Abstract
Peri-partum cardiomyopathy (PPCM) is a frequently fatal disease that affects women near delivery, and occurs more frequently in women with pre-eclampsia and/or multiple gestation. The etiology of PPCM, or why it associates with pre-eclampsia, remains unknown. We show here that PPCM is associated with a systemic angiogenic imbalance, accentuated by pre-eclampsia. Mice that lack cardiac PGC-1α, a powerful regulator of angiogenesis, develop profound PPCM. Importantly, the PPCM is entirely rescued by pro-angiogenic therapies. In humans, the placenta in late gestation secretes VEGF inhibitors like soluble Flt1 (sFlt1), and this is accentuated by multiple gestation and pre-eclampsia. This anti-angiogenic environment is accompanied by sub-clinical cardiac dysfunction, the extent of which correlates with circulating levels of sFlt1. Exogenous sFlt1 alone caused diastolic dysfunction in wildtype mice, and profound systolic dysfunction in mice lacking cardiac PGC-1α. Finally, plasma samples from women with PPCM contained abnormally high levels of sFlt1. These data strongly suggest that PPCM is in large part a vascular disease, caused by excess anti-angiogenic signaling in the peri-partum period. The data also explain how late pregnancy poses a threat to cardiac homeostasis, and why pre-eclampsia and multiple gestation are important risk factors for the development of PPCM.
PPCM affects 1:300 to 1: 3000 pregnancies, with geographic hot spots like Nigeria and Haiti.1,2 The disease is characterized by systolic heart failure presenting in the last month of pregnancy or the 1st 4 months post-partum. Although approximately half of affected women recover cardiac function post-partum, many women progress to chronic heart failure, cardiac transplantation, or death. PPCM can thus devastate otherwise healthy young women and their infants. PPCM remains an orphan disease of unknown etiology. The onset late in gestation does not coincide with increased hemodynamic load on the heart, suggesting other mechanisms. Recent data has suggested that anti-angiogenic prolactin fragments may play an important role in some patients.3 Risk factors for PPCM also include pre-eclampsia and multiple gestation, suggesting potential mechanistic overlap with these processes.1,2
PGC-1α is a transcriptional coactivator that drives mitochondrial biogenesis and other metabolic programs in numerous tissues, including the heart.4,5 PGC-1α is highly expressed in the heart, and mice lacking PGC-1α globally have abnormal cardiac energetic reserves and respond poorly to stressful stimuli such as transverse aortic banding.6,7 In addition to its role in mitochondrial homeostasis, PGC-1α also induces the expression and secretion of pro-angiogenic factors such as vascular endothelial growth factor (VEGF) that leads to formation of new blood vessels.8,9 While the angiogenic function of PGC-1α has been described in skeletal muscle, its role in cardiac tissue remains unexplored.
Cardiac-specific PGC-1α deletion leads to PPCM
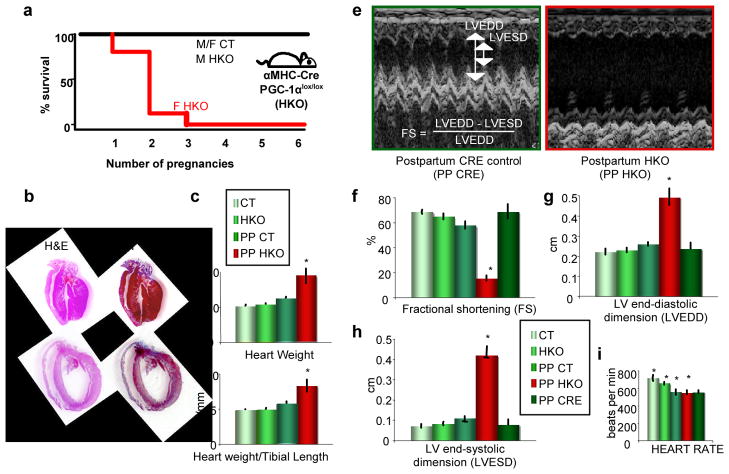
To further study the role of PGC-1α in the heart, we generated cardiac-specific PGC-1α knock-out (HKO) mice (see materials and methods). While studying these mice, we noticed that female HKO mice were fertile, and delivered normal litter sizes (not shown), but invariably died after 1 or 2 pregnancies (Figure 1a). The hearts of these mice were large, dilated, and fibrotic (Figure 1b–d), consistent with a dilated cardiomyopathy. 2-dimensional M-mode echocardiography revealed dilated, poorly contractile hearts in HKO mice after their 2nd delivery (Figure 1e). Left ventricular end-diastolic and end-systolic dimensions (LVEDD and LVESD) were markedly enlarged, and fractional shortening (FS), a direct measure of cardiac contractile function, was profoundly depressed (Figure 1f–i). Nulliparous mice, as well as post-partum control mice, were not affected. Males were also not affected (Figure S1). The absence of PGC-1α in cardiomyocytes thus leads to a profound PPCM in mice.
Figure 1. Mice lacking cardiac PGC-1α develop peri-partum cardiomyopathy.
a, Kaplan-Meier survival curve in female αMHC-Cre: PGC-1αlox/lox mice (F HKO), versus HKO males or control mice of either gender (M/F CT). b, Hematoxylin and Eosin and Masson Trichrome stains of hearts from post-partum HKO mice (PP HKO), versus CT mice (PP CT). c–d, Heart weight (c) and heart weight/tibial length ratios (d) of nulliparous CT and HKO mice, and after two pregnancies (PP). e, Sample M-mode echocardiograms of PP HKO mice, and control mice containing the αMHC-Cre transgene alone (PP CRE). f–i, Echocardiographic measures in mice of the indicated genotypes, either nulliparous or post-partum (PP). n≥5 for all groups. *p<0.05
PGC-1α regulates angiogenesis in cardiac tissue
We have recently shown in skeletal muscle that PGC-1α regulates angiogenesis by driving the expression of angiogenic factors like VEGF.8,9 Anti-angiogenic therapies, including antibodies that neutralize VEGF and small molecule VEGF receptor inhibitors, are being increasingly used in the oncological and ophthalmological settings, and cardiomyopathy and heart failure have recently been recognized as important side effects10,11, demonstrating that anti-angiogenic therapy can be harmful to the heart in humans. Impaired VEGF signaling has also been linked with cardiac dysfunction in mice.12,13 At the same time, late pregnancy is a strong anti-angiogenic environment, in part due to the secretion by the placenta of anti-angiogenic factors like sFlt1 that bind to and neutralize soluble members of the VEGF family.14 These observations led us to postulate that PGC-1α regulates an angiogenic program in cardiomyocytes, and that the absence of this program would leave the hearts defenseless to the anti-angiogenic setting of late pregnancy, thus leading the animals to develop PPCM.
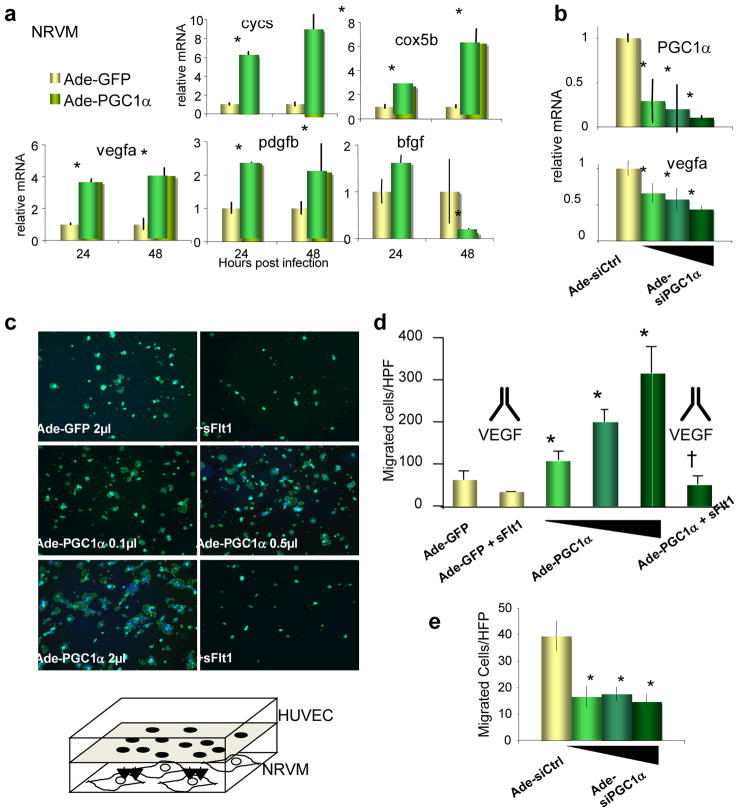
Consistent with the above notion, over-expression of PGC-1α in neonatal rat ventricular myocytes (NRVMs) strongly induced angiogenic genes involved in the activation and recruitment of endothelial cells (e.g., vegfa) and mural cells (e.g., pdgfb), along with, as previously shown15, genes involved in the mitochondrial respiratory chain (cycs, cox5b) (Figure 2a). Interestingly, some angiogenic genes were repressed (e.g., bfgf), a pattern similar to that seen in skeletal muscle cells8, indicating that PGC-1α reprograms the angiogenic program in a stereotypical manner. Conversely, siPGC-1α significantly suppressed the expression of vegfa (Figure 2b). Endothelial activation and migration is a hallmark of angiogenesis. As shown in Figure 2c–d, over-expression of PGC-1α in NRVMs led to a dramatic, dose-dependent, up to six-fold increase in the migration of the adjacent human vein endothelial cells (HUVECs) in a co-culture system. Addition of sFlt1 completely neutralized the induced endothelial migration, indicating that secreted members of the VEGF family, likely vegfa itself, are critical for the effect. Conversely, repression of PGC-1α in NRVMs by siRNA significantly repressed endothelial migration (Figure 2e). PGC-1α thus controls an angiogenic program in cardiomyocytes.
Figure 2. PGC-1α regulates an angiogenic program in cardiomyocytes.
a–b, Relative expression of mitochondrial genes (cycs and cox5b) and angiogenic genes (vegfa, pdgfb, bfgf) in neonatal rat ventricular myocytes (NRVMs) infected with adenovirus expressing PGC-1α versus GFP (a), or siPGC-1α versus siCtrl (b). c–d, PGC-1α expression in NRVMs induces the migration of adjacent endothelial cells, and the migration is blocked by sFlt1. Representative images (c) show phalloidin-stained endothelial cells that have migrated towards the NRVMs. Experimental procedure is schematized in bottom panel. Data are quantified in (d). e, Knockdown of PGC-1α inhibits the migration of endothelial cells. Error bars are +/− SE. *p<0.05 versus control. †p<0.05 versus cells not treated with sFlt1.
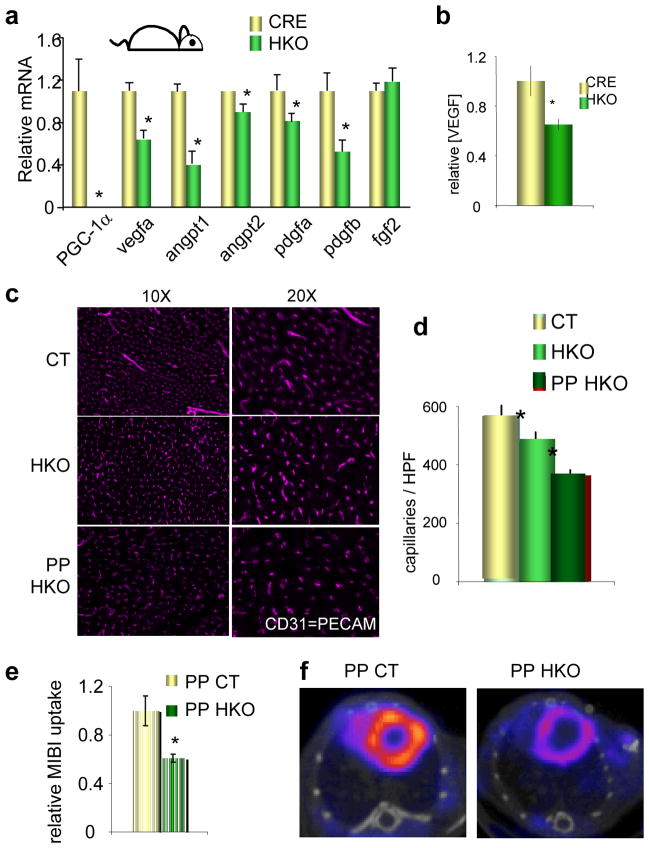
To test if PGC-1α is required for this program in intact animals, levels of vegfa and other angiogenic factors were measured in hearts from PGC-1α HKO mice. As shown in Figure 3a, the expression of vegfa and pdgfb, and a number of other angiogenic factors, was repressed in these hearts by as much as 50%. VEGFA protein was decreased by 30% in HKO hearts (Figure 3b). Levels of VEGFA are normally tightly regulated, and even global haplo-insufficiency is lethal16,17, underscoring the significance of a 30% drop in protein levels. Consistent with these findings, the capillary density of HKO hearts, as measured by staining with the endothelial-specific marker CD31, was decreased by about 15% (Figure 3c–d). PGC-1α thus regulates both vascular density (these data) and mitochondrial function18 in the heart, providing an important regulatory link between the delivery of fuel (blood vessels), and its consumption (mitochondria). These data suggested that PPCM in these mice might be caused by the combination of a heart-specific vascular defect caused by the absence of PGC-1α, and the normal systemic anti-angiogenic environment of late pregnancy. Indeed, the vascular density in HKO hearts decreased by almost 50% post-partum (Figure 3c–d), equivalent to that seen in mice lacking cardiac VEGF.19 Consistent with this vascular rarefaction, perfusion of post-partum HKO hearts was profoundly decreased nearly 50% compared to wild type animals, as determined by MIBI uptake and SPECT/CT (Figure 3e–f).
Figure 3. Mice lacking cardiac PGC-1α have reduced microvascular density that is worsened by pregnancy.
a–b, Relative mRNA expression of the indicated genes (a) and VEGF protein levels (b) in HKO versus control hearts. c–d, Vascular density in hearts from CT and HKO mice nulliparous or post-partum (PP). Representative images stained for CD31 (PECAM) are shown in c, and quantified in d. e–f, Reduced cardiac MIBI uptake in PP HKO versus PP CT animals. Representative SPECT/CT images are shown in f, and quantified in e. n≥5 for all groups. Error bars are +/− SE. *p<0.05 versus control.
Pro-angiogenic therapy rescues PPCM
To test directly the notion that an angiogenic imbalance drives PPCM in HKO mice, rescue experiments were done. In a first series of experiments, breeding mice were administered daily subcutaneous injections of VEGF-121 protein (100mcg/kg), versus vehicle control (Figure S2). The efficacy of VEGF-121 injections was confirmed by the presence of VEGF-121 in the serum, and robust activation of cardiac VEGFR2 phosphorylation within 30 minutes of injection (Figure S2b–c). The VEGF treatment led to improved survival of the breeding HKO females, up to five pregnancies (Figure S2d). However, capillary density was only partly rescued, cardiac contractility was only marginally improved, and the hearts remained quite enlarged (Figure S2e–g). VEGF administration thus partly rescued lethality in multiparous HKO animals, but was insufficient to fully rescue PPCM, suggesting that other pathways are also important.
Hilfiker-Kleiner et al. recently showed that STAT3 regulates mitochondrial superoxide dismutase (MnSOD) and protects against reactive oxygen species (ROS) in the heart.3 Absence of cardiac STAT3 and the consequent increase in ROS led to inappropriate cleavage of prolactin to a potent anti-angiogenic 16kda form, and subsequent PPCM (Figure 4a). Importantly, the PPCM could be rescued by treatment with bromocriptine, which inhibits the secretion of prolactin from the pituitary gland. PGC-1α is known to increase ROS scavenging.20 In cardiomyocytes, over-expression of PGC-1α strongly induced MnSOD mRNA expression and protein (Figure S3a–b). Conversely, MnSOD was repressed in PGC-1α HKO hearts, and levels of reactive oxygen species (ROS) were increased (Figure S3c–d). PGC-1α and STAT3 thus both regulate MnSOD and ROS in cardiomyocytes, suggesting that the absence of PGC-1α in the heart may, like the absence of STAT3, lead to prolactin-mediated anti-angiogenic effects. Prolactin had no direct effects on PGC-1α or VEGF expression in cardiac cells, and prolactin levels did not differ in heart or serum between wild-type and HKO animals (Figure S4).
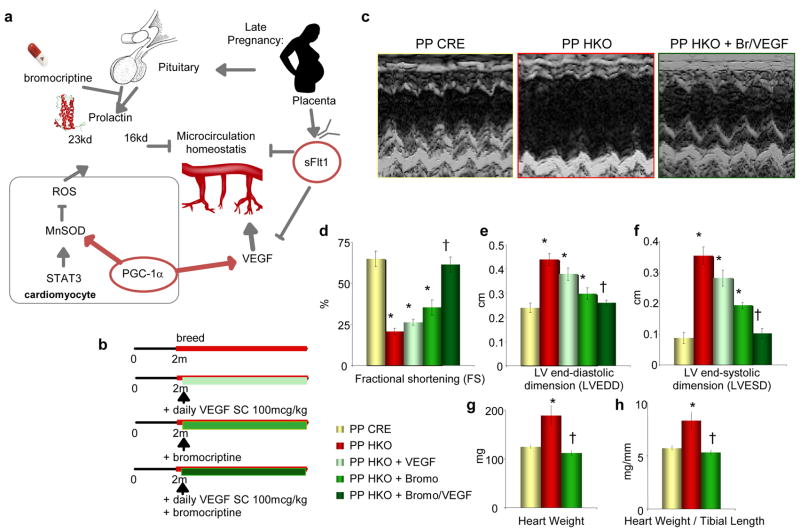
Figure 4. Combined treatment with VEGF and Bromocriptine rescues PPCM in PGC-1α HKO mice.
a, Schema of proposed role of cardiac PGC-1α in the regulation of cardiac angiogenesis, and defending against pregnancy-induced anti-angiogenic factors. b, Experimental outline. c, Sample echocardiograms from PP HKO, PP CRE, and PP HKO mice receiving both bromocriptine and VEGF treatments (PP HKO + Br/VEGF). d–h, Indicated echocardiographic measures (d–f), heart weight (g), and heart weight/tibial length ratio (h), in post-partum mice of the indicated genotypes. n≥5 for all groups. Error bars are +/− SE. *p<0.05 versus PP CRE control. †p<0.05 versus PP HKO.
These observations thus suggested that PGC-1α regulates two separate pro-angiogenic pathways in the heart, a VEGF pathway and a prolactin pathway, and that aberration of both pathways in PGC-1α HKO mice leads to PPCM (Figure 4a). To test this idea directly, both pathways were rescued simultaneously: breeding HKO mice were treated with daily subcutaneous injections of VEGF protein and with bromocriptine supplementation in the water (Figure 4b). This double treatment resulted in complete rescue of the PPCM in HKO females (Figure 4c). After two pregnancies, heart weights and all echocardiographic indices of cardiac function (FS, LVEDD, LVESD) were normal in HKO mice (Figure 4c–h). Bromocriptine alone prevented some of the LV dilation, but provided only minimal rescue of LV function, demonstrating that rescue of both pathways is necessary (Figure 4c–h). Together, these data indicate that PPCM can be caused by an angiogenic imbalance and vascular dysfunction, at least in rodents.
Pre-eclampsia and cardiac dysfunction
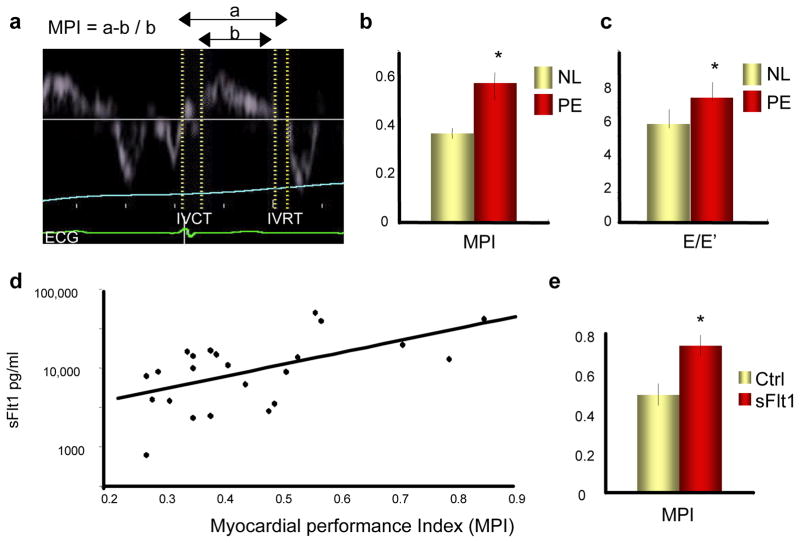
To test the idea that anti-angiogenic signaling can cause cardiac dysfunction in pregnant women, studies were undertaken with women with pre-eclampsia, in whom VEGF signaling is compromised due to high serum levels of anti-angiogenic sFlt1.21 (see Supplementary Table 1 for patient characteristics). Cardiac function was evaluated non-invasively by measuring myocardial performance index (MPI, or Tei index) and other indices of cardiac function with cardiac echocardiography. MPI measures the relative duration of isovolemic contraction and relaxation (Figure 5a), and is a sensitive marker of diastolic function.22–24 Women with pre-eclampsia had markedly increased serum levels of sFlt1 (Figure S5a, p=0.005), as previously shown.21 Strikingly, women with pre-eclampsia also had markedly abnormal MPI (Figure 5b and Supplementary Table 2, p=0.01) and E/E′ (Figure 5c and Supplementary Table 2, p=0.02), another sensitive measure of cardiac dysfunction.25 Moreover, the MPI correlated with levels of circulating sFlt1 (Figure 5d, R=0.59, p=0.003). Elevated blood pressure (BP) in the pre-eclamptic women (Supplementary Table 1) is unlikely to explain the worsening MPI, because MPI is thought to reflect cardiac function independently of BP22, and pregnant women with similar mild elevations of BP but without pre-eclampsia have normal cardiac function.26 Instead, these data suggested that elevated sFlt1 causes the diastolic dysfunction. To test this hypothesis directly, sFlt1 was delivered systemically to pregnant mice by intravenous injection of adenoviruses expressing sFlt1, and MPI was examined using high-resolution murine echocardiography. sFlt1 caused significant increases in MPI in these mice within 10 days (Figure 5e). These data, taken together with published observations in patients receiving anti-angiogenic therapies10,11, thus strongly suggest that elevated sFlt1 causes cardiac dysfunction in women with pre-eclampsia. While the LV dysfunction recovers following delivery in many patients, a second insult in some women likely precipitates PPCM.
Figure 5. Women with pre-eclampsia have depressed cardiac function that correlates with circulating sFlt1 levels, and sFlt1 causes cardiac dysfunction in mice.
a, Sample tracing of tissue Doppler imaging. MPI: myocardial performance index. IVCT: isovolemic contraction time. IVRT: isovolemic relaxation time. b–c, Elevated MPI (b) and E/E′ (c) in women with pre-eclampsia (PE) versus normal (NL) pregnancies. p=0.01 and p=0.02, respectively. d, Elevated MPI correlates with sFlt1 levels. R=.59. p=0.003. e, Elevated MPI in pregnant mice infected with adenovirus expressing sFlt1. p=0.01 n≥5 for all groups. *p<0.05 versus control.
sFlt1 induces cardiomyopathy and is high in PPCM
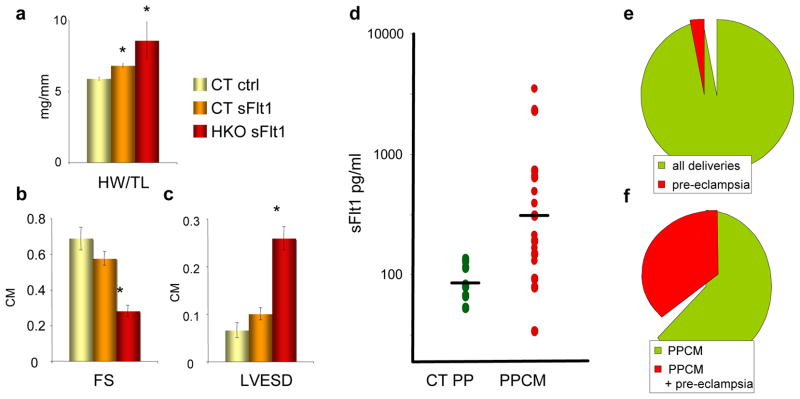
The above observations strongly support the hypothesis that PPCM can be induced by excess anti-angiogenic signaling, including the high expression of sFlt1 during late gestation seen in both women27 and mice (Figure S5c, p=.009). To test this hypothesis directly, sFlt1 was delivered systemically to nulliparous mice, as above. PGC-1α HKO mice that received sFlt1 developed profound cardiac failure within three weeks, as evidenced by increases in cardiac weight and dramatic decreases in fractional shortening on echocardiography (Figure 6a–c). This was accompanied by a striking drop in vascular density (Figure S6), though not in larger vessels (Figure S7). Wild-type mice also revealed significant, though less extensive, decreases in vascular density and cardiac function after exposure to three weeks of sFlt1. sFlt1 alone is thus sufficient, even in the absence of pregnancy, to cause dramatic cardiomyopathy in the setting of a heart unable to withstand the anti-angiogenic insult.
Figure 6. sFlt1 is sufficient to induce cardiomyopathy in HKO mice, women with PPCM have elevated sFlt1 levels, and pre-eclampsia as a risk factor for PPCM.
a–c, Heart weight/tibial length ratios (a), echocardiographic fractional shortening (b), and LV end-systolic dimensions (c) in HKO mice injected with adenovirus expressing sFlt1, versus controls. d, Elevated sFlt1 levels in post-partum women with PPCM. p=0.002. e–f, Prevalence of pre-eclampsia among all deliveries (e) and among women with PPCM (f) at Harvard teaching hospitals in the previous 9 years.
To further investigate in humans if elevated sFlt1 levels could be contributing to PPCM, plasma from women with PPCM was acquired 4–6wks post-partum and sFlt1 levels were measured. sFlt1 levels normally return to normal within 48–72hrs after delivery.28 sFlt1 levels were elevated in a large subset of these PPCM patients (p=0.002), remaining up to 5- or 10-fold higher than the levels in control participants (Figure 6d). Post-partum sFlt1 levels can remain modestly higher in subjects with pre-eclampsia29,30, but the levels found here are notably higher. The findings are thus consistent with the notion that a significant percentage of PPCM subjects have been exposed to pre-eclampsia, and that secretion of sFlt1 persists inappropriately post-partum. Indeed, in our own institution, 33% of the last 75 cases of PPCM were associated with pre-eclampsia (Figure 6e–f), markedly more than the population rate of 3–5%.31 The persisting extra-placental source of sFlt1 in the post-partum period is not known, and may include placental remnants, circulating mononuclear cells32, or shed syncytial microparticles33.
Discussion
Our study shows that angiogenic imbalance in the heart during the peri-partum period may lead to PPCM in mice and in humans. The data strongly suggest that PPCM is caused by a “two-hit” combination of 1) systemic anti-angiogenic signals during late pregnancy, and 2) a host susceptibility marked by insufficient local pro-angiogenic defenses in the heart. The first hit explains why PPCM is a disease of the late gestational period, which is precisely when circulating anti-angiogenic factors such as sFlt1 peak in pregnancy.21,34 Other pathways, such as prolactin or excess angiotensina II signaling, may also be involved.3,35 The first hit is also worse in pre-eclampsia, which is characterized by markedly elevated sFlt1 levels. Associations between pre-eclampsia and PPCM have been well noted in many populations1,2,36–41 (detailed in Supplementary Table 3). Interestingly, some studies involving women of African descent have not found an association between hypertensive disorders of pregnancy and PPCM42, suggesting ethnic variability in the pathogenesis of PPCM. It is also quite possible that PPCM with and without associated pre-eclampsia have different pathogeneses.43 Overall, our data strongly indicate that elevated sFlt1 levels in pre-eclampsia contribute to at least the PPCM that is associated with pre-eclampsia. We further propose that elevated sFlt1 levels in fact present a challenge to the myocardium in all pregnancies, thus explaining why the peri-partum period puts women at risk of developing heart failure, even in the absence of pre-eclampsia. Interestingly, other situations of elevated sFlt1 (twin pregnancies44) and recurrent exposures to sFlt1 (multiple pregnancies) are also strong risk factors for PPCM even in the absence of pre-eclampsia.2,43
Only a minority of women with pre-eclampsia develops PPCM, consistent with the existence of a second “hit”. Abnormal PGC-1α function is such an event in rodents, and may be so in humans as well. A number of previously identified processes may also constitute this second hit, including myocarditis, immune activation, viral infection, and/or autoantibodies.43 Interestingly, PGC-1α expression is repressed by inflammatory states in the heart and elsewhere45,46, suggesting that many of the above processes implicated in PPCM may in part converge on PGC-1α. Consistent with this, we found repressed PGC-1α expression in cardiac samples from women with PPCM (Figure S8). Abnormal STAT3 function and ROS production may also contribute3, as may genetic predispositions.47
In conclusion, the data presented here strongly support the idea that PPCM is in part a two-hit vascular disease due to imbalances in angiogenic signaling, and that anti-angiogenic states such as pre-eclampsia or multiple gestation significantly worsen the process. Our data may explain why pregnancy triggers PPCM, and also explain the longstanding epidemiological observation that pre-eclampsia is a risk factor for developing PPCM. Pro-angiogenic therapies such as exogenous VEGF-121, or removal of sFlt1 itself48, may thus be beneficial in PPCM.
METHODS SUMMARY
All animal experiments were performed according to procedures approved by the Institutional Animal Care and Use Committee. Human soluble VEGF-121 was a kind gift of Scios, Inc CA. Bromocriptine treatments were done as previously described.3 Human studies were approved by the institutional review board of Beth Israel Deaconess Medical Center. Informed consent was obtained from all subjects. Angiogenic factor assays were performed with commercially available ELISAs (R&D systems, Minneapolis, MN).
Full methods and any associated references are available in the online version of the paper at www.nature.com
FULL METHODS
Animal studies
All animal experiments were performed according to procedures approved by the Institutional Animal Care and Use Committee. Mice bearing floxed alleles of PGC-1α flanking exons 3–4, and mice containing the α-MHC::CRE transgene were kind gifts of Bruce Spiegelman49 and Mike Schneider50, respectively. Mice were maintained on a standard rodent chow diet with twelve-hour light and dark cycles. Mice bearing floxed alleles of PGC-1α flanking exons 3–4, and mice containing the α-MHC::CRE transgene were kind gifts of Bruce Spiegelman49 and Mike Schneider50, respectively. For murine echocardiography, the chest hair was removed with a topical depilatory agent, and two-dimensional images were visualized using a Vivid FiVe echocardiography system (GE Medical Systems, Milwaukee, Wisconsin, USA) on unanesthetized mice. Parasternal short-axis projections were visualized, and M-mode recordings at the mid-ventricular level were recorded. Heart rate, left ventricular end-diastolic and end-systolic (LVEDD, LVESD) were measured in at least three beats from at least 3 recordings and averaged, and LV fractional shortening was then calculated (FS = (LVEDD−LVESD)/LVEDD). For the high-resolution MPI studies, a VisualSonics 2100 echocardiography machine was used on mice anesthetized with isoflurane, and the MPI was calculated using the manufacturer software program. SPECT/CT imaging of mice was performed by the Longwood Area Small Animal Imaging Facility (SAIF). For the VEGF treatment studies, human VEGF-121 (100mcg/kg) was injected subcutaneously daily, versus saline control. For the bromocriptine studies, bromocriptine was added to the drinking water. Mice were bred starting at the age of 8 wks while receiving bromocriptine in the drinking water, daily subcutaneous VEGF-121, or both.
Cells and reagents
All reagents were from Sigma, unless otherwise indicated. Human soluble VEGF-121 was a kind gift of Scios, Inc CA. Staining of capillaries was performed using anti-CD31 antibody (BD Pharmingen, CA) or isolectin B4 (Vector Lab). Quantification of capillaries was performed computationally, using Volocity software (Improvision, PerkinElmer), on three random fields chosen from the septum of transverse sections from the mid-heart. Staining of arterioles was performed using anti-SMA antibody (Santa Cruz) and quantified similarly, using random low-power fields. All quantifications were performed blindly. Isolation and culture of primary neonatal rat ventricular myocytes (NRVMs) was performed as described. Cells were infected with adenovirus at a multiplicity of infection of 10–30, and mRNA expression was measured 24 or 48hrs later. The adenovirus expressing PGC-1α51 and sFlt152 have been described. Prolactin, VEGF, and sFlt1 ELISAs were from R&D Systems. The thiobarbituic acid reactive substances (TBARS) assay was performed on cardiac extracts according to manufacturer instructions (Cayman).
Gene expression studies
Total RNA’s were isolated from mouse tissue or cultured cells using the Trizol method (Invitrogen). Samples for real-time PCR analyses were reverse transcribed (Invitrogen), and quantitative real-time PCR reactions were performed on the cDNAs in the presence of fluorescent dye (SYBR green), on a BioRad CFX 384 Touch™ Real-Time PCR Detection System. DNA product of the expected size was confirmed for each primer pair.
Endothelial migration assay
NRVMs in 24-well plates were infected with adenovirus expressing GFP or PGC-1α for 34hrs. BSA or soluble Flt1 (100ng/ml) was added to the media for 12hrs. Then, 5 × 104 cells of human umbilical cord endothelial cells (HUVECs) at 5 × 104 were put on the upper compartment of transwells (8.0um pore size, Corning #3422) pre-warmed with EBM2 media for O/N at 37°C. HUVEC migration to the lower compartment of transwells was measured after 12hrs. Migrated HUVECs were fixed with 4% paraformaldehyde in PBS for 20min at RT, cells remained on the upper compartment were removed with cotton swab. Cells were blocked with 5% BSA in PBST (0.2% Tween) and stained with phallodin-FITC in PBST for 4hrs to visualize filamentous actin. Transwell inserts were washed three times in PBST and mounted unto slides with DAPI mounting medium.
Human studies
The institutional review board of Beth Israel Deaconess Medical Center in Boston approved this study. Eligible women were enrolled after providing written informed consent from November 2009 through May 2010. Pregnant women at least 18 years of age with a singleton pregnancy of at least 24 weeks and less than 41 weeks and either a diagnosis of pre-eclampsia or without any hypertensive disorder of pregnancy were eligible. Exclusion criteria included pre-existing cardiovascular disease, pulmonary disease and non-gestational diabetes mellitus. Participants were recruited upon admission to labor and delivery, the ante-partum floor, or during a routine prenatal visit. All clinical data was abstracted from medical records. The diagnosis of pre-eclampsia was based on the National High Blood Pressure Education Program Working Group definition, also endorsed by the American Congress of Obstetricians and Gynecologists (ACOG). A Maternal-Fetal Medicine specialist confirmed all diagnoses. Archived plasma samples from subjects with PPCM have been previously described.3 Patients in both studies were predominantly Caucasian. Retrospective analyses of PPCM and pre-eclampsia in the Harvard teaching hospitals were performed using the Harvard Shared Health Research Information Network (SHRINE)53, a de-identified repository of aggregate patient information.
Human echocardiography
Bedside transthoracic echocardiogram was performed using a Siemens X-300 (Mountainview, CA) machine by two expert echocardiographers using P5-1 Transducer. Images were obtained with the patient lying in the left lateral decubitus position and reported according to the American Society of Echocardiography guidelines. Images were stored in a cine-loop format with three cardiac cycles of non- compressed data with electrocardiogram information. The echocardiographers performed a comprehensive examination, which included a complete two-dimensional and color flow-Doppler assessment of the left ventricle, right ventricle and intra-cardiac valves. Specifically: a) Ejection Fraction: Visual quantitative estimation; b) Trans-mitral pulse wave Doppler (E and A waves and deceleration time); c) Doppler Tissue Image (DTI): Both medial and lateral mitral annulus were interrogated, final value of peak velocity of E′ was calculated as average of three velocities at each location; d) MPI: The MPI calculation was performed off-line utilizing a Siemens Syngo DICOM viewing station (Mountainview, CA). The echocardiograms were de-identified before calculating MPI. Ejection fraction, MPI and E/E′ ratios were calculated. Each images was analyzed by one of two blinded echocardiographers.
Angiogenic factor assays
Women were consented for a blood draw at the time of the echocardiogram. All samples for the MPI study were collected in the ante-partum prior to the delivery, while samples in the PPCM study were collected 4–6wks post-partum. The samples were centrifuged at 3000 RPM for 8 minutes and plasma was collected and stored at −80° C. Samples were randomly ordered and analyzed by a single person in a blinded fashion. ELISAs for sFlt1 were performed with commercially available kits (R&D systems, Minneapolis, MN). All assays were performed in duplicate and values were averaged. If >20% difference was observed between duplicate values, the samples were reanalyzed.
Data and statistical analysis
SAS 9.2 (SAS institute Inc., Cary, NC) was used for data analysis. All tests were two sided, and P values <0.05 were considered statistically significant. Data are presented as mean +/− standard error, or median and inter-quartile ranges, as indicated. Comparisons were made using two-tailed Student’s t-test or non-parametric Mann-Whitney test, as indicated.
Supplementary Material
Acknowledgments
S.R. is supported by a Harvard Faculty Development and Diversity Award. S.S is supported by John Hedley White grant. G.C.R. is supported by a Merck Fellowship. M.R.H. is supported by the Harvard Catalyst and Clinical and Translational Science Center, and Harvard University and affiliated academic health care centers. S.A.K. is an investigator of the Howard Hughes Medical Institute. Z.A. is supported by the NHLBI, the Smith Family Foundation, the Ellison Medical Foundation, the March of Dimes Foundation, and the Harvard Stem Cell Institute.
Footnotes
AUTHOR CONTRIBUTIONS
I.P. performed the majority of mouse experimental work, with the assistance of G.C.R, L.L., N.K., and C.F. S.R., S.S., J.R., M.H., J.M., F. M., and P. H. performed the clinical MPI study. S.R. performed the sFlt1 measurements. E.K. and S.B. performed the mouse MPI studies. C.J. and L.L. performed the endothelial migration studies. J.B., F.dM. and D.H.K. provided samples from women with PPCM and provided intellectual input. S.A.K. and Z.A. conceived and supervised the study. Z.A. designed the experimental procedures and wrote the manuscript. All authors read and approved the final manuscript.
COMPETING FINANCIAL INTERESTS
S.A.K is a co-inventor on multiple patents held by the Beth Israel Deaconess Medical Center that is related to the use of angiogenic proteins for diagnosis and treatment of pre-eclampsia. S.A.K. has financial interest in Aggamin Therapeutics. All other authors report no competing financial interests.
References
- 1.Pearson GD, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283:1183–1188. doi: 10.1001/jama.283.9.1183. [DOI] [PubMed] [Google Scholar]
- 2.Sliwa K, Fett J, Elkayam U. Peripartum cardiomyopathy. Lancet. 2006;368:687–693. doi: 10.1016/S0140-6736(06)69253-2. [DOI] [PubMed] [Google Scholar]
- 3.Hilfiker-Kleiner D, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 4.Rowe GC, Jiang A, Arany Z. PGC-1 coactivators in cardiac development and disease. Circ Res. 107:825–838. doi: 10.1161/CIRCRESAHA.110.223818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 6.Arany Z, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Arany Z, et al. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci U S A. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arany Z, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 9.Chinsomboon J, et al. The transcriptional coactivator PGC-1{alpha} mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci U S A. 2009;106:21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirk R. Bevacizumab and heart failure. Nat Rev Clin Oncol. 8:124. doi: 10.1038/nrclinonc.2011.11. [DOI] [PubMed] [Google Scholar]
- 11.Uraizee I, Cheng S, Moslehi J. Reversible cardiomyopathy associated with sunitinib and sorafenib. N Engl J Med. 365:1649–1650. doi: 10.1056/NEJMc1108849. [DOI] [PubMed] [Google Scholar]
- 12.May D, et al. Transgenic system for conditional induction and rescue of chronic myocardial hibernation provides insights into genomic programs of hibernation. Proc Natl Acad Sci U S A. 2008;105:282–287. doi: 10.1073/pnas.0707778105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmeliet P, et al. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999;5:495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- 14.Bdolah Y, Sukhatme VP, Karumanchi SA. Angiogenic imbalance in the pathophysiology of preeclampsia: newer insights. Semin Nephrol. 2004;24:548–556. doi: 10.1016/s0270-9295(04)00125-1. [DOI] [PubMed] [Google Scholar]
- 15.Wu Z, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 16.Carmeliet P, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 18.Lehman JJ, et al. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giordano FJ, et al. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc Natl Acad Sci U S A. 2001;98:5780–5785. doi: 10.1073/pnas.091415198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St-Pierre J, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 21.Levine RJ, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 22.Bruch C, et al. Tei-index in patients with mild-to-moderate congestive heart failure. Eur Heart J. 2000;21:1888–1895. doi: 10.1053/euhj.2000.2246. [DOI] [PubMed] [Google Scholar]
- 23.Tei C, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function--a study in normals and dilated cardiomyopathy. J Cardiol. 1995;26:357–366. [PubMed] [Google Scholar]
- 24.Poulsen SH, Jensen SE, Nielsen JC, Moller JE, Egstrup K. Serial changes and prognostic implications of a Doppler-derived index of combined left ventricular systolic and diastolic myocardial performance in acute myocardial infarction. Am J Cardiol. 2000;85:19–25. doi: 10.1016/s0002-9149(99)00599-8. [DOI] [PubMed] [Google Scholar]
- 25.Kasner M, et al. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–647. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 26.Melchiorre K, Sutherland GR, Baltabaeva A, Liberati M, Thilaganathan B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension. 57:85–93. doi: 10.1161/HYPERTENSIONAHA.110.162321. [DOI] [PubMed] [Google Scholar]
- 27.Venkatesha S, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 28.Maynard SE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf M, et al. Preeclampsia and future cardiovascular disease: potential role of altered angiogenesis and insulin resistance. J Clin Endocrinol Metab. 2004;89:6239–6243. doi: 10.1210/jc.2004-0548. [DOI] [PubMed] [Google Scholar]
- 30.Saxena AR, et al. Increased sensitivity to angiotensin II is present postpartum in women with a history of hypertensive pregnancy. Hypertension. 55:1239–1245. doi: 10.1161/HYPERTENSIONAHA.109.147595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 32.Rajakumar A, et al. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26:563–573. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Rajakumar A, et al. Transcriptionally active syncytial aggregates in the maternal circulation may contribute to circulating soluble fms-like tyrosine kinase 1 in preeclampsia. Hypertension. 2012;59:256–264. doi: 10.1161/HYPERTENSIONAHA.111.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 122:478–487. doi: 10.1161/CIRCULATIONAHA.109.895458. [DOI] [PubMed] [Google Scholar]
- 35.Hubel CA, et al. Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension. 2007;49:612–617. doi: 10.1161/01.HYP.0000256565.20983.d4. [DOI] [PubMed] [Google Scholar]
- 36.Cruz MO, Briller J, Hibbard JU. Update on peripartum cardiomyopathy. Obstet Gynecol Clin North Am. 37:283–303. doi: 10.1016/j.ogc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol. 58:659–670. doi: 10.1016/j.jacc.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 38.Demakis JG, Rahimtoola SH. Peripartum cardiomyopathy. Circulation. 1971;44:964–968. doi: 10.1161/01.cir.44.5.964. [DOI] [PubMed] [Google Scholar]
- 39.Witlin AG, Mabie WC, Sibai BM. Peripartum cardiomyopathy: an ominous diagnosis. Am J Obstet Gynecol. 1997;176:182–188. doi: 10.1016/s0002-9378(97)80033-6. [DOI] [PubMed] [Google Scholar]
- 40.Amos AM, Jaber WA, Russell SD. Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J. 2006;152:509–513. doi: 10.1016/j.ahj.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Goland S, et al. Evaluation of the clinical relevance of baseline left ventricular ejection fraction as a predictor of recovery or persistence of severe dysfunction in women in the United States with peripartum cardiomyopathy. J Card Fail. 2011;17:426–430. doi: 10.1016/j.cardfail.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Fett JD, Christie LG, Carraway RD, Murphy JG. Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin Proc. 2005;80:1602–1606. doi: 10.4065/80.12.1602. [DOI] [PubMed] [Google Scholar]
- 43.Ntusi NB, Mayosi BM. Aetiology and risk factors of peripartum cardiomyopathy: a systematic review. Int J Cardiol. 2009;131:168–179. doi: 10.1016/j.ijcard.2008.06.054. [DOI] [PubMed] [Google Scholar]
- 44.Bdolah Y, et al. Twin pregnancy and the risk of preeclampsia: bigger placenta or relative ischemia? Am J Obstet Gynecol. 2008;198:428 e421–426. doi: 10.1016/j.ajog.2007.10.783. [DOI] [PubMed] [Google Scholar]
- 45.Schilling J, et al. Toll-like receptor-mediated inflammatory signaling reprograms cardiac energy metabolism by repressing peroxisome proliferator-activated receptor gamma coactivator-1 signaling. Circ Heart Fail. 2011;4:474–482. doi: 10.1161/CIRCHEARTFAILURE.110.959833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tran M, et al. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011;121:4003–4014. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horne BD, et al. Genome-wide significance and replication of the chromosome 12p11.22 locus near the PTHLH gene for peripartum cardiomyopathy. Circ Cardiovasc Genet. 4:359–366. doi: 10.1161/CIRCGENETICS.110.959205. [DOI] [PubMed] [Google Scholar]
- 48.Thadhani R, et al. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation. 124:940–950. doi: 10.1161/CIRCULATIONAHA.111.034793. [DOI] [PubMed] [Google Scholar]
- 49.Handschin C, et al. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest. 2007;117:3463–3474. doi: 10.1172/JCI31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agah R, et al. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest. 1997;100:169–179. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puigserver P, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 52.Kuo CJ, et al. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci U S A. 2001;98:4605–4610. doi: 10.1073/pnas.081615298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber GM, et al. The Shared Health Research Information Network (SHRINE): a prototype federated query tool for clinical data repositories. J Am Med Inform Assoc. 2009;16:624–630. doi: 10.1197/jamia.M3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.