Abstract
Chronological life span (CLS) has been studied as an aging paradigm in yeast. A few conserved aging genes have been identified that modulate both chronological and replicative longevity in yeast as well as longevity in the nematode Caenorhabditis elegans; however, a comprehensive analysis of the relationship between genetic control of chronological longevity and aging in other model systems has yet to be reported. To address this question, we performed a functional genomic analysis of chronological longevity for 550 single-gene deletion strains, which accounts for approximately 12% of the viable homozygous diploid deletion strains in the yeast ORF deletion collection. This study identified 33 previously unknown determinants of CLS. We found no significant enrichment for enhanced CLS among deletions corresponding to yeast orthologs of worm aging genes or among replicatively long-lived deletion strains, although a trend toward overlap was noted. In contrast, a subset of gene deletions identified from a screen for reduced acidification of culture media during growth to stationary phase was enriched for increased CLS. These results suggest that genetic control of CLS under the most commonly utilized assay conditions does not strongly overlap with longevity determinants in C. elegans, with the existing confined to a small number of genetic pathways. These data also further support the model that acidification of the culture medium plays an important role in survival during chronological aging in synthetic medium, and suggest that chronological aging studies using alternate medium conditions may be more informative with regard to aging of multicellular eukaryotes.
Key words: aging, genomic, screen, lifespan, yeast, C. elegans, pH, chronological, replicative
Introduction
The budding yeast Saccharomyces cerevisiae has emerged as one of the primary non-mammalian model organisms used in aging-related research.1 Two aging paradigms have been described in yeast: replicative and chronological. The yeast RLS (RLS) has been extensively studied and refers to the number of daughter cells an asymmetrically dividing mother cell can produce prior to senescence.2 A molecular cause of replicative aging was described more than a decade ago, with the discovery that extrachromosomal rDNA circles accumulate specifically in the mother cell with replicative age and are sufficient to induce cell senescence.3 In addition to accumulation of extrachromosomal rDNA circles, other molecular determinants of replicative aging have been proposed, including mitochondrial degeneration, proteotoxic stress, oxidative damage and epigenetic changes.4–6
The yeast chronological lifespan (CLS) is an aging model in which lifespan is defined as the length of time a quiescent cell can survive in a non-replicative environment.7 CLS is typically assayed by culturing cells in synthetic defined growth medium with 2% glucose as the initial carbon source and monitoring survival over a time scale of weeks. After an initial growth phase lasting 1–2 days, the cells exit the cell cycle and persist in a metabolically active non-dividing state. Survival in this assay is defined as the fraction of culture that retains the ability to re-enter vegetative growth upon return to nutrient replete conditions.7
There is strong evidence that oxidatively damaged proteins and other macromolecules accumulate during chronological aging and may play a causal role in senescence. In support of this model, overexpression of superoxide dismutase can increase CLS.8 There is also abundant data suggesting that at least a subset of chronologically aging cells induce an apoptotic like cell death pathway, although it remains unclear whether invocation of this pathway is a cause of chronological senescence or a response to the lifespan limiting damage.9,10
Recently, a molecular cause of chronological aging has been described, with the finding that accumulation of acetic acid in the growth medium is sufficient to account for mortality observed under standard aging conditions.11 Acetic acid accumulation in the medium results from fermentation of glucose to ethanol, which is then utilized as a carbon source following glucose depletion. During the catabolic breakdown of ethanol, acetic acid accumulates in the culture medium and is specifically toxic to chronologically aging yeast.11 Acetic acid likely exerts toxicity by inducing the yeast apoptotic-like response,12 and it has been suggested that acetic acid contributes to an increase in reactive oxygen species (ROS).13,14
Prior studies have identified dozens of genetic and environmental modifiers of chronological or replicative longevity, some of which are now known to function similarly to modulate life span in multicellular eukaryotes.15–17 One example of such a conserved longevity intervention is dietary restriction, which has been shown to slow aging in many different species including yeast, nematodes, fruit flies and rodents,18,19 and most recently in rhesus monkeys.20 Dietary restriction can be accomplished in yeast by reducing the glucose concentration of the growth medium from 2 to 0.5% or lower and is sufficient to increase both RLS and CLS.21–24 Our previous findings suggest that prolonged stationary phase survival of chronologically aging yeast in low glucose results, at least in part, from decreased production of acetic acid.11 In addition to dietary restriction, inhibition of the target of rapamycin (TOR) kinase and/or deletion of the gene coding for the ribosomal S6 kinase homolog, SCH9, are both known to increase replicative life span and CLS in yeast,25–28 as well as the lifespan of C. elegans29,30 and D. melanogaster.31
Recently, a functional genomic screen provided quantitative evidence that genetic control of aging has been conserved between C. elegans and the yeast replicative aging model.32 This study found that yeast strains lacking genes homologous to C. elegans genes whose reduced expression leads to lifespan extension (referred to hereafter as C. elegans aging genes) are significantly more likely have enhanced RLS, identifying 25 homolog pairs that modulate life span in both species. Among these, at least eight act in a single pathway linking nutrient availability and mRNA translation, including Tor1 and Sch9, several translation initiation factors and multiple ribosomal proteins. Considering that yeast and worms diverged an estimated 1.5 billion years ago,33 this finding lends credence to the idea that at least a subset of genes modulating life span share a common ancient evolutionary origin.
Here we describe a large-scale, quantitative comparison of CLS for single-gene deletion strains corresponding to three different gene sets: (1) yeast homologs of C. elegans aging genes, (2) replicatively long-lived deletion strains and (3) strains identified from a genome-wide screen for reduced acidification of the culture medium. This analysis includes data for 550 unique single-gene deletion strains in the yeast open reading frame deletion (ORF) collection,34 representing a coverage of 11.6% of the approximately 4,800 viable single-gene deletions in the BY4743 homozygous diploid genetic background (Fig. 1). Among these three sets, 34 lifespan extending mutations were identified; however, only the set containing acidification mutants was significantly enriched for enhanced CLS, relative to randomly selected deletion strains. These findings indicate that, under the standard conditions used in this study, genetic control of CLS is not strongly conserved with either yeast RLS or C. elegans life span. They also further support the idea that culture medium acidification and acetic acid-induced cell death is a major contributor to mortality in this chronological aging system.
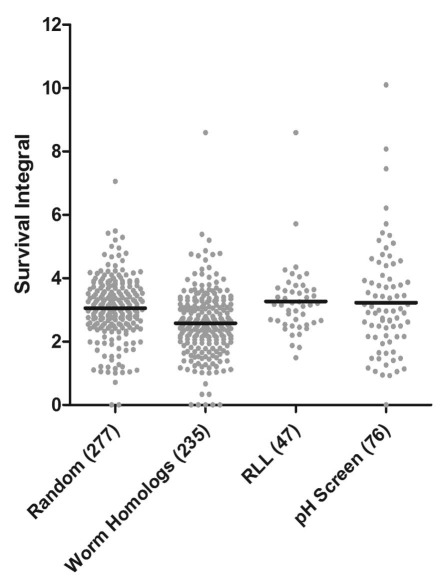
Figure 1.
Scatter plot of survival integrals for all reported long-lived gene deletions. The mean survival integral (SI) for each strain was calculated from at least nine replicate chronological lifespan experiments and plotted by group. The median SI for any of the experimental groups was not found to be significantly greater than an expected median value generated by assaying a randomized list of single-gene deletions (random). The set of genes corresponding to worm homologs, however, had a median SI that was shorter than expected by Dunnett's multiple comparison test (p < 0.05). Whiskers illustrate data within the 1.5 interquartile range.
Results
Quantitative comparison of genetic control of CLS with genetic control of aging in C. elegans.
To determine the degree to which genetic determinants of CLS in yeast overlap with longevity-control in C. elegans, we performed an analysis of CLS for deletion strains lacking homologs of C. elegans aging genes, similar to that previously described by Smith et al.32 for RLS. Of the 264 non-essential yeast genes identified as having high homology to C. elegans aging genes,32 we analyzed CLS for 237 corresponding homozygous deletion strains (Sup. Table 1). CLS was determined for each of these strains in biological triplicate and compared to the wild type BY4743 parental strain. In addition, a haploid strain lacking the gene coding for a ribosomal S6 kinase homolog, SCH9, was included in this analysis, since the deletion mutant is not included in the deletion collection, but has been previously shown to be chronologically long-lived.35,36 Of the 237 strains assayed, two strains (fun12Δ and lys12Δ) were eliminated due to slow growth that prohibited accurate lifespan analysis (see Materials and Methods). For comparison, CLS was determined for 227 randomly selected single gene deletions (Sup. Table 2).
A multiple replication strategy was taken to minimize the frequency of false positive classification of increased CLS. The chronological aging potential of each deletion strain was initially characterized through a single experiment containing three biological replicates aged and analyzed in parallel. From this analysis, 61/227 (26.9%) of randomly selected deletion strains and 63/235 (26.8%) homologs of C. elegans aging genes showed significantly greater survival than experimentally-matched wild type cells (student's t-test, p < 0.05). On initial characterization, the set of genes corresponding to worm aging homologs were not significantly enriched for modifiers of yeast chronological aging compared to the random screen (G-test, one degree of freedom, p = 0.98767).
The CLS of each of these strains was then independently measured in at least two additional replicate experiments performed on different dates. Among these replicate sets, eight deletion strains (3.51%) from the randomly selected set (Fig. 2) and 12 (5.06%) from the C. elegans homolog set (Fig. 3) were found to be significantly long-lived in each replicate analysis (Sup. Tables 1 and 2). This difference in the percent of long-lived deletions between the two sets was not statistically significant (G-test, one degree of freedom, p = 0.40705, Table 1).
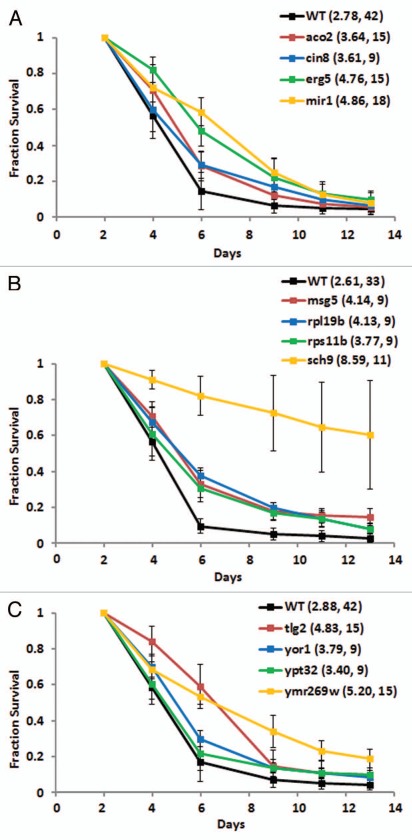
Figure 2.
Survival curves for long-lived strains from the random screen. Eight gene deletions conferring increased chronological life span were identified from 227 randomly selected deletion strains. These data were used to estimate the frequency of long-lived mutations in the deletion collection for comparison against other groups. The survival curves represent pooled lifespan data from multiple experiments with experiment matched wild type (BY4743) lifespan data. The mean survival integral along with the number of biological replicate experiments is shown next to the strain name.
Figure 3.
Survival curves for long-lived strains from the worm homolog screen. Twelve of 235 gene deletions corresponding to C. elegans aging genes confer increased chronological life span. The survival curves represent pooled lifespan data from multiple experiments with experiment matched wild type (BY4743) lifespan data. The mean survival integral along with the number of biological replicate experiments is shown next to the strain name.
Table 1.
Summary of lifespan data
| High stringency (100%) | Low stringency (2/3) | |||||||
| Random | C.e. Aging homologs | Replicative long-lived | pH screen | Random | C.e. Aging homologs | Replicative long-lived | pH screen | |
| Genes Screened | 227 | 235 | 47 | 76 | 227 | 235 | 47 | 76 |
| Long lived | 8 | 12 | 4 | 11 | 32 | 33 | 10 | 19 |
| Percent | 3.5% | 5.1% | 8.5% | 14.5% | 14.0% | 14.0% | 21.3% | 25.0% |
| G (=2∑O*ln(E)) | N/a | 0.70309 | 1.9408 | 9.9258 | N/a | 0.00875 | 1.4392 | 4.6144 |
| p-value | N/a | 0.40175 | 0.16358 | 0.00163* | N/a | 0.92547 | 0.23027 | 0.03170* |
CLS was determined for the indicated number of strains from the following four sets: randomly selected deletions (Random), homologs of C. elegans aging genes (C. e. Aging Homologs), replicatively long-live deletion strains, and strains identified from a screen for reduced medium pH (pH screen). A strain is categorized as chronologically long-lived according to whether it is significantly longer than the experiment matched wild type in every assay (High Stringency) or significantly longer than the experiment matched wild type no less than 2/3 times (Low Stringency), using the student's t-test (p ≤ 0.05). The G-test was used to determine whether a group of genes was significantly enriched for longevity modifiers compared to the expected rate determined by the random screen. Only the pH Screen was enriched for chronological longevity modifiers by both criteria.
A reanalysis of the data including deletion strains that tested as long-lived in at least 2/3 of the replicate experiments results in a similar conclusion. In this case, 32/227 random strains (14.0%) and 33/235 C. elegans homolog strains (14.0%) were classified as long lived (G-test, one degree of freedom, p = 0.92547, Table 1). Taken together, these data suggest that deletion strains corresponding to yeast homologs of C. elegans aging genes are not significantly more likely to modulate yeast CLS than randomly selected deletion strains, at least under standard assay conditions.
Quantitative comparison of the overlap between genetic control of yeast replicative and chronological life span.
Several studies have reported on single-gene deletion strains that extend yeast RLS, and information generated from these screens is being used to understand networks and pathways that potentially affect the processes related to cellular and organismal aging.1 To date, at least 50 single-gene deletion strains from the haploid yeast ORF deletion collection are reported to have increased RLS.26,32,37–39 To determine if the set of genes that extend the RLS also modulate CLS, we assayed CLS for 47 of these strains in the BY4743 homozygous diploid background, as described above for yeast homologs of C. elegans aging genes (Sup. Table 3). Of these 47 strains, four (8.5%) were long-lived in each replicate experiment (Fig. 4) and 10 (21.3%) were long lived in at least 2/3 of replicate experiments. In neither case was this significantly different from the frequency of CLS extension observed among randomly selected deletion strains (G-test, one degree of freedom, p = 0.16358 and p = 0.23027, respectively for each type of analysis, Table 1). We note, however, that there is a trend toward enrichment for increased CLS among replicatively long-lived mutants in both cases.
Figure 4.
Survival curves for yeast deletion strains that are both chronologically and replicatively long-lived. Four of 47 single-gene deletions known to increase replicative lifespan also conferred increased chronological life span. The survival curves represent pooled lifespan data from multiple experiments with experiment matched wild type (BY4743) lifespan data. The mean survival integral along with the number of biological replicate experiments is shown next to the strain name.
Gene deletions that reduce acidification of the culture medium are associated with increased CLS.
Based on the prior observations that acetic acid accumulation in the culture medium limits CLS and that buffering the culture medium to a higher pH is sufficient to extend life span,11 we hypothesized that deletion strains exhibiting reduced acidification of the culture medium during outgrowth may be enriched for increased CLS. A high throughput screen of yeast ORF deletion collection was performed to identify such strains, based on the absorbance of bromophenol blue in four day old cell-free culture supernatant. Bromophenol blue is a useful indicator between a pH of 3.0 and 4.6, which allowed us to identify strains with a culture pH > 3.0 (wild type culture medium for this study exhibited a mean pH of 2.64). The pH of the candidates identified from the high throughput screen was independently verified with a pH meter in 5 ml culture tubes. Following this verification, we identified 80 single-gene deletion strains whose phenotype consisted of a culture pH greater than or equal to 3.0 after 4 days (Sup. Table 4).
Of the 80 strains identified in the pH screen, four strains (cpa2Δ, fun12Δ, hfi1Δ and rvs161Δ) were eliminated due to slow growth that prohibited accurate lifespan determination (Sup. Table 4). Using the same analysis described above, 11 of the remaining 76 strains (14.5%) tested as long-lived in each of the replicate experiments (Fig. 5). This represents a significant enrichment for increased CLS compared to the random selected deletion strains (G-test, one degree of freedom, p = 0.00163, Table 1). Similarly, 19 of the 76 strains (25%) assayed were long-lived in at least 2/3 of the replicate experiments, which also represented a significant enrichment relative to randomly selected strains (G-test, one degree of freedom, p = 0.03170).
Figure 5.
Survival curves for long-lived strains from the screen for reduced acidification of the culture medium. Eleven of 76 deletions strains exhibiting reduced acidification of the culture medium where chronologically long-lived. Deletion of HOM6 (B; S.I. = 8.08) and VPS51 (C; S.I. = 7.65) had a comparable lifespan to deletion of SCH9 (Fig. 3B; S.I. = 8.59). Vps51p, along with Tlg2p identified in the worm homolog screen (Fig. 3C), is involved in endosome docking at the late Golgi. The survival curves represent pooled lifespan data from multiple experiments with experiment matched wild type (BY4743) lifespan data. The mean survival integral along with the number of biological replicate experiments is shown next to the strain name.
Analysis of pooled life span data across multiple experiments.
The analysis of the yeast CLS assays described above was performed through an iterative process that compared the mean of at least three biological replicates of a particular deletion strain to that of at least three experiment-matched wild type controls. Experiment-to-experiment variation in the behavior of wild type lifespans was observed over the course of the screens (Sup. Table 5). To determine if the observed variation was consistent with a Gaussian distribution, the survival integrals for individual wild type lifespans pooled across all experiments was plotted (n = 99), and the distribution was fitted using the sample mean and standard deviation (µ = 3.002263, σ = 0.575539, Sup. Fig. 1). No significant departure from normality was found across the wild type lifespans by applying the Anderson-Darling normality test to the sample distribution (A = 0.4855, p value = 0.2217).
The normal distribution of individual wild type lifespans across multiple experiments allowed for a re-analysis of the sample data by comparing the mean lifespan of deletion strains pooled across multiple experiments to the mean of the pooled wild type data. Initially, a scatter plot of the pooled lifespan data for each gene in the four categories was generated (Fig. 1). In no case was the median survival for an experimental group significantly greater than the expected median survival as determined by a Mann-Whitney U-test; however, the group comprising the worm homologs had a significantly shorter median lifespan than the random set (p < 0.0001). We next evaluated which experimental strains were long-lived compared to the wild type parental strain by using pooled data. To correct for type I error arising from multiple comparisons between the pooled data for an experimental strain and the pooled wild type lifespan, a Bonferroni correction was applied to the student's t-test (α = 0.05/n, number of unique comparisons = 550). This increased the stringency for defining a chronologically long-lived deletion strain by establishing the confidence interval at 0.009% (p ≤ 0.00009). By this analysis, 32 strains (14%) from the random screen were classified as long-lived (Sup. Table 6 and Table 2). Applying the same criteria, 18 of the 235 strains from the C. elegans homolog group (7.7%) were classified as long-lived. Deletion strains known to extend the RLS of yeast were also not significantly enriched for increased CLS by this analysis (10/47 = 21.3%, G-test, one degree of freedom, p = 0.23027, Table 2), although a trend was again evident. On the other hand, 20 of the 76 strains identified in the pH screen were classified as long-lived (26.3%), which represents a significant enrichment of long-lived single-gene deletions (G-test, one degree of freedom, p = 0.01761, Table 2).
Table 2.
Analysis of pooled lifespan data
| Analysis of pools (Bonferroni corrected) | ||||
| Random | C.e. Aging homologs | Replicative long-lived | pH screen | |
| Genes Screened | 227 | 235 | 47 | 76 |
| Long lived | 32 | 18 | 10 | 20 |
| Percent | 14.0% | 7.7% | 21.3% | 26.3% |
| G (=2∑O*ln(E)) | N/a | 5.0097 | 1.4392 | 5.6349 |
| p-value | N/a | 0.02521 | 0.23027 | 0.01761* |
The lifespan data across all experiments was pooled and compared to the distribution of pooled wild type lifespans. A strain is categorized as chronologically long if the pooled experiment data was significantly different from the pooled wild type data by student's t-test (p ≤ 0.00009, corrected for multiple comparisons). Only the pH Screen was enriched for chronological longevity modifiers by this criteria.
Identification of deletion strains with increased chronological life span.
In the course of these studies, we identified 34 single-gene deletions conferring a robust extension in CLS using the most stringent criteria, 33 of which have not been previously described (Table 3 and Figs. 2–5). In addition to the strains that were consistently long-lived, we classified a total of 89 gene deletions that extended CLS in at least 2/3 replicate experiments. The list of 89 genes was submitted to the Saccharomyces Genome Database GO Termfinder (www.yeastgenome.org/cgibin/GO/goTermFinder.pl) to determine whether any common biological function is shared among the candidate genes that can be attributed to the long lifespan phenotype. According to this analysis, GTPase activity was annotated as a function for six of the genes (cdc10Δ, gpa1Δ, mgm1Δ, vps1Δ, ypt32Δ and ypt6Δ), which represented a significant enrichment (p = 0.00369). Interestingly, VPS1, YPT32 and YPT6 are involved in Golgi trafficking and vacuolar protein sorting, which may implicate a role for the secretory pathway in modulating CLS.
Table 3.
Identification of chronologically long-lived deletion strains
| Gene | ORF | Annotation | |
| Random Screen | CBP4 | YGR174C | Mitochondrial respiratory chain complex III assembly |
| RCK2 | YLR248W | Protein kinase involved in response to oxidative and osmotic stress | |
| RPL11B | YGR085C | Component of 60 S ribosomal subunit; involved in ribosome assembly | |
| RPS0B | YLR048W | Component of 40 S ribosomal subunit; required for 18 S RNA maturation | |
| UBA4 | YHR111W | Activating protein of Urm1p prior to urmylation | |
| YDL041W | YDL041W | Dubious ORF. Overlaps with SIR2/YDL042C | |
| YLR064W | YLR064W | Protein of unknown function | |
| YPL105C | YPL105C | Protein of unknown function | |
| Worm Ortholog Screen | ACO2 | YJL200C | Mitochondrial aconitase isozyme; induced by growth on glucose |
| CIN8 | YEL061C | Kinesin motor protein involved in spindle assembly | |
| ERG5 | YMR015C | Ergosterol biosynthetic process; C-22 sterol desaturase | |
| MIR1 | YJR077C | Mitochondrial inorganic phosphate transmembrane transporter | |
| MSG5 | YNL053W | Protein phosphatase; involved in mating pheromone response | |
| RPL19B | YBL027W | Component of 60 S ribosomal subunit | |
| RPS11A | YDR025W | Component of 40 S ribosomal subunit | |
| SCH9* | YHR205W* | Protein kinase involved in cAPK and TORC1 activity; phosphorylates Rps6p | |
| TLG2 | YOL018C | t-SNARE mediates fusion of endosome-derived vesicles with late Golgi | |
| YMR269W | YMR269W | Nucleolar protein of unknown function; implicated in ribosome biogenesis | |
| YOR1 | YGR281W | Multidrug plasma membrane transporter | |
| YPT32 | YGL210W | GTPase involved in exocytic pathway and budding from the trans-Golgi | |
| pH Screen | BIM1 | YER016W | Microtubule binding; involved in spindle assembly checkpoint |
| CCR4 | YAL021C | Component of CCR4-NOT transcriptional complex; regulates gene expression | |
| CTF8 | YHR191C | Involved in mitotic sister chromatid cohesion | |
| GPA1 | YHR005C | α-subunit of heterotrimeric G protein that couples to pheromone receptor | |
| HFM1 | YGL251C | DNA helicase, catalyzes unwinding of Holliday junctions | |
| HOM6 | YJR139C | Homoserine dehydrogenase; methionine and threonine biogenesis | |
| MOT2 | YER068W | Subunit of CCR4-NOT complex; roles in transcription and mRNA degradation | |
| MTO1 | YGL236C | Adds modification to the wobble uridine base in mitochondrial tRNAs | |
| PHM6 | YDR281C | Protein of unknown function | |
| TRP5 | YGL026C | Tryptophan biosynthesis | |
| VPS51 | YKR020W | Component of GARP, required for endosome to late Golgi trafficking | |
| Replicative Long-lived | PKH2 | YOL100W | Ser/Thr protein kinase that controls endocytosis |
| SCH9* | YHR205W* | Protein kinase involved in cAPK and TORC1 activity; phosphorylates Rps6p | |
| SOK1 | YDR006C | Involved in cAMP-mediated signaling | |
| YBR266C | YBR266C | Dubious ORF. Potential role in cytoskeleton organization | |
Thirty-four unique single-gene deletion strains were identified which exhibited prolonged stationary phase survival according to a high-stringency definition.
sch9Δ belongs to both the worm ortholog screen and the list of replicatively long-lived deletions.
vps51Δ, an extremely long-lived mutant identified in the pH screen (Fig. 5C), lacks a component of the Golgi associated retrograde protein complex (GARP), which also contains Vps52p, Vps53p and Vps54p.40 This complex docks endosomes to the late Golgi through the interaction of Vps52p with activated Ypt6-GTP,41 another gene whose deletion results in modest CLS extension (Sup. Table 1). Vps51p mediates tethering of the Vps52/53/54 complex to the fusion apparatus, which suggests a regulatory role in docking.40 The docking is facilitated by the Tlg1p SNARE complex, of which Tlg2p is also a component.42 While deletion of TLG1 is lethal and thus not reported in the screens, tlg2Δ is significantly long lived according to the high stringency criteria (Fig. 3C). Neither vps51Δ nor tlg2Δ resulted in a significant increase in RLS, however, further supporting the idea that chronological and replicative aging in yeast are in part mechanistically distinct (Fig. 6).
Figure 6.
Deletion of VPS51 or TLG2 does not significantly increase RLS. Two gene-deletions (vps51Δ and tlg2Δ) which resulted in extended chronological lifespan mediate Golgi-associated retrograde trafficking. The survival curves represent pooled lifespan data from multiple experiments with experiment matched wild type (BY4742) lifespan data. The mean replicative lifespan along with the number of mother cells examined is shown next to the strain name.
We next examined the lifespan of the vps51Δ in the haploid BY4742 background. Compared to the parental wild type strain, a decrease in the rate of mortality was observed in the haploid deletion strain similar to that observed in the homozygous diploid deletion (Fig. 7), indicating that the effect on lifespan extension is likely not dependent on ploidy. To determine if deletion of any of the other members of the GARP complex increase yeast CLS, we tested the single-gene deletion strains of the remaining three GARP complex members (vps52Δ, vps53Δ and vps54Δ; Fig. 7). While none could extend CLS to the same extent as vps51Δ in the BY4742 background, a modest extension was observed in the vps52Δ strain.
Figure 7.
Deletion of components of the GARP complex increase yeast CLS. vps51Δ, a strain that lacks a regulatory unit of the Golgi-associated retrograde protein complex, was identified as extremely long-lived. Mutation in the genes encoding the remaining complex components (VPS52, VPS53 and VPS54) did not have a similar degree of lifespan extension, indicating a role for the regulation of the Vps52/53/54 complex with the t-SNARE in yeast CLS.
Two gene deletion strains, hom6Δ and trp5Δ, which are involved in amino acid biosynthesis, also showed a dramatic lifespan extension compared to wild type (Fig. 5B and C). HOM6, the yeast homoserine dehydrogenase, is required for the biosynthesis of methionine and threonine from aspartate. Threonine is also an intermediate in the biosynthesis of valine, leucine and isoleucine. Trp5 catalyzes the production of tryptophan from indole-3-glycerol phosphate. Previously, the availability of asparagine and glutamate had been implicated in CLS extension,27 and it will be interesting to determine whether allosteric regulation of amino acid biosynthesis is implicated in the yeast response to acetic acid stress or to aging in other organisms.
Analysis of short-lived deletion strains.
In addition to increased CLS, our analysis also uncovered several short-lived single-gene deletion strains. Using the method of analysis for pooled data described above, 19/227 (8.4%) randomly selected gene deletions were significantly short lived compared to the pooled wild-type lifespan (student's t-test, p ≤ 0.00009, Sup. Table 6). Among yeast strains whose deletion corresponds to a homologous worm aging gene, 40/235 (17%) were determined to be significantly shorter than wild-type (Sup. Table 6). 1/47 (2.1%) and 10/76 (13.2%) of the replicatively long-lived deletions and strains identified from the pH screen, respectively, were chronologically short-lived.
The list of short-lived strains was submitted for analysis using GO Termfinder (see above) to determine if any significant GO terms were enriched in the short-lived strains. Not surprisingly, a large number of terms related to cellular respiration were represented among those gene-deletion strains, including mitochondrial organization, mitochondrial transcription, energy coupled proton transport, ATP synthase activity and holocytochrome-c synthase activity. This is consistent with previous findings indicating that proper respiratory function is required for normal survival in the CLS assay.28
Discussion
Invertebrate model organisms have made a tremendous contribution to understanding the aging process in mammals. While clear evidence exists for conserved longevity pathways that modulate aging in yeast, worms, flies and mice, some aspects of aging are likely to be specific to each organism.1 In order to determine which genes modulating yeast CLS play a similar role in other model systems, we analyzed the CLS of 550 single-gene deletion strains consisting of randomly selected strains, homologs of C. elegans aging genes, replicatively long-lived deletion strains and strains identified from a screen for reduced acidification of the culture medium. This analysis failed to detect significant overlap between strains with increased CLS and strains lacking either homologs of C. elegans aging genes or strains with increased RLS. In contrast, strains that showed reduced acidification of the culture medium were more likely to be chronologically long-lived, relative to randomly selected strains. These data indicate that culture pH is likely to be a predictor of CLS, while RLS or homology to a gene that modulates life span in C. elegans is not.
The observation that yeast deletions corresponding to homologs of C. elegans aging genes are more likely to have increased replicative life span than increased CLS is at first surprising, given that it is reasonable to expect that aging mechanisms would be more likely to be shared between C. elegans and yeast CLS, since both are primarily post-mitotic systems. The apparent lack of conservation between C. elegans aging genes and factors that affect yeast CLS may arise, at least in part, from the fact that several of the known C. elegans aging genes code for proteins important in mitochondrial function.43,44 Most yeast mutants defective for mitochondrial function have reduced CLS due to an inability to appropriately induce respiration upon glucose depletion during the diauxic shift. In this regard, our data are consistent with two recent screens of the yeast ORF deletion collection that have demonstrated a link between compromised mitochondrial function and diminished yeast chronological viability.45,46 Our lifespan data on strains harboring deletions in the GARP complex diverge slightly from those of Fabrizio et al. who show that Vps-dependent protein degradation is essential for stationary phase viability,45 as we have identified vps51Δ as a reproducibly long-lived deletion strain (Fig. 5C). The GARP complex recycles macromolecules from both early and late endosomes to the trans-Golgi network.40
Although we failed to detect a significant overlap between genes that modulate chronological longevity and genes that modulate longevity in C. elegans, it has been noted previously that overlap does exist.16 In particular, specific components of nutrient- and stress-responsive pathways are known to influence aging in both systems. These include the TOR1/let-363 and SCH9/rsks-1 homolog pairs. In both cases, the encoded kinases are thought to play an important role in the response to dietary restriction and both tor1Δ and sch9Δ cells are also replicatively long-lived.47 It will be important to determine whether the downstream mechanisms by which this pathway modulates longevity in each of these systems is also conserved.
The homozygous diploid collection was chosen for this study, rather than one of the haploid mating types, to facilitate comparison with C. elegans, which is a diploid organism. The diploid deletion collection has been used previously for chronological lifespan screens,27 and our prior work has shown that acetic acid toxicity limits CLS in both diploid and haploid cells.11 A limitation to this approach, however, is that yeast deletions with increased replicative life span have been identified, almost exclusively, from the haploid deletion collections. In most cases, it has not been formally demonstrated that homozygous deletion of these genes results in increased replicative life span. Thus, some caution is warranted when evaluating the overlap between genetic control of replicative and chronological aging from this study.
Identification of new regulators of chronological life span.
In the course of this study, we identified 34 single-gene deletion strains that showed increased CLS in at least three independent replicate experiments comprising at least nine biological replicate aging cultures. By loosening the criteria to those strains that exhibited increased CLS in at least 2/3 of the replicate experiments, this list was expanded to include 89 strains. Interestingly, while the tor1Δ strain was identified as long-lived by the latter, lower stringency criteria, the effect of TOR1 deletion was often modest and not significantly different from the wild type parental strain in every experiment (Sup. Table 1). This may be due, in part, to the presence of a second TOR kinase in yeast, which is encoded by the essential TOR2 gene.
Two recent studies report screening of the deletion collection for strains with altered CLS by growing pooled cultures of mutants and identifying disproportionate survivors after fixed periods of time.45,46 Both studies uncovered a trend for reduced CLS among mutants defective for autophagy and mitochondrial respiration, and our data further support the idea that mitochondrial function is critical for ensuring full CLS. Both studies also identified novel long-lived deletion mutants: Fabrizio et al. report that acb1Δ, cka2Δ, trm9Δ, ydr417cΔ and aro7Δ cells are long-lived, and Matetic et al. report increased CLS for fcy2Δ, lcl1Δ, dcw1Δ, snx41Δ, lcl2Δ, ade3Δ, mum2Δ, sas4Δ, uaf30Δ, sds23Δ, lcl3Δ and ade4Δ.45,46 The fact that these two studies did not identify similar gene sets among the strains with increased CLS suggests that many additional long-lived deletion mutants remain to be identified.
There are fundamental differences between these competition microarray-based screens and the quantitative large-scale longevity analysis presented in this report. Most notably, both prior screens were performed under conditions where strains were co-cultured together during the aging assay.45,46 The extracellular milieu is known to be a potent effector of CLS, and it is possible that co-culture of multiple strains alters the environment in unpredictable ways. Pooling strategies may also mask the effect of any long-lived strain whose deletion results in a severe slow growth phenotype or amplify the effect of mutants that grow to a higher density than wild type cells. Among the 17 total deletion strains validated as having increased CLS from Fabrizio et al. and Matecic et al. none were tested as part of our study.
Chronological lifespan and aging in other model systems.
There are several conclusions that can be reached from our findings with regard to the utility of the CLS assay as a means to understand aging in other eukaryotic species. First, acidification of the growth medium is a significant determinant of chronological aging under conditions of growth in synthetic media. We cannot rule out a model whereby a similar molecular mechanism promotes aging in other organisms, although we know of no supporting evidence. We agree with statements from colleagues that the cellular stress induced by media acidification, which may promote oxidative stress and certainly promotes apoptosis, may be similar to that occurring during aging in other organisms.14
Second, it appears that there is not a significant overlap between the genes determining CLS and those determining C. elegans lifespan, at least under the conditions employed here. This may be condition-dependent, and we believe it is now important to determine whether other experimental paradigms (e.g., aging in rich medium such as YEPD, in water, in buffered synthetic medium, or in conditions of amino acid starvation) could be employed to enhance levels of conservation. Indeed, it may be of benefit to assay the yeast chronological lifespan under multiple media conditions. Nothing precludes turning the experimental approach of this study inside out. Instead of determining whether there is conservation in one setting, these ortholog sets could be used to determine which CLS assay conditions most resembles aging in other eukaryotes.
Finally, although we did not detect a significant overlap between genes that modulate chronological aging and those that modulate replicative aging, a trend in this direction was observed. This trend, in combination with the prior observation that chronologically aged cells have a reduced RLS,48 suggests that at least some aspects of aging are shared at a molecular level in both yeast paradigms. Likely candidates for these shared molecular causes of aging include oxidatively damaged proteins and damaged mitochondria, both of which have also been implicated in aging of multicellular eukaryotes. Future studies characterizing the mechanisms linking chronological and replicative aging, and whether this link is independent of media acidification, will likely be informative.
Materials and Methods
Ortholog identification.
Algorithms for determining yeast homologs of C. elegans aging genes have been previously described in reference 32. Briefly, homologs were identified by querying the C. elegans protein sequence in a BLASTp search against the Saccharomyces Genome Database (SGD, www.yeastgenome.org). Because of the lack of definitive parameters in assigning orthologous gene pairs, a multiple stringency strategy was employed to classify candidate yeast aging genes. Candidates that met a reciprocal BLASTp match that satisfied a requirement of 20% sequence identity and 20% sequence alignment to the C. elegans protein were defined as the reciprocal best hit group (RBH). To account for an ancient yeast genomic duplication, any yeast protein that had a BLASTp score within 10% of the best match was also included in this group. The set of yeast aging orthologs referred to as the related protein set (RP) was defined as the top six BLASTp matches that contained at least 20% sequence identity and 10% sequence alignment to the nematode protein sequence. It is important to note that the RP set includes the complete RBH set. The RP set resulted in a list of 393 yeast genes, 264 of which were non-essential. For the analysis presented in this report, 235 of the 264 related proteins were assayed for CLS.
Yeast strains and media.
Unless otherwise stated, all experiments were performed with Saccharomyces cerevisiae diploid strain BY4743 (MATa/α his3Δ1 leu2Δ0 ura3Δ0) and isogenic homozygous deletion strains from the yeast ORF deletion collection.34 CLS assays were performed as previously described using the Bioscreen C MBR automated shaker/incubator/plate reader.23,49,50 All aging cultures were initiated by seeding a 5 ml liquid culture of YEPD with a single colony from a freshly streaked strain grown on YEPD agar at 30°C. A 1:100 dilution of the YEPD culture was made into synthetic complete (SC) medium, containing 2% glucose, unless otherwise noted. Basic medium is 1.7 g/L Yeast Nitrogen Base (-AA/-AS) (BD Difco™) and 5 g/L (NH4)2SO4. Components of the SC medium used in this study have been described elsewhere in detail in reference 23. All strain auxotrophies were compensated with a four-fold excess of amino acids. Cultures were grown and aged in a roller drum enclosed in a water-jacketed incubator at 30°C. YEPD was 20 g/L Bacto Peptone and 10 g/L Yeast Extract (BD Difco™), supplemented with glucose at the indicated concentrations.
Quantitative chronological lifespan analysis.
Lifespan data was collected using a Bioscreen C MBR automated incubator/plate reader to monitor the outgrowth kinetics of chronologically aged cultures in a synthetic complete medium supplemented with 2% glucose, as previously described in reference 23, 49 and 50. Chronological viability was calculated from growth curves of aging cultures using the Yeast Outgrowth Data Analyzer (YODA, www.sageweb.org/yoda).51 The survival integrals were calculated using the “cleaned” algorithm on YODA, meaning that the calculation does not allow for increases in the fraction viable at any age point, and the doubling time was calculated by the “interval” method. The calculation parameters were as follows: Threshold ODs (Min = 0.100, Max = 2.000); Doubling Time Interval OD (Min = 0.200, Max = 0.500); Doubling Time Adjustment [Delay OD = 0.500, Slope = 0.0261, Min Delay (sec) = 500]; Survival Time Shift (OD = 0.300). This method allowed high-throughput determination of the survival integral (SI), defined as the area under the mortality curve, for all strains in biological triplicate. SI provides a quantitative measure of the CLS and allows for statistical analysis between deletion strains and a wild type control.49,50
For all experiments, outgrowth data was normalized to the initial time point collected on the second day of chronological aging (thus day 2 represents 100% culture viability). Though rare, a few deletion strains exhibited outgrowth readings indicating continued growth past day 2 (rising above 100% viability in the mortality curve). Such cases, as well as strains exhibiting significant growth past day 2, were eliminated from the statistical analysis, since the SI is artificially inflated by additional culture growth (indicated by red outline in Sup. Tables 1–6). Data was collected for most slow-growing strains by re-assaying the lifespan; however, in five strains (cpa2Δ, fun12Δ, hfi1Δ, lys12Δ and rvs161Δ) slow growth prohibited determination of lifespan, and these strains were eliminated from the analysis. Increased viability at day 4 was associated with larger doubling-times as calculated from the growth curves (mean doubling-time of excluded runs = 137.4 min, as compared to 103.9 min for included runs, p < 1 × 10−18, Sup. Tables 1–4).
pH screen, random screen and yeast homologs to C. elegans aging genes.
The homozygous diploid ORF deletion collection (Open Biosystems, Huntsville, AL, USA34) was replica-plated into fresh 96-well plates containing 100 ul of YEPD (2% glucose) and incubated overnight at 30°C. 1 µl of the YEPD overnight culture was inoculated into new 96-well plates containing 200 µl of standard SC 2% medium. These plates were incubated at 30°C for four days. Approximately 100 µl of cell free supernatant was removed from each well and transferred to a new 96-well plate. 20 µl of a 0.1% bromophenol blue pH indicator solution was added to 100 µl of the aged culture supernatant and absorbance at 600 nm was recorded using a Wallace Victor2 plate reader (Perkin Elmer, Waltham, MA, USA). An arbitrary absorbance unit was used to identify yeast strains that exhibited reduced acidification of the culture supernatant. Strains identified from the high-throughput screen were grown in 5 ml liquid cultures to verify the culture pH using a pH meter. 80 strains that were determined to have a culture pH ≥ 3.0 were submitted for CLS analysis.
The list of homozygous diploid deletion strains was numbered and a random number generator (MATLAB) was used to randomize the list, using the time of day method as a seed for randomization. The first 227 genes in the randomized table that could be grown to stationary phase were used in the random screen to ascertain the expected frequency of long-lived deletions in the homozygous diploid collection. The identification of yeast deletion strains corresponding to genes that are homologous to C. elegans aging genes was previously published in reference 32. The 237 single-gene deletion strains assayed for CLS corresponded to the “related proteins” (RP), which consists of the top six genes most homologous to a worm aging gene in a BLASTp search, with at least 20% sequence identity and 10% amino acid alignment.
Acknowledgments
The authors would like to acknowledge Dr. M. McCormick and Dr. E. Smith for helpful discussion and B. Robison for technical support. This work was supported by a pilot project grant from the University of Washington Nathan Shock Center of Excellence in the Basic Biology of Aging (NIH P30AG013280), NIH grants R21AG031965 and R01AG025549 and support from the Ellison Medical Foundation to M.K. C.R.B. was supported by the Howard Hughes Medical Institute through the Med into Grad Initiative and by NIH Grant T32AG000057. M.K. is an Ellison Medical Foundation New Scholar in Aging.
Abbreviations
- RLS
replicative lifespan
- CLS
chronological lifespan
- ROS
reactive oxygen species
- SI
survival integral
Supplementary Material
References
- 1.Kaeberlein M. Lessons on longevity from budding yeast. Nature. 2010;464:513–519. doi: 10.1038/nature08981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinkraus KA, Kaeberlein M, Kennedy BK. Replicative aging in yeast: the means to the end. Annu Rev Cell Dev Biol. 2008;24:29–54. doi: 10.1146/annurev.cellbio.23.090506.123509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinclair DA, Guarente L. Extrachromosomal rDNA circles—a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 4.Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- 5.Jazwinski SM. Yeast longevity and aging—the mitochondrial connection. Mech Ageing Dev. 2005;126:243–248. doi: 10.1016/j.mad.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Dang W, Steffen KK, Perry R, Dorsey JA, Johnson FB, Shilatifard A, et al. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature. 2009;459:802–807. doi: 10.1038/nature08085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabrizio P, Longo VD. The chronological life span of Saccharomyces cerevisiae. Aging Cell. 2003;2:73–81. doi: 10.1046/j.1474-9728.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 8.Fabrizio P, Liou LL, Moy VN, Diaspro A, Valentine JS, Gralla EB, et al. SOD2 functions downstream of Sch9 to extend longevity in yeast. Genetics. 2003;163:35–46. doi: 10.1093/genetics/163.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herker E, Jungwirth H, Lehmann KA, Maldener C, Frohlich KU, Wissing S, et al. Chronological aging leads to apoptosis in yeast. J Cell Biol. 2004;164:501–507. doi: 10.1083/jcb.200310014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockenfeller P, Madeo F. Apoptotic death of ageing yeast. Exp Gerontol. 2008;43:876–881. doi: 10.1016/j.exger.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burtner CR, Murakami CJ, Kennedy BK, Kaeberlein M. A molecular mechanism of chronological aging in yeast. Cell Cycle. 2009;8:1256–1270. doi: 10.4161/cc.8.8.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludovico P, Sousa MJ, Silva MT, Leao C, Corte-Real M. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology. 2001;147:2409–2415. doi: 10.1099/00221287-147-9-2409. [DOI] [PubMed] [Google Scholar]
- 13.Ludovico P, Rodrigues F, Almeida A, Silva MT, Barrientos A, Corte-Real M. Cytochrome c release and mitochondria involvement in programmed cell death induced by acetic acid in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:2598–2606. doi: 10.1091/mbc.E01-12-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burhans WC, Weinberger M. Acetic acid effects on aging in budding yeast: are they relevant to aging in higher eukaryotes? Cell Cycle. 2009;8:2300–2302. doi: 10.4161/cc.8.14.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaeberlein M, Burtner CR, Kennedy BK. Recent developments in yeast aging. PLoS Genet. 2007;3:84. doi: 10.1371/journal.pgen.0030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- 18.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Weindruch RH, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield IL: Thomas; 1988. [Google Scholar]
- 20.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 23.Murakami CJ, Burtner CR, Kennedy BK, Kaeberlein M. A method for high-throughput quantitative analysis of yeast chronological life span. J Gerontol A Biol Sci Med Sci. 2008;63:113–121. doi: 10.1093/gerona/63.2.113. [DOI] [PubMed] [Google Scholar]
- 24.Smith DL, Jr, McClure JM, Matecic M, Smith JS. Calorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently of the Sirtuins. Aging Cell. 2007;6:649–662. doi: 10.1111/j.1474-9726.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- 25.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 26.Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 27.Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007;5:265–277. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 30.Pan KZ, Palter JE, Rogers AN, Olsen A, Chen D, Lithgow GJ, et al. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell. 2007;6:111–119. doi: 10.1111/j.1474-9726.2006.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith ED, Tsuchiya M, Fox LA, Dang N, Hu D, Kerr EO, et al. Quantitative evidence for conserved longevity pathways between divergent eukaryotic species. Genome Res. 2008;18:564–570. doi: 10.1101/gr.074724.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang DY, Kumar S, Hedges SB. Divergence time estimates for the early history of animal phyla and the origin of plants, animals and fungi. Proc Biol Sci. 1999;266:163–171. doi: 10.1098/rspb.1999.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- 35.Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, et al. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008;4:13. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madia F, Gattazzo C, Wei M, Fabrizio P, Burhans WC, Weinberger M, et al. Longevity mutation in SCH9 prevents recombination errors and premature genomic instability in a Werner/Bloom model system. J Cell Biol. 2008;180:67–81. doi: 10.1083/jcb.200707154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Managbanag JR, Witten TM, Bonchev D, Fox LA, Tsuchiya M, Kennedy BK, et al. Shortest-path network analysis is a useful approach toward identifying genetic determinants of longevity. PLoS ONE. 2008;3:3802. doi: 10.1371/journal.pone.0003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Genes determining yeast replicative life span in a long-lived genetic background. Mech Ageing Dev. 2005;126:491–504. doi: 10.1016/j.mad.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Steffen KK, MacKay VL, Kerr EO, Tsuchiya M, Hu D, Fox LA, et al. Yeast life span extension by depletion of 60s ribosomal subunits is mediated by Gcn4. Cell. 2008;133:292–302. doi: 10.1016/j.cell.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conibear E, Cleck JN, Stevens TH. Vps51p mediates the association of the GARP (Vps52/53/54) complex with the late Golgi t-SNARE Tlg1p. Mol Biol Cell. 2003;14:1610–1623. doi: 10.1091/mbc.E02-10-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siniossoglou S, Pelham HR. Vps51p links the VFT complex to the SNARE Tlg1p. J Biol Chem. 2002;277:48318–48324. doi: 10.1074/jbc.M209428200. [DOI] [PubMed] [Google Scholar]
- 42.Coe JG, Lim AC, Xu J, Hong W. A role for Tlg1p in the transport of proteins within the Golgi apparatus of Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:2407–2423. doi: 10.1091/mbc.10.7.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, et al. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- 44.Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- 45.Fabrizio P, Hoon S, Shamalnasab M, Galbani A, Wei M, Giaever G, et al. Genome-wide screen in Saccharomyces cerevisiae identifies vacuolar protein sorting, autophagy, biosynthetic and tRNA methylation genes involved in life span regulation. PLoS Genet. 2010;6:1001024. doi: 10.1371/journal.pgen.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matecic M, Smith DL, Pan X, Maqani N, Bekiranov S, Boeke JD, et al. A microarray-based genetic screen for yeast chronological aging factors. PLoS Genet. 2010;6:1000921. doi: 10.1371/journal.pgen.1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashrafi K, Sinclair D, Gordon JI, Guarente L. Passage through stationary phase advances replicative aging in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1999;96:9100–9105. doi: 10.1073/pnas.96.16.9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burtner CR, Murakami CJ, Kaeberlein M. A genomic approach to yeast chronological aging. Methods Mol Biol. 2009;548:101–114. doi: 10.1007/978-1-59745-540-4_6. [DOI] [PubMed] [Google Scholar]
- 50.Murakami C, Kaeberlein M. Quantifying yeast chronological life span by outgrowth of aged cells. J Vis Exp. 2009;27:1156. doi: 10.3791/1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olsen B, Murakami CJ, Kaeberlein M. YODA: Software to facilitate high-throughput analysis of chronological life span, growth rate and survival in budding yeast. BMC bioinformatics. 2010;11:141. doi: 10.1186/1471-2105-11-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.