Abstract
Background
This pilot study examined whether a novel diabetes screening approach using gingival crevicular blood (GCB) could be used to test for hemoglobin A1c (HbA1c) during the periodontal visit.
Methods
At a large periodontics clinic, finger stick blood (FSB) samples from 120 patients as well as GCB samples from those patients with adequate bleeding on probing were collected on special blood collection cards and were analyzed for HbA1c levels in a laboratory. The Pearson correlation coefficient was used to measure correlation between FSB and GCB HbA1c values for 75 paired FSB and GCB samples. A Receiver Operator Characteristic Curve (ROC) analysis was performed to determine an optimal GCB HbA1c criterion value for a positive diabetes screen.
Results
For the 75 paired samples, the Pearson correlation coefficient was .842. The ROC analysis identified a criterion value of 6.3% for the GCB HbA1c test with high sensitivity (.933) and high specificity (.900) corresponding to FSB HbA1c values of 6.5% or greater (in the diabetes range). Using this GCB HbA1c criterion value for 27 additional paired samples in which there was an unidentified component observed to co-elute within the elution window of GCB HbA1c in the laboratory, there was agreement between FSB and GCB values for 24 of the pairs according to whether they were both within, or both outside of the diabetes range.
Conclusions
Using a criterion value of 6.3%, GCB samples are acceptable for HbA1c testing to screen for diabetes in most persons with bleeding on probing at the GCB collection site.
Keywords: diabetes mellitus, periodontitis, public health dentistry
INTRODUCTION
Diabetes has reached epidemic proportions in the United States. In 2010, 18.8 million people in the U.S. had been diagnosed with diabetes, including 1.9 million aged 20 years or older who were newly diagnosed that year.1 Of special concern is that an additional 7 million people living with diabetes in the U.S. are estimated to have been undiagnosed in 2010.1 Notably, early diabetes detection could identify diabetes-related complications at an earlier stage,2–10 suggesting the value of screening to discover unrecognized illness, manage existing complications, and prevent the progression of disease.
Some have therefore advocated for opportunistic screening among at-risk persons who present for health care unrelated to diabetes.11–16 Among those at risk are persons with moderate or severe periodontal disease,17–22 a condition that affects about 1/8 of U.S. adults over 30 years of age.23 In our earlier analysis of National Health and Nutrition Examination Survey (NHANES) data collected from adults who had moderate or severe periodontitis but did not report a past diabetes diagnosis, we found that 93% would have been recommended for diabetes testing according to American Diabetes Association guidelines.24 Many of these adults had recent contact with a dentist (50% in the past year), and therefore could potentially have been screened for diabetes in the dental office. For the many persons with periodontitis who might not be screened elsewhere, the dental visit may be an important setting for opportunistic diabetes screening, with considerable benefit to patients who might not otherwise be screened. In addition, knowing a patient’s diabetes status can help dentists make decisions about treatment to optimize the patient’s oral health care,25 and enhance the role that dental providers play in supporting their patients’ overall health.
Several approaches have been proposed to screen for diabetes at dental visits. One suggested approach has been to use dental and demographic data to identify at-risk patients.26–28 While non-invasive, this approach requires that patients visit their primary care providers for even initial determinations of whether their glucose levels are in the diabetes range. Our earlier work and that of others proposed an alternate diabetes screening approach that involved the use of a hand-held glucose meter to perform point-of-care glucose testing using gingival crevicular blood (GCB) obtained from patients with periodontitis.29–33 This approach capitalized on the fact that the dental clinician’s routine probing to measure periodontal pocket depth typically produces substantial gingival crevicular bleeding in patients with moderate or severe periodontal disease. As dental providers and patients may expect the dental provider to perform intra-oral, rather than extra-oral procedures, it was thought that GCB glucose testing might be a more acceptable and feasible means for diabetes screening in the dental office.34 In fact, in a survey of a national random sample of U.S. dentists, although 76.6% thought it important for dentists to conduct diabetes screening, only 55.9% were willing to collect blood via finger stick.35 Results from glucose testing with hand-held glucose meters generally demonstrated high correlations between GCB glucose values and finger stick blood glucose readings. However, limitations in the accuracy of these readings, and the fact that the readings were affected by what and when the patient last ate, made the value of their use in diabetes screening somewhat limited.
Notably, the hemoglobin A1c (HbA1c) test, recently promoted by the American Diabetes Association for diabetes diagnostic purposes, is often preferred over other recommended diagnostic tests (i.e., Fasting Plasma Glucose or Oral Glucose Tolerance) as it does not require a fasting blood sample. By removing the need for fasting, the HbA1c test eliminates a major barrier to diabetes screening, and can thereby help reduce the number of undiagnosed patients, whatever the venue.36 To facilitate diabetes screening with HbA1c testing in the dental office, a certified, high-complexity, Clinical Laboratory Improvement Amendment [CLIA]-accredited laboratory¶ has developed a diabetes screening approach specifically intended for dental providers that involves finger stick HbA1c testing. Giving highly reliable and valid results that conform to American Diabetes Association recommendations regarding testing for HbA1c, this approach uses a self-contained kit with all of the necessary supplies. It involves collecting a drop of the patient’s finger stick blood (FSB) for HbA1c testing on a specially prepared blood collection card, enclosing the card with the dried blood sample in a sealed, desiccated foil pouch within a waterproof envelope to be sent via U.S. mail to the laboratory, and having the laboratory perform the HbA1c test on the sample. However, consistent with the rationale for collecting GCB for glucose testing using a hand-held glucose meter, some dental providers and patients may be more amenable to the collection of oral blood in the dental venue. Therefore, in addition to using the laboratory’s HbA1c testing approach with FSB, we expanded its approach to HbA1c testing to include the testing of GCB, with the blood collected on a specially prepared blood collection card wand and sent to the laboratory for testing. The dental provider collects the GCB sample; the laboratory, rather than the dental provider, conducts the HbA1c test and provides test results to the patient.
The major objective of the research study was to determine if GCB HbA1c tests are feasible and acceptable for diabetes screening in periodontal patients. Specifically, our research was intended to determine (1) whether periodontal patients will agree to have GCB collected and dental providers will be willing and able to collect GCB for diabetes screening in the course of the dental visit, and (2) whether a criterion value on the GCB HbA1c test in periodontal patients can be determined that results in high sensitivity and specificity relative to an FSB HbA1c value in the diabetes range. This paper focuses on the second of these two aspects of the GCB HbA1c testing approach’s feasibility and acceptability. To do so, we report results from a pilot study involving HbA1c testing of FSB and GCB samples from 120 patients who were recruited from a Periodontics Clinic in a large, urban Dental College. We first examined the HbA1c FSB test results from participating patients with regard to the extent to which they were in the diabetes range (i.e., HbA1c ≥ 6.5%). For patients from whom a GCB sample was also collected, we report the extent to which these GCB HbA1c sample results could be used for comparison with FSB HbA1c sample tests. For patients for whom these comparisons could be made (N=75), we then determined (a) the correlation of FSB and GCB HbA1c values, and (b) the optimal criterion GCB HbA1c value to identify patients with FSB HbA1c values in the diabetes range.
MATERIALS AND METHODS
Study recruitment, participation, and data collection took place at the New York University College of Dentistry (NYUCD) Periodontics Clinic from March through May 2011. All of the recruited subjects were patients either under treatment, or on maintenance for periodontal disease. Patients were eligible for the research if they were ≥ 18 years old and if (1) they did not require antibiotic pre-medication before dental treatment, and did not have a history of severe cardiovascular, hepatic, immunological, renal, hematological, or other organ impairment (consistent with NHANES exclusion criteria37), and (2) had diabetes or were at risk for diabetes according to criteria established by the American Diabetes Association.38 In establishing the study’s recruitment and eligibility criteria and strategy, it was anticipated that while all of the participating periodontal patients would be able to provide a FSB sample for HbA1c testing, not all of these patients would have sufficient bleeding on probing for GCB blood collection. The approach to patient recruitment and eligibility was intended, in part, to assess the extent to which sufficient GCB blood collection for HbA1c testing would be possible for the majority of patients receiving care at a Periodontics Clinic in a large, urban Dental College.
When patients presented for their dental appointment at the Clinic, they were given an NYU IRB-approved flyer that contained information about the study. When a patient expressed interest in learning more about the study and possibly participating, a Research Assistant (RA) who was on site at the NYUCD Periodontics Clinic waiting area, reviewed with the patient the informed consent form for study participation. Individuals who agreed to participate and provided informed consent were first screened for eligibility in accordance with the study inclusion criteria, receiving a $5 gift card for completing this eligibility screening. Eligible patients who participated in the study received an additional $20 gift card. Participants were each given unique study identification codes for all assessment instruments and blood samples; no other information that would disclose the participant’s identity was found on any assessment instruments. A separate secured sheet, accessible only to the PI, Collaborators, and the RA, linked the participant’s name to the identification number. Individuals were assured that their decision of whether or not to participate would not affect services they received at the NYUCD. The IRB at the NYU School of Medicine approved all instruments and procedures. By the end of May, 2011, a total of 134 individuals had been recruited into the study, with 14 who expressed interest in study participation found to be ineligible.
The RA first oversaw the eligible participant’s completion of a 10-minute survey before the dental visit at the NYUCD Periodontics Clinic. The survey gathered socio-demographic and health information, as well as participants’ knowledge and feelings about diabetes and diabetes screening. Blood samples were collected by a dental provider when the patient was seated in a dental chair in the Clinic. For collection of the FSB sample, the patient’s finger tip was cleaned with an alcohol prep pad and the alcohol was allowed to evaporate. The finger tip was then punctured with a sterile lancet. The drop of blood was touched to a blood collection card comprised of a Whatman 903® filter paper medium that was overlayed with a protective inert paper covering to minimize handling and contamination of the filter paper card. A barcode label, unique to each patient, was affixed to the blood collection card. The blood sample was allowed to dry for 15 minutes at ambient temperature, and then transferred to a zip-lock sealed, desiccated foil pouch. The pouch was enclosed with the laboratory’s requisition endorsed by the patient in a pre-addressed, business reply, leak-proof envelope and sent to the laboratory for processing. The participating laboratory in the research has demonstrated 28-day stability of dried blood specimens using this system, which is approved by the U.S. Postal Service for transport of bio-hazardous substances. Patients were informed that they would receive their FSB HbA1c results directly from the laboratory.
Next, an intra-oral exam was performed by a dentist or dental hygienist to determine the extent of periodontal pocket depth and bleeding on probing so that an optimal site for GCB collection could be selected (maxillary anterior whenever possible as this area offered the best access for GCB collection and with minimal contamination with saliva). If there was no indication of bleeding upon probing, only FSB was collected. When it was possible to obtain a GCB sample for HbA1c testing, a dental provider then inserted a Marquis periodontal probe into the gingival sulcus in one of the areas that exhibited erythema and/or edema. The probe was then removed, and the gingival crevice observed for bleeding. After selecting the site, the dental provider isolated it with cotton rolls to limit saliva contamination, scaled the site, dried the area with gauze to eliminate contaminants from the tooth, and then re-probed the site. The tip of a special blood collection card wand, with the wand in the shape of a right trapezoid and affixed with a unique barcode label, was then touched to the bleeding gum, and care was taken to avoid contact with the surface of the tooth or gum. As much blood as wicked onto the tip of the wand was collected and allowed to dry for 15 minutes at ambient temperature. As with the patient’s FSB sample, the wand containing the GCB sample was enclosed in a sealed, desiccated foil pouch, placed in the waterproof envelope created for this purpose, and mailed to the laboratory for HbA1c testing. We note that some of the initial GCB samples that were collected covered less than one third of the tip of the wand and were found by the laboratory to be inadequate for HbA1c testing. Therefore, for subsequent GCB HbA1c tests, we sent the laboratory only those GCB samples that covered at least one third of the tip of the wand. When the amount of GCB was inadequate for HbA1c testing, only the patients’ FSB samples were sent to the laboratory for this testing. We also note that although the barcode labels for the FSB and GCB samples were carefully linked to each patient, the laboratory was blind to this linkage and therefore analyzed the samples as unique blood specimens for HbA1c testing.
The HbA1c tests on the FSB and GCB samples were performed at the laboratory under the direction of a board-certified medical technologist and clinical chemist, and the medical direction of a licensed clinical and anatomical pathologist. At the laboratory, analysis for HbA1c underwent an elution process, which began with punching a 3mm spot using a calibrated hole punch (McGill Inc., Marengo, IL) from a homogeneous region of the dried blood spot on the blood collection card or wand. The punched spot was transferred to a pre-labeled 15 × 100mm borosilicate tube (Fisher Scientific, Pittsburgh, PA). To each tube, 500μL of a proprietary hemolyzing reagent was pipetted. To ensure homogeneity, the eluate tubes were rotated at 1000 rpm for 30 minutes at 25°C. The resulting hemolysate was analyzed for HbA1c using the BioRad Variant II Analyzer (BioRad, Inc, Hercules CA), which employs high-performance liquid chromatography, and exclusively uses BioRad Reagents.39 The BioRad Variant II HbA1c method is certified by the National Glycohemoglobin Standardization Program (NGSP) as being traceable to the HbA1c reference method.40 Analytical runs were quality-controlled with commercially available assayed whole blood control materials from BioRad, and laboratory-prepared dried blood quality control cards prepared on Whatman 903® filter paper using donor blood. Within-run precision and between-run precision of dried blood spot quality control have been established at coefficients of variation of 0.5% and 0.5%, and 1.4% and 1.4% at HbA1c values of 5.3% and 6.4% respectively. Comparison of HbA1c in dried blood spot samples to paired whole blood samples has yielded a correlation coefficient of 0.991 (n = 87), and demonstrated diagnostic accuracy exceeding 98% at a HbA1c diagnostic cutoff of 6.5%. The laboratory also participates in semi-annual proficiency testing challenges to enable blinded assessment of performance compared to a peer group of clinical laboratories, as well as comparison to the NGSP HPLC reference method.
In the current work, descriptive statistics (i.e., means, standard deviations, proportions) were used to report the average FSB HbA1c readings, socio-demographic characteristics, and health-related factors of the participants, especially as they are relevant to diabetes risk. For some GCB samples, an unidentified component was observed to co-elute within the elution window of GCB HbA1c in the laboratory, interfering with the quantification of the HbA1c percentages. Thus, for participants for whom their GCB HbA1c tests did not have such an unidentified component, the Pearson correlation coefficient was used to assess the correlation of FSB and GCB values. A Receiver Operator Characteristic Curve (ROC) analysis was then performed to determine an optimal GCB HbA1c criterion value for a positive diabetes screen. All analyses were conducted using PASW version 18.0.
RESULTS
A total of 120 individuals were eligible and participated in the study and provided a FSB sample for HbA1c testing. Average FSB HbA1c readings were 6.0% (standard deviation = .83%), with 20 (16.7%) in the diabetes range. An additional 66 (55.0%) patients had FSB HbA1c test results in the pre-diabetes range (i.e., between 5.7% and 6.4%), and are at increased risk of developing diabetes in the future.
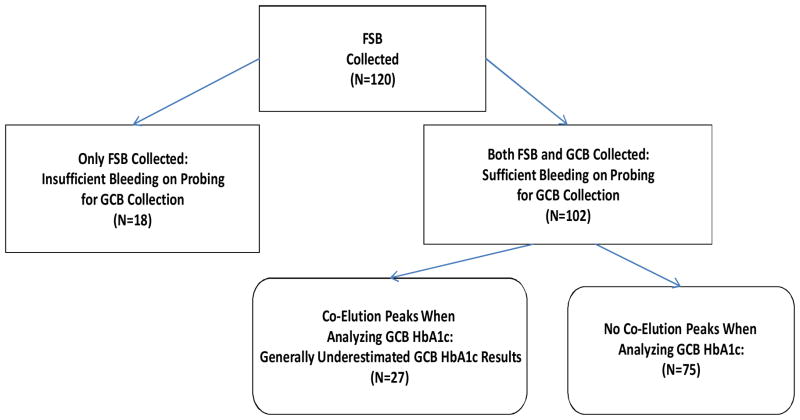
GCB samples were also collected from 102 of the 120 participants; no bleeding on probing or insufficient bleeding to adequately cover the tip of the blood collection wand prevented collection and/or laboratory analysis of GCB samples from the remaining 18 participants. In the case of 27 of the 102 GCB samples, an unidentified component was observed to co-elute within the elution window of HbA1c in the laboratory. The presence of a co-elution peak from this unidentified component interfered with the quantification of HbA1c and generally resulted in underestimation of true HbA1c percentages. Thus, as seen in Figure 1, assessment of the HbA1c percentage without such interference was possible for 75 of the 102 GCB samples.
Figure 1.
Study Participants from Whom Finger Stick Blood (FSB) and Gingival Crevicular Blood (GCB) Were Collected.
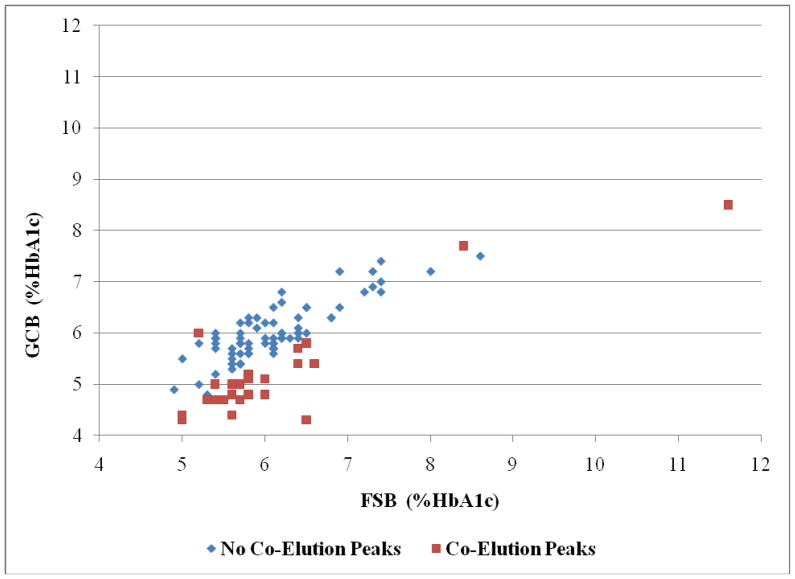
Comparisons between the FSB and GCB HbA1c readings for the 75 participants without co-elution peaks when conducting the GCB HbA1c tests and the 27 participants with co-elution peaks when performing these tests are shown in Figure 2.
Figure 2.
Finger Stick Blood (FSB) Vs. Gingival Crevicular Blood (GCB) %HbA1c (N=102).
Confining our analyses to the 75 participants who had GCB HbA1c samples without co-elution peaks, forty-two (56.0%) of the 75 participants were women, with 44 (58.7%) participants between the ages of 45 and 64, and 16 (21.3%) at least 65 years old. With regard to their ethnicity, 16 (21.3%) were Latino. Participants’ racial distribution included 29 (38.7%) who were Black, African American or Caribbean; 31 (41.3%) who were White; and 5 (6.7%) who were Asian, Native American, American Indian, or Pacific Islander. The remaining participants generally identified their race as Hispanic. Forty-seven (62.7%) of the 75 participants had a body mass index (BMI) ≥ 25 kg/m2, and 35 (46.7%) indicated that they got little exercise during a given day. Seventeen (22.7%) participants had a sibling with diabetes and 27 (36.0%) had a parent with the condition. Two women had a baby of at least 9 pounds at birth. Most (76.0%) indicated that they had regular dental checkups at least annually with a dentist or dental hygienist.
Notably, of the 75 participants, 15 (20.0%) had FSB HbA1c values in the diabetes range (≥ 6.5%), including 8 who indicated that they had never been told by a health care provider that they had diabetes. For these 75 participants, the correlation between FSB and GCB HbA1c values was .842.
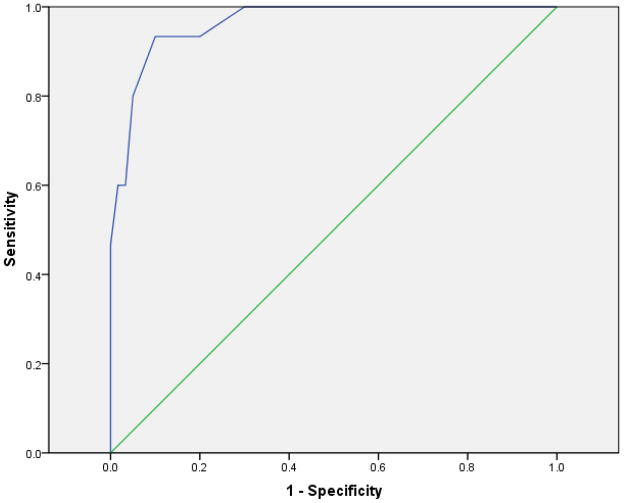
We next performed an ROC analysis to determine the optimal GCB HbA1c value that could serve as a criterion for a positive diabetes screening result corresponding to an FSB HbA1c reading ≥ 6.5% (in the diabetes range). The area under the ROC curve (AUC) was .964, with asymptotic 95% confidence interval (.915, 1.000) (see Figure 3).
Figure 3.
Receiver Operator Charactistic Curve for Gingival Crevicular Blood (GCB) HbA1c Values Corresponding to Finger Stick Blood (FSB) Values ≥ 6.5% (N=75)
Table 1 provides the sensitivities and specificities for different possible criterion GCB HbA1c values that correspond to FSB HbA1c values ≥ 6.5%. As can be seen in Table 1, a criterion value of 6.3% on the GCB HbA1c test provides the optimal combination of sensitivity (.933) and specificity (.900) as it minimizes the value of the sum, (1-sensitivity)2 + (1-specificity)2.
Table 1.
Coordinates of the ROC Curve: GCB HbA1c Cutoff Values for FSB HbA1c Values ≥ 6.5% (N=75)
| Criterion Value for a GCB %HbA1c Cutoff | Sensitivity | 1-Specificity |
|---|---|---|
| 3.80 | 1.000 | 1.000 |
| 4.85 | 1.000 | 0.983 |
| 4.95 | 1.000 | 0.967 |
| 5.10 | 1.000 | 0.950 |
| 5.25 | 1.000 | 0.933 |
| 5.35 | 1.000 | 0.917 |
| 5.45 | 1.000 | 0.833 |
| 5.55 | 1.000 | 0.800 |
| 5.65 | 1.000 | 0.717 |
| 5.75 | 1.000 | 0.600 |
| 5.85 | 1.000 | 0.467 |
| 5.95 | 1.000 | 0.300 |
| 6.05 | 0.933 | 0.200 |
| 6.15 | 0.933 | 0.167 |
| 6.30 | 0.933 | 0.100 |
| 6.40 | 0.800 | 0.050 |
| 6.55 | 0.600 | 0.033 |
| 6.70 | 0.600 | 0.017 |
| 6.85 | 0.467 | 0.000 |
| 6.95 | 0.400 | 0.000 |
| 7.10 | 0.333 | 0.000 |
| 7.30 | 0.133 | 0.000 |
| 7.45 | 0.067 | 0.000 |
| 8.50 | 0.000 | 0.000 |
ROC=Receiver Operating Characteristic; HbA1c=hemoglobin A1c; FSB=finger stick blood; GCB=gingival crevicular blood
With a criterion value of 6.3% on the GCB HbA1c test, of the 15 individuals with a FSB HbA1c value ≥ 6.5%, only 1 individual (whose FSB HbA1c value was 6.5%) would fail to be recognized as having a HbA1c reading in the diabetes range (a false negative; see Table 2). Moreover, with this GCB criterion value, only 6 of the 60 individuals with a FSB HbA1c reading < 6.5% would be falsely classified as having a HbA1c reading in the diabetes range (a false positive; see Table 2). Even for these 6 individuals, FSB HbA1c values fell between 5.8% and 6.4%, all in the pre-diabetes range.
Table 2.
FSB and GCB HbA1c Values: Criterion Cutoff of 6.3% for the GCB HbA1c Values (N=75)
| GCB HbA1c Readings | FSB HbA1c Readings < 6.5% | FSB HbA1c Readings ≥ 6.5% | Total |
|---|---|---|---|
| < 6.3% | 54 | 1 | 55 |
| ≥ 6.3% | 6 | 14 | 20 |
| Total | 60 | 15 | 75 |
HbA1c=hemoglobin A1c; FSB=finger stick blood; GCB=gingival crevicular blood
Notably, for 24 of the 27 GCB samples that had co-elution peaks when analyzing HbA1c, a criterion value of 6.3% on the GCB HbA1c test resulted in correct classification regarding whether a paired FSB HbA1c value was inside, or outside of the diabetes range (see Figure 1). In particular, 22 of the individuals would have been correctly classified as having HbA1c values below the diabetes range (i.e., FSB HbA1c < 6.5%), and 2 would have been correctly classified as having HbA1c values in the diabetes range (i.e., FSB HbA1c ≥ 6.5%). In the 3 discordant cases, the FSB HbA1c values were 6.5% (N=2) or 6.6% (N=1), and the paired GCB HbA1c values were less than 6.3%.
DISCUSSION
In view of the growing number of people with unrecognized diabetes and the increased risk for diabetes among periodontal patients, diabetes screening at the time of the dental visit is a promising public health opportunity. In fact, although not a random sample, it is noteworthy that 16.7% of the 120 patients who participated in our study had FSB HbA1c values in the diabetes range, and 55% of the values were in the pre-diabetes range. Although the laboratory’s approach to diabetes screening using FSB samples may be acceptable to some dental providers and some patients, others may prefer that such screening be performed with a GCB sample. Notably, results from our pilot study suggest that criterion-based testing for periodontal patients’ HbA1c values with dried blood GCB samples using the laboratory’s approach is feasible at the time of the dental visit. Although in need of subsequent research to understand how to avoid or limit co-elution peaks at the time of GCB HbA1c testing, (1) the high correlation (.842) between FSB and GCB HbA1c values among the samples with GCB HbA1c tests not having co-elution peaks, and (2) the high specificity and sensitivity (.933 and .900, respectively) among these samples for a criterion value of 6.3% on the GCB HbA1c tests generates considerable promise regarding the acceptability of our unique approach to diabetes screening at the dental visit. Furthermore, because 76% of these patients visited a dental provider regularly, there is great potential for reaching at-risk periodontal patients for diabetes screening, many of whom exhibit diabetes risk factors such as having a first degree relative with diabetes or having a high BMI.
Together with the advantage of collecting oral blood that is present when probing the pocket depths of periodontal patients, our GCB HbA1c approach to diabetes screening retains many of the advantages of the laboratory’s finger stick HbA1c testing approach. As advocated by the American Diabetes Association, the laboratory’s HbA1c analysis with both FSB and GCB sample testing employs High Pressure Liquid Chromatography (HPLC) technology, widely regarded as the reference method of the National Glycohemoglobin Standardization Program (NGSP). Portable devices used for HbA1c testing at dental visits and elsewhere generally use an immunoassay, which is prone to inaccuracy from abnormal hemoglobin variants, including the common sickling hemoglobins S and C. In addition, portable devices for HbA1c testing are very costly and complex, limiting their practicality for use in a dental venue. They require collecting blood into a capillary tube and inserting it into a vial that is mixed, timed, and transferred to an instrument for testing. There is no indication whether the blood collected was of adequate quantity, or whether the sample mixing steps were performed correctly. In contrast, our method requires only a single drop of blood to be collected by the dental provider, applied to a card, and mailed to the laboratory for HbA1c testing. This quality control testing is performed at a certified clinical laboratory and run by a medical technologist or laboratory technician who analyzes the HbA1c. In addition, use of the portable HbA1c testing devices requires that a Clinical Laboratory Improvement Amendments (CLIA) certificate of waiver be obtained by the dental practice if the testing is to be conducted in the dental office, whereas the blood collection at the point-of-care using our method is CLIA-exempt, with analysis of HbA1c levels conducted by a CLIA-accredited laboratory. Notably, our approach also does not require the dentist to make a diabetes diagnosis, a task with which the dentist may be especially uncomfortable as it is outside of her/his scope of practice.
We acknowledge some limitations and issues in need of future research in using our approach for diabetes screening. First, as was the case with 18 of our 120 participants, not all patients will have adequate oral bleeding to obtain a GCB sample for HbA1c testing. For such patients, the laboratory’s FSB HbA1c testing approach can be used. Another limitation involves the presence of co-elution peaks in the analysis of some GCB samples. In spite of careful debridement of the collection site to remove as much of the contaminants (i.e., saliva, oral effluents) as feasible, some of these oral samples may have been contaminated with high levels of bacteria, or retained elements from a mouth rinse that may have been used prior to the GCB collection. Although this limitation affected 27 samples, only 3 of the 27 samples did not agree with the FSB samples regarding whether or not HbA1c values were in the diabetes range. Nonetheless, further research is needed to understand the reason for these co-elution peaks, and to identify the optimal way to preserve specimen integrity through the use of stabilizing agents that can be incorporated into the specimen transport media. In addition, although the GCB samples were collected from maxillary anterior sites whenever possible, some samples were collected from posterior and mandibular sites when bleeding in the maxillary anterior was limited. To obtain the most contaminant-free GCB samples at all GCB collection sites, site isolation with cotton rolls was followed by scaling, drying with gauze, and re-probing. Future studies will explore whether there is a need to standardize GCB specimen collection sites to best minimize sample contamination and optimize performance. An additional limitation to the research concerns the involvement of patients from only one dental venue: a Periodontics Clinic in an urban Dental College. The feasibility and acceptability of this approach needs to be further examined in community-based and other dental practices. Finally, although dentists appear to be willing to incorporate screening for medical conditions into their practices,35 the extent to which community-based dental providers would be amenable to incorporating our suggested approach to diabetes screening remains to be determined. In spite of these limitations, our pilot study provides data in strong support of further exploration to capitalize on a promising approach to screen periodontal patients for diabetes.
Acknowledgments
The research received financial support from the National Center for Research Resources, National Institutes of Health. Drs. Strauss, Rindskopf, Schoor, Einhorn, Hochstein, Russell, and Rosedale, and Ms. Tuthill, Singh, and Brodsky report no financial relationships related to any products involved in this study. Dr. Maggiore is part owner of Healthy Life Laboratories, which provided clinical analyses for the study.
We dedicate this work to the memory of Alla Wheeler, Clinical Associate Professor of Dental Hygiene at NYU, who played a major role in earlier studies on the link between diabetes and periodontal disease. We also appreciate the support provided by NYU Dental Hygiene students Inna Drayluk, Ariana Bytyci, Krystal Savice, and Alisa Llambiri, an introduction to the finger stick diabetes screening approach for dentists by Dr. Ron Schefdore, and the patients who participated in the research.
We gratefully acknowledge support for this research from grant 1UL1RR029893 from the National Center for Research Resources, National Institutes of Health. Drs. Strauss, Rindskopf, Schoor, Einhorn, Hochstein, Russell, and Rosedale, and Ms. Tuthill, Singh, and Brodsky report no financial relationships related to any products involved in this study. Dr. Maggiore is part owner of Healthy Life Laboratories, which provided clinical analyses for the study.
Footnotes
Healthy Life Laboratories, Bannockburn, IL
References
- 1.Centers for Disease Control and Prevention. [Accessed June 24, 2011];National diabetes fact sheet. 2011 Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf.
- 2.Diabetes Prevention Program Research Group. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med. 2007;24:137–144. doi: 10.1111/j.1464-5491.2007.02043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60:108–111. doi: 10.1212/wnl.60.1.108. [DOI] [PubMed] [Google Scholar]
- 4.Herman WH. Diabetes epidemiology: guiding clinical and public health practice: the Kelly West Award lecture, 2006. Diabetes Care. 2007;30:1912–1919. doi: 10.2337/dc07-9924. [DOI] [PubMed] [Google Scholar]
- 5.Barrett-Connor E, Ferrara A. Isolated postchallenge hyperglycemia and the risk of fatal cardiovascular disease in older women and men. The Rancho Bernardo study. Diabetes Care. 1998;21:1236–1239. doi: 10.2337/diacare.21.8.1236. [DOI] [PubMed] [Google Scholar]
- 6.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 7.Adler AI, Neil HA, Manley SE, Holman RR, Turner RC. Hyperglycemia and hyperinsulinemia at diagnosis of diabetes and their association with subsequent cardiovascular disease in the United Kingdom Prospective Diabetes Study (UKPDS 47) Am Heart J. 1999;138(5 Pt 1):S353–S359. doi: 10.1016/s0002-8703(99)70035-9. [DOI] [PubMed] [Google Scholar]
- 8.The DECODE Study Group. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. European Diabetes Epidemiology Group. Diabetes epidemiology: collaborative analysis of diagnostic criteria in Europe. Lancet. 1999;354:617–621. [PubMed] [Google Scholar]
- 9.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barr ELM, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose and impaired glucose tolerance. The Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Circulation. 2007;116:1–7. doi: 10.1161/CIRCULATIONAHA.106.685628. [DOI] [PubMed] [Google Scholar]
- 11.Borch-Johnsen K, Lauritzen T, Glümer C, Sandbaek A. Screening for Type 2 diabetes--should it be now? Diabet Med. 2003;20:175–181. doi: 10.1046/j.1464-5491.2003.00842.x. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care. 2007;30(Suppl 1):S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 13.Alberti KGMM, Zimmett PZ for a WHO consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organisation; 1999. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. [Google Scholar]
- 14.Diabetes UK. Position Statement. [Accessed June 24, 2011];Early identification of people with Type 2 diabetes. 2008 Available at: www.diabetes.org.uk.
- 15.Diabetes Australia. [Accessed June 24, 2011];National evidence based guideline for case detection and diagnosis of Type 2 diabetes, Part 3, Case detection and diagnosis. 2001 Available at: http://www.nhmrc.gov.au/_files_nhmrc/file/publications/synopses/di9.pdf.
- 16.Wareham NJ, Griffin SJ. Should we screen for Type 2 diabetes? Evaluation against National Screening Committee criteria. BMJ. 2001;322:986–988. doi: 10.1136/bmj.322.7292.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hein C, Cobb C, Iacopino A. [Accessed June 24, 2011];Report of the Independent Panel of Experts of the Scottsdale Project. 2007 Available at: http://downloads.pennnet.com/pnet/gr/scottsdaleproject.pdf.
- 18.Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol. 1998;3:51–61. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- 19.Mealey BL, Rethman MP. Periodontal disease and diabetes mellitus. Bidirectional relationship. Dentistry Today. 2003;22:107–113. [PubMed] [Google Scholar]
- 20.Mealey BL. Periodontal disease and diabetes. A two way street. J Am Dent Assoc. 2006;137(suppl 2):26S–31S. doi: 10.14219/jada.archive.2006.0404. [DOI] [PubMed] [Google Scholar]
- 21.Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol. 2001;6:99–112. doi: 10.1902/annals.2001.6.1.99. [DOI] [PubMed] [Google Scholar]
- 22.Southerland JH, Taylor GW, Offenbacher S. Diabetes and periodontal infection: making the connection. Clin Diabetes. 2005;23:171–178. [Google Scholar]
- 23.Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988–1994. J Periodontol. 1999;70(1):13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- 24.Strauss SM, Russell S, Wheeler AJ, Norman R, Borrell LN, Rindskopf D. The periodontal office visit as a potential opportunity for diabetes screening: an analysis using NHANES 2003–2004 data. J Public Health Dent. 2010;70:156–162. doi: 10.1111/j.1752-7325.2009.00157.x. [DOI] [PubMed] [Google Scholar]
- 25.Vernillo AT. Dental considerations for the treatment of patients with diabetes mellitus. J Am Dent Assoc. 2003;134 (Suppl 1):24S–33S. doi: 10.14219/jada.archive.2003.0366. [DOI] [PubMed] [Google Scholar]
- 26.Lalla E, Kunzel C, Burkett S, Cheng B, Lamster IB. Identification of unrecognized diabetes and pre-diabetes in a dental setting. J Dent Res. 2011;90:841–854. doi: 10.1177/0022034511407069. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Williams PL, Douglass CW. Development of a clinical guideline to predict undiagnosed diabetes in dental patients. J Am Dent Assoc. 2011;142:28–37. doi: 10.14219/jada.archive.2011.0025. [DOI] [PubMed] [Google Scholar]
- 28.Borrell LN, Kunzel C, Lamster I, Lalla E. Diabetes in the dental office: using NHANES III to estimate the probability of undiagnosed disease. J Perio Res. 2007;42:559–565. doi: 10.1111/j.1600-0765.2007.00983.x. [DOI] [PubMed] [Google Scholar]
- 29.Strauss SM, Wheeler AJ, Russell SR, et al. The potential use of gingival crevicular blood for measuring glucose to screen for diabetes: an examination based on characteristics of the blood collection site. J Periodontol. 2009;80:907–914. doi: 10.1902/jop.2009.080542. [DOI] [PubMed] [Google Scholar]
- 30.Parker RC, Rapley JW, Isley W, Spencer P, Killoy WJ. Gingival crevicular blood for assessment of blood glucose in diabetic patients. J Periodontol. 1993;64:666–672. doi: 10.1902/jop.1993.64.7.666. [DOI] [PubMed] [Google Scholar]
- 31.Beikler T, Kuczek A, Petersilka G, Flemmig TF. In-dental-office screening for diabetes mellitus using gingival crevicular blood. J Clin Periodontol. 2002;29:216–218. doi: 10.1034/j.1600-051x.2002.290306.x. [DOI] [PubMed] [Google Scholar]
- 32.Khader YS, Al-Zu’bi BN, Judeh A, Rayyan M. Screening for type 2 diabetes mellitus using gingival crevicular blood. Int J Dent Hyg. 2006;4:179–182. doi: 10.1111/j.1601-5037.2006.00206.x. [DOI] [PubMed] [Google Scholar]
- 33.Müller HP, Behbehani E. Screening of elevated glucose levels in gingival crevice blood using a novel, sensitive self-monitoring device. Med Princ Pract. 2004;13:361–365. doi: 10.1159/000080474. [DOI] [PubMed] [Google Scholar]
- 34.Horowitz AM, Siriphant P, Sheikh A, Child W. Perspectives of Maryland dentists on oral cancer. J Am Dent Assoc. 2001;132:65–72. doi: 10.14219/jada.archive.2001.0027. [DOI] [PubMed] [Google Scholar]
- 35.Greenberg BL, Glick M, Frantsve-Hawley J, Kantor ML. Dentists’ attitudes toward chairside screening for medical conditions. J Am Dent Assoc. 2010;141:52–62. doi: 10.14219/jada.archive.2010.0021. [DOI] [PubMed] [Google Scholar]
- 36.American Diabetes Association. Standards of medical care in diabetes 2010. Diabetes Care. 2010;33:S4–S10. doi: 10.2337/dc12-s004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. [Accessed June 24, 2011];Mobile Exam Center Components Descriptions. Available at: http://www.cdc.gov/nchs/data/nhanes/meccomp.pdf.
- 38.American Diabetes Association. [Accessed June 24, 2011];ADA Diabetes Risk Test. Available at http://www.diabetes.org/diabetes-basics/prevention/diabetes-risk-test/
- 39.Variant II Hemoglobin A1c Instruction Manual: Bio-Rad Laboratories, effective May 2007.
- 40.National Glycohemoglobin Standardization Program (NGSP) [Accessed June 24, 2011]; Available at: http://www.ngsp.org/certified.asp.