Abstract
Carcinogenic human papillomavirus (HPV) infections are very common after sexual debut and nearly all become undetectable (“clear”) within a few years. Following clearance, the long-term risks of type-specific HPV re-appearance and subsequent risk of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) are not well defined.
In the 7-year, population-based cohort study in Guanacaste, Costa Rica, we studied how often type-specific carcinogenic HPV infections re-appeared after clearance, and how often re-appearance led to CIN2+. We considered 1740 carcinogenic HPV infections detected by MY09/11 PCR among 2805 women (18-91 years old, median 34) who were actively followed at 6- or 12-month intervals. We identified women with 1 or more type-specific HPV infections that cleared and re-appeared, and further defined a subgroup of “definite clearance and re-appearance” (≥ 2 intervening negative results over a period of ≥ 1 year). We determined the absolute risk of CIN2+ among the different groups. P values are two sided.
Only 7.7% (81/1052) of HPV-infected women had intervening negative results. Very few (3.7%, 39/1052) had “definite clearance and re-appearance”, of which 5.1% (2/39) subsequently persisted to a diagnosis of CIN2. There were zero CIN3+ lesions.
Extremely few women (2/2805 of women in our cohort) had a type-specific carcinogenic HPV infection clear, re-appear and lead to CIN2+. If confirmed, this argues against vaccination to avoid re-appearance that leads to precursor lesions and against the need of frequent HPV screening after initial negative results.
Keywords: HPV infection re-appearance, CIN2+ risk after re-appearance, HPV infection epidemiology
Introduction
Infections with one or more carcinogenic genotypes of human papillomavirus (HPV) are very common after sexual debut 1-3. The vast majority of carcinogenic HPV infections become undetectable (clear) shortly after first detection 1, 4-6; however, the rate of clearance decreases with time 7, 8. Overt HPV persistence is strongly associated with progression to the necessary precursors of cancer, ie, cervical intraepithelial neoplasia grade 2 or worse (CIN2+) or more stringently cervical intraepithelial neoplasia grade 3 or worse (CIN3+) 9. In fact, CIN2+ can be found in most cases of persistent carcinogenic HPV infections after ~5 years, although the lesions are extremely small initially10.
The subsequent risks of HPV type-specific re-appearance and CIN2+ after infections clear are much less well understood. Because so many women have infections that clear understanding their subsequent risk for neoplasia will determine 1) the potential benefit of prophylactic vaccination among older women to prevent re-appearing infections and subsequent risk of CIN2+ lesions, and 2) the optimal intervals for HPV screening in the general population especially after a first round of testing is negative.
Regarding vaccination, there is controversy about the effectiveness of the immune system to clear subsequent infections with types a woman has previously cleared if she becomes exposed later in life or even due to re-activation of latent infections. There is a theoretical concern that those subsequent exposures or even re-activations of latent infections could carry a substantial risk of CIN2+, which might therefore warrant vaccinating older women. With regard to primary screening using HPV testing, the lower the risk of type-specific re-appearance and subsequent CIN2+, the longer the screening intervals can safely be.
The few longitudinal cohort studies, with a wide age range and type-specific HPV infection data have varied in the frequency of detection of a previously cleared carcinogenic HPV infection, ranging from 3.3% reported by Trottier et al to 8% by Insinga et al 11, 12. Nevertheless, the risk of CIN2+ attributable to type-specific HPV clearance and re-appearance has not been determined. In the population-based cohort study in Guanacaste, Costa Rica we studied the type-specific patterns of HPV infection and estimated the absolute risk of CIN2+ among women with and without infections that re-appeared after one or more negative tests.
Material and methods
Study design and population
After 18 months of recruitment between June 1993 and December 1994, a 10049 woman population-based cohort of women 18 and older was assembled in Guanacaste, Costa Rica to study the natural history of HPV infection and cervical neoplasia; we achieved a participation rate of 93.6% of those eligible. Details regarding the cohort design, methods and written informed consent have been described elsewhere 13. The study protocol was reviewed and re-approved annually by the National Cancer Institute and a Costa Rican Institutional Review Board.
Women were screened with multiple techniques at enrollment: three kinds of cytology (conventional, liquid-based and a computer-assisted method called PapNet which is no longer available), naked-eye visual examination of the cervix, expert evaluation of photographic images of the cervix and detection from exfoliated cervical cells of 11 carcinogenic HPV types at a threshold of 10 pg/mL of HPV DNA by Hybrid Capture Tube Test (earlier version of Hybrid Capture 2, Digene, Gaithersburg, MD, now Qiagen). A total of 2140 women were referred for colposcopic evaluation at enrollment after having one or more abnormal screening test results or if selected as part of the 338 women with negative test results that were sent as referral controls.
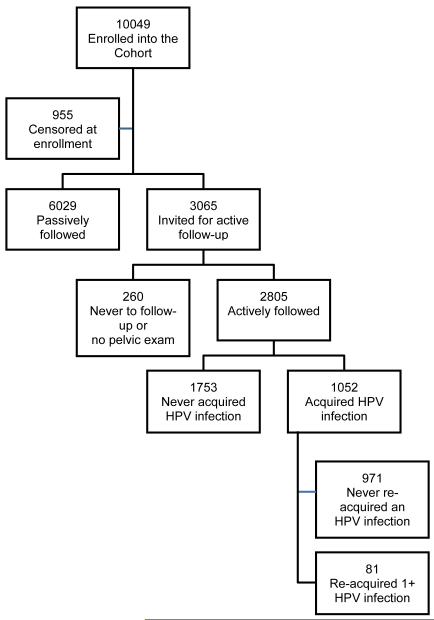
Women found to have prevalent CIN2 or worse disease (CIN2+) were treated accordingly and censored from follow-up (12 with cervical cancer, 73 with histological CIN3 and 57 with CIN2). Also, 151 women with HSIL screening test results but without histological confirmation and 659 who had undergone hysterectomy before enrollment were censored from follow-up (n=955) 8, 14. Women with totally negative screening tests (n = 6029) and considered as having low risk for subsequent CIN2+ were screened again at their 5th-7th year visit after enrollment (passive follow-up) and are excluded from this analysis due to the long period without sampling, see figure 1.
Figure 1.
CONSORT diagram
We actively followed 3065 women (18 to 97 years old, median 34 years), including all women thought to be at elevated risk of incident CIN2+, ie, women with minor screening test abnormalities at enrollment and those who at enrollment reported 5 or more sexual partners in their lifetime. We also included for active follow up women who were younger than 26 years of age and not yet sexually active at enrollment and, a representative sample of women in the passive follow-up, to confirm their low risk 8.
Of the women included in the active follow-up, 260 never contributed samples during follow up (154 were still virginal at the end of follow-up, 96 never came for follow-up visits and 10 women refused pelvic examination) and are also excluded from this analysis. The remainder (n = 2805) were screened yearly or semi-annually for up to 7 years (figure 1) and they constitute the population for this analysis.
During follow-up, women who presented with cytologic low-grade squamous intraepithelial lesions (LSIL), histologic CIN grade 1 (CIN1), or low-grade equivalent cervicographic findings were shifted to a 6-month interval screening schedule. All those with screening test results of HSIL or images suggestive of CIN2 or worse were referred for colposcopic evaluation, treated according to the severity of their lesions and censored from further follow-up. The retention rate over the seven years for those in active follow-up was high (83.5%) with 6 as the median number of attended visits. The main reasons for drop out were; 161 refused further participation, 108 moved out of the study area, 67 died, 50 became too ill to attend screening visits and 44 women never returned (passive refusal). During the course of the study 75 women had a hysterectomy for reasons different than cervical neoplasia and were censored from further follow-up in the study.
Sample collection
During follow-up at each screening visit, exfoliated cervical cells were collected using a Cervex brush (Unimar, Wilton, CT) for conventional and liquid-based cytology (ThinPrep; Hologic Inc, Bedford, MA). Cytologic interpretations were reported using the Bethesda System 15. Additional cells were collected with a Dacron swab for HPV and stored in ViraPap DNA transport medium (Digene); during the course of the study the buffer was switched to Digene’s DNA specimen transport medium (STM). After cell collection, the cervix was rinsed with a 5% acetic acid solution and two magnified photographic images were taken (cervigrams; National Testing Laboratories, Fenton, MO) for later interpretation by a colposcopist. See details elsewhere 13, 14.
HPV DNA testing
All HPV DNA testing by polymerase chain reaction (PCR) and genotyping was performed at the Albert Einstein College of Medicine in New York; details have been described elsewhere16, 17. In brief, HPV DNA extracted from exfoliated cells preserved in Virapap transport medium or STM was amplified using the MY09/MY11 L1 degenerate primer PCR system with AmpliTaq Gold polymerase (TaqGold; Perkin-Elmer-Cetus, Norwalk, CT). After amplification, PCR products were analyzed by electrophoresis and hybridized with radiolabeled generic HPV DNA probes. Dot-blot hybridization was used for HPV typing: probes were specific for types 2, 6, 11, 13, 16, 18, 26, 31-35, 39, 40, 42-45, 51-59, 61 62, 64, 66-74, 81-85, and 89. We considered the following HPV types as carcinogenic: HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, and −68 9, 18-21. HPV testing was done masked to clinical outcomes. Signal strength was determined for each HPV type by three raters, using an ordinal scale (1, 2, 3, 4 or 5) going from weakest to strongest signal strength, based on density and size of dots on an x-ray film. In case of disagreement a final decision was made by consensus. This scale is non-linear but correlated with semi-quantitative methods 20.
Approximately 2000 initially negative specimens, including those with and without cytological abnormalities, were retested with additional PCR primers (ie, GP5+/6+22 and FAP9/6423) to confirm the sensitivity of the main assay. Extremely few additional positive results arose from this confirmatory retesting (n = 207). Untyped HPV DNA-positive samples were uncommon (7.96%, 633 of 7948 HPV positive samples).
Colposcopy and final pathology diagnosis
As mentioned above, during follow-up women were referred to colposcopy if a cytology HSIL result was reported by any of the methods used or if the colposcopist reported “suggestive of high grade lesion” when evaluating the cervicographic images of the cervix. PCR results were considered for colposcopic referral only after the final screening visit was completed.
At colposcopy, the decision of whether to do a colposcopy directed biopsy or a loop electrosurgical excision procedure was based on a formal algorithm considering the colposcopic impression, woman’s age, parity and screening results. Women with cancer were referred to the Social Security System for treatment 14. All pathology slides were read in Costa Rica for clinical management and at the end of follow-up were also read by a second pathologist in the US who was blinded to the first pathologist assessment and clinical outcome. If both pathologists agreed on the diagnosis then it was considered as the final pathology diagnosis, but if they disagreed the case slides were read by a third pathologist in the US and if two of the pathologists agreed then a final diagnosis was assigned. For the few cases where all three pathologists disagreed the final pathology diagnosis was assigned after a joint pathologist review.
Statistical analysis
We looked at the individual history of all carcinogenic HPV infections (n=1740) detected at enrollment or during the 7-year follow-up among women who were actively followed (n=2805) and for whom PCR results were available. Women were classified according to their MY09/11 PCR test results, as “No carcinogenic HPV infection (n=1753)”, “≥ 1 infections that cleared and re-appeared (n=81)”, or “≥ 1 infections without intervening negative test results (n=971)”. We further divided women with ≥ 1 intervening negative results into “possible false-negative intervening result” (1 negative test result between positive results and mostly within a period of < 2 years, n=42) and “definite clearance and re-appearance” (≥ 2 negative test results over a period of ≥ 1 year, n=39), see table 1. Women with multiple infections (simultaneously or consecutively) were considered as “with intervening negative result” even if only one of their infections was categorized as having an intervening negative result, if there was no intervening negative result for any type found, they were classified as “without intervening negative test results”. To categorize infections with multiple periods of clearance/re-appearance we used the longest period of negativity or undetectable period. There were 7 infections that were cleared and re-appeared two times (2 categorized as false negative and 5 as definitive clearance and re-appearance) and 2 infections that had three periods of intervening negative results (1 categorized as false and 1 as definitive clearance and re-appearance) during the course of the study.
Table 1.
Distribution according to number of intervening negative tests and time lag between positive tests
| Time lag between positive tests | ||||
|---|---|---|---|---|
| Number of intervening negative tests |
Number of women |
< 1 year | 1 to 2 years | 2 or more years |
| 1 | 42 | 21* | 15* | 6* |
| 2+ | 39 | 0† | 13† | 26† |
Classified as possible false negative result
Classified as definite intervening negative results
Eight women had one infection with several lapses of intervening negative results (6 had 2 episodes, 2 had 3 episodes). Women were classified according to the longest episode of intervening negative results
The absolute risk of CIN2+ lesion among women was estimated by the categories of HPV infection previously defined. The exact 95% CI was calculated for all proportions.
To further study these re-appearing infections we analyzed, at the type-specific infection level, time of detection (prevalent or newly-detected during follow up), age and genotype distribution and compared their frequencies between those infections that did and did not re-appear. The p-values were estimated using large sample statistics. To understand if infections with intervening negative results were different regarding viral load, we looked at PCR signal strength from different perspectives considering the infection complete history or comparing a specific single-point determination. First we estimated for each carcinogenic HPV infection the average viral load by adding all PCR signal strength scores divided by the number of times it was determined and compared those averages between infections with and without intervening negative results. Second, using a single point in time determination, we compared the signal strength distribution at first detection for incident infections without intervening negative results and first detection after the negative period for those with intervening negative results. We also compared the signal strength at first detection of newly-detected infections without intervening negative results, to first detection of infections with intervening negative results. The Wilcoxon Mann-Whitney test for significance was used for both comparisons. Restricted to infections with intervening negative results we compared the signal strength distributions before and after the negative period and the statistical significance was evaluated with Bowker’s test of symmetry. For analytical purposes we categorized signal strength into three categories: weak (1), medium (2 and 3) and strong (4 and 5).
Results
The 2805 women were followed for a median of 7.01 years (IQR 6.95 – 7.05 years), 1052 (37.5%) were ever HPV positive; with a total of 1740 carcinogenic HPV infections (mean of 1.7 infections per woman, 170 women had 3 or more infections). Overall detection of carcinogenic HPV infections with clearance and re-appearance during follow-up was an uncommon phenomenon with only 81 of 2805 (2.9%) women and 81 of 1052 (7.7%) HPV-infected women ever having one or more detected.
When comparing the age of women with carcinogenic HPV infections with and without intervening negative results, women with intervening negative results were significantly older (p = 0.04). Also women with intervening negative periods tended to acquire more HPV infections in general (simultaneously or sequentially) than those who did not have intervening negative results (p < 0.001). No significant differences were observed between the two groups of women regarding lifetime number of sexual partners at enrollment or at study exit, by use of contraception or by smoking status (data not shown).
The overall absolute risk of CIN2+ among women who were actively-followed was 3.3% (93/2805). In 11 women, despite never having a carcinogenic HPV infection detected during the course of the study, a histologic CIN2+ lesion was confirmed by the pathologist review panel. Acknowledging low statistical power due to a small number of CIN2+ cases, no statistically significant differences in risk for CIN2+ were found among HPV positive groups of women, ie, those with no intervening negative periods, possible false negative intervening results or definite clearance and re-appearance (see table # 2, part A all women and part B restricted to infections with intervening negative results). Of of the two women with definitive intervening negative results and CIN2+ lesions, only in one could the lesion be attributed with some confidence to the infections with a definite intervening negative period (table #2, part C risk). The complete carcinogenic HPV infection history of the 7 women with infections with intervening negative results and CIN2 is presented in table 3; of note no cases of CIN3 were detected among women with definite intervening negative periods.
Table 2.
Part A Risk of CIN2+ among women with carcinogenic HPV infection by detection of intervening negative results. Part B Risk of CIN2+ estimated considering all infections with intervening negative result. Part C Risk estimated considering only infections for which the infection with the intervening negative result appears as causal for the CIN2+ lesion.
| Women | Risk of CIN2+ | ||
|---|---|---|---|
| No. | No. | % | |
| Part A | |||
| All | 2805 | 93 | 3.3 |
| No HPV detected | 1753 | 11 | 0.63 |
| HPV detected, NO intervening negative result | 971 | 75 | 7.7 |
| 1+ Carcinogenic type re-appeared | 81 | 7 | 8.6 |
| Part B | |||
| 1+ Carcinogenic type re-appeared | 81 | 7 | 8.6 |
| Possible false negative | 42 | 5 | 11.9 |
| Definite intervening negative | 39 | 2 | 5.1 |
| Part C | |||
| 1+ Carcinogenic type re-appeared | 81 | 3 | 3.7 |
| Possible false negative | 42 | 2 | 4.8 |
| Definite intervening negative | 39 | 1 | 2.6 |
Table 3.
Carcinogenic HPV infection history for women with re-appeared infections and CIN2+ lesions
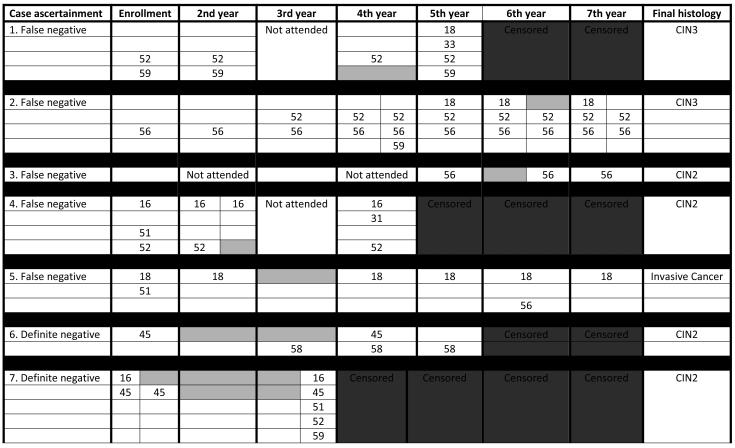
|
Two sets of HPV results are shown within a year for women who were screened every six months. White boxes represent negative test results; gray boxes represent negative intervening results.
When we looked at the infection level and compared infections with intervening negative results and infections without intervening negative results (each group includes all other infections regardless of the final outcome), we observed that infections with intervening negative results were more frequent among prevalent infections found at enrollment than those found as new during follow-up (2.9% vs 7.7%, p < 0.0001). However, prevalent infections were, on average, followed longer than newly-detected infections and thus had more opportunity to have intervening negatives results. A significant association was found between re-appeared infections (compared to infections without intervening negative results) and increasing age of the woman at first detection; the association was stronger among prevalently-detected infections (p for trend < 0.0001 for prevalent infections and p for trend = 0.03 for newly-detected; Wilcoxon Mann-Whitney test using age as continuous). No differences were found regarding HPV genotype distribution between the two groups of infections, although power to find a difference was limited due to the small number of infections with intervening negative results (data not shown).
Regarding signal strength we found a significantly weaker mean signal strength for infections that cleared and re-appeared (averaging the signal strength at all time periods detected) compared to that of infections without negative periods, 2.7 and 3.3 respectively (p < 0.001, Wilcoxon 2-sample test). To explore further the behavior of signal strength among infections with intervening negative results, we looked at the distribution of the signal strength at specific points in time. First, under the assumption that most infections when detected as re-appeared would probably behave as a newly-detected or “incident” infection, we compared the signal strength distribution detected after the negative period to the signal strength of incident infections when first detected but found that re-appearing infections after a negative period had a significantly weaker signal strength than newly-detected infections (p < 0.001 Wilcoxon Mann-Whitney test), see table #4. We also found significant differences (p = 0.007 Wilcoxon Mann-Whitney test) when we compared the signal strength when first detected of newly-detected infections without, to first detection of infections with subsequent intervening negative results (3.2 and 2.8 mean viral load, respectively). Then restricted to infections with intervening negative results, we compared the signal strength distribution at the last detection time before the negative intervening result to that measured immediately after the negative lapse and found that the proportion of infections with strong signal strength was lower after the negative period, 20.5% and 34.1% respectively (p = 0.06, Bowker’s symmetry test), see table #5.
Table 4.
Carcinogenic HPV infections PCR signal strength distribution at first detection for incident newly-detected infections and first detection after negative lapse for infections with intervening negative results.
| Newly-detected infections, first detection |
Intervening negative infections, first detection after negative lapse |
||||
|---|---|---|---|---|---|
| N | % | N | % | p | |
| All infections | 936 | 100 | 88 | 100 | |
| Weak (1) | 219 | 23.4 | 32 | 36.4 | |
| Medium (2 – 3) | 265 | 28.3 | 38 | 43.2 | |
| Strong (4 – 5) | 452 | 48.3 | 18 | 20.5 | <0.01 |
Wilcoxon Mann-Whitney test = 0.0069
Table 5.
PCR signal strength distribution for carcinogenic HPV infections with intervening negative results before and after the intervening negative results
| Before | After | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Weak (1) | Medium (2 – 3) | Strong (4 – 5) | All infections | p-value | |||||
| N | % | N | % | N | % | N | % | ||
| Weak (1) | 10 | 11.4 | 9 | 10.2 | 3 | 3.4 | 22 | 25.0 | |
| Medium (2 – 3) | 8 | 9.1 | 18 | 20.4 | 10 | 11.4 | 36 | 40.9 | |
| Strong (4 – 5) | 14 | 15.9 | 11 | 12.5 | 5 | 5.7 | 30 | 34.1 | |
| All infections | 32 | 36.4 | 38 | 43.2 | 18 | 20.5 | 88 | 100 | 0.065 |
Bowker’s test of symmetry p = 0.065
Discussion
Based on 7-year follow-up of 2805 women aged 18-84, we found that extremely few women had a carcinogenic HPV infection that cleared or became undetectable (ie, 2 or more negative tests within a period of 1 year or longer), later re-appeared and eventually led to a CIN2+ lesion. To our knowledge, these data are novel, and require confirmation in even larger cohorts with greater statistical power.
Understanding the meaning of clearance and re-appearance regarding risk of CIN2+ is an important piece of information required to optimize the use of both prophylactic vaccination and molecular diagnostic testing. The subsequent risk of CIN2+ was no higher for women whose infections re-appeared after 2 or more negative tests than the subsequent risk in HPV infected women without intervening negative results; notably no CIN3 or worse lesions were detected among women with re-appearing infection; only a very few CIN2 cases, which are less reproducible and more prone to regression, were found. Because the absolute frequency of CIN3 due to re-appearing infections was so low, vaccination against HPV among mid-adult or older women to prevent CIN2+ lesions due to re-appearance of previously cleared infections does not seem to be justified.
Frequent re-screening of women in the general population who test HPV negative also seems to be unnecessary. The high sensitivity and negative predictive value for CIN2+ of HPV testing are motivating a switch from cytology-based cervical cancer screening programs to those based on HPV test as the primary detection method. The ideal screening interval to be used is still under debate; although the false negative rate of an HPV test is dependent on the specific assay used. In this study, we confirm that CIN2+ lesions mainly come from overt persistent infections and that the risk for a woman of having an infection clear, re-appear and later have a CIN2+ lesion is very low, therefore extended screening intervals appear safe after an initial negative HPV test. The exact optimal interval is beyond the scope of this analysis, and will depend on societal norms regarding safety and cost.
This study also addressed HPV natural history. Re-appearance of infections is uncommon according to our data and similar to that described by Insinga et al12. Similarly, Trottier et al previously reported a re-appearance rate of 3.3% for carcinogenic HPV infections among women enrolled in the Ludwig-McGill cohort. In a more recent publication from the same cohort, re-appearance of carcinogenic HPV infections was observed to be 5.8 fold less frequent than first time detection of incident infections: 1.2 per 1000 women-months (95% CI 0.7-2.0) and 7.0 (95% CI 6.5-7.5) for reappearance and initial infections, respectively 11, 24. Due to how uncommon infections with intervening negative results are, in spite the long follow-up duration and the size of the Guanacaste cohort we could not estimate type-specific rate of new-detection versus rate of reappearance. However, at the type-specific level including all carcinogenic types, the cumulative rate of reappearance was definitely lower (2.9%, 81 of 2805) than the previously reported cumulative rate of new detections 23.7% (95% CI 22.1 to 25.3)8. We await data from the other large cohorts with longitudinal data.
Trottier et al 24 also found that re-appearance was consistently associated at mid-adult ages with having new sexual partners; however, among women in our cohort infection re-appearance was not associated with having new sexual partners (only 7 of 81 women with re-appearing infections reported having new sexual partners in the year previous to the re-appearance). Lack of association with recent new sexual partners was also reported by Insinga 12. An alternative explanation to new exposure through contact with sexual partners (vaginal intercourse or other kinds of sexual activity) could be re-appearance of HPV infections that were previously acquired but remained in a latent or non-detectable state. In Guanacaste, in a nested case-cohort immunology study among women 45 to 75 years old, with an intensive examination of female and male sexual behavior, it was reported that among 107 women who had previous sexual experience but reported no sexual intercourse during the study period (~2 years) and who were considered as HPV negative based on a single determination at the beginning of the study 53 (49.5%) of them became HPV positive, suggesting that re-appearance from latency could play a major role 25. Of note, this particular PCR test has been widely used and validated as roughly equal in analytic sensitivity to other research-use PCR typing assays and to a widely used commercial test, HC2 (Qiagen)20. Theoretically, a study using a test with different analytic sensitivity might show a different rate of intervening negative results predicting different risks for CIN2+.
Although the average viral signal strength, based on HPV type-specific hybridization, of infections that cleared and later re-appeared was weaker than that of infections that never showed an intervening negative result, Trottier et al reported no significant difference 24. Again, we have no obvious explanation for the discrepancy. We also found a significantly weaker viral load at re-appearance time compared to the signal at first detection of all other newly-acquired or incident infections detected during the 7 years of follow-up. Among infections with intervening negative results the signal strength at the time of re-appearance was weaker, although the difference was borderline non-significant, compared to the signal strength at the last positive test before the negative period.
Clearly, more results are needed to understand the differences in natural history data that Trottier et al 24 and we have produced. If our finding of lower viral loads associated with definite intervening negative results is confirmed, it would suggest different biological characteristics of either the particular virus or the host. Studies with larger number of infections with intervening negative test results would be needed to thoroughly understand the cause for the lower viral load; at the present time, no clinical use for viral load is foreseen.
To control for possible misclassification errors due to false negative tests (e.g., caused by bad sampling or accidents during testing) and under the assumption that repeated false negatives on sensitive PCR-based HPV tests are unlikely for the same women, we classified women according to the number of intervening negative test results. In doing so, we attempted to identify the characteristics and estimate the risk of CIN2+ for those with infections with definite (2+) intervening negative results. Most women (26 out of 39 women) with infections with two or more negative tests had 2 or more years between the flanking positive tests. We could not identify other sociodemographic characteristics, beside older age at the time of first detection and larger total number of infections per woman during the study period, which could differentiate women who presented infections with negative periods from those who had carcinogenic HPV infections without negative intervening results. We have no explanation for this finding.
Few cohorts have ever reported re-appearing infections 11, 12, 24, 26. Large cohorts of women with extensive and frequent testing at the type-specific level for several years are needed to study carcinogenic HPV infections that re-appear after a period of negative test results because of the rarity of these re-appearances. However, we note again that no information was provided regarding risk of CIN2+ precluding any comparison with the results we presented here.
Despite the large size of the Guanacaste cohort and the 7 years of follow-up our main finding of similar absolute risk of CIN2+ among women with re-appearing infections to that of women with infections without intervening negative results might not hold beyond the follow-up time in this study. However, it is re-assuring that our cohort covered, albeit in yearly snapshots for seven years, the full lifespan of women in Guanacaste (women were 18 to 97 years old at enrollment). Also, after re-appearance, infections were followed on average for 3.5 years, a duration where newly-detected infections have already been shown to have increased risk of progression to CIN2+ 8. We considered a woman as having definite intervening negative results despite having overt persistence for other carcinogenic types which could have falsely increased the risk for CIN2+ among those women.
In conclusion, re-appearance or re-acquisition of previously cleared infections that lead to CIN2+ is very rare; the potential benefit of vaccinating women who have previously cleared HPV infections with genotypes included in the current vaccines is much reduced. Prolongation of screening intervals after an initial negative HPV test merits consideration.
Manuscript novelty and impact.
Understanding the frequency of type-specific HPV infection clearance and re-appearance and risk of subsequent CIN2+ is important to fully understand the best use of both prophylactic vaccination and molecular diagnostic testing. We observed that re-appearance of the same HPV type is uncommon and very few women had a type-specific carcinogenic HPV infection clear, re-appear and later persist with evidence of CIN2+.
Our findings argue against the need of frequent HPV screening after initial negative results since re-appearance of the same type is uncommon, and for an extremely low potential benefit of vaccination to avoid the very rare CIN2+ lesions coming from re-appearing infections.
Acknowledgments
The authors thank the women who participated in this study.
Funding:
This work was supported by the National Institutes of Health (N01-CP-21081, N01-CP-33061, N01-CP-40542, N01-CP-50535, N01-CP-81023, and intramural program CA78527 to RB). The Guanacaste cohort (design and conduct of the study, sample collection, management, analysis and interpretation of the data) for the enrollment and follow-up phases were supported by the National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
Footnotes
The authors do not have any conflict of interest to declare.
References
- 1.Brown DR, Shew ML, Qadadri B, Neptune N, Vargas M, Tu W, Juliar BE, Breen TE, Fortenberry JD. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191:182–92. doi: 10.1086/426867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winer RL, Kiviat NB, Hughes JP, Adam DE, Lee SK, Kuypers JM, Koutsky LA. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731–8. doi: 10.1086/427557. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez AC, Burk R, Herrero R, Hildesheim A, Bratti C, Sherman ME, Solomon D, Guillen D, Alfaro M, Viscidi R, Morales J, Hutchinson M, et al. The natural history of human papillomavirus infection and cervical intraepithelial neoplasia among young women in the Guanacaste cohort shortly after initiation of sexual life. Sex Transm Dis. 2007;34:494–502. doi: 10.1097/01.olq.0000251241.03088.a0. [DOI] [PubMed] [Google Scholar]
- 4.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–8. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 5.Moscicki AB, Hills N, Shiboski S, Powell K, Jay N, Hanson E, Miller S, Clayton L, Farhat S, Broering J, Darragh T, Palefsky J. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995–3002. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 6.Goodman MT, Shvetsov YB, McDuffie K, Wilkens LR, Zhu X, Thompson PJ, Ning L, Killeen J, Kamemoto L, Hernandez BY. Prevalence, acquisition, and clearance of cervical human papillomavirus infection among women with normal cytology: Hawaii Human Papillomavirus Cohort Study. Cancer Res. 2008;68:8813–24. doi: 10.1158/0008-5472.CAN-08-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195:1582–9. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME, Solomon D, Guillen D, Alfaro M, Morales J, Hutchinson M, Katki H, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010;102:315–24. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humans. IWGotEoCRt Human Papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1–636. [PMC free article] [PubMed] [Google Scholar]
- 10.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 11.Trottier H, Mahmud S, Prado JC, Sobrinho JS, Costa MC, Rohan TE, Villa LL, Franco EL. Type-specific duration of human papillomavirus infection: implications for human papillomavirus screening and vaccination. J Infect Dis. 2008;197:1436–47. doi: 10.1086/587698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Insinga RP, Perez G, Wheeler CM, Koutsky LA, Garland SM, Leodolter S, Joura EA, Ferris DG, Steben M, Brown DR, Elbasha EH, Paavonen J, et al. Incidence, duration, and reappearance of type-specific cervical human papillomavirus infections in young women. Cancer Epidemiol Biomarkers Prev. 2010;19:1585–94. doi: 10.1158/1055-9965.EPI-09-1235. [DOI] [PubMed] [Google Scholar]
- 13.Herrero R, Schiffman MH, Bratti C, Hildesheim A, Balmaceda I, Sherman ME, Greenberg M, Cardenas F, Gomez V, Helgesen K, Morales J, Hutchinson M, et al. Design and methods of a population-based natural history study of cervical neoplasia in a rural province of Costa Rica: the Guanacaste Project. Rev Panam Salud Publica. 1997;1:362–75. doi: 10.1590/s1020-49891997000500005. [DOI] [PubMed] [Google Scholar]
- 14.Bratti MC, Rodriguez AC, Schiffman M, Hildesheim A, Morales J, Alfaro M, Guillen D, Hutchinson M, Sherman ME, Eklund C, Schussler J, Buckland J, et al. Description of a seven-year prospective study of human papillomavirus infection and cervical neoplasia among 10000 women in Guanacaste, Costa Rica. Rev Panam Salud Publica. 2004;15:75–89. doi: 10.1590/s1020-49892004000200002. [DOI] [PubMed] [Google Scholar]
- 15.The 1988 Bethesda System for reporting cervical/vaginal cytological diagnoses JAMA; National Cancer Institute Workshop; 1989; pp. 931–4. [PubMed] [Google Scholar]
- 16.Herrero R, Castle PE, Schiffman M, Bratti MC, Hildesheim A, Morales J, Alfaro M, Sherman ME, Wacholder S, Chen S, Rodriguez AC, Burk RD. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1796–807. doi: 10.1086/428850. [DOI] [PubMed] [Google Scholar]
- 17.Castle PE, Schiffman M, Gravitt PE, Kendall H, Fishman S, Dong H, Hildesheim A, Herrero R, Bratti MC, Sherman ME, Lorincz A, Schussler JE, et al. Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol. 2002;68:417–23. doi: 10.1002/jmv.10220. [DOI] [PubMed] [Google Scholar]
- 18.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 19.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, Ghissassi F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 20.Gravitt PE, Kovacic MB, Herrero R, Schiffman M, Bratti C, Hildesheim A, Morales J, Alfaro M, Sherman ME, Wacholder S, Rodriguez AC, Burk RD. High load for most high risk human papillomavirus genotypes is associated with prevalent cervical cancer precursors but only HPV16 load predicts the development of incident disease. Int J Cancer. 2007;121:2787–93. doi: 10.1002/ijc.23012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 22.de Roda Husman AM, Walboomers JM, van den Brule AJ, Meijer CJ, Snijders PJ. The use of general primers GP5 and GP6 elongated at their 3′ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995;76(Pt 4):1057–62. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]
- 23.Forslund O, Antonsson A, Nordin P, Stenquist B, Hansson BG. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J Gen Virol. 1999;80(Pt 9):2437–43. doi: 10.1099/0022-1317-80-9-2437. [DOI] [PubMed] [Google Scholar]
- 24.Trottier H, Ferreira S, Thomann P, Costa MC, Sobrinho JS, Prado JC, Rohan TE, Villa LL, Franco EL. Human papillomavirus infection and reinfection in adult women: the role of sexual activity and natural immunity. Cancer Res. 2010;70:8569–77. doi: 10.1158/0008-5472.CAN-10-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez P, Hildesheim A, Rodriguez AC, Schiffman M, Porras C, Wacholder S, Pineres AG, Pinto LA, Burk RD, Herrero R. Behavioral/lifestyle and immunologic factors associated with HPV infection among women older than 45 years. Cancer Epidemiol Biomarkers Prev. 2010;19:3044–54. doi: 10.1158/1055-9965.EPI-10-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winer RL, Hughes JP, Feng Q, Xi LF, Cherne S, O’Reilly S, Kiviat NB, Koutsky LA. Early natural history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev. 2011;20:699–707. doi: 10.1158/1055-9965.EPI-10-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]