Summary
Experimental animal models have demonstrated that one of the primary consequences of prenatal stress is increased fear and anxiety in the offspring. Few prospective human studies have evaluated the consequences of prenatal stress on anxiety during preadolescence. The purpose of this investigation is to determine the consequences of prenatal exposure to both maternal biological stress signals and psychological distress on anxiety in preadolescent children. Participants included 178 mother-child pairs. Maternal psychological distress (general anxiety, perceived stress, depression and pregnancy-specific anxiety) and biological stress signals were evaluated at 19, 25, and 31 gestational weeks. Anxiety was evaluated in the children at 6 to 9 years of age using the Child Behavior Checklist. Analyses revealed that prenatal exposure to elevated maternal cortisol, depression, perceived stress and pregnancy-specific anxiety was associated with increased anxiety in children. These associations remained after considering obstetric, sociodemographic and postnatal maternal psychological distress; factors that could influence child development. When all of the prenatal measures were considered together, cortisol and pregnancy-specific anxiety independently predicted child anxiety. Children exposed to elevated prenatal maternal cortisol and pregnancy-specific anxiety were at an increased risk for developing anxiety problems during the preadolescent period. This project identifies prenatal risk factors associated with lasting consequences for child mental health and raises the possibility that reducing maternal distress during the prenatal period will have long term benefits for child well-being.
Keywords: anxiety, development, fetal programming, prenatal, cortisol, stress, pregnancy
One out of 17 Americans will suffer from a mental illness during their lifetime (Kessler et al., 2005). The origins of mental illness often begin early in life (De Bellis et al., 1999; Nemeroff, 2004; Gunnar & Quevedo, 2008; Green et al., 2010), yet surprisingly little is known about how early life experiences determine risk for mental illness. The prenatal period represents a time of extremely rapid change in brain development including neurogenesis, cell neuronal differentiation, dendritic arborization, axonal elongation, synapse formation, collateralization, pruning and myelination (Huttenlocher, 1994; Bourgeois, 1997; Huttenlocher & Dabholkar, 1997; Levitt, 2003). The rapid neurological advances during this period render the fetus susceptible to beneficial and detrimental influences with life-long implications for mental health. Maternal stress signals are a dominant early life influence contributing to the organization of the nervous system. The purpose of the present study was to investigate the programming influence of prenatal exposure to maternal psychological distress and biological indicators of stress on the development of anxiety during preadolescence.
Prenatal Maternal Glucocorticoids and Child Behavioral and Emotional Regulation
For a number of reasons, glucocorticoids have been proposed as a primary candidate for fetal programming of later health outcomes. Glucocorticoids are steroid hormones that are the end product of the hypothalamic-pituitary adrenocortical (HPA) axis and exert influences on nearly every organ and tissue in the body (Drake et al., 2007). Maternal cortisol increases two to four-fold over the course of normal gestation (Sandman et al., 2006). Fetal exposure to the increasing concentrations of maternal cortisol is regulated by a placental enzyme, 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which oxidizes cortisol to its inactive form cortisone (Brown et al., 1996; Murphy & Clifton, 2003). A portion of active maternal cortisol passes through the placenta resulting in significant correlations between fetal and maternal cortisol levels (Gitau et al., 1998; Gitau et al., 2001). Exposure to maternal cortisol can influence the fetal nervous system because cortisol easily passes through the immature blood-brain barrier and targets glucocorticoid receptors that are present throughout the central nervous system, influencing both emotional and cognitive functioning (Jacobson & Sapolsky, 1991; Sanchez et al., 2000). Glucocorticoids play a critical role in normal brain development, providing further evidence for glucocorticoids as a mechanism for programming the fetus (Trejo et al., 2000; Seckl, 2008).
Animal and human evidence indicate that one of the primary consequences of exposure to excess glucocorticoids may be increased fear and anxiety in the offspring. Results from studies with rodents and non-human primates indicate that fetal exposure to elevated glucocorticoids is associated with increased fearful behaviors and greater stress reactivity (Maccari & Morley-Fletcher, 2007; Kapoor et al., 2008; Seckl, 2008). Consistent with animal work, human fetal exposure to elevated maternal cortisol is associated with greater physiological and behavioral reactivity to stress during infancy and childhood (Gutteling et al., 2005a; Davis et al., 2011a) as well as fussiness, negative behavior and fearfulness during infancy (de Weerth et al., 2003; Davis et al., 2007). Further evidence for the role of glucocorticoids in the development of anxiety disorders comes from studies of prenatal exposure to synthetic glucocorticoid. Children who were exposed to synthetic glucocorticoid treatment during the prenatal period display both impaired HPA axis regulation (Davis et al., 2004; Davis et al., 2011b) and higher levels of behavioral inhibition and social anxiety (Trautman et al., 1995; Lajic et al., 2011). These findings raise the possibility that prenatal exposure to elevated glucocorticoids may increase the risk for developing anxiety problems among children.
Prenatal Maternal Psychological Distress and Child Behavioral and Emotional Regulation
Accumulating evidence has shown that human fetal exposure to maternal psychological distress during pregnancy additionally is associated with increases in fearful and anxious behavior later in life. Prenatal exposure to maternal anxiety, stress and depression has been associated with impaired stress regulation (Van den Bergh et al., 2008; Grant et al., 2009; Davis et al., 2011a), behavioral/emotional problems (Huizink et al., 2002; O’Connor et al., 2002; O’Connor et al., 2003; Van den Bergh & Marcoen, 2004; Gutteling et al., 2005b) and fearful temperament (Davis et al., 2004; Austin et al., 2005; Davis et al., 2007; Bergman et al., 2008; Blair et al., 2011), even after controlling for postnatal maternal psychological distress. Although few prospective studies have evaluated the consequences of gestational stress on child affective problems beyond infancy and early childhood, there is evidence that these effects persist. Prenatal maternal anxiety is associated with behavioral problems during childhood (O’Connor et al., 2003) as well as risk for depressive mood during adolescence (Van den Bergh et al., 2008) after considering postnatal maternal psychological distress. Consistent with these findings, Finnish children whose mothers experienced the stress of the Chernobyl disaster during pregnancy, but did not experience significant exposure to radiation, were at an increased risk for depression during adolescence (Huizink et al., 2007). Not all studies have found that the effects of the prenatal environment persist after considering postnatal influences. In a sample of children conceived by in vitro fertilization who were genetically unrelated to their mothers, Rice and colleagues (2010) found evidence for a non-genetic pathway to the development of anxiety. However, in this study the relation between prenatal stress (analyzed only in late gestation) and child anxiety did not persist after statistically covarying current maternal distress. These studies document the importance of jointly considering both prenatal and postnatal maternal psychological distress in order to understand the development of child anxiety.
Most studies of fetal exposures have focused on general measures of prenatal maternal psychological distress such as perceived stress, state anxiety, and depression. However, more recent studies suggest that pregnancy-specific anxiety may be superior to general measures of distress for predicting developmental outcomes because it more accurately characterizes psychological states specific to a woman during pregnancy (Huizink et al., 2003; DiPietro et al., 2006; Buss et al., 2010; Davis & Sandman, 2010; Sandman & Davis, 2010; Blair et al., 2011; Buss et al., 2011; Tollenaar et al., 2011). For this reason, we have included both generalized distress measures as well as evaluation of pregnancy-specific anxiety.
The present study is unique in the evaluation of exposure to both prenatal maternal cortisol and several psychological indices of maternal distress at multiple gestational periods, in association with child anxiety. The following hypotheses will be tested 1) prenatal exposure to both psychological and biological indicators of maternal stress will be associated with child anxiety and 2) concern or worry that is specific to pregnancy will be the best predictor of child outcomes as compared to generalized distress measures.
Methods
Study Overview
Study participants included mother-child pairs from a prospective longitudinal study of prenatal stress and development. Women with singleton pregnancies less than 16 weeks gestational age were recruited from obstetric clinics in Southern California and assessed throughout gestation and when the child was 6 to 9 years of age.
Participants
The sample included 178 mothers and their 6 to 9 year old children (mean ± SD: 7.3 ± 0.8; 55% female). Initial prenatal recruitment criteria were as follows: Study participants were English-speaking adult women (>18 years age) with singleton, intrauterine pregnancies. Exclusion criteria were tobacco, alcohol, or other drug use in pregnancy; uterine or cervical abnormalities; or presence of any condition potentially associated with dysregulated neuroendocrine function such as endocrine, hepatic or renal disorders or corticosteroid medication use. All children participating in the current investigation were born after 34 gestational weeks (M gestational age = 38.1 weeks, SD = 1.6 weeks) and had a stable neonatal course (all Apgar scores > 8). Descriptive information for this sample is provided in Table 1. Women participating in this study were primarily non-Hispanic white or Latina, married or cohabitating at the time of child assessment and had at least some college education. All methods and procedures were approved by the Institutional Review Board of the participating institutions and all mothers provided written informed consent and all children provided informed assent.
Table 1.
Description of the Study Sample at the Time of Child Assessment
| Study Sample (N = 178) | |
|---|---|
| Maternal Age (yrs) | 38.1a |
| Married or Cohabitating (%) | 82 |
| Maternal Education (%) | |
| High school or equivalent | 30 |
| Associates degree | 12 |
| Bachelors degree | 29 |
| Graduate degree | 23 |
| Annual Household Income (%) | |
| $0 – $30,000 | 5 |
| $30,001 – $60,000 | 17 |
| $60,001 – $100,000 | 30 |
| Over $100,000 | 48 |
| Maternal Race/Ethnicity %) | |
| Non-Hispanic White | 49 |
| Latina | 18 |
| Asian | 13 |
| African American | 10 |
SD = 5.8, range = 25 – 51
Procedures
Overview of Data Collection
Maternal salivary samples were collected for cortisol analysis and maternal psychological state (state anxiety, perceived stress and depression) was assessed at three intervals during pregnancy (Time 1: 19.3 ± 0.8, Time 2: 25.0 ± 0.8, Time 3: 31.0 ± 0.8 weeks of gestation). The sampling intervals were selected based upon evidence that biological markers of stress (e.g., cortisol) increase over the course of human pregnancy (Sandman et al., 2006). Prenatal medical history and risk for adverse obstetric outcomes were obtained from extensive structured medical interviews at each visit in combination with a thorough review of prenatal and hospital medical records. When the child was 6 and 9 years of age, child anxiety and maternal psychological distress were evaluated.
Maternal Salivary Cortisol
Salivary samples were collected in the early afternoon, at least one hour after the participant had eaten, (mean time of day ranged from 1417h to 1446h across the three assessment intervals) using a Salivette sampling device (Sarstedt, Numbrecht, Germany). Within this restricted range of sample collection time, time of day was modestly associated with cortisol levels (r’s ranged from −0.14 to −0.25, p’s ranged from 0.08 to 0.01). Thus time of day was regressed against maternal cortisol and residuals retained for subsequent analysis.
Salivary samples were spun and stored at −70 degrees C until assayed. Thawed samples were centrifuged at 3000 rpm for 15 minutes before assay. Salivary cortisol levels were determined by a competitive luminescence immunoassay (LIA; IBL-America, Minneapolis, MN) with reported detection limits of 0.015 μg/dl. The cross reactivity of the assay was <2.5% with cortisone, prednisone and corticosterone and <0.1% with other naturally occurring steroids. The intra- and inter-assay coefficients of variance were 5.5% and 7.6%, respectively. Data reduction for the LIA assay was done by an automated four-parameter logistics computer program (software Mikro Win 2000; Berthold Microplate Luminometer). Samples were assayed in duplicate and averaged. A log transformation was implemented to reduce skewness. Values reported in the text, tables and graphs are raw μg/dL levels to facilitate interpretation. Cortisol assessments were not available for six of the women participating in the present investigation.
Maternal Psychological Assessments
Maternal psychosocial stress was evaluated using four standardized measures. Generalized or non-specific stress was evaluated using the 12-item version of Cohen’s Perceived Stress Scale (PSS) (Cohen et al., 1983). This measure evaluates participants’ feelings about how they were able to handle day-to-day problems and hassles, how often they felt nervous and stressed and how often they felt things were going well during the past week. Responses were made on a 5-point Likert scale ranging from 0 (never) to 4 (almost always) and the final score ranged from 0 to 48. The short form of the Center for Epidemiological Studies Depression Inventory was used to evaluate maternal depression (Santor & Coyne, 1997). Responses to each of the 9 items in this measure were recorded on a four-point Likert scale with a range of 0 to 3. Anchor points, in terms of days per week during the last week, were “rarely or none of the time (less than 1 day)” to “most or all of the time (5–7 days)”. The final score ranged from 0 to 27, with a higher score indicating greater impairment. The CES-D has been validated in pregnant samples (Marcus et al., 2003) and is commonly used in studies evaluating prenatal depression (e.g., Kramer et al., 2009; Yim et al., 2009; Goedhart et al., 2010). General anxiety was measured using the 10-item State Anxiety subscale of the State-Trait Personality Inventory (Speilberger, 1983). This measure assesses the degree to which participants had experienced anxiety-related symptoms or emotions in the last few days. Responses were made using a 4-point Likert scale ranging from 1 (not at all) to 4 (very much) and scores ranged from 10 to 40. Pregnancy-specific anxiety was measured with the 10 item Pregnancy Related Anxiety scale (Rini et al., 1999). This instrument assesses a woman’s feelings about her health during pregnancy, the health of her baby and her feelings about labor and delivery and has been used in a number of studies evaluating pregnancy-specific anxiety (e.g., Glynn et al., 2008). Answers were given on a 4-point Likert scale ranging from 1 (not at all) to 4 (very much) and included items such as: I am fearful regarding the health of my baby, I am concerned or worried about losing my baby, and I am concerned or worried about developing medical problems during my pregnancy. The final score on this measure ranged from 10 to 40.
Prenatal Course and Birth Outcome
An extensive structured medical interview was conducted by a research nurse at each prenatal visit to assess maternal health and pregnancy related complications. Maternal and infant medical records were reviewed to assess pregnancy complications and birth outcome. A score assessing prenatal obstetric risk was derived (Hobel, 1982).
Child Anxiety
Child anxiety was measured using the Achenbach System of Empirically Based Assessment (ASEBA) which offers a comprehensive approach to assessing adaptive and maladaptive functioning (Achenbach & Rescorla, 2001). The ASEBA is a reliable and valid measure that is widely used in research and clinical practice with children. The parent report form, the Child Behavior Checklist (CBCL) was administered to mothers by a trained interviewer who was directly supervised by a clinical psychologist. The CBCL contains 113 items representing a broad scope of behaviors. It has high test retest stability and good internal consistency. In the present study the Anxiety Problems subscale of the CBCL was used. This standard scale is consistent with the Diagnostic and Statistical Manual of Mental Disorders-IV evaluation of Generalized Anxiety disorder, Separation Anxiety Disorder and Specific Phobia (Achenbach & Rescorla, 2001). The anxiety problems scale consists of six statements. Responses were made on a 3 point Likert scale ranging from 0 (not true) to 2 (very true). Thus, scores on this measure could range from 0 to 12.
Data Reduction and Analysis
Preliminary analyses were carried out using correlations and t-tests to identify sociodemographic (maternal race/ethnicity, maternal age, cohabitating with child’s father, and years of maternal education), pregnancy-related (obstetric medical risk, gestational age at birth), and child (sex, current age, birth order) variables that might affect child anxiety. Only gestational age at birth was significantly correlated with child anxiety [r (178) = −0.20, p<0.05] and thus was included as a covariate in all subsequent analyses. Maternal perceived stress [r (178) = 0.23, p<0.05], general anxiety [r (178) = 0.34, p<0.05] and depression [r (178) = 0.28, p<0.05] at the time of child assessment were significantly associated with report of child anxiety. To reduce the effects of maternal reporting bias maternal current psychological state was included as a covariate in all analyses. In addition to considering maternal psychological state, all analyses adjusted for maternal education as a proxy for socioeconomic status and child sex in order to statistically account for these factors known to be associated with child anxiety (Essex et al., 2010; Lewis et al., 2011).
Primary analyses evaluated the association between cumulative exposure to maternal prenatal cortisol, perceived stress, depression, general anxiety and pregnancy-specific anxiety over the course of gestation and child anxiety. Hierarchical linear regression analyses were performed to assess the association between average prenatal maternal cortisol (adjusted for time of day), perceived stress, depression, general anxiety and pregnancy-specific anxiety and child anxiety. Child sex, gestational age at birth, maternal education, and maternal psychosocial stress at the time of child assessment were entered in the first step. Prenatal predictors were entered separately in step 2 resulting in four models (prenatal cortisol, perceived stress, depression, general anxiety and pregnancy-specific anxiety). When significant relations were observed between prenatal predictors and child outcomes two sets of subsequent analyses were performed. First, post hoc tests were implemented to determine effects of timing of exposure. Second, logistic regressions were implemented to determine if prenatal measures predicted “clinically significant” levels of anxiety. For these analyses, child anxiety status was dummy coded (none vs. borderline or clinically significant using standard CBCL cut off scores) and entered as the dependent variable, covariates were entered in the first step and prenatal predictors were entered separately in step 2. A final model was implemented to determine which of the four gestational measures best predicted child anxiety and whether these measures had independent associations with child anxiety. In this model prenatal cortisol, perceived stress, depression, general anxiety and pregnancy-specific anxiety were entered step-wise in level 2. Diagnostic tests described by Cohen and colleagues (2003) indicated that multicollinearity was not a problem for this model.
Results
Maternal Cortisol
Maternal salivary cortisol levels increased from 0.24 μg/dl at 19 gestational weeks to 0.29 μg/dl at 25 weeks and to 0.34 μg/dl at 31 gestational weeks (p<0.01). Intercorrelations among cortisol measures ranged from 0.17 to 0.29, p’s < 0.05.
Maternal Psychological State
Means and standard deviations for perceived stress, depression, general anxiety and pregnancy-specific anxiety are shown in Table 2. Perceived stress and depression did not significantly change over the course of pregnancy (p’s > 0.3). Consistent with prior work, a non significant trend for state anxiety to decrease (p=0.058) and a significant decrease in pregnancy-specific anxiety (p<0.01) were observed as pregnancy advanced (see Sandman et al., 2011 for review).
Table 2.
Psychological and endocrine stress measures during gestation
| Mean (SD) | |
|---|---|
| Average Depression | 16.0 (4.5) |
| Depression at 20 wks’ GA | 15.8 (5.4) |
| Depression at 25 wks’ GA | 15.8 (4.8) |
| Depression at 30 wks’ GA | 16.4 (5.4) |
| Average Perceived Stress | 26.9 (6.8) |
| Perceived Stress at 20 wks’ GA | 27.2 (7.7) |
| Perceived Stress at 30 wks’ GA | 26.5 (7.7) |
| Average General Anxiety | 20.5 (5.5) |
| General Anxiety at 20 wks’ GA | 21.0 (6.6) |
| General Anxiety at 25 wks’ GA | 20.4 (6.2) |
| General Anxiety at 30 wks’ GA | 19.8 (6.6) |
| Average Pregnancy-Specific Anxiety | 18.7 (4.6) |
| Pregnancy-Specific Anxiety at 20 wks’ GA | 19.9 (5.8) |
| Pregnancy-Specific Anxiety at 25 wks’ GA | 18.3 (4.8) |
| Pregnancy-Specific Anxiety at 30 wks’ GA | 17.9 (4.7) |
| Average Cortisol (μg/dl) | 0.29 (0.14) |
| 20 wks’ GA | 0.24 (0.23) |
| 25 wks’ GA | 0.29 (0.19) |
| 30 wks’ GA | 0.34 (0.23) |
The correlation of each of the four maternal psychological distress measures over the three prenatal assessment time points ranged from 0.51 to 0.81. As expected, within each gestational interval, measures of state anxiety, perceived stress and depression were significantly intercorrelated (r’s ranged from 0.64 to 0.81, p’s < 0.01). Associations between the general distress measures and pregnancy-specific anxiety were significant, but lower (r’s ranged from 0.31 to 0.47, p’s <0.01). Prenatal psychological distress measures were additionally correlated with psychological distress at the time of child assessment (r’s ranged from 0.35 to 0.53, p’s <0.01).
Prenatal Maternal Cortisol and Maternal Psychological state
Maternal cortisol was not significantly associated with concurrent measures of maternal perceived stress, depression, general anxiety, or pregnancy-specific anxiety at any of the three prenatal assessment time points (all p’s > 0.24).
Child Anxiety
Child anxiety scores ranged from 0 to 12 (M =1.5 SD = 2.2). The vast majority of children (n=150) scored within the normal range for anxiety using standard cut off scores as described in the ASEBA manual. Of the remaining 28 children, 17 were in the borderline range and 11 were in the clinical range for anxiety. Child anxiety was analyzed two ways: 1) using continuous data and 2) dummy coded for comparison of children scoring in the normal range versus children in the borderline or clinical range.
Does Prenatal Maternal Cortisol Predict Child Anxiety?
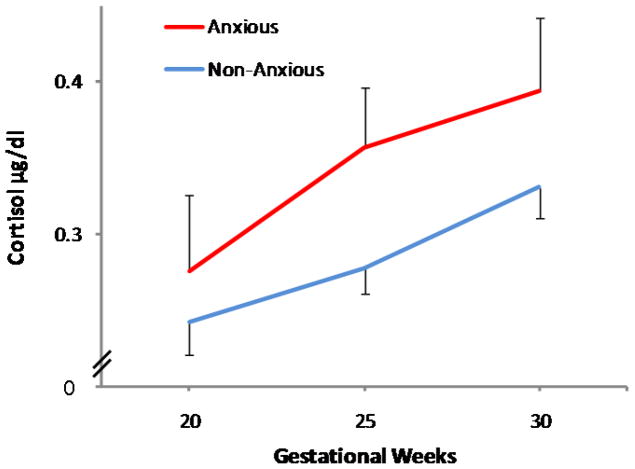
Elevated average maternal gestational cortisol was associated with higher child anxiety at 6 to 9 years of age after considering relevant covariates including current maternal psychological distress (beta=0.16, p<0.05; F(4,167)= 6.7, p<0.05). No significant gestational timing effects of cortisol exposure were observed. Next, the relation between prenatal cortisol and child anxiety status (normal range vs. borderline/clinically significant) was considered. Logistic regression confirmed group differences in prenatal cortisol exposure based on the clinical status of child anxiety. Children with anxiety ratings within the borderline/clinically significant range were twice as likely to have been exposed to higher maternal cortisol during gestation compared to children with ratings in the normal range (odds ratio = 2.1, 95% confidence interval = 1.1 to 3.9, p < 0.05). The relation between prenatal maternal cortisol and child anxiety status is illustrated in Figure 1.
Figure 1.
Gestational exposure to maternal cortisol is associated with anxiety among preadolescent children. Children who are in the normal range for anxiety were exposed to lower maternal cortisol during gestation. In contrast, children who were exposed to elevated maternal cortisol during gestation are significantly more likely to be rated in the anxious/borderline anxious range.
Does Prenatal Maternal Psychological Distress Predict Child Anxiety?
High average perceived stress, depression and pregnancy-specific anxiety during gestation were associated with higher child anxiety at 6 to 9 years of age, after consideration of relevant covariates including postnatal maternal distress at the time of child assessment (beta=0.20, F(4,177)= 7.1, p<0.05, beta=0.19, F(4,177)= 5.7, p<0.05, beta=0.20, F(4,177)=7.3, p<0.05 respectively). Prenatal maternal general anxiety was not significantly associated with child anxiety (p=0.12). Post hoc tests evaluating the effects of timing of exposure indicated that for perceived stress and depression, fetal exposure at 20 weeks’ GA emerged as the strongest predictor of child anxiety (beta=0.20, F(4,158)= 5.5, p<0.05, beta=0.24, F(4,158)= 5.8, p<0.05 respectively). For pregnancy-specific anxiety fetal exposure at 25 weeks’ GA was the strongest predictor of child anxiety (beta=0.22, F(4,158)= 5.8, p<0.05).
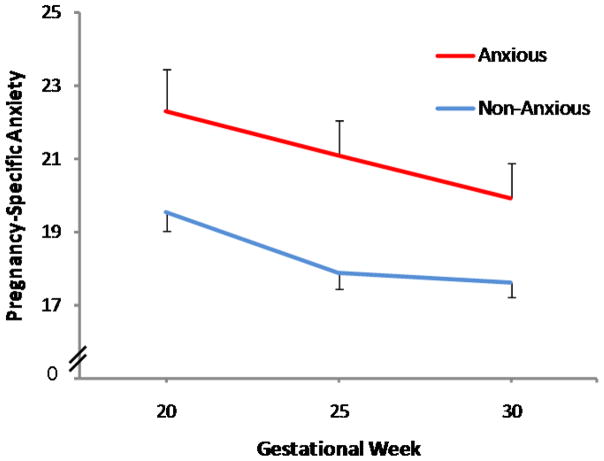
Next analyses evaluated the relation between the psychosocial measures and child anxiety status. Logistic regression revealed that among all of the psychosocial measures, only pregnancy-specific anxiety was associated with child anxiety status (normal range vs. borderline/clinically significant). Children with anxiety ratings within the normal range were exposed to significantly lower maternal pregnancy-specific anxiety during gestation compared to children in the borderline/clinically significant group (odds ratio = 1.1, 95% confidence interval = 1.0 to 1.2, p = 0.05). This indicates that a 1 point change in pregnancy anxiety (range 10 to 40) is associated with a 10% increase in risk for child anxiety. The relation between maternal pregnancy-specific anxiety and child anxiety status is illustrated in Figure 2.
Figure 2.
Pregnancy-specific anxiety predicts anxiety classification during preadolescence. Children who are in the normal range for anxiety were exposed to lower pregnancy-specific anxiety. In contrast, children who were exposed to elevated pregnancy-specific anxiety during gestation are significantly more likely to be rated in the anxious/borderline anxious range.
Do Maternal Psychological and Endocrine Factors Exert an Independent or Joint Influence on Child Anxiety?
A hierarchical regression model determined whether psychosocial (perceived stress, depression, pregnancy-specific anxiety) and endocrine (cortisol) measures independently or jointly predicted portions of the variance in child anxiety. After adjusting for covariates, pregnancy-specific anxiety (beta=0.22, F(4,165)= 6.4, p<0.05) and prenatal maternal cortisol (beta=0.16, F(5,164)= 6.1, p<0.05) independently predicted child anxiety. After accounting for the variance associated with pregnancy-specific anxiety and prenatal maternal cortisol, perceived stress and depression no longer significantly contributed to the model.
Discussion
In a prospective study with multiple prenatal assessments we document that prenatal maternal cortisol and prenatal psychosocial distress measures are associated with anxiety in preadolescent children. These are among the first prospective findings to document an association between both biological indicators of stress and psychological distress during gestation and risk for anxiety during preadolescence.
Prenatal Maternal Cortisol and Child Anxiety
The results of this study indicate that elevated maternal cortisol levels during gestation are associated with childhood anxiety as long as nine years later and that this association cannot be explained by critical prenatal or obstetric risk factors or by postnatal influences such as maternal psychological well being and socioeconomic status. Specifically, children who were exposed to elevated levels of maternal cortisol during gestation are significantly more likely to fall in the borderline or clinically significant range for anxiety. Interestingly, in the current study the consequences of prenatal cortisol are not determined by timing of exposure. The association between prenatal exposure to elevated maternal cortisol and child anxiety is consistent with experimental animal studies indicating lifelong consequences of prenatal exposure to elevated glucocorticoids (Kapoor et al., 2008; Seckl, 2008) and with human studies that link maternal cortisol with increased fearful or reactive behavior during infancy (de Weerth et al., 2003; Davis et al., 2007; Davis et al., 2011a). Given the documented influence of postnatal maternal psychological well being on child mental health (e.g., Rice et al., 2010), it is important that the observed associations remained after considering current maternal psychological distress.
Exposure to maternal cortisol during gestation may influence the development of anxiety by modifying fetal development in regions such as the amygdala (Herman & Cullinan, 1997; Joels & Baram, 2009) that are particularly sensitive to excessive levels of glucocorticoids (Rodrigues et al., 2009) and play a role in the regulation of anxious behavior (Schulkin, 2006). Findings from animal models illustrate that prenatal stress exposures including excess glucocorticoids alter the density of cortisol receptors (Kapoor et al., 2006) and increase the production of CRH in the amygdala (Cratty et al., 1995; Mueller & Bale, 2008). Further, exposure to stress during the prenatal period is associated with an increase in amygdala volume (Salm et al., 2004) suggesting a plausible mechanism by which prenatal cortisol may influence vulnerability to the development of anxiety.
Maternal Psychosocial Stress and Child Anxiety
Higher maternal perceived stress, depression and pregnancy-specific anxiety also are associated with elevated child anxiety after considering relevant covariates including current maternal psychological distress. This finding is consistent with experimental animal studies indicating that prenatal maternal stress is associated with increased fear/anxiety (Maccari & Morley-Fletcher, 2007; Kapoor et al., 2008; Seckl, 2008; Markham & Koenig, 2011). The present findings add to human work linking prenatal maternal psychological distress with increased fearful or anxious behavior during infancy/toddlerhood (Davis et al., 2004; Bergman et al., 2007; Davis et al., 2007), and are conceptually consistent with two prospective studies showing persisting effects into childhood (O’Connor et al., 2005) and adolescence (Van den Bergh et al., 2008). In a large sample of children, O’Connor and colleagues (2005) found that maternal anxiety measured only in late gestation was associated with increased behavioral and emotional problems at 6.5 years of age. Van den Berg and colleagues (2008) demonstrated that prenatal anxiety predicts depressive mood in 14 to 15 year old children. Our data support these findings and add new information indicating that pregnancy-specific anxiety is the type of maternal anxiety most strongly related to child anxiety.
Pregnancy-specific anxiety measures a woman’s fears and beliefs about pregnancy, delivery, and health of the baby. This construct has been shown to be an independent entity, separate from measures of generalized anxiety or other psychological distress measures. As reviewed elsewhere, measures of pregnancy-specific anxiety predict a wide variety of developmental outcomes including fetal behavior, birth outcome, infant and toddler cognitive and motor development, early childhood temperament and child brain development (Huizink et al., 2004; Sandman et al., 2011). The finding that pregnancy-specific anxiety exerts a more potent influence on fetal development compared to generalized measures of distress may be because stress related to events, beliefs or fears proximal to pregnancy are more relevant to the pregnant woman and therefore more consequential to the fetus.
The mechanism by which maternal psychosocial stress is communicated to the fetus is unknown. Consistent with prior work, it is unlikely that maternal cortisol mediates the effect of maternal report of psychosocial stress on child anxiety (e.g., de Weerth & Buitelaar, 2005; Harville et al., 2009; Davis & Sandman, 2010). As reported here, maternal cortisol and psychosocial distress are not correlated and they exert independent effects on child mental health. The report in humans that fetal exposure to maternal depression is associated with increased methylation of the glucocorticoid receptor gene in the neonate and with increased HPA responses to a challenge, suggests an epigenetic mechanism that may underlie both the maternal communication of adversity to the fetus and the persistent influence of the exposure (Oberlander et al., 2008). This possibility is supported by animal models demonstrating that very early experiences exert lifelong consequences on methylation (Champagne & Curley, 2009; Szyf, 2011). It has additionally been shown that prenatal anxiety alters placental functioning providing an additional mechanism by which psychosocial distress may impact fetal development (O’Donnell et al., 2011). Further, maternal psychosocial stress exerts widespread influences on a number of stress sensitive systems other than the HPA axis including the immune and vascular systems (Dunkel Schetter & Glynn, 2011). The present findings illustrate that the evaluation of both psychological and biological indicators of maternal stress during gestation will significantly improve our understanding of the influence of the prenatal environment on child outcomes.
Limitations
This study relied on naturally occurring variations in maternal biological stress signals and psychological distress, rather than experimental manipulations. It is difficult, therefore, to separate the effects of prenatal maternal psychological state from the consequences of other factors that might contribute to this association including genetic factors. Genetically informed study designs involving children conceived by in vitro fertilization who were not genetically related to their mothers provide strong evidence that the environment contributes to poor child mental health including anxiety risk (Rice et al., 2010; Lewis et al., 2011). Studies evaluating the consequences of random exposure to extreme stressors during gestation further suggest that prenatal exposures to stress exerts a lasting influence on child development (King & Laplante, 2005; Huizink et al., 2007; Li et al., 2010). An additional limitation of the present investigation is the reliance on maternal report of child behavior. Notably, in the present study, all significant associations between prenatal exposures and child anxiety remain after statistically covarying maternal psychological state at the time of reporting on child anxiety and sociodemographic factors that might contribute to child anxiety. A strength of the study is that mother-child pairs were assessed using a prospective and serial longitudinal design beginning in gestation which allowed for greater temporal resolution to examine the programming effects of prenatal maternal stress on child anxiety. The assessment of maternal cortisol during gestation, in addition to self report of psychological distress, is a strength and circumvents methodological issues related to shared methods variance (i.e, mother’s reporting on their own stress and on child behavior) that are prevalent in the existing literature.
Implications
There is growing recognition that early experiences are a primary factor contributing to mental illness. It has been recently estimated that early adversity may explain over 30% of the risk for developing mental illness (De Bellis et al., 1999; Nemeroff, 2004; Gunnar & Quevedo, 2008; Green et al., 2010). The presence of anxiety during childhood suggests lifetime vulnerability to poor mental health; over half of mentally ill adults exhibit symptoms before the age of 14 (Kessler et al., 2005). Greater understanding of the developmental origins of mental illness is a critical step for the development of new diagnostic methods and improved treatments. The current findings indicate that the understudied prenatal biological and psychological indicators of maternal distress shape the construction of the fetal nervous system with consequences for the development of anxiety in preadolescent children. Effect sizes in the current investigation were similar to those observed in studies evaluating the association between prenatal stress and birth and child outcomes (see Talge et al., 2007 for review). Associations were observed in a healthy, low risk sample of mother-child pairs and thus it is plausible that more profound effects would be observed among children of clinically anxious or depressed pregnant women. It is, however, possible that evaluation of a low risk sample that did not include women who smoked or used alcohol or illegal drugs during their pregnancy may have increased our power to detect an association between prenatal stress indices and child anxiety. This is so because the known consequences of these teratogens on health and development (e.g., Stroud et al., 2009; Shankaran et al., 2011) may mask the effects of maternal stress and anxiety on child anxiety.
We found that prenatal maternal cortisol and pregnancy-specific anxiety independently predict anxiety at 6 to 9 years of age. These data suggest that it would be beneficial for clinicians to consider these factors when working with pregnant women. Data indicating the importance of pregnancy-specific anxiety as compared to generalized distress measures are particularly encouraging because a woman’s concerns and fears about pregnancy are likely modifiable with early intervention. Approaches focused on cognitive reassessment of the risks associated with pregnancy may be most effective at promoting the psychological health of both mother and child. Specifically, reducing pregnancy-specific anxiety may benefit a wide range of developmental outcomes including childhood anxiety, resulting in more optimal psychological functioning
Conclusions
There is increasing evidence that the fetal environment plays a role in the development of psychiatric disorders (Schlotz & Phillips, 2009). There are important studies demonstrating associations between maternal psychological distress during pregnancy and risk for poor mental health during childhood and adolescence (O’Connor et al., 2003; Huizink et al., 2007; Van den Bergh et al., 2008). To our knowledge these are the first findings linking both biological and psychosocial measures of maternal stress to mental health in preadolescence. Future studies with these children will consider the implications of prenatal stress exposures for the transition to adolescence.
Acknowledgments
This research was supported by awards from the NIH to EPD (HD-50662) and CAS (HD-51852 and NS-41298). The authors wish to thank the families who participated in this project. The assistance of Megan Faulkner, Christina Canino, Cheryl Crippen and Natalie Hernandez of the Women and Children’s Health and Well-Being Project, Department of Psychiatry & Human Behavior, University of California is gratefully acknowledged.
ROLE OF THE FUNDING SOURCE: Funding for this study was provided by awards from the NIH to EPD (HD-50662) and CAS (HD-51852 and NS-41298). The NIH had no further role in study design; in the collection analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
DECLARATION OF INTEREST: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
CONTRIBUTERS: EPD participated in the study design, oversight of data collection, data analysis, and manuscript preparation. CAS participated in the study design, oversight of data collection, and provided critical feedback during the processes of manuscript preparation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles: An Integtated System of Multi-Informant Assessment. University of Vermont, Reaserch Center for Children, Youth, & Families; Burlington, VT: 2001. [Google Scholar]
- Austin MP, Hadzi-Pavlovic D, Leader L, Saint K, Parker G. Maternal trait anxiety, depression and life event stress in pregnancy: relationships with infant temperament. Early Hum Dev. 2005;81:183–90. doi: 10.1016/j.earlhumdev.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, Glover V, O’Connor TG. Quality of child-parent attachment moderates the impact of antenatal stress on child fearfulness. J Child Psychol Psychiatry: Child Adolesc Mental Health. 2008;49:1089–1098. doi: 10.1111/j.1469-7610.2008.01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, O’Connor TG, Modi N, Glover V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J Am Acad Child Adolesc Psychiatry. 2007;46:1454–1463. doi: 10.1097/chi.0b013e31814a62f6. [DOI] [PubMed] [Google Scholar]
- Blair MM, Glynn LM, Sandman CA, Davis EP. Prenatal maternal anxiety and early childhood temperament. Stress. 2011;14:644–51. doi: 10.3109/10253890.2011.594121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JP. Synaptogenesis, heterochrony and epigenesis in the mammalian neocortex. Acta Paediatr Suppl. 1997;422:27–33. doi: 10.1111/j.1651-2227.1997.tb18340.x. [DOI] [PubMed] [Google Scholar]
- Brown RW, Diaz R, Robson AC, Kotelevtsev Y, Mullins JJ, Kaufman MH, Seckl JR. The ontogeny of 11β-hydroxysteroid dehydrogenase type 2 and mineralicorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology. 1996;137:794–797. doi: 10.1210/endo.137.2.8593833. [DOI] [PubMed] [Google Scholar]
- Buss C, Davis EP, Hobel CJ, Sandman CA. Maternal pregnancy-specific anxiety is associated with child executive function at 6–9 years age. Stress. 2011;14:665–76. doi: 10.3109/10253890.2011.623250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Muftuler LT, Head K, Sandman CA. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrino. 2010;35:141–53. doi: 10.1016/j.psyneuen.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Curley JP. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neurosci Biobehav Rev. 2009;33:593–600. doi: 10.1016/j.neubiorev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Laurence Erlbaum Associates Inc; Mahwah, New Jersey: 2003. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- Cratty MS, Ward HE, Johnson EA, Azzaro AJ, Birkle DL. Prenatal stress increases corticotropin-releasing factor (CRF) content and release in rat amygdala minces. Brain Res. 1995;675:297–302. doi: 10.1016/0006-8993(95)00087-7. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-DeMet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry. 2007;46:737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry. 2011a;52:119–29. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Snidman N, Wadhwa PD, Dunkel Schetter C, Glynn L, Sandman CA. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy. 2004;6:319–331. [Google Scholar]
- Davis EP, Townsend EL, Gunnar MR, Georgieff MK, Guiang SF, Ciffuentes RF, Lussky RC. Effects of prenatal betamethasone exposure on regulation of stress physiology in healthy premature infants. Psychoneuroendocrino. 2004;29:1028–36. doi: 10.1016/j.psyneuen.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Davis EP, Waffarn F, Sandman CA. Prenatal treatment with glucocorticoids sensitizes the hpa axis response to stress among full-term infants. Dev Psychobiol. 2011b;53:175–83. doi: 10.1002/dev.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. Developmental traumatology Part I: Biological stress systems. Biol Psychiat. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- de Weerth C, Buitelaar JK. Physiological stress reactivity in human pregnancy--a review. Neurosci Biobehav Rev. 2005;29:295–312. doi: 10.1016/j.neubiorev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- de Weerth C, van Hees Y, Buitelaar J. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Hum Dev. 2003;74:139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Novak MFSX, Costigan KA, Atela LD, Ruesing SP. Maternal psychological distress during pregnancy in relation to child development at age two. Child Dev. 2006;77:573–587. doi: 10.1111/j.1467-8624.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Tang JI, Nyirenda MJ. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin Sci. 2007;113:219–232. doi: 10.1042/CS20070107. [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C, Glynn L. Stress in pregnancy: Empirical evidence and theoretical issues to guide interdisciplinary research. In: Contrada R, Baum A, editors. The Handbook of Stress Science. Springer Publishing Company, LLC; New York, NY: 2011. [Google Scholar]
- Essex MJ, Klein MH, Slattery MJ, Goldsmith HH, Kalin NH. Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. Am J Psychiatry. 2010;167:40–6. doi: 10.1176/appi.ajp.2009.07010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. Lancet. 1998;352:707–8. doi: 10.1016/S0140-6736(05)60824-0. [DOI] [PubMed] [Google Scholar]
- Gitau R, Fisk NM, Teixeira JM, Cameron A, Glover V. Fetal hypothalamic-pituitary-adrenal stress responses to invasive procedures are independent of maternal responses. J Clin Endocrinol Metab. 2001;86:104–9. doi: 10.1210/jcem.86.1.7090. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Dunkel Schetter C, Hobel C, Sandman CA. Pattern of perceived stress and anxiety in pregnancy predict preterm birth. Health Psychol. 2008;27:42–51. doi: 10.1037/0278-6133.27.1.43. [DOI] [PubMed] [Google Scholar]
- Goedhart G, Snijders AC, Hesselink AE, van Poppel MN, Bonsel GJ, Vrijkotte TG. Maternal depressive symptoms in relation to perinatal mortality and morbidity: results from a large multiethnic cohort study. Psychosom Med. 2010;72:769–76. doi: 10.1097/PSY.0b013e3181ee4a62. [DOI] [PubMed] [Google Scholar]
- Grant K, McMahon C, Austin M, Reilly N, Leader L, Ali S. Maternal prenatal anxiety, postnatal caregiving and infants’ cortisol responses to the still-face procedure. Dev Psychobiol. 2009;51:625–637. doi: 10.1002/dev.20397. [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:113–23. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo KM. Early care experiences and HPA axis regulation in children: a mechanism for later trauma vulnerability. Prog Brain Res. 2008;167:137–149. doi: 10.1016/S0079-6123(07)67010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendcrino. 2005a;30:541–549. doi: 10.1016/j.psyneuen.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Willemsen-Swinkels SH, Huizink AC, Mulder EJ, Visser GH, Buitelaar JK. The effects of prenatal stress on temperament and problem behavior of 27-month-old toddlers. Eur Child Adolesc Psychiatry. 2005b;14:41–51. doi: 10.1007/s00787-005-0435-1. [DOI] [PubMed] [Google Scholar]
- Harville EW, Savitz DA, Dole N, Herring AH, Thorp JM. Stress questionnaires and stress biomarkers during pregnancy. J Womens Health (Larchmt) 2009;18:1425–33. doi: 10.1089/jwh.2008.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamio - pituitary - adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hobel CJ. Identifying the patient at risk. In: Bolognese RJ, Schwartz RH, Schneider J, editors. Perinatal medicine: Management of the high risk fetus and neonate. Williams & Wilkins; Baltimore, MA: 1982. pp. 3–28. [Google Scholar]
- Huizink AC, De Medina PG, Mulder EJ, Visser GH, Buitelaar JK. Psychological measures of prenatal stress as predictors of infant temperament. J Am Acad Child Adolesc Psychiatry. 2002;41:1078–1085. doi: 10.1097/00004583-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Dick DM, Sihvola E, Pulkkinen L, Rose RJ, Kaprio J. Chernobyl exposure as stressor during pregnancy and behaviour in adolescent offspring. Acta Psychiatr Scand. 2007;116:438–46. doi: 10.1111/j.1600-0447.2007.01050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJ, Robles de Medina PG, Visser GH, Buitelaar JK. Is pregnancy anxiety a distinctive syndrome? Early Hum Dev. 2004;79:81–91. doi: 10.1016/j.earlhumdev.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Robles de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry. 2003;44:810–8. doi: 10.1111/1469-7610.00166. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptogenesis, synapse elimination, and neural plasticity in human cerebral cortex. In: Nelson CA, editor. Threats to Optimal Development: Integrating Biological, Psychological, and Social Risk Factors. Lawrence Erlbaum; Mahwah, NJ: 1994. pp. 35–54. [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic pituitary adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–66. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572:31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Petropoulos S, Matthews SG. Fetal programming of hypothalamic–pituitary–adrenal (HPA) axis function and behavior by synthetic glucocorticoids. Brain Res Rev. 2008;57:586–595. doi: 10.1016/j.brainresrev.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, Wang P, Wells KB, Zaslavsky AM. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352:2515–23. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S, Laplante DP. The effects of prenatal maternal stress on children’s cognitive development: Project ice storm. Stress. 2005;8:35–45. doi: 10.1080/10253890500108391. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Lydon J, Seguin L, Goulet L, Kahn SR, McNamara H, Genest J, Dassa C, Chen MF, Sharma S, Meaney MJ, Thomson S, Van Uum S, Koren G, Dahhou M, Lamoureux J, Platt RW. Stress pathways to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. Am J Epidemiol. 2009;169:1319–26. doi: 10.1093/aje/kwp061. [DOI] [PubMed] [Google Scholar]
- Lajic S, Nordenstrom A, Hirvikoski T. Long-term outcome of prenatal dexamethasone treatment of 21-hydroxylase deficiency. Endocr Dev. 2011;20:96–105. doi: 10.1159/000321228. [DOI] [PubMed] [Google Scholar]
- Levitt P. Structural and functional maturation of the developing primate brain. J Pediatr. 2003;143:S35–45. doi: 10.1067/s0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Lewis G, Rice F, Harold GT, Collishaw S, Thapar A. Investigating environmental links between parent depression and child depressive/anxiety symptoms using an assisted conception design. J Am Acad Child Adolesc Psychiatr. 2011;50:451–459. e1. doi: 10.1016/j.jaac.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Olsen J, Vestergaard M, Obel C, Baker JL, Sorensen TI. Prenatal stress exposure related to maternal bereavement and risk of childhood overweight. PLoS One. 2010;5:e11896. doi: 10.1371/journal.pone.0011896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccari S, Morley-Fletcher S. Effects of prenatal restraint stress on the hypothalamus-pituitary-adrenal axis and related behavioural and neurobiological alterations. Psychoneuroendocrino. 2007;32(Suppl 1):S10–5. doi: 10.1016/j.psyneuen.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Marcus SM, Flynn HA, Blow FC, Barry KL. Depressive symptoms among pregnant women screened in obstetrics settings. J Women’s Health. 2003;12:373–380. doi: 10.1089/154099903765448880. [DOI] [PubMed] [Google Scholar]
- Markham JA, Koenig JI. Prenatal stress: role in psychotic and depressive diseases. Psychopharmacology (Berl) 2011;214:89–106. doi: 10.1007/s00213-010-2035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–65. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy VE, Clifton VL. Alterations in human placental 11beta-hydroxysteroid dehydrogenase type 1 and 2 with gestational age and labour. Placenta. 2003;24:739–44. doi: 10.1016/s0143-4004(03)00103-6. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Early-life adversity, CRF dysregulation, and vulnerability to mood and anxiety disorders. Psychopharmacol Bull. 2004;38:14–20. [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180:502–8. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Glover V. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry. 2003;44:1025–1036. doi: 10.1111/1469-7610.00187. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal Anxiety Predicts Individual Differences in Cortisol in Pre-Adolescent Children. Biol Psychiat. 2005;58:211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- O’Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O’Connor TG, Glover V. Maternal prenatal anxiety and downregulation of placental 11beta-HSD2. Psychoneuroendocrino. 2011 doi: 10.1016/j.psyneuen.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Boivin J, van den Bree M, Hay DF, Thapar A. The links between prenatal stress and offspring development and psychopathology: disentangling environmental and inherited influences. Psychol Med. 2010;40:335–45. doi: 10.1017/S0033291709005911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini CK, Dunkel Schetter C, Wadhwa PD, Sandman CA. Psychological adaptation and birth outcomes: The role of personal resources, stress, and sociocultural context during pregnancy. Health Psychol. 1999;18:333–345. doi: 10.1037//0278-6133.18.4.333. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Salm AK, Pavelko, Krose EM, Webster W, Kraszpulski M, Birle DL. Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Dev Brain Res. 2004;148:159–167. doi: 10.1016/j.devbrainres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: Relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Davis EP. Gestational stress influences cognition and behavior. Future Neurol. 2010;5:675–690. [Google Scholar]
- Sandman CA, Davis EP, Buss C, Glynn LM. Exposure to Prenatal Psychobiological Stress Exerts Programming Influences on the Mother and Her Fetus. Neuroendocrinology. 2011 doi: 10.1159/000327017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Glynn LM, Dunkel Schetter C, Wadwha PD, Garite T, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): Priming the placental clock. Peptides. 2006;27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Santor DA, Coyne JC. Shortening the CES-D to improve its ability to detect cases of depression. Psychol Assessment. 1997;9:233–243. [Google Scholar]
- Schlotz W, Phillips DI. Fetal origins of mental health: evidence and mechanisms. Brain Behav Immun. 2009;23:905–16. doi: 10.1016/j.bbi.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Schulkin J. Angst and the amygdala. Dialogues Clin Neurosci. 2006;8:407–16. doi: 10.31887/DCNS.2006.8.4/jschulkin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoids, developmental ‘programming’ and the risk of affective dysfunction. Prog Brain Res. 2008;167:17–34. doi: 10.1016/S0079-6123(07)67002-2. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Das A, Bauer CR, Bada HS, Lester BM, Wright LL, Higgins RD, Poole WK. Prenatal cocaine exposure and small-for-gestational-age status: Effects on growth at 6years of age. Neurotoxicol Teratol. 2011;33:575–81. doi: 10.1016/j.ntt.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speilberger C. State-Trait Anxiety Inventory. Mind Garden; Redwood City, CA: 1983. [Google Scholar]
- Stroud LR, Paster RL, Goodwin MS, Shenassa E, Buka S, Niaura R, Rosenblith JF, Lipsitt LP. Maternal smoking during pregnancy and neonatal behavior: a large-scale community study. Pediatrics. 2009;123:e842–8. doi: 10.1542/peds.2008-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M. The early life social environment and DNA methylation; DNA methylation mediating the long-term impact of social environments early in life. Epigenetics. 2011;6 doi: 10.4161/epi.6.8.16793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: How and why? J Child Psychol Psychiatry. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollenaar M, Beijers R, Jansen J, Riksen-Walraven J, de Weerth C. Maternal prenatal stress and cortisol reactivity to stressors in human infants. Stress. 2011;14:53–65. doi: 10.3109/10253890.2010.499485. [DOI] [PubMed] [Google Scholar]
- Trautman PD, Meyer-Bahlburg HFL, Postelnek J, New MI. Effects of early dexamethasone on the cognitive and behavioural development of young children: Results of a pilot study. Psychoneuroendocrino. 1995;20:439–449. doi: 10.1016/0306-4530(94)00070-0. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Cuchillo I, Machin C, Rua C. Maternal adrenalectomy at the early onset of gestation impairs the postnatal development of the rat hippocampal formation: effects on cell numbers and differentiation, connectivity and calbindin-D28K immunoreactivity. J Neurosci Res. 2000;62:644–667. doi: 10.1002/1097-4547(20001201)62:5<644::AID-JNR4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev. 2004;75:1085–97. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacol. 2008;33:536–45. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- Yim IS, Glynn LM, Dunkel-Schetter C, Hobel CJ, Chicz-DeMet A, Sandman CA. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry. 2009;66:162–9. doi: 10.1001/archgenpsychiatry.2008.533. [DOI] [PMC free article] [PubMed] [Google Scholar]