Abstract
Background
Therapies exist for acute organophosphate (OP) exposure but mortality rates remain high (10% to 20%). Currently, treatment focuses on reversing the resultant cholinergic excess effects through the use of atropine. Intralipid fat emulsion (IFE) has been used to treat lipophilic drug ingestions and theoretically would be beneficial for some OP agents.
Objectives
The hypothesis was that IFE would decrease the acute respiratory depressant effects following lethal OP exposure using a lipophilic OP agent (parathion).
Methods
The authors used a previously validated animal model of OP poisoning with detailed physiologic respiratory recordings. The model consisted of Wistar rats anesthetized but spontaneously breathing 100% oxygen. Airflow, respiratory rate, tidal volume, mean arterial pressure, and pulse rate were digitally recorded for 120 minutes following OP exposure or until respiratory failure. Three study groups included parathion alone (n = 6), parathion and IFE 5 minutes after poisoning (n = 6), and parathion and IFE 20 minutes after poisoning (n = 6). In all groups, parathion was given as a single oral dose of 54 mg/kg (4 times the rat oral 50% population lethal dose [LD50]). Three boluses of IFE (15 mg/kg/min) were given over 3 minutes, 20 minutes apart, starting either 5 or 20 minutes after poisoning. Timing of IFE was based on parathion kinetics. In one study group IFE was initiated 5 minutes after poisoning to coincide with initial absorption of parathion. In another study group IFE was given at 20 minutes to coincide with peak intravenous parathion concentration. Primary outcome was percent of animals with apnea. Secondary outcome was time to apnea.
Results
Animals exposed to parathion alone demonstrated a steady decline in respiratory rate and tidal volume post-exposure, with apnea occurring a mean of 51.6 minutes after poisoning (95% CI = 35.8 min to 53.2 min). Animals treated with IFE 5 minutes post-exposuredemonstrated no difference in mean time to apnea (44.5 minutes vs. 51.6 minutes, p = 0.29), or number of animals with respiratory arrest (100% vs. 100%, p = 1.00). Animals treated with IFE 20 minutes post-exposure demonstrated a significantly prolonged mean time to apnea (95.3 minutes vs. 51.6 minutes, p = 0.002), but there was no difference in number of animals with respiratory arrest (100% vs. 66.7%, p = 0.45).
Conclusions
All animals exposed to 4x LD50 of oral parathion demonstrate apnea and respiratory arrest. IFE given immediately after oral parathion does not prolong time to apnea. IFE given 20 minutes after oral exposure to parathion decreases the acute effects of the OP, and prolongs the time to apnea.
INTRODUCTION
Organophosphate (OP) pesticide exposure is responsible for more than three million poisonings worldwide each year, and results in an estimated 250,000 deaths annually.1,2 Mortality from OP exposure is multifactorial, but both central apnea and pulmonary failure occur following lethal exposure to OP agents.3 OP compounds are used globally in agriculture, and the intentional ingestion of these chemicals is a significant public concern, particularly in developing countries in and around Asia.4 Related compounds also pose a risk from terrorist attack, such as the 1995 sarin gas attack on the Tokyo city subway.5 Although treatments exist for OP poisoning, the mortality rate remains unacceptably high, with rates of up to 40% in even the most sophisticated intensive care hospital.6 The effects of OP exposure disproportionally affect developing world countries, where availability of OP agents is high and medical care may be less available.
Despite advances in other areas of medicine, the treatment of acute OP poisoning has not changed substantially in over 50 years. OPs function by inhibiting acetylcholinesterase. Currently, treatment focuses on reversing the resultant cholinergic excess effects through the use of atropine. Adjunctive treatments include reversing cholinesterase inhibition through the use of oximes, and suppressing OP-induced neurotoxicity via benzodiazepines. Medical therapy alone is commonly insufficient for severe OP poisoning, as patients often require mechanical ventilation following the primary resuscitation.7,8 One promising alternative to treating the post-exposure effects is to reduce the effects of the OP by increasing the elimination or degradation of the pesticide. Such enzymatic degradation of OPs has been successful in animal models,9,10 but this research is still in the pre-clinical stage. Another possible alternative is the use an agent such as intralipid fat emulsion (IFE) to sequester the OP intravenously after ingestion.
Although the specific mechanism of IFE action is unknown, it has been suggested that IFE acts as a “lipid sink,” absorbing lipophilic drugs and separating them from the primary site of toxicity.11,12 IFE has conventionally been used to administer calories in the form of fatty acids to patients requiring parenteral nutrition, or as a vehicle for the delivery of highly lipid soluble drugs such as propofol, paclitaxel, etomidate, and diazepam.13,14 More recently, it has been found to be beneficial in treating severe overdoses of some lipophilic drugs such as local anesthetics and calcium channel blockers.15 As many OP agents are lipophilic, IFE potentially represents a novel therapeutic agent for OP ingestions. We hypothesized that IFE would decrease the acute respiratory depressant effects following lethal OP exposure using a lipophilic OP agent (parathion).
METHODS
Study Design
This was a laboratory study using a murine model of OP poisoning. The study protocol was approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School.
Animal Handling and Preparation
Male Wistar rats obtained from Charles River Laboratories (Wilmington, MA) were pair housed and maintained on a 12-hour light and dark cycle. Food and water was available ad libitum prior to sedation at the start of the study. All animals were housed and cared for in accordance with the guidelines of the National Institute of Health. We used a previously validated animal model of OP poisoning with detailed physiologic respiratory recordings, which are described in the following sections.
Anesthesia
All animals were anesthetized, spontaneously breathing 100% oxygen via a 3/32 inch kynar barbed y-fitting (Small Parts, Inc., Miami Lakes, FL). The gas mixture was delivered through one arm (inspiratory), while the second (exhalatory) was connected to a low-pressure scavenger system (Surgivet, Waukesha, WI). An end-tidal CO2 detector (Columbus Instruments, Columbus, OH) and pneumotach (HSE, March-Hugstetten, Germany) were connected in series with the tracheal tube. The dead space of the entire system totaled 0.33 cm3, or approximately 10% of the animals’ tidal volume.
Animals were anesthetized using a titrated dose of isoflurane (Webster Veterinary, Sterling, MA) between 1.5% and 2.5%. Anesthetic efficacy was determined by the lack of withdrawal from painful stimulus on the foot or tail. Anesthesia was titrated prior to initiating the experimental protocol to a level sufficient to sustain a respiratory rate between 45 and 60 breaths per minute. No changes in anesthesia occurred from 10 minutes prior to the OP exposure to termination of the study protocol.
Study Protocol
Surgical Setup
A mid-cervical tracheostomy was performed using polyethylene tubing (0.24 mm). Polyethylene tubing (PE50) was placed under direct visualization into the femoral artery and connected to a pressure transducer. Polyethylene tubing (PE50) was placed under direct visualization into the femoral vein and connected to a stopcock. A rigid gavage tube (0.05 mm inner diameter) was temporarily inserted into the stomach through the mouth to administer the parathion. The gavage tube was removed immediately following administration of the parathion.
Respiratory and Hemodynamic Recordings
End-tidal pressure of CO2 (PCO2), airflow, and mean arterial pressure (MAP) were recorded continuously during the experiment. Respiratory rate was calculated from the airflow signal using peak of inspiration as the mark of an individual breath. Volume of expired gas was calculated through integration of the airflow tracing. Minute ventilation was calculated by multiplying the respiratory rate and volume of expiration. Pulse rate was calculated from the arterial tracing. A non-invasive pulse oximeter (MouseOx, Starr Life Sciences Corp., Oakmont, PA) attached to the right back paw measured arterial oxygen saturation.
Data Acquisition
Signals were digitally recorded and displayed in real time using a data acquisition system (PowerLab, ADInstruments, Inc., Colorado Springs, CO) and a computer (Dell, Round Rock, TX). Airflow recordings were filtered and amplified using the CyberAmp (AutoMate Scientific, Berkeley, CA), and all data were sampled at 400Hz. A separate amplifier (ADInstruments, Colorado Springs, CO) filtered and amplified the arterial pressure signal. Data were recorded for later analysis.
Drug Exposure
Animals were divided into three study groups: parathion (Sigma-Aldrich, St. Louis, MO) alone (Group 1, n = 6), parathion followed by IFE 5 minutes post-exposure (Group 2, n = 6), and parathion followed by IFE 20 minutes post-exposure (Group 3, n = 6). Parathion was given as a single oral dose of 54 mg/kg (approximately 4x rat oral LD50), over 20 seconds. Parathion was suspended in peanut oil to a concentration of 10 mg/ml. Each dose of IFE consisted of 20% IFE (Fresenius Kabi, Uppsala, Sweden) given intravenously at a rate of 15 ml/kg via syringe pump (CMA 402, CMA Microdialysis, North Chelmsford, MA) over an average of three minutes. The dose of IFE is based on previous experiments using IFE in rats.14,16 Animals in Group 2 received three doses of IFE separated by 20 minutes, starting 5 minutes post-exposure (i.e. at 5, 25, and 45 minutes post-exposure). Animals in Group 3 received three doses of IFE separated by 20 minutes, starting 20 minutes post-exposure (i.e. at 20, 40, and 60 minutes post-exposure). The timing of the administration of the IFE was chosen to coincide with either the initial effects of parathion following oral exposure (5 minutes),17 or the peak concentrations of parathion following oral exposure (20 minutes).18 According to Yamamoto et al., the initial measurable levels of parathion following oral exposure to parathion occur 6 to 8 minutes after ingestion.17 According to Miyamoto et al., the peak effect following oral parathion occurs 30 minutes post-exposure.18 We chose to start the IFE infusion before these times to ensure serum levels of IFE at the stated initial and peak levels. The peak concentration of ethyl-parathion (the compound used in this study) following oral exposure is unknown, so the peak concentration of methyl-parathion was used. The primary endpoint was the percent of animals with apnea.
Data Analysis
A review of our data using the Shapiro-Wilk test for normality demonstrated that our data were non-parametric. Comparison between groups was performed using a Mann-Whittney test or a Kruskal-Wallis test where appropriate. In comparisons that involved multiple groups, the Kruskal-Wallis test was followed by a Dunns multiple comparison test to compare each individual study group. Respiratory variables were analyzed with analysis of variance (ANOVA) techniques.
Assuming 100% respiratory failure following parathion exposure with an alpha of 0.05 and a beta of 0.2, our study was powered to detect an absolute difference of 60% between any two groups. As there are no previously published data to use for our power calculation, we used a 60% difference between groups based on the effect of IFE in previous studies of verapamil poisoning, another lipophilic compound.16 We assume a familywise error rate of 0.0975%, and did not adjust for multiple comparisons. A further data analysis was performed to calculate the rate of decline of the respiratory rate for each group of animals. Using a best-fit curve of breaths per minutes vs. time, a constant for each animal’s rate of respiratory decline was determined and defined as breaths per minute.
RESULTS
Baseline respiratory and circulatory parameters were similar between groups at baseline (Table 1). Animals exposed to parathion alone (Group 1) demonstrated apnea characterized by a steady decline in respiratory rate, tidal volume, and minute ventilation post-exposure. Apnea occurred a mean 44.5 minutes after parathion exposure (range 31 to 54 minutes). No animals demonstrated a decrease of pulse or blood pressure below baseline values post-exposure and prior to apnea. Animals treated with IFE 5 minutes post-exposure (Group 2) demonstrated a similar clinical course with all animals demonstrating apnea followed by circulatory collapse and death. Animals in Group 2 demonstrated no difference in mean time to apnea (44.5 min, 95% CI = 35.78 to 53.22 vs. 51.6 min, 95% CI = 43.55 to 59.79; p = 0.29) or number of animals with respiratory arrest (100% in both groups). Animals treated with IFE 20 minutes post-exposure (Group 3) demonstrated no difference in mortality compared to control (100% vs. 66.7%, p = 0.45). The mean time to apnea in these animals was prolonged compared to Group 1 (51.6 min, 95% CI = 43.55 to 59.79 vs. 95.3 min, 95% CI = 71.65 to 119.02; p = 0.002). The times to apnea for all animals in Group 1 with no treatment (range 31 to 54 minutes) and Group 2 with IFE at 5 minutes (range 41 to 65 minutes) were less than for any individual animal treated with IFE at 20 minutes (range 68 to 120 minutes).
Table 1.
Baseline parameters of study groups
| Variable | Group 1 Parathion Alone (n=6) |
Group 2 Parathion then IFE at 5 minutes (n=6) |
Group 3 Parathion then IFE at 20 minutes (n=6) |
|---|---|---|---|
| Respiratory rate | 49.3 | 50.9 | 49.5 |
| Tidal volume | 3.1 | 2.7 | 2.7 |
| Minute ventilation | 153.3 | 138.9 | 135.4 |
| Mean arterial pressure | 88.1 | 73.8 | 90.9 |
| Pulse rate | 372.0 | 376.9 | 357.0 |
| Pulse oxygenation | 92.0 | 98.5 | 98.6 |
There were no significant differences of respiratory or cardiovascular values at baseline between groups (p = ns all comparisons). Due to technical failures, one animal in the control group did not have an arterial pressure recording and a separate animal in an experimental group did not have a pulse oximetry recording.
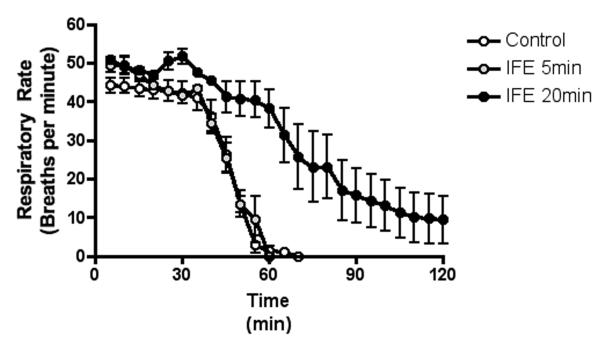
Respiratory variables demonstrated no difference between animals that received no therapy and animals that were treated with IFE 5 minutes post-exposure (p = ns, by ANOVA), but animals treated with IFE 20 minutes post-exposure demonstrated a prolonged time to respiratory failure compared to either group (p < 0.001, by ANOVA). All three groups demonstrated similar changes in respiratory variables until roughly 30 minutes post-exposure (Figure 1). The rate of respiratory decline was significantly steeper in animals with no treatment and those treated with IFE at 5 minutes post-exposure when compared to animals treated with IFE 20 minutes post-exposure (Table 2).
Figure 1.
The decline in respiratory rate after parathion exposure
The data were averaged into 5-minute bins starting from the time of OP introduction to the 2-hour endpoint of the study. Data are presented as mean (dot) with standard deviation (error bars).
Table 2.
Rate of respiratory decline of study groups
| Parameters | Group 1 Control |
Group 2 IFE at 5 minutes |
Group 3 IFE at 20 minutes |
|---|---|---|---|
| Rate of respiratory decline, mean | −2.65 | −2.295 | −0.795 |
| Standard deviation | ±1.35 | ±0.58 | ±0.55 |
| P value | - | 0.661 | 0.0179 |
Rate of respiratory decline was calculated by calculating a constant for each animal’s rate of respiratory decline in breaths per minute using a best-fit curve of breaths per minute vs. time.
DISCUSSION
Data from this study indicate that administration of IFE administered at 20, 40, and 60 minutes after exposure mitigates the respiratory depressant effects of acute parathion poisoning in a rat model. When IFE was given 5, 25, and 45 minutes after oral parathion, there were no statistically significant differences in any cardiopulmonary measures. However, none of the study groups (initial IFE at 5 or 20 minutes post-exposure) demonstrated any increase in survival. It is possible that this is related to dose, timing, or the inability of IFE to fully protect against OP exposure. Further studies are required.
The mechanism of action of IFE in decreasing OP-induced respiratory depression is unknown. Because of the lipophilic nature of the parathion (log octanol/water partition coefficient [log Kow] of 3.83), it is possible that the IFE absorbed a portion of the circulating parathion, thereby sequestering the OP and preventing its inhibition of acetylcholinesterase (AChE). This speculative mechanism has been described as a “lipid sink,” and was initially described by Weinberg et al.15 The IFE may effectively provide a circulating intravenous lipid compartment that produces a gradient for diffusion of lipophilic substances out of surrounding tissue. The concept of a “lipid sink,” although unproven, provides a possible explanation for the increased effectiveness of IFE when given at the time of peak parathion effect. It raises an interesting question concerning the clinical dosing of IFE, because if true, the time of maximal efficacy of IFE would vary depending on the specific compound, route of poisoning, and possibly the presence of other co-ingestants.
It is not clear why IFE first given 5 minutes after parathion poisoning demonstrated no effect on the respiratory decline post-exposure, but IFE initially given 20 minutes after parathion poisoning demonstrated an effect. IFE for both treatment groups was administered every 20 minutes, so at 30 minutes post-exposure, both groups had received IFE. Animals receiving IFE starting at 5 minutes post-exposure had received twice the IFE as animals with IFE starting 20 minutes post-exposure. There were no respiratory effects from parathion demonstrated prior to 30 minutes, so it is possible that IFE given at 5 minutes was given too early, when serum levels were lower. Animals with IFE started at 5 minutes received one dose when serum levels of parathion were presumably higher, while animals with IFE started at 20 minutes received two doses. This however does not explain why animals with IFE started at 5 minutes died before they could receive a second dose. This raises the possibility that the IFE is only effective when given at a specific time related to peak concentration.
Alternatively, the IFE may have contributed to higher serum levels of parathion, accelerating OP-induced respiratory depression. It is possible that the early administration of IFE provides a higher gradient of diffusion from the stomach to a circulating lipid compartment in the vasculature, resulting in increased early tissue exposure to parathion. This theory is less likely, as we demonstrated no early respiratory depression in animals treated with IFE at 5 minutes post-exposure when compared to controls.
Limited previous work has been published on the effect of IFE on OP toxicity, with only a single published abstract found on the subject. Bania et al. investigated the use of IFE on paraoxon (the active metabolite of parathion) toxicity in mice.19 While their results failed to demonstrate an increase in the LD50 of paraoxon with the use of IFE, it is possible that the extreme rapidity of paraoxon’s toxicity, as well as differences in kinetics between parathion and paraoxon, account for the differences between the current study and that of Bania et al. Parathion requires P450-mediated metabolism to paraoxon in the liver before it is able to inhibit acetylcholinesterase.17 This metabolic step provides a period of time where a medication that mitigates tissue exposure can be administered. Because paraoxon requires no metabolic conversion to be physiologically active, there may be insufficient time to administer IFE for it to have any beneficial effects following paraoxon exposure.
Despite several decades of research, no methods have been proven effective in reducing the amount of OPs absorbed after poisoning in humans.20 Because standard treatment with anti-cholinergics and oximes has severe limitations (as demonstrated by the high morbidity and mortality post-OP ingestion despite aggressive therapy) alternative therapies are urgently needed.20,21 A novel strategy such as sequestering the OP after ingestion has the potential to fundamentally change the management of acute OP poisoning. Our results demonstrate a potential role for IFE post-OP exposure, but more research is needed to determine the timing of administration and the role of IFE in other OP agents.
LIMITATIONS
We did not quantify parathion concentrations in plasma in any group of animals. If IFE truly sequesters OPs (as is theorized for other lipophilic drugs19), then an increase in parathion within the intravascular compartment would be expected. In addition, quantifying the concentration of IV parathion during IFE administration may have helped elucidate the mechanism of action of IFE. Quantification of IV parathion could help determine if early IFE increases the exposure of parathion after oral ingestion.
The use of an anesthetized model to monitor OP-induced respiratory effects produces significant limitations. Isoflurane causes respiratory depression and could have contributed to the OP-induced apnea. It is unlikely that the use of isoflurane is responsible for differences in respiratory effects between study groups for a number of reasons. First, each group of animals received a similar level of isoflurane, so differences in respiratory changes should not be attributed to the anesthetic. Second, in previously published animals models of OP poisoning with isoflurane from our lab, we demonstrated longer periods of anesthesia without respiratory depression. Third, isoflurane can prevent seizures, a known effect of OP ingestion. These effects of isoflurane limit the generalizability of our findings.
It is unclear what effect IFE has on the traditional therapies for acute OP exposure. The current mainstay of therapy includes anti-cholinergic agents, oximes, and benzodiazepines. In an effort to study the isolated effects of IFE, we intentionally did not include standard therapeutic agents, so it remains unclear if IFE affects the efficacy of these agents. Studies of combination treatment with all of these agents should be considered prior to recommending the clinical use of IFE in patients exposed to OP agents.
The use of IFE for acute OP poisoning is also complicated by the fact that there are hundreds of unique OP agents with a wide range of physiochemical properties. OP pesticides are a group of physicochemically distinct compounds. They can differ substantially in such properties as potency,20 lipophilicity, need for bioactivation, response to oximes,20 and persistence in the environment. Future trials with alternative OP agents should be considered.
CONCLUSIONS
The use of intralipid fat emulsion post-exposure partially mitigates the respiratory depressant effects of parathion. Further research is required before intralipid fat emulsion therapy should be considered for clinical use after organophosphate pesticide exposure. Optimal intralipid fat emulsion dosing, rate of administration, total or duration of dosing, and the pharmacokinetic and pharmacodynamic effects of intralipid fat emulsion on atropine and oximes remain unknown.
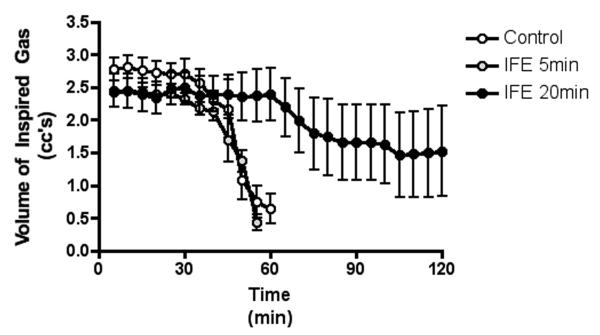
Figure 2.
The decline in tidal volume after parathion exposure
The data were averaged into 5-minute bins starting from the time of OP introduction to the 2-hour endpoint of the study. Data are presented as mean (dot) with standard deviation (error bars).
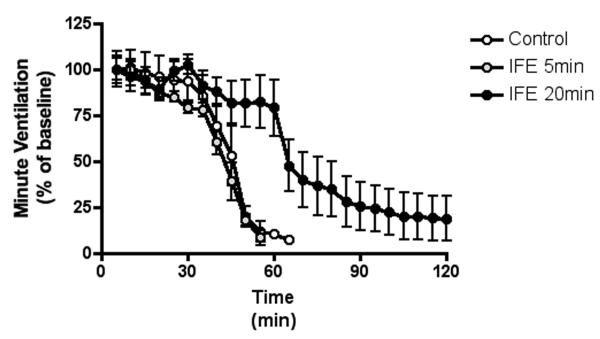
Figure 3.
The decline in minute ventilation after parathion exposure
The decrease in minute ventilation (MV) is shown as percent of baseline to normalize differences between individual animals within groups. The data were averaged into 5-minute bins starting from the time of OP introduction to the 2-hour endpoint of the study. Data are presented as mean (dot) with standard deviation (error bars).
Acknowledgments
Funding: This study was supported in part by the National Institutes of Health (grant 1K08NSO48857).
Footnotes
Disclosures: The authors have no disclosures or conflicts of interest to report.
Presentations: Society for Academic Emergency Medicine Annual Meeting, Boston MA, June 2011
References
- 1.Eddleston M, Buckley NA, Eyer P, Dawson AH. Management of acute organophosphorus pesticide poisoning. Lancet. 2008;371(9612):597–607. doi: 10.1016/S0140-6736(07)61202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization [Accessed Feb 10, 2012];The impact of pesticides on health: preventing intentional and unintentional deaths from pesticide poisoning. Available at: http://www.who.int/mental_health/prevention/suicide/en/PesticidesHealth2.pdf.
- 3.Gaspari RJ, Paydarfar D. Pathophysiology of respiratory failure following acute dichlorvos poisoning in a rodent model. Neurotoxicology. 2007;28(3):664–71. doi: 10.1016/j.neuro.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. QJM. 2000;93(11):715–31. doi: 10.1093/qjmed/93.11.715. [DOI] [PubMed] [Google Scholar]
- 5.Okumura T, Takasu N, Ishimatsu S, et al. Report on 640 victims of the Tokyo subway sarin attack. Ann Emerg Med. 1996;28(2):129–35. doi: 10.1016/s0196-0644(96)70052-5. [DOI] [PubMed] [Google Scholar]
- 6.Eyer F, Meischner V, Kiderlen D, et al. Human parathion poisoning. A toxicokinetic analysis. Toxicol Rev. 2003;22(3):143–63. doi: 10.2165/00139709-200322030-00003. [DOI] [PubMed] [Google Scholar]
- 7.Goswamy R, Chaudhuri A, Mahashur AA. Study of respiratory failure in organophosphate and carbamate poisoning. Heart Lung. 1994;23(6):466–72. [PubMed] [Google Scholar]
- 8.Sungur M, Guven M. Intensive care management of organophosphate insecticide poisoning. Crit Care (London) 2001;5(4):211–5. doi: 10.1186/cc1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bird SB, Sutherland TD, Gresham C, Oakeshott J, Scott C, Eddleston M. OpdA, a bacterial organophosphorus hydrolase, prevents lethality in rats after poisoning with highly toxic organophosphorus pesticides. Toxicology. 2008;247(2-3):88–92. doi: 10.1016/j.tox.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bird SB, Dawson A, Ollis D. Enzymes and bioscavengers for prophylaxis and treatment of organophosphate poisoning. Front Biosci (Schol Ed) 2010;2:209–20. doi: 10.2741/s58. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, Zhan C, Li Y, Zhong Q, Pan H, Yang G. Intravenous lipid emulsions combine extracorporeal blood purification: a novel therapeutic strategy for severe organophosphate poisoning. Med Hypotheses. 2010;74(2):309–11. doi: 10.1016/j.mehy.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Cave G, Harvey M. Intravenous lipid emulsion as antidote beyond local anesthetic toxicity: a systematic review. Acad Emerg Med. 2009;16:815–24. doi: 10.1111/j.1553-2712.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 13.Davis SS. Coming of age of lipid-based drug delivery systems. Adv Drug Deliv Rev. 2004;56(9):1241–2. doi: 10.1016/j.addr.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg GL, VadeBoncouer T, Ramaraju GA, Garcia-Amaro MF, Cwik MJ. Pretreatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine-induced asystole in rats. Anesthesiology. 1998;88(4):1071–5. doi: 10.1097/00000542-199804000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Arroyo AM, Kao LW. Calcium channel blocker toxicity. Pediatr Emerg Care. 2009;25(8):532–8. doi: 10.1097/PEC.0b013e3181b0a504. [DOI] [PubMed] [Google Scholar]
- 16.Tebbutt S, Harvey M, Nicholson T, Cave G. Intralipid prolongs survival in a rat model of verapamil toxicity. Acad Emerg Med. 2006;13:134–9. doi: 10.1197/j.aem.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto T, Egashira T, Yoshida T, Kuroiwa Y. Comparative metabolism of fenitrothion and methylparathion in male rats. Acta Pharmacol Toxicol (Copenh) 1983;53(2):96–102. doi: 10.1111/j.1600-0773.1983.tb01875.x. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto J, Sato Y, Kadota T, Fujianmi A, Endo M. Studies on the mode of action of organophosphorous compounds. Part I. Metabolic fate of P32 labeled sumithion and methylparathion in guinea pig and white rat. Agtic Biol Chem. 1963;28:411–21. [Google Scholar]
- 19.Bania T, Chu J, Stolback A. The effect of intralipid on organophosphate toxicity in mice. Acad Emerg Med. 2005;12:15. [Google Scholar]
- 20.Bird SB, Dawson A, Ollis D. Enzymes and bioscavengers for prophylaxis and treatment of organophosphate poisoning. Front Biosci. 2010;S2:209–20. doi: 10.2741/s58. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum C, Bird SB. Non-muscarinic therapeutic targets for acute organophosphorus poisoning. J Med Toxicol. 2010;6(4):408–12. doi: 10.1007/s13181-010-0093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]