage.
11. Run gel to completion by allowing the loading dye to run off the bottom of the gel. Run time is approximately 35 - 45 min.
12. Turn off power and prepare for transfer.
Transfer
13. Equilibrate gel in transfer buffer for 15 min.
14. Cut one piece of PVDF membrane (3-5mm larger than the gel) and six pieces of Whatman filter paper (same size as the gel).
15. Soak the PVDF membrane in 100% methanol for 5 – 10 sec and then thoroughly rinse with water.
16. Soak the sponges and PVDF membrane in transfer buffer during the last 5 min of the gel equilibration period (see step 13).
17. Assemble the transfer sandwich as indicated below and place in the transfer tank. It is important to do everything possible to avoid air bubbles while assembling. Handle PVDF by edges using gloves. Hold the PVDF over the gel at a 45 degree angle and lower gradually. If air bubbles do form between the gel and the PVDF, remove by rolling a 10 ml glass pipette over the PVDF.
BLACK
Sponge
3 pieces of filter paper (immerse in transfer buffer as sandwich is being assembled)
GEL
PVDF Membrane
3 pieces of filter paper
Sponge
WHITE
18. Once sandwich is placed in the transfer tank, with cooling coil in place and tank filled with transfer buffer (to the level of the plastic screws), turn the power supply on and set current conditions (1.15 Amps, constant current, 1.25 h). Once transfer has begun, start cold tap water circulating through the cooling coil.
For best transfer results, it is important to use the large Trans Blot cell specified in Materials that holds 3 L of transfer buffer and has a larger plate electrode and a cooling coil (cat # 170-3939). For best transfers using 1.15 A constant current, we strongly recommend the Bio-Rad PowerPac HC power supply which can go up to 3.0 A (Bio-Rad cat # 164-5052). The older power supplies are fine for electrophoresis but not for transfers.
Over time, the insulating material that coats the cooling coil will wear. This will cause an electrical grounding effect that will stop the transfer. If this occurs, you can put a new coil in and continue the transfer. Although this is rare, it can happen. Therefore, check the transfer approximately every 20 min to make sure it has not stopped.
19. When time is up, turn off power, disassemble transfer sandwich and remove membrane. Prepare for immunostaining. Alternatively, membrane can be air dried at RT, wrapped in plastic wrap, and stored @ 4°C for several weeks.
To verify that transfer has occurred, check that the visible (blue) molecular weight standards (Precision Plus Prestained SDS-PAGE standards, listed above in Materials) have transferred to the PVDF. The gel should be clear of blue standards. When setting up the Protocol for the first time, it is also advisable to perform control experiment 2 described under Troubleshooting to check that efficient transfer but not “over-transfer” has occurred. If drying membrane for storage, membrane must be COMPLETELY dry prior to wrapping in plastic wrap. Depending on the ambient humidity, this can take up to 1 h at RT.
Immunostaining
20. Rinse transblotted membrane in distilled water (twice) and TBS-T (twice), protein side up, on plate shaker for 2 min at room temperature (RT).
We recommend that this step and all subsequent incubations can be done in covered plastic containers specifically designed for immunoblotting (PerfectWestern, GenHunter.com).
21. Block membrane in Blocking Buffer on plate rocker for 1 h at RT.
22. After 1 h, decant Blocking Buffer and briefly rinse membrane in TBS-T.
23. Prepare 1° antibody according to desired concentration in TBS-T. Use 0.3 mL diluted antibody volume/cm2 of membrane, e.g., for an 8 × 8 cm membrane, use ~20mL of diluted antibody.
At the time of publication, the following dilutions were optimal for antibodies listed in Materials: GluR1, 1/1000; GluR2, 1/200; GluR2/3, 1/2000; GluR3, 1/500 dilution.
24. Add diluted antibody to membrane and incubate at 4°C on plate shaker overnight.
25. Wash membrane 6 times in TBS-T, changing wash solution every 5 min, at RT.
26. Prepare 2° antibodies at desired working concentrations in TBS-T (Goat anti-Rabbit or Goat anti-Mouse anti-HRP, 1/10000 dilution). Use 0.3 mL diluted antibody volume/cm2 of membrane, e.g., for an 8 × 8 cm membrane, use ~20mL of diluted antibody
27. Add diluted antibody to membrane, incubate at RT on plate shaker for 1 h.
28. Wash membrane 6 times in TBS-T, changing wash solution every 5 min, at RT.
Previous protocols from our lab may include TBS and water washes prior to chemiluminescence detection, but changes in PVDF formulation have made these steps unnecessary.
Chemiluminescence detection
The following steps apply to using ECL Western blotting detection reagents (Amersham Biosciences) with either film or the VersaDoc System (BioRad). Other detection reagents have been tried, but results may not be as quantitative.
29. During the last water wash, prepare the ECL substrate
30. Mix 1:1, ECL1:ECL2 (working substrate).
31. Remove membrane from water and immediately place the working substrate directly onto the membrane. Incubate for 1 min.
32. Blot off excess working substrate, place membrane between 2 sheets of transparency paper, plastic wrap, or page protector sheets and expose to film.
33. Develop film after desired exposure time, and analyze film with Total Lab® or comparable data analysis program. Alternatively, analyze using VersaDoc system (Biorad).
Determination of total protein per lane using Ponceau S (for normalization of samples)
It is also possible to use a loading control (e.g., actin or GADPH) but we prefer the Ponceau S method because the normalization is based on multiple proteins instead of a single protein.
34. Remove membrane from transparency sandwich and rinse thoroughly in ddH2O.
35. Immerse membrane in Ponceau S solution for 5 min.
36. Rinse Ponceau S stained membrane for 6 min in ddH2O, changing water every 2 min.
37. Place membrane between 2 sheets of transparency paper, plastic wrap, or page protector sheets and capture image on a scanner. Scan the entire lane of each sample to determine “total protein in the lane” for normalization (see Data analysis, below).
Reagents and Solutions
Components for lysis buffer
25 mM HEPES, pH 7.4
500 mM NaCl
2 mM EDTA
1 mM DTT
1 mM Phenylmethyl sulfonyl fluoride (PMSF)
20 mM NaF
1 x Protease inhibitor cocktail tablet; Calbiochem cat # 539131 (see product insert for constituents)
0.1% Nonidet P-40 (v/v)
1 uM Okadaic acid (Calbiochem, Cat.# 459620)
1 uM Microsystin-LF (Calbiochem, Cat # 475815)
1 mM Sodium orthovanadate (NaOV)
Prepare the lysis buffer just prior to use. Store on ice.
Sodium Citrate Buffer, 5mM in ddH2O, pH 5.0
1mL (100mM citric acid) + 19mL ddH2O = 5mM acid
1mL (100mM Na-citrate) + 19ml ddH2O = 5mM base
Combine 20.5mL 5mM acid + 29.5mL 5mM base = 5mM Na-citrate buffer, pH 5.0
Na-citrate buffer must be prepared fresh on the morning of the experiment. Accurate pH is critical.
Artificial CSF (aCSF), pH 7.4
10x CaCl2 = 12mM
10x HEPES = 200mM, pH 7.4
10X Salt = 1.47M NaCl, 27mM KCl, 10mM MgCl2
100x Dextrose = 1M
Combine 1mL of 10x CaCl2, 1mLof 10x HEPES, 1mL of 10x Salt, 100μL of 100x dextrose, 6.9mL of ddH2O.
We recommend HEPES, but alternate buffers may be used for aCSF (e.g. phosphate-based buffers) depending on experimental requirements provided the buffer is devoid of primary and secondary amines (3° amines are acceptable).
10X Tris Buffered Saline (10x TBS)
200mM Tris base
1370mM NaCl in ddH2O
pH to 7.4
Tris Buffered Saline/Tween-20 (TBS-T)
1xTBS
0.05% (v/v) Tween-20
Blocking Buffer
5% (w/v) NFDM
1% (v/v) NGS in TBS-T
Sample Treatment Buffer (2x STB)*
0.01% (w/v) Bromophenol blue
62.5mM Tris –HCl/pH 6.8, 2% (w/v)
200 mM Dithiothreitol (DTT)
2% (w/v) Sodium dodecylsulfate (SDS)
25% (v/v) Glycerol
*Commercially available STB: BioRad, cat # 161-0737
Electrophoresis running buffer (1x)*
25mM Tris-base
192mM glycine
0.1% (w/v) SDS, pH 8.3
*Commerically available running buffer (10x stock): BioRad, cat # 161-0732
Transblotting buffer (1x)*
25mM Tris-base
192mM glycine
20% (v/v) methanol
0.037% (w/v) SDS
*Commericially available Transblotting buffer (10x stock w/o MEOH or SDS): BioRad, cat # 161-0734
COMMENTARY
Background information
A number of groups have used the BS3 assay to monitor changes in glutamate receptor distribution in dissociated cells (Hall et al., 1997; Hall and Soderling, 1997a,b; Archibald et al., 1998; Gao and Wolf, 2008; Sun et al., 2008) and brain slices (Broutman and Baudry, 2001; Clayton et al., 2002; Grosshans et al., 2002a,b; Gerges et al., 2004). The BS3 crosslinking reaction in these prior studies is identical to the reaction in our Protocol. Our contribution was to adapt the assay to freshly dissected brain tissue, in order to detect changes in receptor surface expression produced by a prior in vivo treatment (Basic Protocol 1), and optimize conditions for quantifying both surface-expressed and intracellular receptor pools in the same gel (Fig. 1). The latter is achieved through the use of gradient gels and specific transfer conditions that enable efficient transfer of high molecular weight crosslinked proteins (Basic Protocol 2). If gradient gels are not used, the high molecular weight crosslinked protein will not enter the separating gel, and the amount of surface-expressed protein must be inferred from measuring the intracellular band (e.g., a decrease in the intracellular band indicates redistribution of receptor to the surface). However, this is only valid if total receptor expression (surface + intracellular) is unchanged, which may not be the case, especially for chronic treatments (e.g., Conrad et al., 2008). Other groups have also utilized gradient gels to detect the crosslinked band (Archibald et al., 1998; Gerges et al., 2004).
Biotinylation and chymotrypsin digestion have also been used to monitor glutamate receptor surface expression. These methods, like BS3 crosslinking, are based on selective modification of surface-expressed proteins. Studies comparing the three methods have found that they yield nearly identical estimates of the percent of glutamate receptors on the cell surface in dissociated cultures (Hall et al., 1997; Hall & Soderling, 1997a,b). In selecting a method to adapt to in vivo studies, we chose the BS3 assay over chymotrypsin digestion or biotinylation for several reasons.
Chymotrypsin digestion occurs at 37°C, which raises the possibility of receptor redistribution during the incubation. Furthermore, changes in surface-expressed receptors are inferred from quantification of the intracellular (intact) receptor, which is potentially problematic, as noted above. The biotinylation method can be used to monitor in vivo receptor trafficking in exactly the same way described for BS3 (e.g., see Ferrario et al., 2011). In fact, in an unpublished study in which we crosslinked nucleus accumbens tissue from one hemisphere and biotinylated accumbens tissue from the other hemisphere (30 min at 4°C, n= 6 rats), we obtained very similar measures for the GluR1 surface/intracellular ratio (BS3: 5.3 ± 1.6; biotinylation: 6.7 ± 2.8; N.S., paired t-test). However, the BS3 assay is preferred for several reasons.
Biotin-treated samples must be purified into biotinylated (surface) and nonbiotinylated (intracellular) fractions before analysis. Then, the biotinylated and nonbiotinylated fractions must be measured in separate SDS-PAGE lanes and normalized to total protein for comparison. Error may be introduced at the purification and normalization steps. In contrast, BS3 crosslinked samples can be analyzed directly by SDS-PAGE without prior purification, and surface and intracellular bands are measured in the same lane, eliminating the need for normalization. These features increase accuracy and efficiency, and decrease cost. However, some proteins are not recognized by available antibodies after they are crosslinked with BS3. In this case, biotinylation must be used. The biotin group is small, so its attachment to a protein is much less likely to interfere with recognition by an antibody. Unfortunately, we have yet to find an antibody that reliably recognizes BS3 crosslinked NR1. Therefore, if NMDA receptors are the major focus of an experiment, biotinylation is recommended.
There are other methods for detecting plasticity produced in vivo. One approach is to conduct a manipulation in the live animal and then monitor changes in receptor levels in synaptosomes or synaptoneurosomes. However, receptor redistribution to these fractions does not necessarily mean that the receptors are expressed on the cell surface. Electrophysiological methods are very powerful for monitoring synaptic targeting of receptors, but a biochemical approach is more efficient. For example, the nucleus accumbens from one rat yields enough tissue for ~40 Western blots, generating a tissue bank that can be used to analyze surface expression of multiple receptors as well as levels of intracellular signaling pathway proteins (since intracellular proteins are not affected by crosslinking). The major limitation of BS3 and biotinylation approaches is that they cannot distinguish synaptic from extrasynaptic receptors. This is important not only because synaptic receptors mediate most neurotransmission, but also because synaptic incorporation of AMPA and NMDA receptors can be regulated by surface trafficking between extrasynaptic and synaptic compartments (Triller & Choquet, 2005), a form of trafficking that our assay would not detect. Another concern is that receptors may redistribute, away from their original in vivo state, during tissue preparation or during the incubation with BS3 or biotin. This is minimized by maintaining tissue at 4°C as much as possible. Most importantly, it will occur to the same extent in all experimental groups, so relative group differences should be preserved.
Critical Parameters
Tissue preparation
Freshly dissected brain tissue must be used. Freezing and/or fixation lead to membrane permeabilization, defeating the purpose of using a membrane-impermeant crosslinking reagent to selectively modify surface-expressed proteins.
Time and temperature dependence
In preliminary studies, we examined the temperature and time-dependence of BS3 crosslinking of the AMPA receptor subunit GluR1. As expected, crosslinking is faster at room temperature than at 4°C and increases over time. However, to our surprise, we observed that the amount of crosslinked GluR1 did not reach a plateau, even after BS3 incubations lasting 4 h (Fig. 2, top panel). The same failure to plateau was observed during long-term incubation with a biotinylating reagent (not shown). We hypothesized that AMPA receptors on the cell surface at the time of brain slice preparation do react fully with BS3 or biotin, but crosslinked product continues to accumulate because new receptors are still being delivered to the surface, where they replenish the pool available for crosslinking or biotinylation. Supporting this, it has long been known that membrane trafficking slows but does not stop at 4°C (Stackpole et al., 1974) and we have observed constitutive insertion of new AMPA receptors onto the surface of cultured nucleus accumbens neurons at 4°C (Mangiavacchi & Wolf, 2004).
Fig. 2.
Time course of GluR1 crosslinking in nucleus accumbens tissue incubated with BS3 at two temperatures, 4°C and room temperature (RT) (*p<0.05, RT vs. 4°C). The full time course is shown in the top panel, with early times expanded in the bottom panels. Crosslinking at both temperatures occurred in three apparent phases indicated by boxed numbers 1-3 that correspond to bracketed numbers in the text.
Closer examination of early incubation times (Fig. 2, middle and lower panels) supports this hypothesis by revealing three apparent phases of crosslinking (bracketed numbers in text correspond to boxed numbers in the figure): [1] an early phase (~0-10 min), which we believe reflects BS3 distribution through the slice, [2] a second phase (~10-30min) during which BS3 crosslinks receptors initially present on the cell surface, and [3] a prolonged phase (30 min and on) during which BS3 crosslinks new receptors that are continually trafficking to the cell surface. Crosslinking of new receptors may “contaminate” phase [2]. All phases are faster at RT but the difference is most marked for [3], as would be expected, because low temperatures should affect membrane trafficking [3] more strongly than distribution through the slice [1] or the crosslinking reaction [2].
The idea that externalization of new receptors is responsible for phase [3] is consistent with data showing that both BS3 crosslinking (Hall & Soderling, 1997a) and biotinylation reactions (Thomas-Crusells et al., 2003) do saturate when homogenates or fixed slices are used (trafficking is not possible in these “dead” preparations). At both temperatures, incubation times between 15 and 30 min probably come closest to estimating absolute levels of surface receptor at the time of decapitation. Our recommended conditions (4°C, 30 min) fall within this optimal window.
Two main conclusions can be drawn from these results. First, crosslinking and biotinylation are best for capturing relative differences between groups, which should be preserved regardless of the duration of the crosslinking or biotinylating reaction, although early times are preferable because the contribution of “new” receptors is minimized. Second, all steps in Basic Protocol 1 should be completed as quickly as possible and timing of the dissection should be kept consistent between all rats. The need to minimize the time between decapitation and placement of a tissue sample in BS3 (typically 3-5 min in our hands) places a limit on the number of brain regions that can be harvested from a single rat. If too much time elapses, the surface to intracellular protein ratio can become variable for rats within an experimental group. Even when the above steps are completed very rapidly, we cannot rule out the possibility that receptors redistribute during the interval between decapitation and placement of the tissue into BS3. However, if each rat is treated identically, the same amount of redistribution should occur in all groups such that relative group differences should be preserved.
Brain region
Data obtained from the BS3 assay are most interpretable if the brain region analyzed has a relatively homogeneous cellular composition. For example, the nucleus accumbens is ideal for BS3 experiments because the majority of accumbens neurons (~90%) are medium spiny GABA neurons (Meredith and Totterdell, 1999). If the brain region is heterogeneous with respect to cellular composition, the BS3 assay will give an aggregate read-out. Thus, changes in receptor surface expression in a particular cell population may be obscured by lack of changes in other populations.
SDS-PAGE and immunoblotting
Many labs that routinely use SDS-PAGE and immunoblotting to analyze non-crosslinked proteins may attempt to use their own standard procedures to analyze crosslinked proteins. This may lead to problems. Crosslinked proteins are very large. For example, we estimate that the surface band detected with AMPA receptor subunit antibodies is between 400 and 600kDa. A similar estimate was reached previously (Archibald et al., 1998). These estimates are consistent with crosslinking of subunits within one tetrameric AMPA receptor (four GluR subunits of ~100 kDa plus small auxiliary subunits such as TARPs or cornichon proteins). The length of the BS3 spacer arm (11.4 angstroms) is also consistent with crosslinking within a tetrameric AMPA receptor (Safferling et al., 2001). As a result of the large size of crosslinked proteins, gradient gels must be used to enable both the crosslinked proteins and much smaller non-crosslinked proteins to be detected on the same gel. Furthermore, large proteins are more difficult to transfer from the gel to the PVDF membrane than small proteins. To achieve successful transfer, we recommend using the equipment and transfer conditions specified in Basic Protocol 2. We also recommend conducting the control experiments described below (Anticipated results and troubleshooting) in order to verify successful crosslinking and transfer.
Data analysis
We determine diffuse densities for surface and intracellular bands in each lane, and then determine total receptor levels by summing surface and intracellular values. Surface (S), intracellular (I), and total receptor values are then normalized to total protein in the lane, as determined by Ponceau S staining, to control for variation in loading. Group means for each value are compared using an appropriate statistical test. We recommend reporting means for normalized surface, intracellular and total receptor values (e.g., Boudreau et al., 2007). Comparison of surface bands provides the most straightforward way to determine if receptor surface expression has changed. Intracellular and total receptor values provide complementary information. For example, if S increases and I decreases, this indicates redistribution of receptors to the surface. If S increases and I remains the same, it could suggest an increase in protein expression, with new receptors preferentially expressed on the cell surface.
In some cases, it is useful to report the surface/intracellular ratio (S/I), which is independent of protein loading and therefore does not require normalization to total protein in the lane. However, it cannot be assumed that an increased S/I ratio reflects receptor trafficking to the surface and a decreased S/I ratio reflects receptor internalization. For example, the S/I ratio could decrease due to an increase in I, but this would not indicate a change in receptor surface expression. Another important point is that the S/I ratio depends on experimental conditions (most importantly, the duration of crosslinking) as well as antibody characteristics, and therefore cannot be used as an absolute measure of the proportion of the total receptor pool expressed in surface versus intracellular compartments. For example, consider two receptors, X and Y, which are similarly distributed between surface and intracellular compartments (e.g., in both cases, 50% of the receptor is on the surface). If antibody to X recognizes its crosslinked form less avidly than the unmodified (intracellular) form, whereas antibody to Y recognizes crosslinked and unmodified forms equally well, the measured S/I ratio will be lower for X than Y, even though the proportion of each protein on the surface is actually the same. Despite these caveats, the S/I ratio can provide a useful measure of relative differences in receptor distribution when comparing the same receptor between different brain regions or treatment groups, as long as the same antibody and experimental conditions (e.g., duration of crosslinking) are used.
In the case of multi-subunit receptors such as AMPA receptors, the BS3 assay measures surface expression of individual subunits. Correlations between changes in distribution of individual subunits can help to infer the subunit composition of the receptor that is affected by the treatment (e.g., Boudreau and Wolf, 2005). More generally, a change in surface expression of an AMPA receptor subunit can be taken to indicate a change in AMPA receptor surface expression because tetramerization of AMPA receptor subunits (forming a functional receptor) is one of the requirements for exit from the endoplasmic reticulum (Greger and Esteban, 2007).
Anticipated Results and Troubleshooting
Prior to running unknown samples, we recommend conducting the following control experiments: 1) Obtain properly crosslinked tissue from a lab that is already utilizing the BS3 assay. Analyze the tissue using SDS-PAGE and immunoblotting as described in Basic Protocol 2. When the blot is probed with antibody to a surface-expressed receptor, two bands should be detected: an intracellular band with the predicted molecular weight for the unmodified receptor, and a higher molecular weight band that corresponds to surface-expressed crosslinked receptor (Fig. 1). Next, check that you obtain the same results when you crosslink your own tissue samples using Basic Protocol 1.
2) Using properly crosslinked tissue, check for complete transfer of crosslinked proteins by staining the gel with Coomassie blue after transfer. If transfer of crosslinked proteins was complete, no high molecular weight proteins should remain on the gel. In addition, it is advisable to check for “over-transfer” by placing a second PVDF membrane in the sandwich. If transfer conditions are optimal, protein should be detected only on the first sheet of PVDF.
3) Run equal amounts of crosslinked and non-crosslinked tissue in different lanes of the same gel (at least 3 lanes of each type of tissue). When immunoblotted and probed with antibody to a surface-expressed receptor, the crosslinked tissue should yield two bands on the immunoblot (intracellular and surface) while the non-crosslinked tissue will yield only the lower molecular weight band. The density of the intracellular band should be less in the crosslinked tissue because the surface-expressed portion of the total protein pool should now be present in the surface band. Furthermore, the sum of surface and intracellular bands in the crosslinked sample should approximately equal the total protein in the single band of the non-crosslinked sample (see Fig. 1).
4) Using the same crosslinked and non-crosslinked samples used for control experiment 3, verify that probing with antibodies to intracellular proteins (e.g., tyrosine hydroxylase in Fig. 1) yields only a single band at the predicted molecular weight. This confirms that BS3 did not have access to the intracellular compartment during incubation of brain slices. (Note: If tissue is lysed prior to incubation with BS3, intracellular proteins will also be crosslinked).
5) Load increasing amounts of crosslinked protein in different lanes of the same gel (5-50μg) and verify that density of both surface and intracellular bands in the immunoblot increases in a linear manner. This establishes that the surface/intracellular ratio (S/I) is independent of protein loading. It will also help to determine the optimal amount of protein for subsequent experiments (see Basic Protocol 2, step 6).
6) If the antigen peptide used to raise the antibody is available, verify that preabsorption of the antibody with the peptide prior to immunostaining eliminates both the surface and intracellular bands.
What if immunoblotting of crosslinked tissue for a surface-expressed protein reveals an intracellular band but no higher molecular weight crosslinked band? One explanation is that the protein may not have been crosslinked. Compare equal amounts of crosslinked and non-crosslinked control tissue. If crosslinking has occurred, the intracellular band should decrease in intensity after crosslinking, even if no surface band appears (see control experiment 3). If you determine that crosslinking has NOT occurred, and you are working with a protein studied previously using the BS3 assay (e.g., GluR1), the problem most likely lies in your execution of Basic Protocol 1. However, if you are working with a novel protein, consider the possibility that it lacks extracellular lysine groups and therefore cannot react with BS3. The sequence should be checked before beginning experiments. Alternatively, the protein may not be crosslinked because it is not in close enough proximity to another protein. As noted above, the length of the BS3 spacer arm is 11.4 angstroms, which is sufficient to crosslink AMPA receptor subunits within a single tetrameric receptor (Safferling et al, 2001), but may not be sufficient to crosslink a monomeric surface-expressed protein to a neighboring protein.
If, on the other hand, you verify that crosslinking has occurred, but you cannot detect a crosslinked band, two possible explanations should be considered. First, the antibody selected may not recognize its target protein after BS3 crosslinking. This possibility should be considered even if the same antibody has been previously reported to recognize the crosslinked protein, because commercially available antibodies may change dramatically from one lot to another. Unreliability is a particular problem for G-protein coupled receptor antibodies. For example, the dopamine receptor antibodies used successfully in BS3 experiments conducted several years ago (Conrad et al., 2010) are no longer useful. Second, it is possible that a crosslinked band is not detected because the crosslinked protein has not been transferred to the PVDF membrane. Perform control experiment 2, described in the previous paragraph.
What if the antibody detects an intracellular band of the predicted molecular weight, but also detects other nonspecific bands? If the protein in the nonspecific band is an intracellular protein, it is not a problem. However, if the protein in the nonspecific band is partly expressed on the cell surface, then it will contribute to the surface band, leading to an over-estimate of surface expression of your protein of interest. If this is the case, the antibody cannot be used. To determine if the protein in the nonspecific band is surface-expressed, compare the nonspecific intracellular band in crosslinked and non-crosslinked samples (load equal amounts of protein) as described in control experiment 3. If the intensity of the nonspecific band is not altered by crosslinking, then it is a purely intracellular protein.
Analysis of intracellular and surface bands is complicated when studying a protein with isoforms that differ in molecular weight. In this case, more than one isoform may contribute to the surface band. This situation was encountered when analyzing the D2 dopamine receptor. For details, see Conrad et al. (2010).
Time Considerations
As noted in Strategic Planning, achieving accurate timing in BS3 crosslinking experiments will probably require a team of three people and practice runs prior to conducting real experiments. In our lab, tissue from one or two brain regions from 20-30 rats can be crosslinked in ~2.5-4 h, assuming ~7-8 min per rat. The time required for SDS-PAGE will depend on the number of proteins to be analyzed.
Acknowledgements
We thank Dr. Jose Esteban for bringing BS3 to our attention many years ago, Dr. Michael Browning for sending us his protocols, Dr. Michela Marinelli and Kerstin Ford for help in early experiments, and Dr. Jessica Loweth for insightful comments on the manuscript. The development of this protocol was supported by DA009621, DA015835 and DA000453 (M.E.W.), predoctoral NRSA DA019762 (A.C.B.) and predoctoral NRSA DA021488 (K.L.C.).
Footnotes
Conflict of interest: Marina E. Wolf and Kelly L. Conrad have no biomedical financial interests but have a patent on A Possible Therapy For Cue-Induced Cocaine Craving Leading to Relapse in Abstinent Cocaine Abusers Based on Blockade of GluR2-lacking AMPA Receptors in the Nucleus Accumbens. The other authors report no biomedical financial interests or potential conflicts of interest.
Literature Cited
- Archibald K, Perry MJ, Molnar E, Henley JM. Surface expression and metabolic half-life of AMPA receptors in cultured rat cerebellar granule cells. Neuropharmacol. 1998;37:1345–1353. doi: 10.1016/s0028-3908(98)00135-x. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J. Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize upon cocaine challenge in association with altered activation of mitogen-activated protein kinases. J. Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Ferrario CR, Glucksman M, Wolf ME. Signaling pathway adaptations and novel protein kinase A substrates related to behavioral sensitization to cocaine. J. Neurochem. 2009;110:363–377. doi: 10.1111/j.1471-4159.2009.06140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broutman G, Baudry M. Involvement of the secretory pathway for AMPA receptors in NMDA-induced potentiation in hippocampus. J. Neurosci. 2001;21:27–34. doi: 10.1523/JNEUROSCI.21-01-00027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton DA, Grosshans DR, Browning MD. Aging and surface expression of hippocampal NMDA receptors. J. Biol. Chem. 2002;277:14367–14369. doi: 10.1074/jbc.C200074200. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat. Rev. Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Ford KA, Marinelli M, Wolf ME. Dopamine receptor expression and distribution dynamically change in the nucleus accumbens after withdrawal from cocaine self-administration. Neuroscience. 2010;169:182–194. doi: 10.1016/j.neuroscience.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME. The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacology. 2010;35:818–833. doi: 10.1038/npp.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galiñanes GL, Heng L-J, Tseng KY, Wolf ME. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2+-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011;61:1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KA, Wolf ME, Hu X-T. Plasticity of L-type Ca2+ channels after cocaine withdrawal. Synapse. 2009;63:690–697. doi: 10.1002/syn.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Wolf ME. Dopamine receptors regulate NMDA receptor surface expression in prefrontal cortex neurons. J. Neurochem. 2008;106:2489–2501. doi: 10.1111/j.1471-4159.2008.05597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerges NZ, Tran IC, Backos DS, Harrell JM, Chinkers M, Pratt WB, Esteban JA. Independent functions of hsp90 in neurotransmitter release and in the continuous synaptic cycling of AMPA receptors. J. Neurosci. 2004;24:4758–4766. doi: 10.1523/JNEUROSCI.0594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Esteban JA. AMPA receptor biogenesis and trafficking. Curr. Opin. Neurobiol. 2007;17:289–297. doi: 10.1016/j.conb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat. Neurosci. 2002a;5:27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. Analysis of glutamate receptor surface expression in acute hippocampal slices. Sci STKE. 2002b;(137):pl8. doi: 10.1126/stke.2002.137.pl8. 2002. [DOI] [PubMed] [Google Scholar]
- Hall RA, Hansen A, Andersen PH, Soderling TR. Surface expression of the AMPA receptor subunits GluR1, GluR2, and GluR4 in stably transfected baby hamster kidney cells. J. Neurochem. 1997;68:625–630. doi: 10.1046/j.1471-4159.1997.68020625.x. [DOI] [PubMed] [Google Scholar]
- Hall RA, Soderling TR. Differential surface expression and phosphorylation of the N-methyl-D-aspartate receptor subunits NR1 and NR2 in cultured hippocampal neurons. J. Biol. Chem. 1997a;272:4135–4140. doi: 10.1074/jbc.272.7.4135. [DOI] [PubMed] [Google Scholar]
- Hall RA, Soderling TR. Quantitation of AMPA receptor surface expression in cultured hippocampal neurons. Neurosci. 1997b;78:361–371. doi: 10.1016/s0306-4522(96)00525-8. [DOI] [PubMed] [Google Scholar]
- Li X, Wolf ME. Brain-derived neurotrophic factor rapidly increases AMPA receptor surface expression in rat nucleus accumbens. Eur. J. Neurosci. 2011;34:190–198. doi: 10.1111/j.1460-9568.2011.07754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Totterdell S. Microcircuits in nucleus accumbens’ shell and core involved in cognition and reward. Psychobiology. 1999;27:165–186. [Google Scholar]
- Mickiewicz AL, Napier TC. Repeated exposure to morphine alters surface expression of AMPA receptors in the rat medial prefrontal cortex. Eur. J. Neurosci. 2011;33:259–265. doi: 10.1111/j.1460-9568.2010.07502.x. [DOI] [PubMed] [Google Scholar]
- Mangiavacchi S, Wolf ME. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J. Neurochem. 2004;88:1261–1271. doi: 10.1046/j.1471-4159.2003.02248.x. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Milovanovic M, Wetter JB, Ford KA, Wolf ME. Behavioral sensitization to amphetamine is not accompanied by changes in glutamate receptor surface expression in the rat nucleus accumbens. J. Neurochem. 2009;109:35–51. doi: 10.1111/j.1471-4159.2009.05911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safferling M, Tichelaar W, Kümmerle G, Jouppila A, Kuusinen A, Keinänen K, Madden DR. First images of a glutamate receptor ion channel: oligomeric state and molecular dimensions of GluRB homomers. Biochemistry. 2001;40:13948–13953. doi: 10.1021/bi011143g. [DOI] [PubMed] [Google Scholar]
- Stackpole CW, De Milo LT, Hammerling U, Jacobson JB, Lardis MP. Hybrid antibody-induced topographical redistribution of surface immunoglobulins, alloantigens, and Concanavalin A receptors on mouse lymphoid cells. Proc. Natl. Acad. Sci. USA. 1974;71:932–936. doi: 10.1073/pnas.71.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons co-cultured with prefrontal cortex neurons. J. Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Crusells J, Vieira A, Saarma M, Rivera C. A novel method for monitoring surface membrane trafficking on hippocampal acute slice preparation. J. Neurosci. Meth. 2003;125:159–166. doi: 10.1016/s0165-0270(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Triller A, Choquet D. Surface trafficking of receptors between synaptic and extrasynaptic membranes: and yet they do move! Trends Neurosci. 2005;28:133–139. doi: 10.1016/j.tins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]