Abstract
It has long been known that excesses of glucose and branched chain amino acids, such as leucine, lead to insulin resistance in skeletal muscle. A recent study in incubated rat muscle suggests that both molecules may do so by virtue of their ability to downregulate the fuel sensing and signaling enzyme AMP-activated protein kinase (AMPK) and activate mTOR/p70S6 kinase (p70S6K) signaling. The results also demonstrated that inhibition of mTOR/p70S6K with rapamycin prevented the development of insulin resistance but had no effect on AMPK activity (Thr172 phosphorylation of its catalytic subunit). In contrast, activation of AMPK by both AICAR and α-lipoic acid led to the phosphorylation of specific molecules that diminished both mTOR/p70S6K signaling and insulin resistance. These findings suggest that downregulation of AMPK precedes mTOR/p70S6K activation in mediating glucose and leucine-induced insulin resistance, although the mechanism by which it does so remains to be determined. Also requiring study is how an excess of the two nutrients leads to AMPK downregulation.
Key words: insulin resistance, AMP-activated protein kinase, mTOR/p70S6K, protein synthesis, branched chain amino acids
It has long been appreciated that nutrient excess leads to insulin resistance in many tissues. This has perhaps best been illustrated in rodent skeletal muscle. Thus, prior incubation with either an elevated concentration of glucose,1 or leucine added to a normal glucose medium,2 decreases the ability of insulin to activate Akt and stimulate glucose uptake and/or its incorporation into glycogen. In the case of leucine, this effect has been attributed to activation of mTOR/p70S6K, which, in turn, leads to the phosphorylation of serine residues on IRS1 (S307, S635) 2,3 that impair insulin signaling. In contrast, the inhibitory effect of high glucose has been attributed to IRS phosphorylation caused by activation of one or more protein kinase C (PKC) isoforms.1,4 The latter, in turn, has been attributed to a decrease in the fuel-sensing enzyme AMPK5 that leads to impaired fatty acid oxidation and increases in the concentration of the PKC activator diacylglycerol.6 Implicit in these explanations is that high glucose and leucine initiate insulin resistance by different mechanisms, but with serine phosphorylation of IRS1 a likely terminal occurrence.
In a recent paper,7 we compared the events associated with insulin resistance in rat extensor digitorum longus (EDL) muscles preincubated with a high concentration of glucose (25 vs. 5 mM) or a medium containing 5 mM glucose ± 100 or 200 µM leucine for 30–60 min. The results strongly suggested that glucose and leucine initiate insulin resistance by a common mechanism. Thus, both molecules increased mTOR/p70S6 kinase phosphorylation, and rapamycin inhibited these changes as well as the insulin resistance. Likewise, they each decreased AMPK activity, and coincubation with two distinct AMPK activators, AICAR and α-lipoic acid, prevented the insulin resistance and increase in mTOR/p70S6K phosphorylation. Finally, both glucose and leucine increased the release of lactate and pyruvate from the muscle as well as the lactate/pyruvate ratio, suggesting that they had also enhanced the generation of ATP by glycolysis and the NADH/NAD ratio in the cytosol.
A logical next question is how do the above-mentioned events, altered by glucose and leucine, interact to cause insulin resistance. One possibility is that they do so by decreasing AMPK activity, which, in turn, increased the activation of mTOR/p70S6K. In keeping with this notion, AMPK activation has been demonstrated to phosphorylate TSC2 (Ser1387) and mTOR (Ser 2448),8 leading to inhibition of mTOR/p70S6K signaling, and both of the AMPK activators used in this study increased phosphorylation at these sites. Furthermore, decreased AMPK is associated with insulin resistance in numerous situations in vivo,9,10 and AMPK activation has been shown to enhance insulin sensitivity by effects on multiple factors, including DAG/PKC, ceramide, inflammation, ER stress and mitochondrial function as well as mTOR/p70S6K.11–13 Against this notion, we did not find diminished phosphorylation of TSC2 (Ser1387) and mTOR (Ser 2448) in muscle incubated with high glucose or leucine despite the decrease in AMPK activity. On the other hand, the low baseline levels of phosphorylation of these molecules could have caused us to miss a small decrease. Thus, the question of whether AMPK downregulation is responsible for, or at least a major contributor to, mTOR/p70S6K activation by high glucose or leucine is still open.
Irrespective of whether the decrease in AMPK is a proximal event in leucine-as well as glucose-induced insulin resistance, an intriguing question is how do incubations of muscle with excesses of either fuel lead to a decrease in AMPK activity? One possibility is that they do so by increasing the energy state of the cell and another that they alter its redox state and, secondarily, SIRT1. In addition, they could have diminished AMPK by effects on specific phosphatases or kinases. Against the notion that glucose and leucine increase the muscle's energy state, no decreases in the AMP/ATP ratio or increases in creatine-PO4 were found in the EDL. On the other hand, the finding that lactate and pyruvate release were elevated is compatible with such an explanation, since it suggests more ATP was generated in the cytosol by glycolysis. This, as well as a decrease in AMP, could have been missed in whole tissue measurements.
A second possibility is that increases in the redox state of the cell diminished AMPK activity by decreasing the activity of the NAD+-dependent histone/protein deactylase SIRT1, an enzyme that has been shown to activate the AMPK kinase LKB1, and, secondarily, AMPK, in other cells.14–16 In keeping with this notion, we have found that incubation with both high glucose and glucose plus leucine results in an increase in the lactate/pyruvate ratio (Saha, Diabetes, 2010) and a decrease in the activity of the rate-limiting enzyme for SIRT1 activation, nicotinamide phosphoribosyltransferase (NAMPT). On the other hand, the decrease in NAMPT appeared to follow rather than precede the decrease in AMPK activity, suggesting, if anything, that it is the result rather than the cause of AMPK downregulation (Saha et al., unpublished).
With respect to the potential role of phosphatases in regulating AMPK activity, both PP2A and PP2C have been shown to dephosphorylate and deactivate AMPK in vitro.17,18 In addition, it has been demonstrated that TNFα suppresses AMPK activity by transcriptionally upregulating PP2C when it causes insulin resistance in rodent skeletal muscle in vivo,19 and elevating plasma palmitate has been shown to have similar effects on PP2A and AMPK activity in rat aorta.20 Likewise incubation of pancreatic β cells in a high-glucose medium has been demonstrated to decrease AMPK phosphorylation by activating both PP2A21 and PP1, the latter by phosphorylating its regulatory subunit.22 Whether glucose has similar effects in muscle and other tissues is not known nor has the effect of leucine on phosphatases been examined.
Finally, increased phosphorylation of AMPK at Ser485/491 of its α subunit has been shown to correlate with inhibition of AMPK activity during both insulin and cAMP-mediated signaling.23 It has been hypothesized that phosphorylation of AMPKα1/α2 at these sites diminishes its interactions with upstream kinases, such as LKB1, that phosphorylate and activate it.24,25 Whether such phosphorylation and inhibition of AMPK occurs in muscle incubated with an excess of glucose or leucine also has not been studied.
Many questions remain about these studies in incubated muscle. One of them is their physiological relevance. With respect to glucose, it has clearly been shown that prolonged (3–8 h) hyperglycemia (ca 13 mM), produced by infusing glucose into a rat, downregulates AMPK activity and subsequently causes insulin resistance in its liver, muscle and adipose tissue (ref. 9 and 26 and Gauthier MS, unpublished). Also, where examined these changes have been associated with increases in mTOR/p70S6K, DAG content, PKC activity and impaired Akt activation by insulin.26 Whether they are also associated with changes in sirtuins or kinases and phosphatases that alter AMPK activity has not been reported. Studies in humans are even more limited. Excess calorie ingestion for 30 d leads to a sustained weight gain and insulin resistance in muscle of human volunteers;27 however, the relative roles of excesses of glucose and amino acids were not specifically studied. On the other hand, insulin resistance has been demonstrated in humans following a 68 h glucose infusion at a rate sufficient to maintain plasma glucose levels at 12.6 mM.28 In neither, this nor the earlier 30 d study were AMPK activity or mTOR/p70S6K measured.
Whether an excess of leucine or branched chain amino acids (BCAA) can similarly cause insulin resistance in vivo is less clear. It has long been known that increased levels of BCAA in humans accompany insulin resistance and obesity,29 and more recently, they have been associated with a predisposition to diabetes.30 Whether high levels of BCAA were a causal factor or the result of insulin resistance was not examined in either study, nor were mTOR/p70S6K and AMPK evaluated. On the other hand, infusion of an amino acid mixture (25% BCAA) into normal human volunteers has been shown acutely to cause insulin resistance and elevate S6 kinase in skeletal muscle.31 To our knowledge, AMPK activity was not measured in these subjects. Likewise, in a chronic study, in which branched chain amino acids were administered to Wistar rats fed a high-fat diet, insulin resistance accompanied by chronic phosphorylation of mTOR and of IRS1 on Ser307, all of which were reversed by rapamycin, was observed.32 In contrast, in a study performed on C57Bl6 mice, insulin resistance caused by ingesting a high-fat diet was diminished by doubling dietary leucine intake.33 Interestingly, leucine did so despite the fact that it caused an increase in insulin-stimulated phosphorylation of p70S6K in muscle, liver and perigonadal fat, although it did increase AMPK phosphorylation on T172 (activation) in muscle, the only tissue in which it was measured.
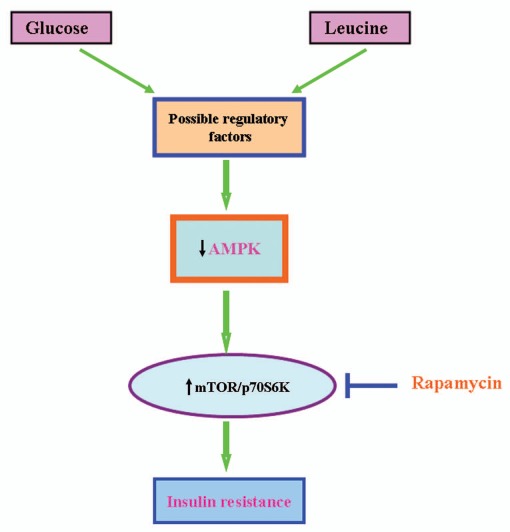
A schematic depiction of the hypothetical mechanisms by which nutrient excess (glucose or leucine) rapidly leads to decreased AMPK activity and, secondarily, mTOR/p70S6K activation and insulin resistance in incubated rat extensor digitorum longus muscle is presented in Figure 1. Multiple aspects of this schema have yet to be clarified. On the other hand, it is clear that mTOR/p70S6K activation mediates insulin resistance, and that it is associated with decreased AMPK activity and is prevented by AMPK activation. Whether other events attributable to decreased AMPK activity, such as increased DAG/PKC and JNK signaling,34 ceramide accumulation or mitochondrial dysfunction, contribute to the insulin resistance observed here, whether or they do so after longer periods of nutrient excess, remains to be determined.
Figure 1.
Hypothetical mechanisms by which excess glucose or leucine lead to decreased AMPK activity and, secondarily, mTOR/p70S6K activation. High glucose and leucine modulate several regulatory factors that could downregulate AMPK, including cellular energy and redox state. In addition, they could activate phosphatases and kinases that lead to decreased AMPK activity. Activation of mTOR/p70S6K can lead to insulin resistance by several mechanisms, including serine phosphorylation of key sites on IRS-1, activation of genes regulating glycolysis that could alter cellular energy and redox state, increased lipid synthesis47,48 and oxidative stress. The inhibition of insulin resistance by rapamycin strongly suggests that in the time frame studied here, changes in mTOR/p70S6K are likely the key factor responsible for insulin resistance. Finally, for reasons described in the text, we believe that decreased AMPK activity, is a major factor responsible for the activation of mTOR/p70S6K by nutrient excess; however, definitive proof of this is still lacking.
It must be emphasized that the implications of these findings go well beyond skeletal muscle. Fuel-sensing mechanisms involving glucose and leucine are also present in pancreatic β cells35,36 and selected regions of the hypothalamus.37,38 Furthermore, where studied, they appear to involve mTOR36,37 and AMPK.35,38 In the case of the hypothalamus, excesses of glucose and leucine decrease AMPK activity, leading to diminished food intake38,39 and hepatic glucose production,40 effects that would theoretically counter the direct actions of a fuels excess on muscle, pancreatic β cells and, presumably, other tissues.
Finally of relevance to this review, an excess of other fuel's (e.g., FFA, high-fat diet) has been shown to impair insulin action41 and other processes regulated by AMPK, including autophagy.42 Conversely, the potential ability of caloric restriction to delay aging has been linked both to activation of SIRT1 43 and AMPK44 and downregulation of mTOR.45,46 Future studies are needed to define the interrelation of these observations to those described here.
Acknowledgments
The work in the author's laboratory was supported by US PHS grants RO1DK19514 and RO1DK67509 and a Mentor-Based fellowship from the American Diabetes Association (to X.J.X.).
References
- 1.Kurowski TG, Lin Y, Luo Z, Tsichlis PN, Buse MG, Heydrick SJ, et al. Hyperglycemia inhibits insulin activation of Akt/protein kinase B but not phosphatidylinositol-3-kinase in rat skeletal muscle. Diabetes. 1999;48:658–663. doi: 10.2337/diabetes.48.3.658. [DOI] [PubMed] [Google Scholar]
- 2.Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. J Clin Invest. 1998;101:1519–1529. doi: 10.1172/JCI1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yip CK, Murata K, Walz T, Sabatini DM, Kang SA. Structure of the human mTOR complex I and its implications for rapamycin inhibition. Mol Cell. 2010;38:768–774. doi: 10.1016/j.molcel.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müssig K, Fiedler H, Staiger H, Weigert C, Lehmann R, Schleicher ED, et al. Insulin-induced stimulation of JNK and the PI 3-kinase/mTOR pathway leads to phosphorylation of serine 318 of IRS-1 in C2C12 myotubes. Biochem Biophys Res Commun. 2005;335:819–825. doi: 10.1016/j.bbrc.2005.07.154. [DOI] [PubMed] [Google Scholar]
- 5.Itani SI, Saha AK, Kurowski TG, Coffin HR, Tornheim K, Ruderman NB. Glucose autoregulates its uptake in skeletal muscle: involvement of AMP-activated protein kinase. Diabetes. 2003;52:1635–1640. doi: 10.2337/diabetes.52.7.1635. [DOI] [PubMed] [Google Scholar]
- 6.Ruderman NB, Saha AK, Vavvas D, Witters LA. Malonyl-CoA, fuel sensing and insulin resistance. Am J Physiol. 1999;276:1–18. doi: 10.1152/ajpendo.1999.276.1.E1. [DOI] [PubMed] [Google Scholar]
- 7.Saha AK, Xu XJ, Lawson E, Deoliveira R, Brandon AE, Kraegen EW, et al. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes. 2010;59:2426–2434. doi: 10.2337/db09-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraegen EW, Saha AK, Preston E, Wilks D, Hoy AJ, Cooney GJ, et al. Increased malonyl-CoA and diacylglycerol content and reduced AMPK activity accompany insulin resistance induced by glucose infusion in muscle and liver of rats. Am J Physiol Endocrinol Metab. 2006;290:471–479. doi: 10.1152/ajpendo.00316.2005. [DOI] [PubMed] [Google Scholar]
- 10.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3:340–351. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- 11.Cacicedo JM, Yagihashi N, Keaney JF, Jr, Ruderman NB, Ido Y. AMPK inhibits fatty acid-induced increases in NFkappaB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;324:1204–1209. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- 12.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 13.Dong Y, Zhang M, Wang S, Liang B, Zhao Z, Liu C, et al. Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes. 2010;59:1386–1396. doi: 10.2337/db09-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suchankova G, Nelson LE, Gerhart-Hines Z, Kelly M, Gauthier MS, Saha AK, et al. Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. Biochem Biophys Res Commun. 2009;378:836–841. doi: 10.1016/j.bbrc.2008.11.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies SP, Helps NR, Cohen PT, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2Calpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–425. doi: 10.1016/00145793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 18.Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS, et al. Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta. 1996;1301:67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, et al. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006;4:465–474. doi: 10.1016/j.cmet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem. 2007;282:9777–9788. doi: 10.1074/jbc. M608310200. [DOI] [PubMed] [Google Scholar]
- 21.Gimeno-Alcañiz JV, Sanz P. Glucose and type 2A protein phosphatase regulate the interaction between catalytic and regulatory subunits of AMP-activated protein kinase. J Mol Biol. 2003;333:201–209. doi: 10.1016/j.jmb.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Haro L, Garcia-Gimeno MA, Neumann D, Beullens M, Bollen M, Sanz P. The PP1-R6 protein phosphatase holoenzyme is involved in the glucose-induced dephosphorylation and inactivation of AMP-activated protein kinase, a key regulator of insulin secretion, in MIN6 beta cells. FASEB J. 2010;24:5080–5091. doi: 10.1096/fj.10-166306. [DOI] [PubMed] [Google Scholar]
- 23.Pulinilkunnil T, He H, Kong D, Asakura K, Peroni OD, Lee A, et al. Adrenergic regulation of AMP-activated protein kinase in brown adipose tissue in vivo. J Biol Chem. 2011;286:8798–8809. doi: 10.1074/jbc.M111.218719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurley RL, Barré LK, Wood SD, Anderson KA, Kemp BE, Means AR, et al. Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J Biol Chem. 2006;281:36662–36672. doi: 10.1074/jbc.M606676200. [DOI] [PubMed] [Google Scholar]
- 25.Soltys CL, Kovacic S, Dyck JR. Activation of cardiac AMP-activated protein kinase by LKB1 expression or chemical hypoxia is blunted by increased Akt activity. Am J Physiol Heart Circ Physiol. 2006;290:2472–2479. doi: 10.1152/ajp-heart.01206.2005. [DOI] [PubMed] [Google Scholar]
- 26.Brandon AE, Hoy AJ, Wright LE, Turner N, Hegarty BD, Iseli TJ, et al. The evolution of insulin resistance in muscle of the glucose infused rat. Arch Biochem Biophys. 2011;509:133–141. doi: 10.1016/j.abb.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sims EA, Bray GA, Danforth E, Jr, Glennon JA, Horton ES, Salans LB, et al. Experimental obesity in man. VI. The effect of variations in intake of carbohydrate on carbohydrate, lipid and cortisol metabolism. Horm Metab Res. 1974;4:70–77. [PubMed] [Google Scholar]
- 28.Boden G, Ruiz J, Kim CJ, Chen X. Effects of prolonged glucose infusion on insulin secretion, clearance and action in normal subjects. Am J Physiol. 1996;270:251–258. doi: 10.1152/ajpendo.1996.270.2.E251. [DOI] [PubMed] [Google Scholar]
- 29.Felig P, Marliss E, Cahill GF., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281:811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- 30.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005;54:2674–2684. doi: 10.2337/diabetes.54.9.2674. [DOI] [PubMed] [Google Scholar]
- 32.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macotela Y, Emanuelli B, Bång AM, Espinoza DO, Boucher J, Beebe K, et al. Dietary leucine—an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS ONE. 2011;6:21187. doi: 10.1371/journal.pone.0021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belgardt BF, Mauer J, Brüning JC. Novel roles for JNK1 in metabolism. Aging (Albany NY) 2010;2:621–626. doi: 10.18632/aging.100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuboi T, da Silva Xavier G, Leclerc I, Rutter GA. 5′-AMP-activated protein kinase controls insulin-containing secretory vesicle dynamics. J Biol Chem. 2003;278:52042–52051. doi: 10.1074/jbc.M307800200. [DOI] [PubMed] [Google Scholar]
- 36.Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ. The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J Biol Chem. 2007;282:10341–10351. doi: 10.1074/jbc.M610631200. [DOI] [PubMed] [Google Scholar]
- 37.Stefater MA, Seeley RJ. Central nervous system nutrient signaling: the regulation of energy balance and the future of dietary therapies. Annu Rev Nutr. 2010;30:219–235. doi: 10.1146/annurev.nutr.012809.104723. [DOI] [PubMed] [Google Scholar]
- 38.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 39.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 40.Ono H, Pocai A, Wang Y, Sakoda H, Asano T, Backer JM, et al. Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. J Clin Invest. 2008;118:2959–2968. doi: 10.1172/JCI34277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banks AS, Kon N, Knight C, Matsumoto M, Gutiérrez-Juárez R, Rossetti L, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armour SM, Baur JA, Hsieh SN, Land-Bracha A, Thomas SM, Sinclair DA. Inhibition of mammalian S6 kinase by resveratrol suppresses autophagy. Aging (Albany NY) 2009;1:515–528. doi: 10.18632/aging.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guarente L. Sirtuins in aging and disease. Cold Spring Harb Symp Quant Biol. 2007;72:483–488. doi: 10.1101/sqb.2007.72.024. [DOI] [PubMed] [Google Scholar]
- 44.Fulco M, Sartorelli V. Comparing and contrasting the roles of AMPK and SIRT1 in metabolic tissues. Cell Cycle. 2008;7:3669–3679. doi: 10.4161/cc.7.23.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pani G. P66SHC and ageing: ROS and TOR? Aging (Albany NY) 2010;2:514–518. doi: 10.18632/aging.100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blagosklonny MV. Calorie restriction: decelerating mTOR-driven aging from cells to organisms (including humans) Cell Cycle. 2010;9:683–688. doi: 10.4161/cc.9.4.10766. [DOI] [PubMed] [Google Scholar]
- 47.Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol Metab. 2011;22:94–102. doi: 10.1016/j.tem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yecies JL, Manning BD. Transcriptional control of cellular metabolism by mTOR signaling. Cancer Res. 2011;71:2815–2820. doi: 10.1158/0008-5472.CAN-10-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]