Abstract
Bone marrow stromal cells (BMSC) have shown significant promise in the treatment of disease, but their therapeutic efficacy is often limited by inefficient homing of systemically-administered cells, which results in low numbers of cells accumulating at sites of pathology. BMSC home to areas of inflammation where local expression of integrins and chemokine gradients are present. We demonstrated that non-destructive pulsed focused ultrasound (pFUS) exposures that emphasize the mechanical effects of ultrasound-tissue interactions induced local and transient elevations of chemoattractants (i.e., cytokines, integrins, and growth factors) in the murine kidney. pFUS-induced upregulation of cytokines occurred through approximately 1 day post-treatment and returned to contralateral kidney levels by day 3. This window of significant increases in cytokine expression was accompanied by local increases of other trophic factors and integrins that have been shown to promote BMSC homing. When BMSC were administered intravenously following pFUS treatment to a single kidney, enhanced homing, permeability, and retention of BMSC was observed in the treated kidney versus the contralateral kidney. Histological analysis revealed up to 8 times more BMSC in the peritubular regions of the treated kidneys on days 1 and 3 post-treatment. Furthermore, cytokine levels in pFUS-treated kidneys following BMSC administration were found to be similar to controls, suggesting modulation of cytokine levels by BMSC. pFUS could potentially improve cell-based therapies as a noninvasive modality to target BMSC homing by establishing local chemoattractant gradients and increasing expression of integrins to enhance tropism of BMSC toward treated tissues.
Keywords: mesenchymal stem cells, pulsed focused ultrasound, high intensity focused ultrasound, mechanotransduction, stem cell migration, cytokines, enhanced homing permeability and retention (EHPR), ICAM, VCAM, integrins, ferumoxides, protamine
Introduction
Stem cell therapy has emerged as a promising therapeutic alternative for tissue regeneration and potential treatment of diseases [1, 2]. Bone marrow stromal cells (BMSC), also known as mesenchymal stem cells, have been shown to down-regulate inflammation or stimulate endogenous stem cell proliferation through paracrine signaling. BMSC have been administered by direct transplantation into tissues, and intra-arterial, or intravenous (IV) injection in experimental and clinical studies [3, 4]. Systemic administration of BMSC requires the trafficking of cells through the vasculature and subsequent margination across endothelial barriers to the site of damaged or inflamed parenchyma [5]. Homing of BMSC to pathology has been defined as the arrest of cells within the vasculature of tissue followed by trans-endothelial migration into the parenchyma analogous to that of inflammatory cells [6]. While mechanisms of BMSC homing remain poorly understood, evidence suggests that local up-regulation of chemo-attractants and integrins on endothelia stimulate BMSC adhesion and the subsequent transmigration from the vasculature into the parenchyma [7, 8].
A significant limitation to the effectiveness of IV administration of BMSC is pulmonary trapping of BMSC [3, 9] that results in the inability to target these cells into tissues. Maximizing the homing efficiency of BMSC to pathology requires orchestrating the precise timing of cell delivery, dosing regime, and/or route of administration to coincide with regional micro-environmental changes (i.e., release of chemo-attractants) within the tissue [6]. Although elevations in cytokines and growth factors are acutely associated with inflammation and injury, it is unclear when to administer the cell products to maximize homing efficiency and therapeutic effectiveness [10–12]. Since directed homing and tissue integration may be key to improving the therapeutic applications of BMSC, the development of a non-destructive, non-invasive modality to trigger release of chemo-attractants in the local microenvironment within or around the periphery of pathology would be valuable for cellular therapies.
Focused ultrasound (FUS) is an emerging non-invasive therapeutic modality to treat solid tumors. It concentrates the ultrasound energy at the targeted site without generating effects in the intervening tissues. Continuous FUS exposures can safely deliver high rates of energy to a targeted region, causing substantial temperature increases (≥80°C) resulting in coagulative necrosis. Precise targeting and monitoring of treatment is possible using diagnostic ultrasound or MRI for image guidance of FUS [13]. Pulsed FUS (pFUS) (i.e., non-continuous focused ultrasound exposures) minimizes heat generation by lowering the rate of energy deposition, and may produce non-destructive effects in both the vasculature and the parenchyma. These can occur as a result of non-thermal mechanisms such as acoustic cavitation and acoustic radiation forces that are mediated through the activity of microbubbles or the local displacement of tissue. These mechanical phenomena can also be utilized for various clinical applications such as enhancing drug delivery by increasing permeability and temporarily altering endothelial cellular interaction in tissues [14]. Although this has yet to be shown, the mechanical effects of pFUS are thought to occur through mechanotransduction - a change in the chemical activity of cells and tissues in response to a mechanical stimulus [15, 16]. We have recently demonstrated in murine skeletal muscle, that similar pFUS exposures can stimulate short-lived increases (~24 hr) in local expression of cytokines, growth factors, and integrins, without tissue destruction [17].
In this study, pFUS was employed to create localized and non-destructive changes in the nude murine kidney leading to transient and local elevations of chemoattractants (cytokines, trophic factors, and integrins) known to increase tropism of IV-injected BMSC. Kidneys are an excellent model given that IV-infused BMSC do not routinely home to or engraft in this tissue (<0.1% of injected dose) [8], and the contralateral kidney serves as internal control. We demonstrated greater numbers of IV-injected BMSC appear in the extravascular space of pFUS-treated kidneys compared to untreated controls suggesting pFUS enhances homing, permeability, and retention (EHPR) of BMSC to targeted tissues and therefore, may represent a valuable tool to improve the efficacy of cellular therapies in the treatment of a variety of pathologies.
Materials and Methods
pFUS System and Exposures
pFUS exposures were administered using a custom-built system modified from a Sonoblate 500 (Focus Surgery, Indianapolis, IN). The probe was comprised of a single element, 1-MHz concave therapeutic transducer with a focal length of 4 cm and an aperture of 5 cm. The focal zone of the transducer was in the shape of an elongated ellipsoid with axial diameter (−3 dB) of 7.20 mm and radial diameter (−3 dB) of 1.38 mm, confirmed by Schelieren measurements previously determined by the manufacturer. An M-Turbo, portable ultrasound imaging system (SonoSite, Bothell, WA) with a SLax imaging transducer (25-mm linear array, broadband frequency 6–13 MHz, max depth of 6 cm) was used for guidance and monitoring of pFUS exposures. For precise targeting, the imaging transducer was mechanically coupled to the pFUS probe such that the focal region of the beam lay in the ultrasound-imaging plane.
All experimental studies were performed in compliance with guidelines provided by the institutional Animal Care and Use Committee (ACUC). Female Balb/C nude mice were anesthetized with isoflurane and pFUS exposures were carried out as previously described [18, 19]. Mice were placed in a custom-built holder, attached to a 3D positioning stage. The mouse was then inserted vertically, up to its neck, in heated (36 °C) degassed water for coupling with the pFUS transducer. Using real-time image guidance with diagnostic ultrasound, the mouse was positioned so that the right kidney was in the center of the focal zone of the pFUS transducer (Figure 1 and Supplemental Fig. 1). For pFUS exposures, the rastering sequence was a 2 × 3 matrix, with 2 mm between the raster points. The pFUS exposure parameters were as follows: spatial average, temporal peak intensity (ISATP) = 2667 W/cm2; duty cycle = 5% (10 ms ON; 190 ms OFF); pulses per raster point = 100. The treatment time for treating one kidney with these parameters was 2 minutes. After pFUS treatment, the mouse was removed from the water bath, dried, and placed in warmed recovery cage.
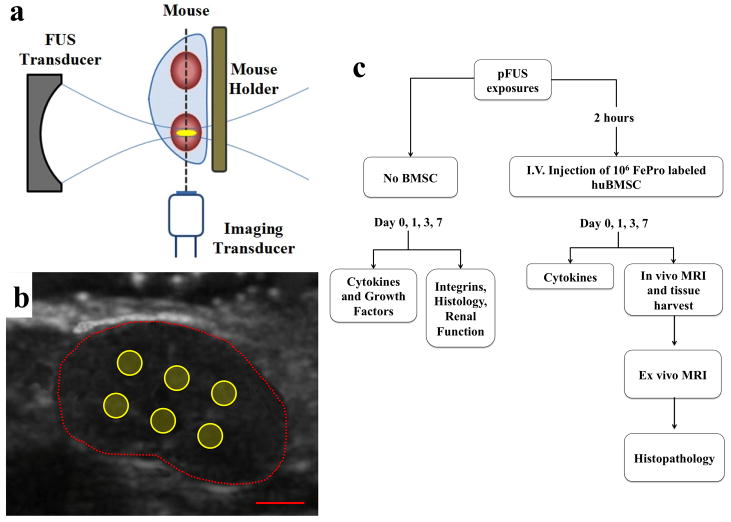
Figure 1.
Schematic of pFUS treatments and experimental design. (a) Diagram of pFUS experimental setup showing position of the imaging and therapeutic transducers relative to the mouse. (b) Ultrasound image of mouse kidney roughly indicated by dotted red line. Solid yellow circles represent position of pFUS raster points (scale bar = 2 mm). (c) Flow chart outlining experimental use of animals.
BMSC Administration
Approximately two hours after pFUS exposures, mice that were to receive BMSC were first given an IV injection of 25 μg sodium nitroprusside (Roche, USA) [4] diluted in 100 μL PBS followed by an IV injection of 106 FEPro-labeled human cells in 100 μL of HBSS.
Cell Culture and labeling
Human BMSC (provided and characterized by Bone Marrow Stromal Cells Transplantation Center at our institution) were obtained from volunteers undergoing bone marrow biopsy under an approved institutional review board protocol at our institution. BMSC were cultured in α-Minimum Essential Medium (α-MEM) supplemented with 2 mM L-glutamine, 100 U/ml Penicillin, 100 μg/ml streptomycin sulfate (Biofluids, Rockville, MD) and 20% fetal bovine serum (Equitech-Bio, Kerrville, TX) at 37 °C, under an atmosphere containing 5% CO2 and 1% O2. Early passages of BMSC (1–5) were used for this study. The BMSC were labeled with ferumoxides (Feridex IV®, Berlex Laboratories, Wayne, NJ) complexed to protamine sulfate (10 mg/ml, American Pharmaceuticals Partner, Schaumburg, IL) as previously described [20]. Briefly, ferumoxides (FE, 100 μg/ml) and protamine sulfate (Pro, 6 μg/ml) were mixed in fresh serum-free Roswell Park Memorial Institute (RPMI) 1640 medium (Biosource, Camarillo, Ca) supplemented with HEPES, MEM non-essential amino acids, sodium pyruvate, and L-glutamine at room temperature for 5–10 min then added to cultured BMSC and incubated for two hours at 37 °C. After 2 hours, an equal volume of serum-containing medium was added making the final concentration of ferumoxides 50 μg/ml, and BMSC were incubated overnight. Cells were washed 3 times with Hank’s Balanced Salt Solution (HBSS) containing 10 U/ml of heparin (Abraxis, Schaumburg, IL), trypsinized, and suspended in heparinized HBSS at 107 cells/ml. This labeling technique has previously been shown not to alter stemness of BMSC [21]. Cell viability was determined by trypan blue exclusion and cells were assayed for surface expression of standard human BMSC markers (CD29, CD44, CD73, CD90, and CD105) and non-BMSC markers (CD34) by flow cytometry (Accuri, Detroit, MI).
MRI
Mice were scanned by MRI at 3 Tesla (T) (Achieva, Philips Medical System, Netherlands, V.V.) on days 1, 3, and 7 post-treatment using a 4 cm solenoid receive-only coil (Philips Research Laboratories, Germany). MRI was performed using a T1-weighted, turbo spin echo (TSE) sequence with the following parameters: repetition time (TR), 600 ms; echo time (TE), 8.4 ms; number of acquisitions (NA), 2; field of view (FOV), 50 mm axial and 60 mm coronal. T2*-weighted gradient echo (GE) images were also acquired with the following parameters: TR, 825 ms; TE, 15 ms; flip angle, 30°; NA, 8; FOV, 50 mm. All images were acquired with a 0.5 mm slice thickness and were reconstructed at with a 100×100 μm in-plane resolution. Ex vivo MRI of treated and control kidneys (n = 5 mice per time point) was performed at 7T on an MR micro-imaging system (Bruker Biospec, Bilirica, MA) using a 20 mm radiofrequency coil and gradients of 100 G/cm. Kidneys were immersed in susceptibility matching fluid (Fomblin, Solvay Solexis, Inc, West Deptford, NJ). 3D multi-slice multi-echo T2-weighted images at 7T were acquired with the following parameters: effective echo time (TEeff), 45.5 ms; echo train length (ETL), 6 (first TE=13 ms); TR, 2.5 s; voxel size, 156×156×195 μm. 3D GRE T2*-weighted images at 7T were acquired with the following parameters: TEeff, 16.1 ms; ETL, 6 (first TE= 3.6 ms); TR, 90 ms; voxel size, 78×78×95 μm.
Prussian Blue Staining
Mice were euthanized and perfused with cold heparinized (10 U/ml) paraformaldehyde (4%). Kidneys, spleens, and lungs were harvested, dehydrated in graded ethanol, embedded in paraffin, and sectioned at thicknesses of 6–10 μm. Prussian blue (PB) staining of histological sections was performed to identify labeled BMSC. Paraffin-embedded sections were heated at 65 °C for 1 hr, deparaffinzed, and rehydrated prior to staining. Sections were washed with deionized water and stained with 2% potassium-ferrocyanide (Sigma-Aldrich, St. Louis, MO) in 3.7% HCl for 30 minutes. Tissues were washed again and counterstained with nuclear fast red (NFR).
Immunohistochemistry
Immunohistochemistry (IHC) was performed (n = 3–5 mice per time point) to detect human BMSC, mouse macrophages, and integrins (ICAM-1 and VCAM-1). Tissue sections were deparaffinized with xylene and rehydrated in graded ethanol concentrations. Sections were microwaved for 5 min in heat-induced epitope retrieval (HIER) citrate buffer for antigen retrieval. Non-specific binding was blocked using Super Block (ScyTek, Logan, UT) for 5 min, and then, the kidney sections were washed in PBS containing Tween-20 (0.1% v/v) (TPBS) and co-incubated with a mouse anti-human mitochondrial (HuMito) IgG (Abcam, Cambridge, MA) for human BMSC detection, and with a rat anti-mouse F4/80 IgG for macrophage detection at a final dilution of 1:50 for both antibodies. To localize the human BMSCs with respect to the renal vasculature, sections were incubated with a rabbit anti-mouse CD31 IgG at dilution of 1:50. In addition, consecutive tissue sections were co-incubated with rat, anti-mouse ICAM-1 IgG (Abcam, Cambridge, MA) and rabbit anti-mouse VCAM-1 IgG (Santa Cruz Biotechnology, Santa Cruz, CA) at a final dilution of 1:50 for both antibodies. All primary antibodies were incubated overnight at 4°C. Slides were washed with TPBS and then incubated with secondary antibodies in the dark for 2 hours at room temperature (AlexaFluor 546-conjugated goat anti-mouse IgG and AlexaFluor 488-conjugated goat anti-rat IgG for HuMito and F4/80; AlexaFluor 488-conjugated goat anti-rat IgG and AlexaFluor 546-conjugated goat anti-rabbit IgG (Invitrogen, Carlsbad, CA) for PECAM-1, ICAM-1, and VCAM-1). Tissues were again washed with PBS and coverslips were applied using ProLong Gold anti-fade reagent with DAPI (Invitrogen, Carlsbad, CA).
Fluorescence immunohistochemistry (IHC) for HuMito was used for quantitative analysis to determine the number of human BMSC in the pFUS-treated and contralateral kidneys at days 1, 3 and 7 post-treatment. Using confocal microscopy, ten different fields of view (FOV) were selected randomly in high power field (63×) and the number of huMito-positive cells was counted in each FOV. Quantitative image analysis for ICAM-1 and VCAM-1 expression was done using Zen LE 2009 software (Carl Zeiss, Oberkochen, Germany). Briefly, intensity histograms for the channels corresponding to each integrin were extracted from images from control kidneys for days 1 and 3 (n = 3 per integrin per day). Four average control histograms (1 for each integrin on each day) were constructed. The mean + 1 SD of each averaged control histogram was calculated and used as the minimum intensity threshold. Control and treated kidney images for each integrin on each day (n = 3) were then reanalyzed applying the appropriate threshold value and the number of pixels above the threshold were counted in each image.
Microscopy
For bright field imaging, slides were examined using an Axioplan Imaging II microscope (Carl Zeiss). Digital images were captured with an AxioCam MRc color CCD camera, equipped with a motorized stage and automated image acquisition system provided by the manufacturer. Kidney sections were also examined using an upright laser scanning confocal microscope (series 710, Carl Zeiss) using Plan-Apochromat objectives (20× air, N.A.=0.8; 63× oil-immersion, N.A.=1.4). Illumination was provided by an argon-ion (Lasos, Jena, Germany), diode, and diode-pumped solid-state lasers (Roithner Lasertechnik, Vienna, Austria). Excitation for DAPI, AlexaFluor488, and AlexFluor546 was performed using laser lines at 405 nm, 488 nm, and 561 nm, respectively and images were acquired sequentially to minimize crosstalk.
Cytokine Analysis
Two cohorts of mice (both receiving pFUS and only one receiving BMSC infusion following pFUS) were euthanized at 3 hours, day 1, day 3, and day 7 post pFUS for cytokine analysis. Cytokines were measured using an ELISA-based array which probed for the following cytokines: IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-17, MCP-1, IFNγ, TNFα, MIP-1α, GMCSF, and RANTES (Quansys Biosciences, Logan, UT). Following euthanasia both treated and contralateral control kidneys were snap-frozen in liquid nitrogen and stored −80 °C. Kidneys were homogenized mechanically on ice in tubes containing cell lysis buffer (Cell Signaling Technology, Danvers, MA) and a protease inhibitor cocktail (Santa Cruz Biotechnology, Santa Cruz, CA). Insoluble material was removed by centrifugation at 14000 RPM for 15 min at 4 °C. Total protein measurements were performed using a bicinchoninic acid (BCA) protein assay (Thermo Scientific, Waltham, MA) according to the manufacturer’s instructions. Thirty microliters of tissue lysate (1 mg/ml total protein) was added to the 96-well microplate supplied with the kit, and analysis was done according to the manufacturer’s protocol and imaged on a LI-COR Odyssey imaging system (LICOR, Lincoln, NE). Quantitative image analysis was performed using Q-view software (Quansys Biosciences, Logan, UT).
Western Blotting
Protein samples (25 μg) (n = 6 per time point) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions on Novex Bis-Tris gels (4–12% acrylamide, Invitrogen) and then transferred to polyvinyldiene fluoride (PVDF) membranes. Membranes were blocked using 5% bovine serum albumin (BSA) in Tris-buffered saline (TBS) containing 0.05% Tween-20 (TTBS) at room temperature for 1 hr. Membranes were hybridized with rabbit anti-mouse IgG primary antibodies against VEGF, FGF, PlGF, HGF, and SDF-1α overnight at 4 °C in TTBS containing 5% BSA. All primary antibodies were purchased from Abcam (Cambridge, MA) and were used at a 1:1000-fold dilution from manufacturer’s stocks. Secondary antibody hybridization was done for 1 hr at room temperature using a horseradish peroxidase (HRP)-conjugated, donkey anti-rabbit-IgG at a 1:5000 dilution from manufacturer’s stock (GE Healthcare, Little Chalfont, UK). Blots were developed by incubation with enhanced chemiluminescence reagents (Invitrogen, Carlsbad, CA) for 2 min at room temperature and luminescence was captured with an ImageStation 4000R Pro (Carestream Molecular Imaging, Woodbridge, CT) with various exposure times. Loading controls were performed with Ponceau-S staining of the membranes and imaged using the “trans-illumination” mode on the ImageStation 4000R Pro. Densitometry was performed on the ImageStation 4000R Pro using manufacturer supplied software and reported values are normalized to loading controls.
Statistical Analysis
All values are presented as mean ± SD. Descriptive statistics and means comparisons were done using GraphPad Prism software (version 5, GraphPad Inc, La Jolla, CA). Means were compared with two-tailed, unpaired t-tests using a Bonferroni corrections for multiple comparisons. A p value < 0.05 was considered significant.
Results
Cytokines, Growth factors, and Integrins
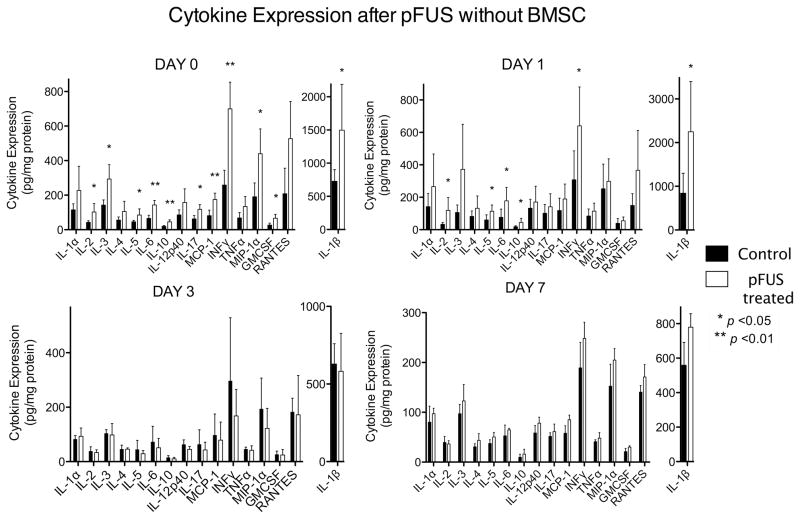
To investigate the effects of pFUS in the kidney and its ability to upregulate the appropriate molecular cues to induce homing of BMSC, mice (n = 6) were treated with pFUS alone (no BMSC) and kidneys were analyzed on days 0, 1, 3, and 7 post-pFUS using an ELISA-based cytokine array. Significant increases (p<0.05) in the following cytokines were observed on days 0 or 1 post-pFUS in the treated kidney compared to the contralateral: Interleukin- (IL) 1β, IL-2, IL-3, IL-5, IL-6, IL-10, IL-17, interferon-γ (IFNγ), monocyte chemotactic protein-1 (MCP-1), granulocyte macrophage colony-stimulating factor (GMSCF), and regulated upon activation, normal T-cell expressed and secreted (RANTES). Cytokine expression in pFUS-treated kidneys returned to control kidney levels by day 3 (Fig. 2, Supplemental Table 1).
Figure 2.
Cytokine expression in pFUS-treated and control kidneys without BMSC on days 0 and 1 post-treatment. Significant increases of cytokines were detected in treated kidneys compared to control kidneys that did not receive pFUS (n = 6; *p < 0.05; see Supplemental Table 1). Note different scale for IL-1β.
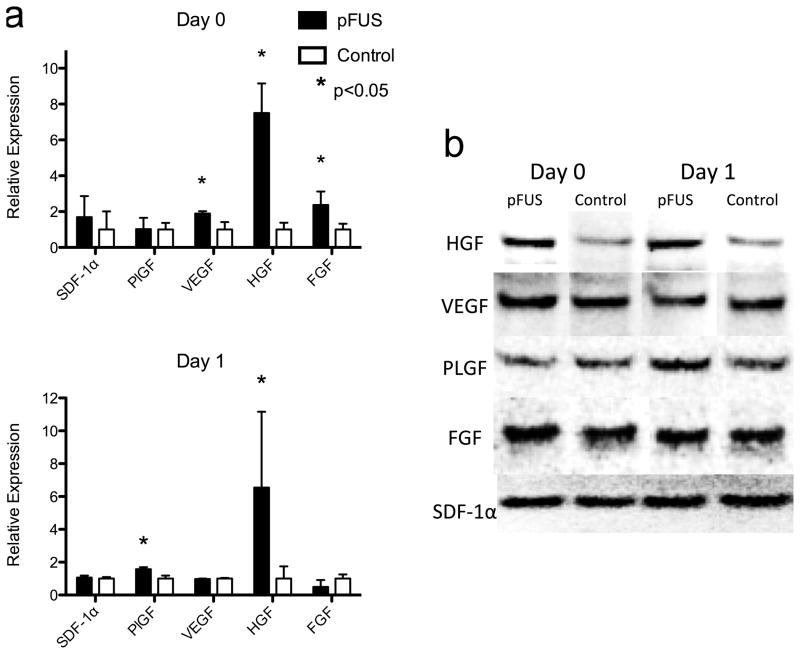
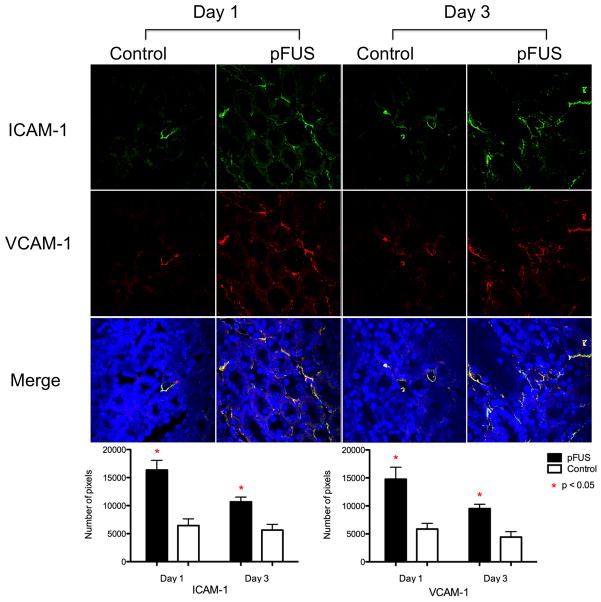
During the window of cytokine elevation in pFUS-treated kidneys (days 0 and 1), levels of trophic factors and integrins were also elevated. Kidneys treated with pFUS alone (no BMSC) were analyzed by western blot and showed significant increases in vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and hepatocyte growth factor (HGF) on day 0 (p<0.05) and increased levels of placental growth factor (PlGF) and HGF (p<0.05) on day 1 compared to contralateral controls (n = 6) (Fig. 3). pFUS treatment alone also resulted in increased expression of ICAM-1 and VCAM-1 on kidney vasculature on days 1 and 3 in pFUS-treated kidneys (Fig. 4). Each integrin was detected with ~3 times greater abundance in pFUS-treated kidneys on day 1 post-treatment, and expression of each was ~2 times greater than controls on day 3.
Figure 3.
Expression of growth factors on day 0 and 1 post-pFUS in the kidneys that received pFUS, but not BMSC. (a) summary of densitometric analyses (n = 6; *p < 0.05; Supplemental Table 3) and (b) representative western blots.
Figure 4.
ICAM-1 and VCAM-1 expression on days 1 and 3 post-treatment. ICAM-1 (green) and VCAM-1 (red) expression was more abundant in pFUS-treated kidneys on days 1 and 3. Yellow indicates merged signals. Images from each for each integrin from each day (n = 3) were thresholded (see Materials and Methods) and the number of pixels above the threshold were counted and compared between pFUS-treated and control images for each group. Approximately 3 times more expression of both is seen in pFUS-treated kidneys on day 1 and approximately 2 times more expression is still seen at day 3.
Renal Function and Apoptosis
TUNEL staining was performed on kidneys that received pFUS alone without BMSC and did not detect apoptotic nuclei in the pFUS-treated or contralateral kidney through day 7 post-treatment (data not shown). To assess the impact of pFUS treatment on renal function, blood urea nitrogen (BUN) and serum creatinine levels were measured at on days 0, 1, 3, and 7 post-pFUS and there were no differences between naïve mice and pFUS-treated mice that did not receive BMSC. Moreover, when mice were given BMSC following pFUS, there was no measureable difference in BUN or serum creatinine levels compared to mice that did not receive BMSC or naïve controls (Supplemental Fig. 5).
Renal Histology Following pFUS Alone
Histological examination of kidney tissue treated with pFUS alone (no BMSC) was performed in a blinded fashion and revealed no hemorrhage, necrosis, or changes in renal architecture upon hematoxylin and eosin (H&E). Periodic acid- Schiff (PAS) staining demonstrated rare and transient disorganization of the brush borders of the proximal tubules on day 1 post-pFUS that was no longer detectable on days 3 or 7. Trichrome staining did not show fibrosis in the pFUS treated or contralateral kidneys (Supplemental Fig. 4).
BMSC labeling
Human BMSC were labeled with superparamagnetic iron-oxide nanoparticles (SPION), and Prussian blue (PB) staining for intracellular iron revealed approximately 100% labeling efficiency with ferumoxide-protamine complexes (FePro) [20]. FePro labeling did not alter viability or surface marker expression, e.g., cells are positive for CD29, CD44, CD73, CD90, CD105, and negative for CD34 (Supplemental Fig. 2). One day 1 post-injection, human BMSC present in the spleen were positive for human mitochondrial markers (HuMito) and negative for murine macrophage-specific F4/80 upon immunohistochemistry (IHC) (Supplemental Fig. 3) demonstrating they can be distinguished from macrophages in vivo.
MRI Tracking of BMSC to pFUS-Treated Kidneys
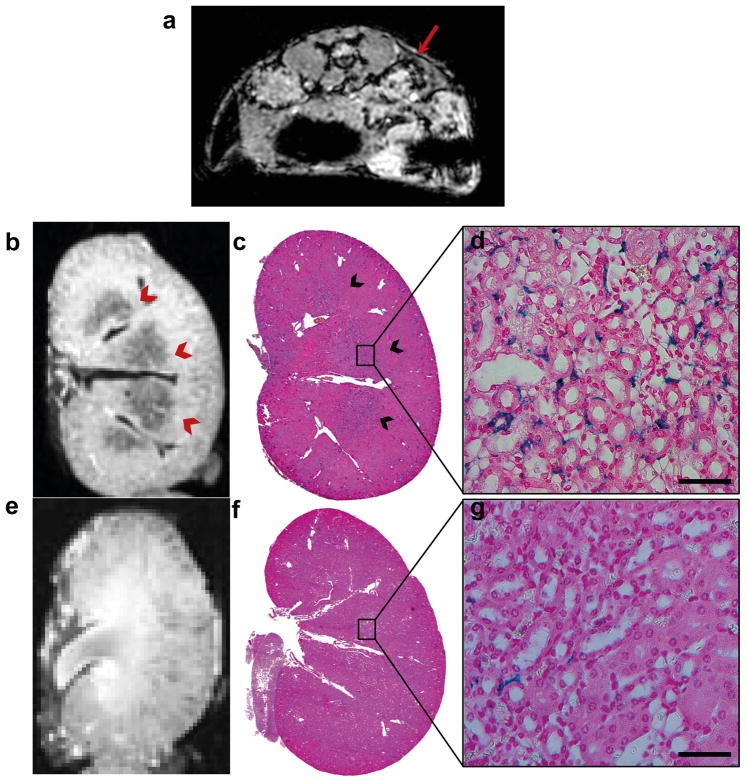
For MRI tracking of iron-labeled cells, mice were given a single treatment of pFUS to the right kidney. In vivo, T2*-weighted MRI at 3T revealed focal hypointense voxels that were distinguished from vessels in the cortical regions of pFUS-treated kidneys that were not observed in control kidneys on. Hypointense voxels consistent with the presence of SPION-labeled BMSC were detected in pFUS-treated kidneys on days 1 (Fig. 5a) and 3, but rarely on day 7. T2-weighted MRI in vivo revealed kidneys of normal appearance bilaterally. Ex vivo, T2*-weighted MRI at 7T also demonstrated hypointense voxels primarily in the juxtacortical regions and renal medulla (Fig. 5b, e) of treated kidneys on days 1 and 3, and showed no presence of BMSC in the contralateral kidney.
Figure 5.
T2*-weighted-MRI and Prussian blue staining of kidneys following pFUS and infusion of BMSC (a) Representative example of in vivo, T2*-weighted MRI at 3T on day 1. Mice were given pFUS to the right kidney and hypointense voxels were detected in the treated kidney (red arrows). (b) Ex vivo T2*-weighted-MRI at 7T of the pFUS treated kidney on day 1 post-treatment shows hypointense regions in the juxtacortical and medulla regions. (c) PB staining of the treated kidney day 1 post-treatment shows PB-positive regions (blue) which correlate with MRI findings. (d) High magnification (63×) of (c) shows an abundance of BMSC engrafted in peritubular spaces. (e) MRI and (f) PB staining of control kidneys do not reveal the presence of FePro labeled BMSC. (g) High power magnification of (f) reveals only small numbers of BMSC are present in control kidneys (scale bar = 50 μm).
Histological Detection of BMSC in pFUS-Treated Kidneys
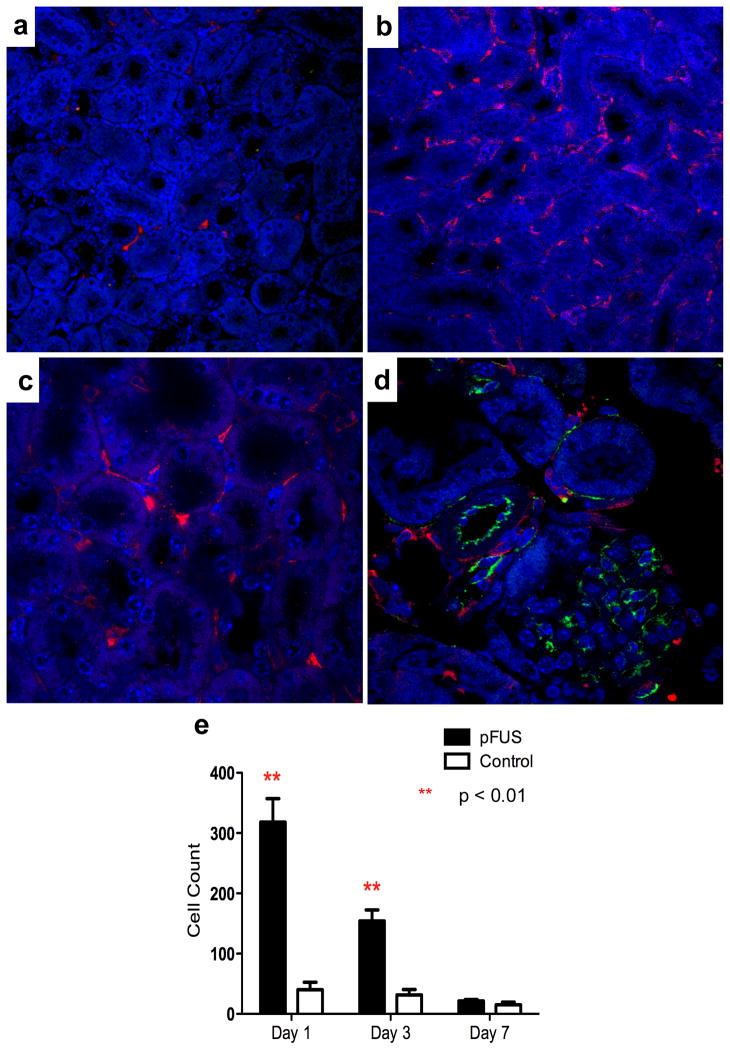
Prussian blue (PB) stain was performed to detect the presence of SPION-labeled BMSC in the kidneys. PB-positive BMSC were found in the pFUS-treated kidneys on days 1 and 3 post-infusion and were primarily localized in the peritubular regions of the medulla and rarely observed in the vasculature or glomeruli (Fig. 5c,d,f,g). PB-positive cells identified as BMSC appeared in serial kidney sections and were positive for HuMito and negative for F4/80 upon immunofluorescence (Fig. 6a–c). Immunofluorescence staining of platelet endothelial cell adhesion molecule (PECAM-1 or CD31) and HuMito showed that BMSC in the pFUS-treated kidney were primarily located in the extravascular space (Fig. 2d). Surveying 10 high-power fields-of-view (HPF) per kidney in three mice at each time point, the number of BMSC in pFUS-treated kidneys (318 ± 40 cells) was approximately 8× greater than the contralateral control kidney (40 ± 12 cells) 1 day post-treatment (p<0.01). Approximately 5× more cells were still retained in the treated kidney (154 ± 18 cells) compared to the contralateral (30 ± 9 cells) on day 3 (p<0.01). By day 7 post-treatment, the number of cells in the treated kidney (22 ± 6 cells) declined to numbers that were similar to contralateral controls (15 ± 4 cells) (p>0.05) (Fig. 2e).
Figure 6.
Representative photomicrographs of the kidneys following infusion of magnetically labeled BMSC on day 1 post-treatment. (a) Confocal microscopy following immunofluorescence for huMito and F4/80 revealed few human BMSC (red) or murine macrophages (green) in the contralateral kidney. (b) Large numbers of human BMSC and few F4/80 cells were detected in the pFUS-treated kidney. (c) High power field-of-view (63×) of panel b. (d) Immunofluorescence for PECAM-1 showed the presence of human BMSCs (red) outside the blood vessels (green). (e) Significantly greater numbers of BMSC (p<0.01) were found following pFUS treatment on days 1 and 3, but not day 7 compared to control kidneys. Quantification surveyed 10 fields-of-view in 3 sections per animal using 3 animals per time point.
Cytokine Levels Following pFUS and BMSC Infusion
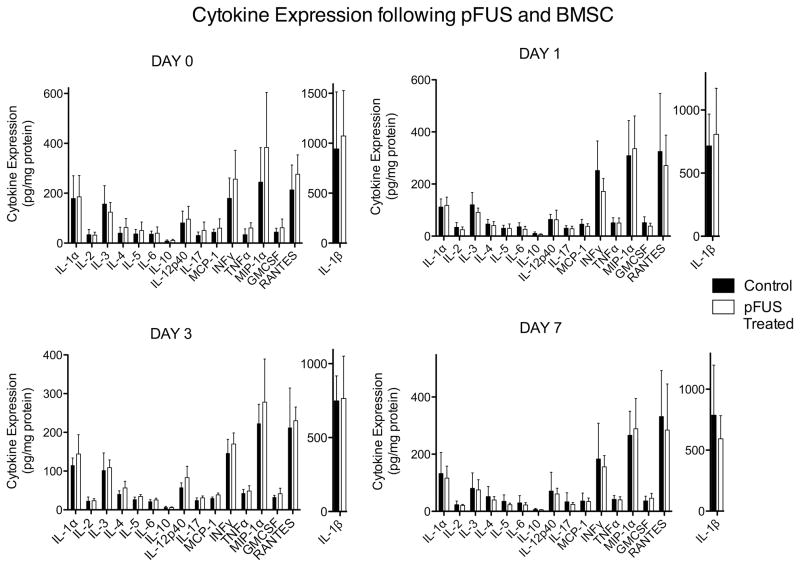
In mice treated with pFUS to a single kidney followed by intravenous BMSC, kidneys were harvested 6 hrs after stem cell infusion (Day 0) and on Days 1, 3, and 7. Tissue from pFUS-treated kidneys were analyzed for cytokine expression and compared to contralateral kidneys that did not receive pFUS. No statistically significant increases were observed for any cytokine measured at any day, however trends for increased expression of several cytokines existed in pFUS-treated kidneys on Day 0 (Fig. 7, and Supplemental Table 2).
Figure 7.
Cytokine expression in pFUS-treated and control kidneys followed by intravenous administration of BMSC. Statistically insignificant trends of increased cytokine expression were detected in treated kidneys compared to kidneys that did not receive pFUS (n = 6; *p < 0.05; see Supplemental Table 2). Note different scale for IL-1β.
Discussion
The underlying mechanisms by which infused BMSC home to sites of pathology are not well understood, but their behavior in the microcirculation is thought to be analogous to the leukocyte adhesion cascade that defines T-cell homing [6–8]. Active and/or passive homing may contribute to BMSC transmigration across endothelial barriers to sites of injury or inflammation. Passive homing may involve slowing capillary blood flow due to BMSC size, followed by transmargination in the presence of cytokine gradients in areas of damaged tissue [3]. Active homing presumably involves the rolling and tethering of BMSC to areas of upregulated integrin expression on endothelial cells followed by diapedesis into the parenchyma as a result of locally produced cytokines and trophic factors [6]. A combination of both active and passive processes that include slowing of blood flow, cellular rolling and adherence to integrins, and establishing a chemo-attractant gradient may be required to target enhanced homing, permeability, and retention of BMSC to pathology.
In the current study, we investigated the local effects induced by pFUS on the morphology and molecular biological profile of the kidney. BMSC are rarely found in the parenchyma of the kidney following IV injection in experimental models [22]. Moreover, pFUS treatment of one kidney allows the contralateral to serve as an internal control. Histological examination demonstrated that pFUS did not alter the cyto-architecture, induce apoptosis, or affect renal clearance of BUN and serum creatinine in the treated kidney. Moreover, macrophages were rarely seen in peritubular spaces on day 1 following pFUS. MRI performed after the administration of FEPro-labeled BMSC revealed focal hypointense voxels within pFUS-treated kidneys compared to contralateral kidneys. These MRI findings were consistent with the T2* shortening by intracellular SPION. BMSC were detected in mice up to three days following pFUS. PB and HuMito staining demonstrated significantly greater numbers of SPION-labeled BMSC in the peritubular spaces of pFUS-treated kidneys, with rare BMSC in the vasculature or glomeruli or in the contralateral kidney.
pFUS alone also increased levels of cytokines, growth factors, and integrins in treated kidneys. Transient elevations of cytokines were observed that are known induce tropism of infused BMSC into the renal parenchyma. Increased expression of VCAM-1 and ICAM-1 in the vasculature, and increased expression of VEGF, PlGF, FGF and HGF were also observed in the pFUS-treated kidneys compared to untreated controls. Alternatively, when cytokine levels were measured following both pFUS and BMSC infusion, increased expression of cytokines was not observed. BMSC have been shown to suppress cytokine expression and is one of their beneficial effects in clinical applications [8, 23]. These results suggest that the enhanced tropism of BMSC using pFUS had direct effects on cytokine expression and highlight the importance of coupling pFUS with cells to induce the homing, permeability, and retention into targeted tissues. While the apparent suppression of increased cytokine levels following pFUS and BMSC infusion compared to pFUS alone is promising, it warrants further investigation. Two potential issues confound interpretation of this data. First, potential physiological effects on cytokine expression following BMSC administration create additional variability and reduce the power of the statistical analysis. Even though sample sizes for cytokine measurements with and without BMSC are identical, the increased variability in the group receiving BMSC creates potential for real differences in expression to be undetectable. Second, it is possible that BMSC are not exclusively responsible the altered molecular profile after they are injected. Cell-adhesion molecules (CAM), including ICAM and VCAM mediate homing of many stem cell and immune cell types [24–27]. Therefore, endogenous cells may also tether to vessels in pFUS-treated and extravasate into the surrounding parenchyma. It is unknown specifically which types of endogenous cells might be present following pFUS or if interactions between endogenous stem/immune cells (e.g. M2 macrophages) and infused BMSC are responsible for attenuation of cytokine expression.
Several other approaches have been developed to enhance stem cell homing to areas of pathology. Image-guided or directed implantation of stem cells into and around areas of damaged tissue is commonly used. However, not all organs are amenable, and increased morbidity was associated with these invasive approaches. Although direct implantation bypasses the initial pulmonary trapping of BMSC [3], long-term survival of cells in areas of inflammation may also be problematic with >90% of cell death occurring within the first 3–7 days [28–30]. Catheter or intra-arterial injections are less invasive and depend upon perfusion to areas of pathology or areas surrounding the penumbra for the delivery of cell products. Intra-arterial injection of BMSC may cause small areas of ischemia, or micro-infarcts, with passive trapping of cells slowing blood flow and subsequent dilation of capillary beds [31]. Previous studies have shown SPION-labeled BMSC were detected in the parenchyma by MRI after intra-renal arterial administration [32]. This study observed increased numbers of BMSC in the kidney through 1–2 days post-injection, however, BMSC did not persist and could not be detected in the majority of animals after 2 days.
Increased tropism of infused BMSC has also been associated with acute elevations in chemoattractants in models of ischemia, inflammation, and infarction within 1–3 days [3, 6, 33–35]. However, IV administration of BMSC following acute inflammation may not result in large numbers of cells migrating to areas of pathology [2, 3, 36] due to vascular and necrotic changes within the parenchyma. Active targeting of BMSC to distant sites in the body has been accomplished by local radiation therapy, therapeutic ultrasound (TUS), and magnetic direction using SPION-labeled cells. Radiation therapy to the abdomen following traumatic brain injury increased engraftment of infused human BMSC to various organs outside the radiation field including the brain compared to mice that only received cells [37]. Moreover, low dose radiation to tumors induced BMSC tropism to target areas, and has been suggested as a possible treatment for satellite micrometastases [38]. Ionizing radiation can produce irreversible damage and cannot be readily translated into the clinic. Coupling SPION-labeled cells with external magnetic fields placed over target tissues resulted in significant enhancement of homing and retention to vascular stents in vessels or in the liver compared to animals without magnetic targeting [39–41].
Non-focused therapeutic ultrasound (TUS) (i.e., used in physical therapy) has been used in tissue regeneration, soft-tissue repair [42] and induction of angiogenesis in hind limb ischemia [43, 44]. Low-intensity TUS following IV infusion of microbubble (MB) contrast agents and direct implantation of bone marrow mononuclear cells caused significant increases in blood flow and augmented neovascularization by inducing expression of VEGF mRNA in moderate and severe ischemic limbs in rodent models [43, 44]. Moreover, multiple low-intensity TUS treatments to ischemic limbs resulted in a 30% increase in the amount of neovascularization compared to animals receiving only one exposure. Although the interaction of non-focused ultrasound with biological tissues was shown to induce enhance vascular permeability and release of growth factors, these effects were superficial and diffuse, and required infusions of MB prior to TUS. The coupling of TUS with MB results in significant thermal increases and or shearing effects due to enhanced acoustic cavitation which may result in unwanted tissue damage [45]. pFUS generates local mechanical effects (e.g., widening of intercellular gaps) that can increase the permeability of tissues for enhancing local drug and gene delivery with high spatial resolution. These non-invasive exposures have been successfully employed with clinically relevant agents used for treating tumors and blood clots in preclinical models [14].
In the current study, pFUS to the kidney induced cytokine expression and changes in endothelial cells over a relatively short time window of 24 hours. These findings could potentially be explained by mechanotransduction resulting in the discrete interactions between the ultrasound energy (i.e., radiation forces) and biological tissues that are reversible and nondestructive. External mechanical forces (fluid shear stress, hydrostatic pressure, and stretching) exerted on cells, have been shown to create changes in cellular signaling, gene expression, and overall function through mechanotransduction [15, 16]. The non-thermal effects of ultrasound, based on the transformation of energy from an ultrasound field into expansions and contractions of the intra-membrane space, were postulated to affect membrane proteins and similarly result in the activation of signaling pathways, leading to changes in gene expression, cellular function, and release of chemoattractive factors [46]. The observed molecular changes in the kidney in response to pFUS were most likely the stimuli that enhanced the observed cellular transmigration of BMSC across vascular endothelial barriers. Similar pFUS exposures in skeletal muscle have shown increased vascular permeability to proteins and nanoparticles 100 nm in diameter, but limited vascular permeability for nanoparticles with diameters of 200 nm [47]. Furthermore, histopathological analysis in the current study did not reveal any evidence of extravascular red blood cells. This indicates the unlikelihood that the renal vasculature is physically permeable to BMSC which are often 25 μm or larger in diameter [48] and migration is instead mediated by the chemoattractant gradients, trophic factors and increased expression of integrins induced by pFUS.
BMSC are usually infused concurrently with, or immediately after injury in ischemic or inflammatory models to capitalize on cytokine elevations thereby maximizing tropism of infused cells [3, 35]. Since pFUS induces local molecular mircoenvironmental changes that resulted in the increased tropism of BMSC and potentially other cell products, the precise spatial and temporal application of pFUS into or circumventing areas of pathology provides flexibility for timing stem cell infusions. Since pFUS can be performed at any time after tissue damage or injury, it should be feasible to synchronize induction of local chemo-attractant gradients with the infusion of cell products (e.g., hematopoietic stem cells, neural stem cells, endothelial precursor cells, cardiac progenitor cells, induced pluripotential stem cells, T-cells, and genetically engineered stem cells), resulting in enhanced homing (through increases in cytokines and growth factors), permeability (i.e., upregulation of integrins and transmigration) and retention (EHPR) of BMSC to a pathological site after the acute inflammation has resolved [49]. The EHPR effect is analogous to the enhanced permeability and retention (EPR) effect of tumors that can be further enhanced by coupling pFUS with chemotherapy [14]. pFUS creates a noninvasive platform to investigate two aspects of cellular therapy that currently need further investigation. First, does cell dose intensity (i.e., increased numbers) localizing to pathology improve therapeutic efficacy or potentially result in undesirable or detrimental outcomes (i.e., increased fibrosis or gliosis)? Secondly, will coupling multiple courses of pFUS with lower doses of cells following the acute inflammatory period improve therapeutic outcome? The administration of multiple courses of pFUS with cells over extended periods of time would be analogous to metronomic chemotherapy that is often used to inhibit neo-vascular formation in tumors [50], but in the case of cell therapy could provide prolonged paracrine effects to stimulate repair of targeted pathology. While this study was performed in healthy tissue, attempting to direct BMSC migration during pathology may be complicated by endogenous inflammation that is associated with increased expression of homing cues. However, this study provides a sufficient basis to explore whether pFUS can induce similar molecular changes during active pathological inflammation or after its resolution.
FUS units have been interfaced with ultrasound devices or built into CT or MRI scanners for treatment of diseases. FUS units are currently FDA-approved to treat uterine fibroids, and were used in early phase clinical trials for noninvasive ablative treatment of malignancy and venous occlusion [51, 52]. In the future, following appropriate regulatory approvals, it should be possible to translate and deliver single or multiple pFUS treatments at the bedside to coincide with infusion(s) of cell products. This approach should provide the flexibility of increasing the numbers of various cell products delivered to targeted pathology over time and offer the possibility of treating inflammation, ischemia (e.g., myocardial infarction and stroke), or stimulate repair as part of a regenerative medicine approach in the treatment of diseases or traumatic injury [1]. Further studies are needed to determine if multiple pFUS exposures and dosing schedules can have additive or synergistic effects on increasing the number of therapeutic cells (e.g. stem cells, immune cells, or genetically engineered cells) homing to various stages or time points in the development of acute or chronic pathology. This study provides the basis to explore the combination of pFUS with stem cell infusions outside the innate inflammatory window to enhance homing and delivery of cellular therapeutics.
Summary
We demonstrated that nondestructive pFUS resulted in increased tropism of infused BMSC to treated kidneys due to the mechanical effects of ultrasound energy inducing local elevations in cytokines, growth factors, and integrins, leading enhanced homing, permeability, and retention to the targeted parenchyma. Our results indicate that coupling pFUS with systemic delivery of BMSC potentially allows novel investigations into whether localizing greater numbers of cells to pathological sites will improve therapeutic outcome in the treatment of pathology. Further investigations are needed to determine if pFUS can elicit homing of other types of cells and in an EHPR effect in targeted tissues. In the future, following preclinical evaluation and clinical regulatory approvals, it may be possible to translate and deliver single or multiple pFUS treatments at the bedside to coincide with infusion(s) of cell products. It is important to note that since pFUS does not modify the infused cell product, but rather alters the microenvironment of the target tissue, it is possible that regulatory restrictions to coupling this approach may require limited modification to the existing IND or IRB protocol for the use in cell therapy clinical trials. The results from this study provide the basis to explore the combination of pFUS with stem cell infusions outside the innate inflammatory window to enhance homing and delivery of cell products as part of a therapeutic or regenerative medicine strategy.
Supplementary Material
Acknowledgments
Funded by the Intramural Research Program at the National Institutes of Health.
This work was supported by the Intramural Research Program in the Clinical Center at the National Institutes of Health. Bone Marrow Stromal Cells were provided by the Center for Bone Marrow Stromal Cell Transplantation at the NIH.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Author Contribution: A.Z, S.R.B: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; E.M.G, B.K.L, A.C: collection and/or assembly of data, M.J.M: data analysis and interpretation, J.A.F., V.F: conception and design, data analysis and interpretation, financial support, manuscript writing, final approval of manuscript.
References
- 1.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nature reviews. Immunology. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 2.Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 3.Harting MT, Jimenez F, Xue H, et al. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg. 2009;110:1189–1197. doi: 10.3171/2008.9.JNS08158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrepfer S, Deuse T, Reichenspurner H, et al. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Baer PC, Geiger H. Mesenchymal stem cell interactions with growth factors on kidney repair. Curr Opin Nephrol Hypertens. 2010;19:1–6. doi: 10.1097/MNH.0b013e328333062c. [DOI] [PubMed] [Google Scholar]
- 6.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Steingen C, Brenig F, Baumgartner L, et al. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J Mol Cell Cardiol. 2008;44:1072–1084. doi: 10.1016/j.yjmcc.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Deak E, Seifried E, Henschler R. Homing pathways of mesenchymal stromal cells (MSCs) and their role in clinical applications. Int Rev Immunol. 2010;29:514–529. doi: 10.3109/08830185.2010.498931. [DOI] [PubMed] [Google Scholar]
- 9.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ley K, Laudanna C, Cybulsky MI, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature reviews. Immunology. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 11.Sordi V. Mesenchymal stem cell homing capacity. Transplantation. 2009;87:S42–45. doi: 10.1097/TP.0b013e3181a28533. [DOI] [PubMed] [Google Scholar]
- 12.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 13.Tempany CM, McDannold NJ, Hynynen K, et al. Focused ultrasound surgery in oncology: overview and principles. Radiology. 2011;259:39–56. doi: 10.1148/radiol.11100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv Drug Deliv Rev. 2008;60:1193–1208. doi: 10.1016/j.addr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CS. Mechanotransduction - a field pulling together? J Cell Sci. 2008;121:3285–3292. doi: 10.1242/jcs.023507. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20:551–556. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burks SR, Ziadloo A, Hancock HA, et al. Investigation of cellular and molecular responses to pulsed focused ultrasound in a mouse model. PLoS One. 2011;6:e24730. doi: 10.1371/journal.pone.0024730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dromi S, Frenkel V, Luk A, et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res. 2007;13:2722–2727. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poff JA, Allen CT, Traughber B, et al. Pulsed high-intensity focused ultrasound enhances apoptosis and growth inhibition of squamous cell carcinoma xenografts with proteasome inhibitor bortezomib. Radiology. 2008;248:485–491. doi: 10.1148/radiol.2482071674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arbab AS, Yocum GT, Kalish H, et al. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 2004;104:1217–1223. doi: 10.1182/blood-2004-02-0655. [DOI] [PubMed] [Google Scholar]
- 21.Balakumaran A, Pawelczyk E, Ren J, et al. Superparamagnetic iron oxide nanoparticles labeling of bone marrow stromal (mesenchymal) cells does not affect their “stemness”. PLoS One. 2010;5:e11462. doi: 10.1371/journal.pone.0011462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vilalta M, Degano IR, Bago J, et al. Biodistribution, long-term survival, and safety of human adipose tissue-derived mesenchymal stem cells transplanted in nude mice by high sensitivity non-invasive bioluminescence imaging. Stem Cells Dev. 2008;17:993–1003. doi: 10.1089/scd.2007.0201. [DOI] [PubMed] [Google Scholar]
- 23.Yagi H, Soto-Gutierrez A, Parekkadan B, et al. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell transplantation. 2010;19:667–679. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hart AL, Ng SC, Mann E, et al. Homing of immune cells: role in homeostasis and intestinal inflammation. Inflamm Bowel Dis. 2010;16:1969–1977. doi: 10.1002/ibd.21304. [DOI] [PubMed] [Google Scholar]
- 25.Gurish MF, Boyce JA. Mast cells: ontogeny, homing, and recruitment of a unique innate effector cell. J Allergy Clin Immunol. 2006;117:1285–1291. doi: 10.1016/j.jaci.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Pelus LM, Fukuda S. Chemokine-mobilized adult stem cells; defining a better hematopoietic graft. Leukemia. 2008;22:466–473. doi: 10.1038/sj.leu.2405021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller FJ, Serobyan N, Schraufstatter IU, et al. Adhesive interactions between human neural stem cells and inflamed human vascular endothelium are mediated by integrins. Stem Cells. 2006;24:2367–2372. doi: 10.1634/stemcells.2005-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pawelczyk E, Jordan EK, Balakumaran A, et al. In vivo transfer of intracellular labels from locally implanted bone marrow stromal cells to resident tissue macrophages. PLoS One. 2009;4:e6712. doi: 10.1371/journal.pone.0006712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rieck B, Schlaak S. Measurement in vivo of the survival rate in autologous adipocyte transplantation. Plast Reconstr Surg. 2003;111:2315–2323. doi: 10.1097/01.PRS.0000060797.59958.55. [DOI] [PubMed] [Google Scholar]
- 30.Kondziolka D, Steinberg GK, Cullen SB, et al. Evaluation of surgical techniques for neuronal cell transplantation used in patients with stroke. Cell Transplant. 2004;13:749–754. doi: 10.3727/000000004783983350. [DOI] [PubMed] [Google Scholar]
- 31.Lee PH, Kim JW, Bang OY, et al. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther. 2008;83:723–730. doi: 10.1038/sj.clpt.6100386. [DOI] [PubMed] [Google Scholar]
- 32.Bos C, Delmas Y, Desmouliere A, et al. In vivo MR imaging of intravascularly injected magnetically labeled mesenchymal stem cells in rat kidney and liver. Radiology. 2004;233:781–789. doi: 10.1148/radiol.2333031714. [DOI] [PubMed] [Google Scholar]
- 33.Chanda D, Kumar S, Ponnazhagan S. Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in diseases of the skeleton. J Cell Biochem. 2010;111:249–257. doi: 10.1002/jcb.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunner S, Engelmann MG, Franz WM. Stem cell mobilisation for myocardial repair. Expert Opin Biol Ther. 2008;8:1675–1690. doi: 10.1517/14712598.8.11.1675. [DOI] [PubMed] [Google Scholar]
- 35.Capoccia BJ, Robson DL, Levac KD, et al. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood. 2009;113:5340–5351. doi: 10.1182/blood-2008-04-154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004;55:1185–1193. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- 37.Francois S, Bensidhoum M, Mouiseddine M, et al. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells. 2006;24:1020–1029. doi: 10.1634/stemcells.2005-0260. [DOI] [PubMed] [Google Scholar]
- 38.Klopp AH, Spaeth EL, Dembinski JL, et al. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res. 2007;67:11687–11695. doi: 10.1158/0008-5472.CAN-07-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arbab AS, Jordan EK, Wilson LB, et al. In vivo trafficking and targeted delivery of magnetically labeled stem cells. Hum Gene Ther. 2004;15:351–360. doi: 10.1089/104303404322959506. [DOI] [PubMed] [Google Scholar]
- 40.Polyak B, Fishbein I, Chorny M, et al. High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surfaces of steel stents. Proc Natl Acad Sci U S A. 2008;105:698–703. doi: 10.1073/pnas.0708338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kyrtatos PG, Lehtolainen P, Junemann-Ramirez M, et al. Magnetic tagging increases delivery of circulating progenitors in vascular injury. JACC Cardiovasc Interv. 2009;2:794–802. doi: 10.1016/j.jcin.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 42.Paliwal S, Mitragotri S. Therapeutic opportunities in biological responses of ultrasound. Ultrasonics. 2008;48:271–278. doi: 10.1016/j.ultras.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Barzelai S, Sharabani-Yosef O, Holbova R, et al. Low-intensity ultrasound induces angiogenesis in rat hind-limb ischemia. Ultrasound Med Biol. 2006;32:139–145. doi: 10.1016/j.ultrasmedbio.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Mizrahi N, Seliktar D, Kimmel E. Ultrasound-induced angiogenic response in endothelial cells. Ultrasound Med Biol. 2007;33:1818–1829. doi: 10.1016/j.ultrasmedbio.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Yu T, Hu D, Xu C. Microbubbles improve the ablation efficiency of extracorporeal high intensity focused ultrasound against kidney tissues. World J Urol. 2008;26:631–636. doi: 10.1007/s00345-008-0290-z. [DOI] [PubMed] [Google Scholar]
- 46.Krasovitski B, Frenkel V, Shoham S, et al. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3258–3263. doi: 10.1073/pnas.1015771108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hancock HA, Smith LH, Cuesta J, et al. Investigations into pulsed high-intensity focused ultrasound-enhanced delivery: preliminary evidence for a novel mechanism. Ultrasound in medicine & biology. 2009;35:1722–1736. doi: 10.1016/j.ultrasmedbio.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furlani D, Ugurlucan M, Ong L, et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res. 2009;77:370–376. doi: 10.1016/j.mvr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 49.English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell. 2010;7:431–442. doi: 10.1016/j.stem.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 50.Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 51.Orsi F, Zhang L, Arnone P, et al. High-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locations. AJR Am J Roentgenol. 2010;195:W245–252. doi: 10.2214/AJR.09.3321. [DOI] [PubMed] [Google Scholar]
- 52.Hwang JH, Zhou Y, Warren C, et al. Targeted venous occlusion using pulsed high-intensity focused ultrasound. IEEE Trans Biomed Eng. 2010;57:37–40. doi: 10.1109/TBME.2009.2029865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.