Abstract
Cardiovascular diseases are among the leading causes of death in the developed world. Developing novel therapies for diseases like heart failure is crucial, but this is hampered by the high attrition rate in drug development. The withdrawal of drugs at the final hurdle of approval is mostly due to their unpredictable effects on normal cardiac rhythm. The advent of cardiac computational modeling in the last six decades has significantly aided the understanding of heart function. Recently, these models have been increasingly applied towards designing and understanding therapies for cardiac disease. This article will discuss how cellular models of electrophysiology, cell signaling and metabolism have been used to investigate pharmacological therapies for cardiac diseases including arrhythmia, ischemia and heart failure.
Keywords: computational modeling, electrophysiology, metabolism, cell signaling, arrhythmia
I. Introduction
Computational modeling has played an important role in understanding heart physiology and pathology since the first model of the cardiac action potential 50 years ago (1). These models have been invaluable in understanding an organ whose function is the result of a complex system of nonlinear feedback loops that span single ion channels, cardiac cells and the whole heart (2). Despite its remarkable robustness, the heart is afflicted by various diseases that are complex and multifaceted (3). Heart disease remains the leading cause of premature death in developed societies. Approximately 2300 Americans die of cardiovascular disease each day, an average of one death every 38 seconds (4). The failure of many therapies targeting cardiac arrhythmia (5), contrasted with the success of counter-intuitive therapies like beta blockers in heart failure (6), highlights the difficulty in developing therapies for cardiovascular disease. A common causes of drug withdrawal from the market is due to their propensity to cause fatal cardiac arrhythmia (7) resulting in significant financial loss (8) and complicating new drug development (9).
Computational modeling can serve as a useful tool both for developing new therapies and evaluating current treatment of cardiovascular diseases and drugs with cardiovascular consequences. This idea is beginning to gain traction with the formation of the preDICT project, a consortium of pharmaceutical companies and academic institutions charged with the mission to model, simulate and predict the impact of pharmacological compounds on heart rhythm (10). Computational modeling has also been identified as a key component of the Critical Path Initiative, a project launched by the US Food and Drug Administration to improve drug and medical device development (11). The Critical Path Initiative lists six broad areas where innovation is needed including harnessing bioinformatics. Clinical trial simulation using in silico disease models was identified as a specific scientific opportunity to aid in this endeavor (12). This represents a shift in the drug approval process from requiring only empirical evidence to an increased emphasis on mechanistic understanding of drug action (13).
Models now exist for many aspects of cellular cardiac biology including electrophysiology, metabolism and signaling networks. These processes do not function in isolation, and there are significant efforts to integrate these models to simulate heart function in increasing detail (14). This review will discuss how models at cellular and tissue scales of cardiac biology can be used to assess current therapies, test therapies in development and suggest new targets (Table 1).
Table 1.
Examples of cardiac computational models used to investigate the effect of drugs and therapies.
| Target | Pathology | Drug/Treatment | Reference |
|---|---|---|---|
| INa | LQT3 | mexiletine | 31 |
| INa | LQT3 | ranolazine | 39 |
| IKr | acquired LQTS | E-4031 | 43 |
| IKr | LQTS | sotalol (pro-arrhythmic) | 48 |
| IKr | LQTS | NS1643 | 50 |
| IK-ATP | ischemia | pinacidil | 65 |
| INa-H | ischemia | blocker of Na-H exchanger | 67 |
| INa(f,L) | ischemia | blocker of late sodium channel | 69 |
| Cr,Adenine, Pi | heart failure | elevating all 3 pools | 75 |
| CaMKII | T-wave alternans | CaMKII inhibitor | 83 |
| CaMKII | myocardial infarct | CaMKII inhibitor | 87 |
| AC | heart failure | AC overexpression | 92 |
| CaMKII | heart failure | inhibition of CaMKII phosporylation of RyR | 96 |
| β-adrenergic receptor | LQT1 | β-adrenergic receptor blocker | 98 |
| β-adrenergic receptor | LQT3 | propranalol | 101 |
| LCC | LQT8 | reduction of LCC current | 104 |
| α-adrenergic receptor | pathological hypertrophy | α-adrenergic receptor blocker | 109 |
II. Therapies targeted against arrhythmias
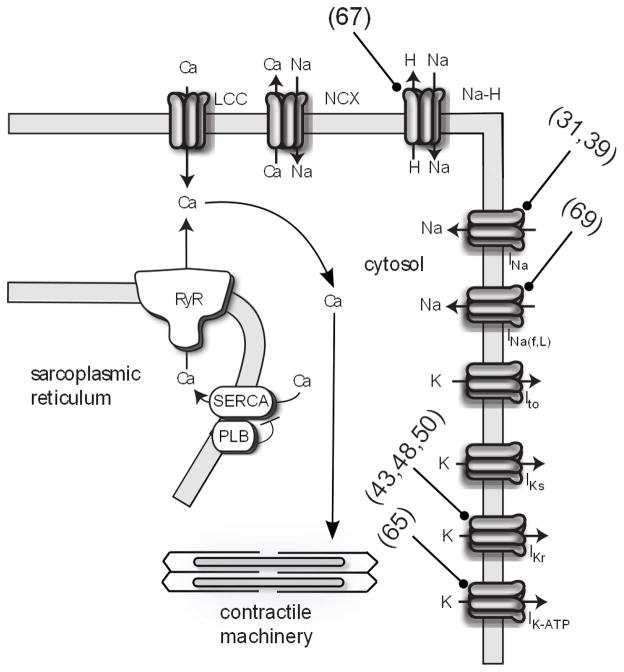
Modeling the electrical activity of the heart (cardiac electrophysiology) is perhaps the most advanced area of cardiac computational biology, and it has been the most successful in drug development (15). The role of cardiac electrophysiology is to coordinate the mechanical pumping of the heart in a process called excitation-contraction coupling (16). The key emergent property of cellular electrophysiology is the action potential, which reflects an intricate interplay between an array of ion channels (Figure 1). Fast activating sodium channels in the cell membrane are the first to open when a cardiac myocyte is slightly depolarized above a threshold. Increased sodium current (INa) strongly depolarizes the membrane, which activates L-type calcium channels (LCC) to increase calcium current (ICa). This influx of calcium induces ryanodine receptors to release an even larger amount of calcium from the sarcoplasmic reticulum, specialized calcium stores, into cytosolic space; a process called calcium-induced calcium release (CICR). It is this rise in cytosolic calcium that activates a myocyte’s contractile machinery (Figure 2). Relaxation occurs when calcium is re-sequestered into the sarcoplasmic reticulum by the sarcoplasmic reticulum calcium ATPase (SERCA) and the cell’s membrane potential is repolarized by potassium currents.
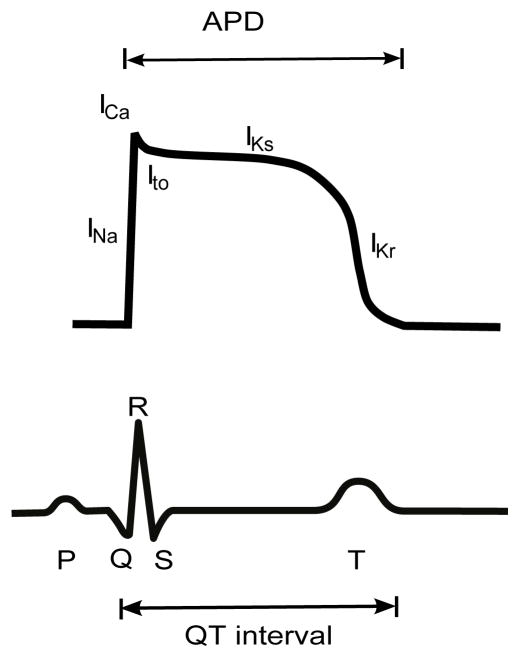
Figure 1.
Cardiac ventricular action potential and electrocardiogram. Predominant ion fluxes at different phases of the action potential are indicated. APD, action potential duration.
Figure 2.
Schematic of cardiac electrophysiology and contraction. Targets of computational models are indicated with blunt arrows (references in parenthesis). INa(f,l), late sodium current; IK-ATP, ATP dependent potassium current; PLB, phospholamban; NCX, sodium-calcium exchanger.
There are multiple channels with different properties that carry the repolarizing potassium current including the transient outward potassium current (Ito), which influences the early repolarization phase, and the delayed rectifier potassium currents (i.e. IKs and IKr.) Alterations in the orchestrated changes in ion flow can cause severe pathology. One such example is Long QT syndrome (LQTS), a family of diseases (LQT1-8) characterized by prolonged action potential duration (APD), which is measured as an increased QT interval on an electrocardiogram (17). LQTS is caused predominantly by either a reduction of potassium current or an increase in sodium current. This can occur due to genetic mutations or drugs acting on channels that carry both currents. Without treatment, 13% of individuals carrying gene mutations that cause LQTS will suffer cardiac arrest or sudden death due to the development of ventricular tachycardia (18). Understanding the mechanistic bases of arrhythmias and applying such knowledge to improve therapy is a great challenge in cardiac electrophysiology (19).
The earliest computational model of cardiac electrophysiology by Noble was based on the Hodgkin-Huxley model for the neuron action potential, predating the discovery of the calcium current (1). The first ventricular cell model, developed using experimental data from a variety of mammals, was published in 1977 by Beeler and Reuter and included the effect of intracellular calcium dynamics on the action potential (20). Luo and Rudy subsequently developed biophysically detailed models of a guinea pig ventricular myocyte by taking advantage of improved measurement of ionic currents from patch clamping (21, 22). By replacing the description of the calcium current in the Luo-Rudy models with detailed models of the L-type calcium channel and ryanodine receptor, Jafri et al. (23) were able to mechanistically represent calcium-induced calcium release. A notable feature of the Jafri-Rice-Winslow model was the inclusion of Markov state models of the L-type calcium channel and ryanodine receptor. This is a departure from Hodgkin and Huxley’s approach which used phenomenological descriptions of ion channel function (24). Ion channels are composed of protein subunits whose conformational changes “gate” ionic currents, causing shifts between inactive, closed or open states (25). The probability of a channel being in a certain state often depends on the previous state (26). Experimental observations of channel gating and aspects of channel structure can be replicated by modeling this channel property as a probabilistic Markov process (27).
Markov models can simulate the molecular basis of arrhythmias caused by mutations in ion channels and the effect of pharmaceutical compounds (27). There is evidence that drug binding affinity is often determined by the conformational state of the ion channel (28). This level of drug-channel interaction is important when developing drugs for patients suffering from LQT3, the most lethal subtype of LQTS (29). LQT3 is caused by mutations in the gene SCN5A, which encodes the α-subunit for the sodium channel. One such mutation results in deletion of three amino acid residues in the channel protein (ΔKPQ mutants) which causes a small persistent current during the plateau phase of the action potential, delaying repolarization (30). Clancy et al. (31) examined the mechanisms of drug block of the sodium channel by modifying Markov models of both the wild type and the ΔKPQ mutant channels (32). The ΔKPQ channel model was able to simulate the experimentally observed faster recovery from inactivation compared to the wild-type channel. By including additional model states for drug binding, they investigated the drugs mexiletine (an open state blocker) and lidocaine (an inactivation state blocker). The model predicted that lidocaine induces a rightward shift in voltage-dependent sodium channel availability, consistent with experimental data. The Markov models for SCN5A were then integrated with the Luo-Rudy model of the ventricular action potential. Simulations suggested that low doses of mexiletine can shorten the action potential duration in LQT3 without affecting the peak sodium current, which underlies the action potential upstroke. Conversely, lidocaine was predicted to have a lesser effect on action potential duration but blocked the peak current at high doses. This study illustrated how the specific mechanisms of drug action can have a significant impact on cellular physiology.
Electrophysiology models have now been developed for a variety of animal species, allowing representation of species-specific propensity for arrhythmia and other aspects of pathophysiology (24). The scarcity of human data, due to the lack of non-invasive techniques, increases the need for computational models of the human heart to gain further insight into cardiac disease (33). Models of the human ventricular myocyte have been developed by Priebe et al. (34), ten Tusscher et al. (35), Iyer et al. (36) and Grandi et al.(37). Like most models in biology, these models are constrained by limited available experimental data and each has specific applications for which its use is appropriate. The ten Tusscher-Noble-Noble-Panfilov (TNNP) model has the advantages of being based mostly on data obtained from human ventricular myocytes and is less complex due to the use of Hodgkin-Huxley-type equations for ion channels (38). Fredj et al. (39) replaced the sodium channel equations in the TNNP model with the Clancy-Rudy SCN5A Markov model (32) to investigate the potential therapeutic effect of ranolazine, an antianginal drug, on LQT3 human patients with ΔKPQ mutant sodium channels. In vitro experiments with a cell line expressing mutant channels confirmed their hypothesis that ranolazine is a use-dependent blocker that reduces sustained sodium current without affecting peak current. Simulations with the model containing the mutant ΔKPQ sodium channel showed a decrease in ventricular cell action potential duration in the presence of ranolazine. This suggests its potential as a treatment for LQT3 arrhythmias.
The rapid delayed rectifier potassium current (IKr) is encoded by the Human Ether-a-go-go Related Gene (hERG) and its mutations cause LQT2 (40). Drugs that inhibit this repolarizing potassium current prolong action potential duration and the QT interval (causing acquired LQTS), which can increase the risk to develop arrhythmia (41). hERG channels can exist as heteromers consisting of hERG 1a and 1b or as homomers of hERG1a. Despite evidence that the hERG 1a/b heteromers underlie the cardiac repolarizing current (42), little is known about how hERG hetero/homomers differ and how the hERG 1b subunit influences channel properties (especially in the context of pharmaceutical channel block). In-vitro experiments by Sale et al. showed that hERG heteromers have a quicker transition to an open state than hERG homomers (43). This suggests that an open channel blocker should be more effective against hERG heteromers but paradoxically, experimental results showed that hERG homomers were more inhibited by open channel block. To explain this mechanistically, the authors turned to the TNNP model, replacing the original IKr model with a Markov model that represents both hERG heteromers and homomers. They discovered that the additional channel gating mode of hERG 1a homomers results in more complete block by the open channel blocker E-4031, thus increasing the risk for acquired LQTS.
One of the challenges of understanding arrhythmia and developing effective therapies is linking molecular mechanisms from single cell to tissue levels (33). A cross-section of the ventricular wall reveals at least three different cell types: epicardial, endocardial and midmyocardial (44). These cells have different electrophysiological characteristics due to the differential expression of various channel proteins (45) including the repolarizing potassium currents (46). This heterogeneity results in tissue level characteristics including delay of repolarization across the ventricular wall or transmural dispersion of repolarization (47). Drugs that delay repolarization produce a substrate for reentrant arrhythmias (44). The electrical signal measured transmurally across the wall reflects properties of the whole body electrocardiogram with respect to QT interval and T wave morphology (3). This tissue heterogeneity can be modeled in its most basic form by using a 1D cable electrophysiology model. This consists of a string of cells with changes made to channel expression in order to simulate the different ventricular cell types (3). Brennan et al.(48) used a 1D fiber model to investigate sotalol, an anti-arrhythmic drug that targets IKr. They developed a Markov model of human hERG to represent the binding kinetics of sotalol (49), which was then included in the TNNP human myocyte model. Single cell simulations indicated that sotalol prolongs action potential duration, similar to experimental data obtained from human ventricular tissue, and is thus pro-arrhythmic. By inserting the modified ventricular models into a fiber model, the authors showed that sotalol increased T-wave dispersion and amplitude (all pro-arrhythmia markers), linking molecular properties of sotalol with observed tissue level phenomena. A similar approach was used by Pietersen et al. to investigate NS1643, a hERG channel opener (50). NS1643 causes an increase in channel conductance (51) and a depolarizing shift in voltage dependency of inactivation (52) which is anti-arrhythmic at the cellular level. Predicting whether NS1643 can be pro-arrhythmic at the tissue level or which of its effect on channel conductance and inactivation is crucial is difficult. Using simulations of a string of 100 human ventricular cells, the authors determined that NS1643 can be pro-arrhythmic at the tissue level (by increasing the vulnerable window for reentry) with the effect on channel inactivation having a larger contribution. However, simulations suggest that at low external potassium concentrations, the effect on channel conductance is more pronounced and increases the drug’s anti-arrhythmic properties by causing a greater reduction of the action potential duration. This indicates a specific condition (hypokalemia) in which the drug would be more beneficial in preventing arrhythmia and illustrates how models can be used to explain drug effects in different experimental conditions. Electrophysiology models can also be used in screening lead compounds as demonstrated by Mirams et al. who use a variety of ventricular action potential models to predict the arrhythmic risk of 31 drugs (53). Using drug IC50 values and therapeutic concentrations to calculate channel properties under drug influence, the authors were able to identify simulation of action potential duration prolongation as an improved measure of potential risk for Torsade-de-Pointes arrhythmias. The study is also a good example of the utility of collaboration between academia and industry in leveraging computational modeling for drug discovery.
III. Therapies targeted against ischemia and metabolic disorders
Computational models of cardiac metabolism have been built to investigate the changes in the metabolic state of a myocyte during pathological conditions like myocardial ischemia (54) and heart failure (55), and its effect on cardiac electrophysiology. Deficiencies in energy input and waste removal results in alterations to the cardiac action potential, contributing to failure of contraction (56). This condition is most pronounced during ischemia, the block of blood flow to the heart, and is a major trigger for arrhythmias (57). The heart consumes more energy than any other organ (58). To acquire this energy, it uses fatty acids and glucose to produce adenosine triphosphate (ATP) in the mitochondria, which is then shuttled to the contractile machinery through the utilization of creatine (59). When ischemia occurs after complete cessation of blood flow, the concentration of oxygen drops drastically (anoxia) resulting in decreased production of ATP (60). This is accompanied by elevated extracellular potassium concentration and increased blood acidity (61). Electrophysiological changes during the initial stages of ischemia are extremely rapid, making it difficult to study experimentally (54). The large number of simultaneous perturbations to the ischemic myocyte and the difficulty developing in vitro models of ischemia also emphasize the need for computational models in this area.
Expanding on the Luo-Rudy model, Shaw et al. (60) studied the ionic mechanisms underlying changes in the action potential in acute ischemia. By increasing the extracellular potassium concentration and changing the channel properties of the ATP sensitive potassium current, L-type calcium current and the sodium current, the authors were able to replicate the shortened action potential and conduction failure seen experimentally during ischemia.
The ATP-sensitive potassium channel plays a key role in the effects of ischemia on the heart (62). The effects of potassium channel openers in ischemia are controversial with conflicting views on whether they are protective or arrhythmogenic (63, 64). Trenor et al.(65) used a model of the ATP-sensitive potassium channel (66), incorporating the effect the potassium channel opener pinacidil, and integrating it with the Luo-Rudy model. The single cell model was able to replicate the experimentally observed reduction in action potential duration due to pinacidil. To study the effects of pinacidil in acute regional ischemia, the authors implemented a two-dimensional virtual heart tissue model. They noticed that the effect of pinacidil was strongly dose-dependent and had a strong anti-arrhythmic effect at high doses.
Ch’en et al. (67) opted to concentrate on the effects of acidosis and ATP depletion in ischemia. They incorporated equations describing ATP hydrolysis and intracellular pH into the DiFrancesco-Noble action potential model (68). The combined model, despite its limited scope, was able to correctly simulate ischemia-induced sodium overload (due to activation of the sodium-hydrogen exchanger, which causes calcium overload through activation of the sodium-calcium pump) seen experimentally. From their simulations, the authors suggested that cardiac arrhythmia can be attenuated by blocking the sodium-hydrogen exchanger. The cardiac action potential is also influenced by a late sodium current which is small but may contribute significantly to sodium/calcium overload during ischemia. Simulations performed by Noble et al. (69) suggest that blocking the late sodium current in conditions where the action potential is prolonged (e.g. with drugs affecting hERG) would be protective against arrhythmia.
The link between metabolism and calcium handling in a myocyte was modeled by Michailova et al.(70). Specifically, they investigated the effect of calcium and magnesium buffering by ATP and ADP and included ATP regulation of ion transporters. However, they did not explicitly simulate mitochondrial activity (71). An integrated cardiac cell model that linked electrophysiology and contraction with mitochondrial energy generation was developed by Cortassa et al. (72). This model was composed of an earlier model of cardiac mitochondrial metabolism which matched experimental data obtained from rat mitochondria (73). Another biophysically detailed model of cardiac mitochondria was developed by Beard et al.(74) and was used by Wu et al. (75) to investigate the evolution of the metabolic state in pathological hypertrophy, a precursor to heart failure. Heart failure, the inability of the heart to supply the body with enough blood (76), is characterized by a complex blend of changes to normal function including reduced mitochondrial ATP synthesis (55). The model is able to correctly predict the drop in cytoplasmic inorganic phosphate concentration seen in moderate left ventricular hypertrophy. They explored possible metabolic therapies by changing levels of creatine, adenine and exchangeable phosphate and discovered that altering levels of creatine alone was not sufficient, consistent with experimental studies (77). Their simulations suggest elevating levels of all three metabolites could be a beneficial metabolic therapy.
IV. Therapies targeted against signaling disorders
Modeling of disorders of cell signaling networks in myocytes is still in its infancy (33) despite the fact that drugs targeting cardiac signaling pathways are among the most heavily prescribed (78). Intracellular signaling pathways manage cellular processes like cell growth and contractility (79). A unifying feature of some signaling pathways in myocytes is the ubiquity of calcium as a second messenger (80). In addition to its role in contraction, calcium can also signal through Ca/calmodulin dependent protein kinase (CaMKII), a serine/threonine protein kinase, which is activated when bound by the calcium/calmodulin complex (Figure 3). CaMKII regulates cardiac gene expression through phosphorylation of transcription factors like CREB and HDAC (81) but also has an important role in calcium mobilization through phosphorylation of proteins like ion channels (82). By modeling the influence of CaMKII on calcium handling, Livshitz et al. (83) were able to show its potential effect on T wave alternans. T wave alternans are beat-to-beat variations in the amplitude of the T wave of the electrocardiogram and is associated with dispersion of repolarization, ventricular arrhythmia and sudden death (83). This is hypothesized to originate from variation in action potential duration at the cellular level coupled with variation in calcium transient amplitude. Livshitz et al. reformulated the CICR kinetics in the Hund-Rudy model (84), a canine ventricular action potential model that includes the CaMKII regulatory pathway. Their model was able to reproduce the experimentally observed frequency-dependent activation of CaMKII and the effect of CaMKII inhibition on calcium transients. Futher simulations showed that increased CaMKII activity causes increased alternans. They identify combined CaMKII inhibition and modulation of repolarizing currents as a possible intervention for suppressing T wave alternans.
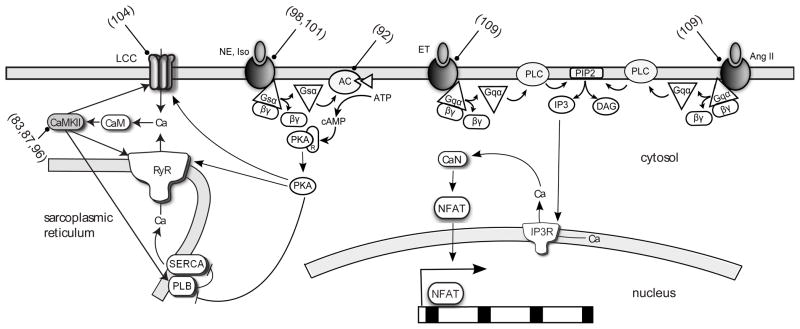
Figure 3.
Schematic of β-adrenergic, α-adrenergic and Ang receptor signaling network. Targets of computational models are indicated with blunt arrows (references in parenthesis). AC, adenylate cyclase; CaM, calmodulin; CaN, calcineurin; ET; endothelin; NE, norepinepherine; IP3R, IP3 receptor; Iso, isoproterenol.
CaMKII can auto-phosphorylate in order to retain kinase activity in the absence of calcium (85). Oxidation can also cause persistent activation independent of auto-phosphorylation (86). Levels of reactive oxygen species, hydrogen peroxide and superoxide are elevated following myocardial infarction resulting in increased levels of oxidated CaMKII. In order to ascertain the impact of oxidative activation of CaMKII following a myocardial infarct, Christensen et al. (87) used the Hund-Rudy model with the inclusion of an oxidized active state for CaMKII in addition to the calcium and autophosphorylation-mediated active states. Their cardiac fiber model described two regions of the epicardium: the normal and the border zone. The border zone of a myocardial infarct is the region of the epicardium that survives a transmural infarct and is often the site of reentrant excitation, which causes ventricular tachycardia (88). Enhanced CaMKII activity was predicted to increase recovery from inactivation of the sodium channel which promotes reduced conduction velocity, a marker for increased risk of arrhythmia (89). Based on their simulations, one may anticipate that inhibition of CaMKII or upstream redox pathways in the infarct border zone could reduce re-entrant arrhythmia and ventricular tachycardia. Indeed, overexpression of a CaMKII inhibitor in mice has been shown to protect from cardiac remodeling following myocardial infarction (90).
Extracellular stimuli for essential processes reach the cell via primary messengers, including catecholamines and hormones, and activate a diverse array of cellular receptors on the plasma membrane (79). These include β-adrenergic receptors, a family of G-protein coupled receptors found predominantly in cardiac, airway smooth muscle and adipose tissue. Binding of the catecholamines epinephrine or norepinepherine results in the coupling of the receptor to the guanine nucleotide binding protein Gs, with the subsequent release of the Gsα subunit following hydrolysis of guanosine triphosphate. Gsα then activates adenylate cyclase resulting in the production of cyclic AMP. Cyclic AMP causes the dissociation of the regulatory and catalytic subunits of protein kinase A (PKA). This allows PKA’s catalytic subunit to phosphorylate various protein targets including L-type calcium channel, phospholamban (inhibitor of SERCA), ryanodine receptor (RyR) and troponin. The key result of classical β-adrenergic receptor activation in the heart is increased cardiac output (91). Saucerman et al. (92) developed a model describing the β1-adrenergic receptor pathway, the most dominant receptor isoform, integrated with the Luo-Rudy model and modified for the rat ventricular myocyte. The model was able to reproduce the experimentally observed temporal response to β1-adrenergic stimulation including cAMP, PKA activity and phospholamban phosphorylation. It then served as a platform for investigating possible therapeutics for heart failure where desensitization of the β-adrenergic receptor system occurs (93). Their model predicted that adenylate cyclase overexpression, which can be achieved through gene therapy, increased the generation of cyclic AMP which in turn increased the β1-adrenergic response with minimal side effects compared with β1-adrenergic receptor or Gs overexpression. They also suggested that a hypothetical drug that increases the affinity of Gsα and adenylyl cyclase could be effective.
CaMKII is overexpressed in heart failure (94) and its activity appears to contribute to some aspects of β-adrenergic signaling (95). Soltis et al. (96) investigated the consequences of integrated β-adrenergic and CaMKII signaling by developing a combined model of both pathways and their regulation of excitation-contraction coupling. The model was validated against key experimental readouts including CaMKII-mediated phosphorylation of the ryanodine receptor and phospholamban and effects of CaMKII on Ca dynamics such as ICa facilitation and acceleration of relaxation. Their model predicted that increased CaMKII expression coupled with β-adrenergic stimulation is pro-arrythmogenic due to a synergy of PKA and CaMKII effects on ICa, RyR, and phospholamban. CaMKII-mediated increase in RyR phosphorylation played a key role and was necessary to predict spontaneous delayed after-depolarizations during β-adrenergic signaling. Thus blocking the ability of CaMKII to phosphorylate the RyR could be a potential therapy in such conditions.
Patients with LQT1 are susceptible to sudden cardiac death during β-adrenergic stimulation due to mutations in KCNQ1, a gene that encodes the repolarizing potassium current IKs (17). One particular KCNQ1 mutation observed clinically is KCNQ1-G589D, which disrupts the channel’s ability to form a signaling complex with PKA and phosphatase 1, mediated by the scaffolding protein yotiao (97). Saucerman et al. (98) investigated the whole-cell and tissue-level consequences of this disruption by integrating the Saucerman-McCulloch model of β-adrenergic signaling with a model of the rabbit ventricular myocyte integrated into a heterogenous 3D ventricular wedge model. The functional consequences of β-adrenergic signaling predicted by the model agreed with literature from rabbit ventricular myocytes including kinetics and dose response to isoproterenol, enhanced current through the KCNQ1 channel, and decreased action potential duration. Single cell model simulations suggested that the KCNQ1 mutation promotes early after depolarization (triggers for arrhythmia) only in the context of β-adrenergic stimulation. This effect was amplified at the tissue level with the appearance of T-wave abnormalities in simulated ECG’s, including dispersion of repolarization and T-wave inversion. Thus these simulations helped explain the in vivo consequences of KCNQ1 mutation and why β-blockers are effective for LQT1 patients. Indeed, a subsequent clinical report illustrated remarkably similar “Himalayan” T-waves in an LQT1 patient, who was subsequently treated with a β-blocker and implantation of a cardioverter-defibrillator (99).
In another subtype of the LQT syndrome, LQT3, it is unclear whether activation of the β-adrenergic system is beneficial or deleterious (100). In order to investigate this discrepancy, Ahrens-Nicklas et al. (101) incorporated a description of the human SCN5A-ΔKPQ mutation associated with LQT3 into a modified version of the Luo-Rudy (102)and used the Saucerman-McCulloch model of β-adrenergic signaling to predict phosphorylation levels of channels for IKs and ICa. This allowed the investigation of the pharmacology of LQT3 mutant channels in the context of a human-like action potential. The tissue effect of the various pharmacological agents was observed by performing transmural fiber simulations. In order to model the effects of β-blockade, decreased phosphorylation of IKs and ICa channels were predicted based on the decreased cyclic AMP production seen with the β-blocker propranolol. In addition, the authors incorporated the separate sodium channel blocking effects of propranolol into a Markov model of the ΔKPQ sodium current. Computational modeling allowed the authors to investigate various β-blocker levels and pacing protocols that have produced apparently contradicting results in published experiments. Isoproterenol decreased action potential duration and suppressed early after depolarization for all 3 ventricular cell types (endocardial, midmyocardial and epicardial). The fiber simulations showed that transmural dispersion of repolarization was decreased in the model following isoproterenol stimulation consistent with in vivo observations (100). An important observation was that this beneficial effect of isoproterenol was pacing–pattern dependent, explaining the discrepancies observed in vivo. Low doses of propranalol increased transmural dispersion and action potential duration, worsening the LQT3 phenotype. However, the high doses of propranolol had the opposite effect on the LQT3 phenotype which suggested to the authors that this beneficial effect was due to increased late sodium current blockage.
Timothy syndrome is a form of long QT syndrome (LQT8) caused by a mutation in the CACNA1C gene that encodes for the α1C-subunit of the L-type calcium channel (17). This mutation eliminates voltage-dependent inactivation of the channel, thus leaving it open (17). The resulting susceptibility to arrhythmia is ultimately deadly and patients rarely survive past 3 years (103). Sung et al. (104) investigated the influence of β-adrenergic stimulation on arrythmogenesis in Timothy Syndrome by making modifications to a Markov model of the L-type calcium channel in a modified version of the Luo-Rudy model (105). β-adrenergic modulation of various ion channels was simulated by changing channel parameter values to mimic saturating concentrations of a β-adrenergic agonist. The authors discovered that β-adrenergic stimulation, when combined with CACNA1C mutation, increases the occurrence of alternans, delayed after-depolarizations and early after-depolarizations (all triggers for arrhythmia). These predictions help validate the use of β-blockers as treatment for Timothy syndrome patients. Further simulations identified reduction of the L-type calcium current as the most effective target for reducing β-adrenergic-stimulated arrhythmia. The authors also identified reduction of SERCA-mediated Ca uptake as another potential target.
Several signaling pathways have been implicated in pathological hypertrophy, the abnormal growth that often leads to heart failure (106), including the inositol-4-5-biphosphate (IP3)-calcineurin pathway (107). IP3 is produced in response to the activation of members of the Gq family of G-protein coupled receptors including the α-adrenergic, endothelin and angiotensin receptors. Activation of these receptors results in liberation of Gqα which activates phospholipase C. Phospholipase C causes hydrolysis of phosphatidyl inositol-4-5-biphosphate to form the second messenger IP3. IP3 is thought to contribute to activation of the phosphatase calcineurin, which causes changes in cardiac gene expression through its effect on the transcription factor NFAT (108). In order to understand the control mechanisms underlying pathway activation, a computational model of the IP3 pathway described above was developed by Cooling et al. (109). Model parameters were fit to match the relatively fast kinetics of IP3 in response to endothelin-1 seen experimentally. Then, by changing only the parameters for receptor activation, the model was able to predict the experimentally-observed slower kinetics in response to angiotensin. Global sensitivity analysis suggested that the most sensitive parameter controlling the IP3 transient was the rate constant for phosphorylation of the active receptor. Despite both agonists stimulating IP3 production via an identical signal transduction pathway, the IP3 responses are remarkably distinct. From their simulation results, an effective strategy at combating pathological hypertrophy influenced by IP3 can be achieved by targeting the receptors for endothelin-1 and angiotensin II. This could be more successful than attempts at attenuating hypertrophy with calcineurin inhibitors(110), especially since G-protein coupled receptors are among the most druggable protein targets(111). Indeed, angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers are widely used for heart failure.
V. Future directions
The rate of attrition in drug discovery represents one of the most serious challenges to the pharmaceutical industry (9). A main reason is the difficulty in extrapolating pre-clinical data to predict clinical efficacy (9). Computational modeling can help bridge this gap at various stages in the drug discovery pipeline (112). Species-specific models of the cellular cardiac action potential allows the quantification of properties of potential drugs in various species and, with the help of human models, gain further insight into how the drugs might function in a clinical setting (33). In addition, the further integration of electrophysiology, signaling and metabolic models will allow the accurate simulation of complex diseases like heart failure with multiple etiologies.
Improvement of current signaling and metabolic models and the development of disease-specific models will require the integration of data made available by the recent emergence of cardiac specific proteomic (113) and metabolic (114) data sets. For example, data on expression levels of calcium handling proteins in heart failure myocytes (115) was used by Winslow et al. (116) to develop a heart failure ventricular cell model. By changing the expression levels of four proteins, namely SERCA, sodium-calcium exchanger, inward rectifying potassium channel and the calcium independent transient outward potassium channel, they were able to simulate the observed changes in calcium transients and action potential duration seen in failing cardiac cells (117). These approaches will need to be extended further, because for example in dilated cardiomyopathy, one of the leading causes of heart failure, over 100 proteins are differentially expressed in humans (118).
Unraveling the complexity of disease states will require development of new systems modeling approaches that leverage the breadth of available ‘omics data (15). For example, Berger et al. (119) combined protein-protein interaction and drug databases to identify a signaling network consisting of 1629 gene products that regulates ion channels involved in LQTS. Comprehensive cardiac models such as these may help predict off-target effects and unexpected connections. Simulation of multi-target treatment can also be achieved (as opposed to the identification of a single “silver bullet”), to overcome disease network properties like redundancy, crosstalk and robustness (120). This has led to the development of successful drug therapies for complex diseases affecting other organ systems including cancer and depression (121).
Cardiac phenomena like arrhythmias depend on changes at the molecular and cellular level but they are fatal because of their effect on whole organ function (122). In addition to 1D fiber, 2D sheet and 3D ventricular wedge models, whole heart models have been developed to examine the role of heart anatomy on arrhythmia (3). Multiscale electromechanical models of the heart have also been developed (123) and, if coupled with molecularly-detailed ion channel and signaling models, present a unique opportunity to explore drug intervention for conditions associated with structural remodeling such as myocardial infarction and hypertrophy. A limiting feature of multiscale models is the high computational requirements, with the simulation of one cardiac cycle in a human whole-heart model developed by Potse et al.(124) requiring approximately 2 days on 32 processors. Future advances in CPU performance and the use of alternative tools including GPU-aided simulations (125) will make multi-scale computational models more feasible, presenting an opportunity to merge tissue modeling with physiologically based pharmacokinetic models (126). Pharmacokinetics, which determines how rapidly and for how long a drug is available at a particular organ (127), is crucial for predicting clinical efficacy (128). Integrating multi-scale models with pharmacokinetic models will result in a modeling platform that is as close to clinical trials as possible. A recent example of the utility of this strategy is provided by Wu et al.(129) who use a multi-scale tissue model to investigate soluble VEGF receptor’s potential as an anti-angiogenic therapeutic.
It is important not to underestimate the challenges involved in applying computational models to drug discovery and development. A key step in every model’s development is the choice of the appropriate level of mechanistic detail. Most models are carefully optimized to address a particular set of biological questions or applications, often employing Albert Einstein’s advice to “make everything as simple as possible, but not simpler.” But when repurposing a model for drug discovery or integrating models to tackle multi-scale phenomena, one must carefully re-evaluate the underlying model assumptions and revalidate for the new experimental system. Parameter selection is particularly difficult when integrating multiple models, as most models are based on data from a range of experimental systems and animal species (10, 129). And as modeling efforts are generally data-limited, the majority of models described above were validated only under a limited set of available experimental conditions. While fully comprehensive model validation is not feasible, a key challenge is to identify the extent of validation that builds sufficient confidence in new model predictions to guide the next experimental or clinical phase. Systematic efforts to validate fundamental aspects of myocyte physiology across a range of models are likely to help in this regard (130).
Despite these challenges, cellular computational modeling has already informed the development of several drugs including ranolazine and ivabradine (10). Such modeling approaches have until recently been primarily used in academia. For the goals of FDA’s Critical Path Initiative to be realized, it will require increased collaboration between academia and industry to harness the potential of cardiac computational modeling for drug discovery and development. The rewards of this collaboration should help reduce the clinical burden of cardiovascular disease.
References
- 1.Noble D. Cardiac action and pacemaker potentials based on the Hodgkin-Huxley equations. Nature. 1960 Nov 5;188:495–7. doi: 10.1038/188495b0. [DOI] [PubMed] [Google Scholar]
- 2.Fink M, Noble D. Pharmacodynamic effects in the cardiovascular system: the modeller’s view. Basic Clin Pharmacol Toxicol. 2010 Mar;106(3):243–9. doi: 10.1111/j.1742-7843.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- 3.Reumann M, Gurev V, Rice JJ. Computational modeling of cardiac disease: potential for personalized medicine. Pers Med. 2009 Jan;6(1):45–66. doi: 10.2217/17410541.6.1.45. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart Disease and Stroke Statistics-2010 Update A Report From the American Heart Association. Circulation. 2010 Feb 23;121(7):E46–E215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 5.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obiasmanno D, Barker AH, et al. Mortality and Morbidity in Patients Receiving Encainide, Flecainide, or Placebo - the Cardiac-Arrhythmia Suppression Trial. New Engl J Med. 1991 Mar 21;324(12):781–8. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 6.Foody JM, Farrell MH, Krumholz HM. beta-blocker therapy in heart failure - Scientific review. Jama-JAm Med Assoc. 2002 Feb 20;287(7):883–9. doi: 10.1001/jama.287.7.883. [DOI] [PubMed] [Google Scholar]
- 7.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004 Mar 4;350(10):1013–22. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 8.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003 Mar;22(2):151–85. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 9.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004 Aug;3(8):711–5. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez B, Burrage K, Gavaghan D, Grau V, Kohl P, Noble D. The Systems Biology Approach to Drug Development: Application to Toxicity Assessment of Cardiac Drugs. Clin Pharmacol Ther. 2010 Jul;88(1):130–4. doi: 10.1038/clpt.2010.95. [DOI] [PubMed] [Google Scholar]
- 11.Woodcock J, Woosley R. The FDA critical path initiative and its influence on new drug development. Annu Rev Med. 2008;59:1–12. doi: 10.1146/annurev.med.59.090506.155819. [DOI] [PubMed] [Google Scholar]
- 12.FDA. FDA’s Critical Path Initiative. 2004. [cited 2010 August] [Google Scholar]
- 13.Lesko LJ. Paving the critical path: How can clinical pharmacology help achieve the vision? Clin Pharmacol Ther. 2007 Feb;81(2):170–7. doi: 10.1038/sj.clpt.6100045. [DOI] [PubMed] [Google Scholar]
- 14.Hunter PJ, Viceconti M. The VPH-Physiome Project: Standards and Tools for Multiscale Modeling in Clinical Applications. Biomedical Engineering, IEEE Reviews. 2009;2:40–53. [Google Scholar]
- 15.Cho CR, Labow M, Reinhardt M, van Oostrum J, Peitsch MC. The application of systems biology to drug discovery. Curr Opin Chem Biol. 2006 Aug;10(4):294–302. doi: 10.1016/j.cbpa.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002 Jan 10;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 17.Modell SM, Lehmann MH. The long QT syndrome family of cardiac ion channelopathies: A HuGE review. Genet Med. 2006 Mar;8(3):143–55. doi: 10.1097/01.gim.0000204468.85308.86. [DOI] [PubMed] [Google Scholar]
- 18.Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, et al. Risk stratification in the long-QT syndrome. New Engl J Med. 2003 May 8;348(19):1866–74. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 19.Jordan PN, Christini DJ. Therapies for ventricular cardiac arrhythmias. Crit Rev Biomed Eng. 2005;33(6):557–604. doi: 10.1615/critrevbiomedeng.v33.i6.20. [DOI] [PubMed] [Google Scholar]
- 20.Beeler GW, Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo CH, Rudy Y. A model of the ventricular cardiac action potential. Depolarization, repolarization, and their interaction. Circ Res. 1991 Jun;68(6):1501–26. doi: 10.1161/01.res.68.6.1501. [DOI] [PubMed] [Google Scholar]
- 22.Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. II. Afterdepolarizations, triggered activity, and potentiation. Circ Res. 1994 Jun;74(6):1097–113. doi: 10.1161/01.res.74.6.1097. [DOI] [PubMed] [Google Scholar]
- 23.Jafri MS, Rice JJ, Winslow RL. Cardiac Ca2+ dynamics: the roles of ryanodine receptor adaptation and sarcoplasmic reticulum load. Biophys J. 1998 Mar;74(3):1149–68. doi: 10.1016/S0006-3495(98)77832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams GS, Smith GD, Sobie EA, Jafri MS. Models of cardiac excitation-contraction coupling in ventricular myocytes. Math Biosci. 2010 Jul;226(1):1–15. doi: 10.1016/j.mbs.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gadsby DC. Ion channels versus ion pumps: the principal difference, in principle. Nat Rev Mol Cell Biol. 2009 May;10(5):344–52. doi: 10.1038/nrm2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudy Y, Silva JR. Computational biology in the study of cardiac ion channels and cell electrophysiology. Q Rev Biophys. 2006 Feb;39(1):57–116. doi: 10.1017/S0033583506004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fink M, Noble D. Markov models for ion channels: versatility versus identifiability and speed. Philos Transact A Math Phys Eng Sci. 2009 Jun 13;367(1896):2161–79. doi: 10.1098/rsta.2008.0301. [DOI] [PubMed] [Google Scholar]
- 28.Pugsley MK. Antiarrhythmic drug development: Historical review and future perspective. Drug Develop Res. 2002 Jan;55(1):3–16. [Google Scholar]
- 29.Zareba W, Moss AJ, Locati EH, Lehmann MH, Peterson DR, Hall WJ, et al. Modulating effects of age and gender on the clinical course of long QT syndrome by genotype. J Am Coll Cardiol. 2003 Jul 2;42(1):103–9. doi: 10.1016/s0735-1097(03)00554-0. [DOI] [PubMed] [Google Scholar]
- 30.Bennett PB, Yazawa K, Makita N, George AL. Molecular Mechanism for an Inherited Cardiac-Arrhythmia. Nature. 1995 Aug 24;376(6542):683–5. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- 31.Clancy CE, Zhu ZI, Rudy Y. Pharmacogenetics and anti-arrhythmic drug therapy: a theoretical investigation. Am J Physiol Heart Circ Physiol. 2007 Jan;292(1):H66–75. doi: 10.1152/ajpheart.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clancy CE, Rudy Y. Na(+) channel mutation that causes both Brugada and long-QT syndrome phenotypes: a simulation study of mechanism. Circulation. 2002 Mar 12;105(10):1208–13. doi: 10.1161/hc1002.105183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno JD, Clancy CE. Using computational modeling to predict arrhythmogenesis and antiarrhythmic therapy. Drug Discovery Today: Disease Models. 2009 Autumn;6(3):71–84. doi: 10.1016/j.ddmod.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Priebe L, Beuckelmann DJ. Simulation study of cellular electric properties in heart failure. Circ Res. 1998 Jun 15;82(11):1206–23. doi: 10.1161/01.res.82.11.1206. [DOI] [PubMed] [Google Scholar]
- 35.ten Tusscher KH, Noble D, Noble PJ, Panfilov AV. A model for human ventricular tissue. Am J Physiol Heart Circ Physiol. 2004 Apr;286(4):H1573–89. doi: 10.1152/ajpheart.00794.2003. [DOI] [PubMed] [Google Scholar]
- 36.Iyer V, Mazhari R, Winslow RL. A computational model of the human left-ventricular epicardial myocyte. Biophys J. 2004 Sep;87(3):1507–25. doi: 10.1529/biophysj.104.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grandi E, Pasqualini FS, Bers DM. A novel computational model of the human ventricular action potential and Ca transient. J Mol Cell Cardiol. 2010 Jan;48(1):112–21. doi: 10.1016/j.yjmcc.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ten Tusscher KH, Bernus O, Hren R, Panfilov AV. Comparison of electrophysiological models for human ventricular cells and tissues. Prog Biophys Mol Biol. 2006 Jan-Apr;90(1–3):326–45. doi: 10.1016/j.pbiomolbio.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Fredj S, Sampson KJ, Liu H, Kass RS. Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. Br J Pharmacol. 2006 May;148(1):16–24. doi: 10.1038/sj.bjp.0706709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995 Mar 10;80(5):795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 41.Recanatini M, Cavalli A, Masetti M. Modeling HERG and its interactions with drugs: recent advances in light of current potassium channel simulations. Chem Med Chem. 2008 Apr;3(4):523–35. doi: 10.1002/cmdc.200700264. [DOI] [PubMed] [Google Scholar]
- 42.London B, Trudeau MC, Newton KP, Beyer AK, Copeland NG, Gilbert DJ, et al. Two isoforms of the mouse ether-a-go-go-related gene coassemble to form channels with properties similar to the rapidly activating component of the cardiac delayed rectifier K+ current. Circ Res. 1997 Nov;81(5):870–8. doi: 10.1161/01.res.81.5.870. [DOI] [PubMed] [Google Scholar]
- 43.Sale H, Wang JL, O’Hara TJ, Tester DJ, Phartiyal P, He JQ, et al. Physiological properties of hERG 1a/1b heteromeric currents and a hERG 1b-specific mutation associated with long-QT syndrome. Circ Res. 2008 Sep 26;103(7):E81–E95. doi: 10.1161/CIRCRESAHA.108.185249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antzelevitch C. Role of transmural dispersion of repolarization in the genesis of drug-induced torsades de pointes. Heart Rhythm. 2005 Nov;2(2 Suppl):S9–15. doi: 10.1016/j.hrthm.2004.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell SG, Flaim SN, Leem CH, McCulloch AD. Mechanisms of transmurally varying myocyte electromechanics in an integrated computational model. Philos Transact A Math Phys Eng Sci. 2008 Sep 28;366(1879):3361–80. doi: 10.1098/rsta.2008.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pereon Y, Demolombe S, Baro I, Drouin E, Charpentier F, Escande D. Differential expression of KvLQT1 isoforms across the human ventricular wall. Am J Physiol Heart Circ Physiol. 2000 Jun;278(6):H1908–15. doi: 10.1152/ajpheart.2000.278.6.H1908. [DOI] [PubMed] [Google Scholar]
- 47.Antzelevitch C. M cells in the human heart. Circ Res. 2010 Mar 19;106(5):815–7. doi: 10.1161/CIRCRESAHA.109.216226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brennan T, Fink M, Stokeley D, Rodriguez B, Tarassenko L. Modelling effects of sotalol on T-wave morphology. 2007; 34th Annual Computers in Cardiology Conference; Durham, NC, USA. 2007. pp. 249–52. [Google Scholar]
- 49.Brennan T, Fink M, Rodriguez B, Tarassenko L. 11th Mediterranean Conference on Medical and Biomedical Engineering and Computing 2007. Springer; Berlin Heidelberg: 2007. Modelling effects of Sotalol on Action Potential morphology using a novel Markov model of the HERG channel; pp. 50–3. [Google Scholar]
- 50.Peitersen T, Grunnet M, Benson AP, Holden AV, Holstein-Rathlou NH, Olesen SP. Computational analysis of the effects of the hERG channel opener NS1643 in a human ventricular cell model. Heart Rhythm. 2008 May;5(5):734–41. doi: 10.1016/j.hrthm.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 51.Hansen RS, Diness TG, Christ T, Demnitz J, Ravens U, Olesen SP, et al. Activation of human ether-a-go-go-related gene potassium channels by the diphenylurea 1,3-bis-(2-hydroxy-5-trifluoromethyl-phenyl)-urea (NS1643) Mol Pharmacol. 2006 Jan;69(1):266–77. doi: 10.1124/mol.105.015859. [DOI] [PubMed] [Google Scholar]
- 52.Casis O, Olesen SP, Sanguinetti MC. Mechanism of action of a novel human ether-a-go-go-related gene channel activator. Mol Pharmacol. 2006 Feb;69(2):658–65. doi: 10.1124/mol.105.019943. [DOI] [PubMed] [Google Scholar]
- 53.Mirams GR, Cui Y, Sher A, Fink M, Cooper J, Heath BM, et al. Simulation of multiple ion channel block provides improved early prediction of compounds’ clinical torsadogenic risk. Cardiovasc Res. 2011 Feb 7; doi: 10.1093/cvr/cvr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez B, Trayanova N, Noble D. Modeling cardiac ischemia. Ann N Y Acad Sci. 2006 Oct;1080:395–414. doi: 10.1196/annals.1380.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol. 2004 Feb 15;555(Pt 1):1–13. doi: 10.1113/jphysiol.2003.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest. 2005 Sep;115(9):2305–15. doi: 10.1172/JCI26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev. 1999 Jul;79(3):917–1017. doi: 10.1152/physrev.1999.79.3.917. [DOI] [PubMed] [Google Scholar]
- 58.Doenst T, Bugger H, Schwarzer M, Faerber G, Borger MA, Mohr FW. Three good reasons for heart surgeons to understand cardiac metabolism. Eur J Cardiothorac Surg. 2008 May;33(5):862–71. doi: 10.1016/j.ejcts.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 59.Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007 Mar 15;356(11):1140–51. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 60.Shaw RM, Rudy Y. Electrophysiologic effects of acute myocardial ischemia: a theoretical study of altered cell excitability and action potential duration. Cardiovasc Res. 1997 Aug;35(2):256–72. doi: 10.1016/s0008-6363(97)00093-x. [DOI] [PubMed] [Google Scholar]
- 61.Cascio WE, Johnson TA, Gettes LS. Electrophysiologic changes in ischemic ventricular myocardium: I. Influence of ionic, metabolic, and energetic changes. J Cardiovasc Electrophysiol. 1995 Nov;6(11):1039–62. doi: 10.1111/j.1540-8167.1995.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 62.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8–14;305(5930):147–8. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 63.D’Alonzo AJ, Darbenzio RB, Hess TA, Sewter JC, Sleph PG, Grover GJ. Effect of potassium on the action of the KATP modulators cromakalim, pinacidil, or glibenclamide on arrhythmias in isolated perfused rat heart subjected to regional ischaemia. Cardiovasc Res. 1994 Jun;28(6):881–7. doi: 10.1093/cvr/28.6.881. [DOI] [PubMed] [Google Scholar]
- 64.D’Alonzo AJ, Zhu JL, Darbenzio RB, Dorso CR, Grover GJ. Proarrhythmic effects of pinacidil are partially mediated through enhancement of catecholamine release in isolated perfused guinea-pig hearts. J Mol Cell Cardiol. 1998 Feb;30(2):415–23. doi: 10.1006/jmcc.1997.0605. [DOI] [PubMed] [Google Scholar]
- 65.Trenor B, Ferrero JM, Jr, Rodriguez B, Montilla F. Effects of pinacidil on reentrant arrhythmias generated during acute regional ischemia: a simulation study. Ann Biomed Eng. 2005 Jul;33(7):897–906. doi: 10.1007/s10439-005-3554-4. [DOI] [PubMed] [Google Scholar]
- 66.Ferrero JM, Jr, Saiz J, Ferrero JM, Thakor NV. Simulation of action potentials from metabolically impaired cardiac myocytes. Role of ATP-sensitive K+ current. Circ Res. 1996 Aug;79(2):208–21. doi: 10.1161/01.res.79.2.208. [DOI] [PubMed] [Google Scholar]
- 67.Ch’en FFT, Vaughan-Jones RD, Clarke K, Noble D. Modelling myocardial ischaemia and reperfusion. Prog Biophys Mol Bio. 1998;69(2–3):515–38. doi: 10.1016/s0079-6107(98)00023-6. [DOI] [PubMed] [Google Scholar]
- 68.DiFrancesco D, Noble D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos Trans R Soc Lond B Biol Sci. 1985 Jan 10;307(1133):353–98. doi: 10.1098/rstb.1985.0001. [DOI] [PubMed] [Google Scholar]
- 69.Noble D, Noble PJ. Late sodium current in the pathophysiology of cardiovascular disease: consequences of sodium-calcium overload. Heart. 2006 Jul;92(Suppl 4):iv1–iv5. doi: 10.1136/hrt.2005.078782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michailova A, McCulloch A. Model study of ATP and ADP buffering, transport of Ca(2+) and Mg(2+), and regulation of ion pumps in ventricular myocyte. Biophys J. 2001 Aug;81(2):614–29. doi: 10.1016/S0006-3495(01)75727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Puglisi JL, Wang F, Bers DM. Modeling the isolated cardiac myocyte. Prog Biophys Mol Biol. 2004 Jun-Jul;85(2–3):163–78. doi: 10.1016/j.pbiomolbio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 72.Cortassa S, Aon MA, O’Rourke B, Jacques R, Tseng HJ, Marban E, et al. A computational model integrating electrophysiology, contraction, and mitochondrial bioenergetics in the ventricular myocyte. Biophys J. 2006 Aug 15;91(4):1564–89. doi: 10.1529/biophysj.105.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cortassa S, Aon MA, Marban E, Winslow RL, O’Rourke B. An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys J. 2003 Apr;84(4):2734–55. doi: 10.1016/S0006-3495(03)75079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beard DA. Modeling of oxygen transport and cellular energetics explains observations on in vivo cardiac energy metabolism. Plos Computational Biology. 2006 Sep;2(9):1093–106. doi: 10.1371/journal.pcbi.0020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu F, Zhang JY, Beard DA. Experimentally observed phenomena on cardiac energetics in heart failure emerge from simulations of cardiac metabolism. P Natl Acad Sci USA. 2009 Apr 28;106(17):7143–8. doi: 10.1073/pnas.0812768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mudd JO, Kass DA. Tackling heart failure in the twenty-first century. Nature. 2008 Feb 21;451(7181):919–28. doi: 10.1038/nature06798. [DOI] [PubMed] [Google Scholar]
- 77.Wallis J, Lygate CA, Fischer A, ten Hove M, Schneider JE, Sebag-Montefiore L, et al. Supranormal myocardial creatine and phosphocreatine concentrations lead to cardiac hypertrophy and heart failure - Insights from creatine transporter-overexpressing transgenic mice. Circulation. 2005 Nov 15;112(20):3131–9. doi: 10.1161/CIRCULATIONAHA.105.572990. [DOI] [PubMed] [Google Scholar]
- 78.Salazar NC, Chen J, Rockman HA. Cardiac GPCRs: GPCR signaling in healthy and failing hearts. Biochim Biophys Acta. 2007 Apr;1768(4):1006–18. doi: 10.1016/j.bbamem.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schaub MC, Hefti MA, Zaugg M. Integration of calcium with the signaling network in cardiac myocytes. Journal of Molecular and Cellular Cardiology. 2006 Aug;41(2):183–214. doi: 10.1016/j.yjmcc.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 80.Bers DM, Guo T. Calcium signaling in cardiac ventricular myocytes. Ann Ny Acad Sci. 2005;1047:86–98. doi: 10.1196/annals.1341.008. [DOI] [PubMed] [Google Scholar]
- 81.Maier LS. CaMKII delta overexpression in hypertrophy and heart failure: cellular consequences for excitation-contraction coupling. Braz J Med Biol Res. 2005 Sep;38(9):1293–302. doi: 10.1590/s0100-879x2005000900002. [DOI] [PubMed] [Google Scholar]
- 82.Bers DM, Grandi E. Calcium/Calmodulin-dependent Kinase II Regulation of Cardiac Ion Channels. J Cardiovasc Pharm. 2009 Sep;54(3):180–7. doi: 10.1097/FJC.0b013e3181a25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Livshitz LM, Rudy Y. Regulation of Ca2+ and electrical alternans in cardiac myocytes: role of CAMKII and repolarizing currents. Am J Physiol-Heart C. 2007 Jun;292(6):H2854–H66. doi: 10.1152/ajpheart.01347.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hund TJ, Rudy Y. Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model. Circulation. 2004 Nov 16;110(20):3168–74. doi: 10.1161/01.CIR.0000147231.69595.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hund TJ, Rudy Y. A role for calcium/calmodulin-dependent protein kinase II in cardiac disease and arrhythmia. Handb Exp Pharmacol. 2006;171:201–20. doi: 10.1007/3-540-29715-4_7. [DOI] [PubMed] [Google Scholar]
- 86.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008 May 2;133(3):462–74. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Christensen MD, Dun W, Boyden PA, Anderson ME, Mohler PJ, Hund TJ. Oxidized calmodulin kinase II regulates conduction following myocardial infarction: a computational analysis. PLoS Comput Biol. 2009 Dec;5(12):e1000583. doi: 10.1371/journal.pcbi.1000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ursell PC, Gardner PI, Albala A, Fenoglio JJ, Jr, Wit AL. Structural and electrophysiological changes in the epicardial border zone of canine myocardial infarcts during infarct healing. Circ Res. 1985 Mar;56(3):436–51. doi: 10.1161/01.res.56.3.436. [DOI] [PubMed] [Google Scholar]
- 89.Veeraraghavan R, Poelzing S. Mechanisms underlying increased right ventricular conduction sensitivity to flecainide challenge. Cardiovasc Res. 2008 Mar 1;77(4):749–56. doi: 10.1093/cvr/cvm090. [DOI] [PubMed] [Google Scholar]
- 90.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005 Apr;11(4):409–17. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 91.Saucerman JJ, McCulloch AD. Cardiac beta-adrenergic signaling: from subcellular microdomains to heart failure. Ann N Y Acad Sci. 2006 Oct;1080:348–61. doi: 10.1196/annals.1380.026. [DOI] [PubMed] [Google Scholar]
- 92.Saucerman JJ, Brunton LL, Michailova AP, McCulloch AD. Modeling beta-adrenergic control of cardiac myocyte contractility in silico. J Biol Chem. 2003 Nov 28;278(48):47997–8003. doi: 10.1074/jbc.M308362200. [DOI] [PubMed] [Google Scholar]
- 93.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003 Nov 14;93(10):896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 94.Hoch B, Meyer R, Hetzer P, Krause EG, Karczewski P. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999 Apr 2;84(6):713–21. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 95.Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007 Feb 16;100(3):391–8. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- 96.Soltis AR, Saucerman JJ. Synergy between CaMKII substrates and β-adrenergic signaling in regulation of cardiac myocyte Ca2+ handling. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marx SO, Kurokawa J, Reiken S, Motoike H, D’Armiento J, Marks AR, et al. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002 Jan 18;295(5554):496–9. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 98.Saucerman JJ, Healy SN, Belik ME, Puglisi JL, McCulloch AD. Proarrhythmic consequences of a KCNQ1 AKAP-binding domain mutation: computational models of whole cells and heterogeneous tissue. Circ Res. 2004 Dec 10;95(12):1216–24. doi: 10.1161/01.RES.0000150055.06226.4e. [DOI] [PubMed] [Google Scholar]
- 99.Darbar D, Roden DM, Ali MF, Yang T, Wathen MS. Himalayan T waves in the congenital Long-QT syndrome. Circulation. 2005 Mar 22;111(11):E161–E. doi: 10.1161/01.CIR.0000159092.92366.CD. [DOI] [PubMed] [Google Scholar]
- 100.Shimizu W, Antzelevitch C. Differential effects of beta-adrenergic agonists and antagonists in LQT1, LQT2 and LQT3 models of the long QT syndrome. J Am Coll Cardiol. 2000 Mar 1;35(3):778–86. doi: 10.1016/s0735-1097(99)00582-3. [DOI] [PubMed] [Google Scholar]
- 101.Ahrens-Nicklas RC, Clancy CE, Christini DJ. Re-evaluating the efficacy of beta-adrenergic agonists and antagonists in long QT-3 syndrome through computational modelling. Cardiovasc Res. 2009 Jun 1;82(3):439–47. doi: 10.1093/cvr/cvp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Faber GM, Rudy Y. Action potential and contractility changes in [Na(+)](i) overloaded cardiac myocytes: a simulation study. Biophys J. 2000 May;78(5):2392–404. doi: 10.1016/S0006-3495(00)76783-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, et al. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci U S A. 2005 Jun 7;102(23):8089–96. doi: 10.1073/pnas.0502506102. discussion 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sung RJ, Wu YH, Lai NH, Teng CH, Luo CH, Tien HC, et al. Beta-adrenergic modulation of arrhythmogenesis and identification of targeted sites of antiarrhythmic therapy in Timothy (LQT8) syndrome: a theoretical study. Am J Physiol Heart Circ Physiol. 2010 Jan;298(1):H33–44. doi: 10.1152/ajpheart.00232.2009. [DOI] [PubMed] [Google Scholar]
- 105.Faber GM, Silva J, Livshitz L, Rudy Y. Kinetic properties of the cardiac L-type Ca2+ channel and its role in myocyte electrophysiology: A theoretical investigation. Biophysical Journal. 2007 Mar 1;92(5):1522–43. doi: 10.1529/biophysj.106.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006 Aug;7(8):589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 107.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002 Apr;109( Suppl):S67–79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 108.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998 Apr 17;93(2):215–28. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cooling M, Hunter P, Crampin EJ. Modeling hypertrophic IP3 transients in the cardiac myocyte. Biophys J. 2007 Nov 15;93(10):3421–33. doi: 10.1529/biophysj.107.110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leinwand LA. Calcineurin inhibition and cardiac hypertrophy: a matter of balance. Proc Natl Acad Sci U S A. 2001 Mar 13;98(6):2947–9. doi: 10.1073/pnas.051033698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002 Sep;1(9):727–30. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 112.Kumar N, Hendriks BS, Janes KA, de Graaf D, Lauffenburger DA. Applying computational modeling to drug discovery and development. Drug Discovery Today. 2006 Sep;11(17–18):806–11. doi: 10.1016/j.drudis.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 113.McGregor E, Dunn MJ. Proteomics of the heart -Unraveling disease. Circ Res. 2006 Feb 17;98(3):309–21. doi: 10.1161/01.RES.0000201280.20709.26. [DOI] [PubMed] [Google Scholar]
- 114.Mervaala E, Biala A, Merasto S, Lempiainen J, Mattila I, Martonen E, et al. Metabolomics in Angiotensin II-Induced Cardiac Hypertrophy. Hypertension. 2010 Feb 1;55(2):508–15. doi: 10.1161/HYPERTENSIONAHA.109.145490. [DOI] [PubMed] [Google Scholar]
- 115.Winslow RL, Cortassa S, Greenstein JL. Using models of the myocyte for functional interpretation of cardiac proteomic data. J Physiol-London. 2005 Feb 15;563(1):73–81. doi: 10.1113/jphysiol.2004.080457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Winslow RL, Rice J, Jafri S, Marban E, O’Rourke B. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, II -Model studies. Circ Res. 1999 Mar 19;84(5):571–86. doi: 10.1161/01.res.84.5.571. [DOI] [PubMed] [Google Scholar]
- 117.O’Rourke B, Kass DA, Tomaselli GF, Kaab S, Tunin R, Marban E. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I -Experimental studies. Circ Res. 1999 Mar 19;84(5):562–70. doi: 10.1161/01.res.84.5.562. [DOI] [PubMed] [Google Scholar]
- 118.McGregor E, Dunn MJ. Proteomics of heart disease. Hum Mol Genet. 2003 Oct 15;12:R135–R44. doi: 10.1093/hmg/ddg278. [DOI] [PubMed] [Google Scholar]
- 119.Berger SI, Ma’ayan A, Iyengar R. Systems Pharmacology of Arrhythmias (vol 3, ra30, 2010) Sci Signal. 2010 May 11;3(121) doi: 10.1126/scisignal.2000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pujol A, Mosca R, Farres J, Aloy P. Unveiling the role of network and systems biology in drug discovery. Trends Pharmacol Sci. 2010 Mar;31(3):115–23. doi: 10.1016/j.tips.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 121.Ma XH, Shi Z, Tan CY, Jiang YY, Go ML, Low BC, et al. In-Silico Approaches to Multi-target Drug Discovery. Pharm Res-Dordr. 2010 May;27(5):739–49. doi: 10.1007/s11095-010-0065-2. [DOI] [PubMed] [Google Scholar]
- 122.Noble D. Modeling the heart. Physiology (Bethesda) 2004 Aug;19:191–7. doi: 10.1152/physiol.00004.2004. [DOI] [PubMed] [Google Scholar]
- 123.Trayanova NA. Whole-heart modeling: applications to cardiac electrophysiology and electromechanics. Circ Res. 2011 Jan 7;108(1):113–28. doi: 10.1161/CIRCRESAHA.110.223610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Potse M, Dube B, Richer J, Vinet A, Gulrajani RM. A comparison of monodomain and bidomain reaction-diffusion models for action potential propagation in the human heart. Ieee T Bio-Med Eng. 2006 Dec;53(12):2425–35. doi: 10.1109/TBME.2006.880875. [DOI] [PubMed] [Google Scholar]
- 125.Sato D, Xie YF, Weiss JN, Qu ZL, Garfinkel A, Sanderson AR. Acceleration of cardiac tissue simulation with graphic processing units. Med Biol Eng Comput. 2009 Sep;47(9):1011–5. doi: 10.1007/s11517-009-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vicini P. Multiscale modeling in drug discovery and development: future opportunities and present challenges. Clin Pharmacol Ther. 2010 Jul;88(1):126–9. doi: 10.1038/clpt.2010.87. [DOI] [PubMed] [Google Scholar]
- 127.Katzung BG, Masters SB, Trevor AJ. Basic and Clinical Pharmacology. 11. 2009. [Google Scholar]
- 128.Michelson S, Sehgal A, Friedrich C. In silico prediction of clinical efficacy. Curr Opin Biotech. 2006 Dec;17(6):666–70. doi: 10.1016/j.copbio.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 129.Wu FT, Stefanini MO, Mac Gabhann F, Popel AS. A compartment model of VEGF distribution in humans in the presence of soluble VEGF receptor-1 acting as a ligand trap. PLoS One. 2009;4(4):e5108. doi: 10.1371/journal.pone.0005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Niederer SA, Fink M, Noble D, Smith NP. A meta-analysis of cardiac electrophysiology computational models. Exp Physiol. 2009 May;94(5):486–95. doi: 10.1113/expphysiol.2008.044610. [DOI] [PubMed] [Google Scholar]