Abstract
Background
The pharmacology of traumatic memory extinction has not been fully characterized despite its potential as a therapeutic target for established, acquired anxiety disorders, including post-traumatic stress disorder (PTSD). Here we examine the role of endogenous glucocorticoids in traumatic memory extinction.
Methods
Male C57BL/6J mice were injected with corticosterone (10 mg/kg, i.p.) or metyrapone (50 mg/kg, s.c.) during re-activation of a contextual fear memory, and compared to vehicle groups (N = 10–12 per group). To ensure that metyrapone was blocking corticosterone synthesis, we measured corticosterone levels following re-activation of a fear memory in metyrapone- and vehicle-treated animals.
Results
Corticosterone administration following extinction trials caused a long-lasting inhibition of the original fear memory trace. In contrast, blockade of corticosteroid synthesis with metyrapone prior to extinction trials enhanced retrieval and prevented extinction of context-dependent fear responses in mice. Further behavioral analysis suggested that the metyrapone enhancement of retrieval and prevention of extinction were not due to non-specific alterations in locomotor or anxiety-like behavior. In addition, the inhibition of extinction by metyrapone was rescued by exogenous administration of corticosterone following extinction trials. Finally, we confirmed that the rise in corticosterone during re-activation of a contextual fear memory was blocked by metyrapone.
Conclusions
We demonstrate that extinction of a classical contextual fear memory is dependent on endogenous glucocorticoid synthesis during re-activation of a fear memory. Our data suggest that decreased glucocorticoids during fear memory re-activation may contribute to the inability to extinguish a fear memory, thus contributing to one of the core symptoms of PTSD.
Keywords: Glucocorticoids, Extinction, Fear conditioning, Post-traumatic stress disorder, Corticosterone, Metyrapone
1. Introduction
The formation of a fear memory following a traumatic event is an important mechanism for the subsequent development of anxiety disorders, such as post-traumatic stress disorder (PTSD) or phobias (LeDoux, 2003; Mineka & Oehlberg, 2008; Phelps & LeDoux, 2005; Pitman, 1989; Yehuda & LeDoux, 2007). For most people, intrusive fear memory retrieval extinguishes or declines over time. Unfortunately, in people that develop a chronic anxiety disorder (like PTSD) the fear memory does not extinguish and thus, can remain easily recalled by a trauma-associated cue (de Quervain, Aerni, Schelling, & Roozendaal, 2009). Indeed, the inability to extinguish fear responses is a common feature for most anxiety disorders, and PTSD in particular (Blechert, Michael, Grossman, Lajtman, & Wilhelm, 2007; Michael, Blechert, Vriends, Margraf, & Wilhelm, 2007; Yang, Chao, & Lu, 2006). Thus, it is necessary to identify factors that modulate the progressive extinction of fear memories.
There is growing evidence from both human and rodent studies that glucocorticoids released from the adrenal cortex play a key role in learning and memory. Glucocorticoids (corticosterone in animals and cortisol in humans) can dose-dependently enhance the consolidation of new fear memoires (Abercrombie, Kalin, Thurow, Rosenkranz, & Davidson, 2003; McGaugh & Roozendaal, 2002; Okuda, Roozendaal, & McGaugh, 2004; Roozendaal, 2002; Sandi & Rose, 1994). Similarly, pharmacological blockade of corticosterone or cortisol synthesis with metyrapone impairs memory consolidation in both animals and humans (Maheu, Joober, Beaulieu, & Lupien, 2004; Roozendaal, Bohus, & McGaugh, 1996). Evidence from both animals and humans suggests that glucocorticoids can act in the amygdala to modulate the memory consolidation of emotional arousing events (Roozendaal, McEwen, & Chattarji, 2009). For instance, lesions of the basolateral amygdala (BLA) in particular block the memory-modulating effects of posttraining systemic injections of glucocorticoids (McGaugh, 2004; Roozendaal & McGaugh, 1996). Furthermore, infusion of a glucocorticoid agonist into the BLA enhances memory consolidation while infusion of a glucocorticoid antagonist into the BLA blocks memory consolidation (Conrad et al., 2004; Donley, Schulkin, & Rosen, 2005). In addition to the amygdala, glucocorticoids infused into the hippocampus (Roozendaal & McGaugh, 1997) or medial prefrontal cortex (mPFC) (Barsegyan, Mackenzie, Kurose, McGaugh, & Roozendaal, 2010) also enhance consolidation. Therefore, it appears that glucocorticoids can act within multiple different brain regions to modulate fear memory consolidation (Barsegyan et al., 2010; Roozendaal & McGaugh, 1997).
As in consolidation of fear memories, the neural circuitry of extinction learning involves the amgydala, mPFC and hippocampus (Chhatwal, Myers, Ressler, & Davis, 2005; Markram, Lopez Fernandez, Abrous, & Sandi, 2007; Milad, Rauch, Pitman, & Quirk, 2006; Sierra-Mercado, Padilla-Coreano, & Quirk, 2010; Zimmerman, & Maren, 2010). Extinction of a fear conditioned response is a type of learning that results from presentation of a conditioned stimulus in the absence of the unconditioned stimulus (an electric shock in the rodent model), leading to a reduced fear response to the conditioned stimulus. Recently, glucocorticoids have been shown to facilitate the consolidation of extinction of fear learning (Abrari, Rashidy-Pour, Semnanian, & Fathollahi, 2008; Cai, Blundell, Han, Greene, & Powell, 2006; Yang et al., 2006). These effects are mediated in part through the amygdala since intraamygdalar infusion of glucocorticoid receptor (GR) agonist was shown to facilitate extinction learning, whereas infusion of a GR antagonist blocked extinction learning (Yang et al., 2006). In addition, multiple studies have shown that human patients with PTSD, a disorder which requires persistent recollection of the traumatic memory as one of the diagnostic criteria, showed decreased cortisol levels (Heim, Ehlert, Hanker, & Hellhammer, 1998; Kellner et al., 2000; Mason, Giller, Kosten, Ostroff, & Podd, 1986; Yehuda, 2002; Yehuda, 2009; Yehuda, McFarlane, & Shalev, 1998; Yehuda et al., 1990), suggesting that low cortisol levels may contribute to a hyper- retrieval of fear memories or an inability to extinguish the fear memory (de Quervain et al., 2009). Taken together, these data from humans and animals support the hypothesis that an endogenous glucocorticoid surge is necessary during fear memory re-activation for normal extinction, and predict that, in the absence of this endogenous glucocorticoid surge, fear memories would persist (with no extinction). In addition, these preclinical studies suggest that glucocorticoids may play a critical role in the development and subsequent treatment of acquired anxiety disorders such as PTSD.
To further understand the therapeutic utility of glucocorticoids, our experiments first aim to determine whether the modulatory effect of glucocorticoids on extinction learning is long-lasting. We next test the hypothesis that an endogenous glucocorticoid surge is necessary during fear memory re-activation for normal extinction. We demonstrate that corticosterone augments multiple- trial extinction in a long-lasting manner and that endogenous corticosterone synthesis is required for normal extinction learning and might also act as a constraint to the retrieval of contextual fear memory.
2. Materials and methods
A total of 156 male, 8-weeks-old C57BL/6 J mice from Jackson Laboratories (Bar Harbor, ME) were used in these experiments. The mice were housed four/cage in a temperature-, humidity-controlled, and IACUC-approved environment at UT Southwestern Medical Center with a 12-h light/12-h dark cycle and free access to food and water. Mice were moved within the animal facility to the testing room and allowed to habituate to the new location for at least 1 h prior to behavioral testing.
2.1. Behavior
Fear conditioning was performed essentially as described previously (Blundell, Kouser, & Powell, 2008; Cai et al., 2006). Briefly, mice were placed in a Plexiglas shock box with clear front and rear walls (MedAssociates, Georgia, VT) for 2 min, and then a 30 s, 90 dB tone coterminating in a 2 s, 0.5 mA foot-shock was delivered twice with a 1 min interstimulus interval. Mice remained in the context for 2 min before being returned to their home cage. After different intervals (varying across the seven experiments as described below), freezing behavior in the training context was monitored every 10 s for 5 min by an observer blind to the experimental manipulation to measure contextual fear conditioning. Extinction training involved daily 5 min exposures to the training context, and re-activation sessions were identical to extinction sessions. The apparatus was wiped with 70% ethanol and air-dried between mice. Student’s t-test was used for two-group comparisons, whereas ANOVA and student’s t-test or post hoc Fisher LSD test were used in experiments with multiple groups over days. Significance was taken as p < 0.05.
2.2. Experiment 1
We trained 24 male C57BL/6J mice in a classical fear conditioning paradigm in which a novel environment was paired with foot-shock. One day later, all mice were re-exposed to the training environment for 5 min. Two to 5 min after re-activation, mice were injected with corticosterone (10.0 mg/kg, n = 12) or vehicle (n = 12). This procedure was repeated for 3 days. Following the last injection (day 4), all mice were returned to their home cages and left undisturbed for 1 week. Seven days later (day 11), all mice were re-exposed to the training environment and fear memory was assessed. Four hours later, all mice were placed back in the training environment and given a subthreshold reminder shock (0.2 mA × 1). The reminder shock of 0.2 mA × with a 4 h delay was empirically determined and does not result in significant contextual fear conditioning in naïve mice and can robustly reverse an established extinguished contextual fear memory (Blundell et al., 2008; Cai et al., 2006). One day later, all mice were re-exposed to the training environment (day 12).
2.3. Experiment 2
We trained 20 male C57BL/6J mice in a classical fear conditioning paradigm in which a novel environment was paired with foot-shock. One day later, mice were injected subcutaneously with metyrapone (50 mg/kg, n = 10) or vehicle (n = 10) and 90 min later, placed back in the training environment for 5 min (without the shock). One day later, all mice were re-exposed to the training environment and fear memory was assessed.
2.4. Experiment 3
We examined the effect of metyrapone on locomotor activity and anxiety-like behaviors. Twenty-four mice received a subcutaneous injection of metyrapone (50 mg/kg, n = 12) or vehicle (n = 12) 90 min prior to testing. Elevated plus maze, open field, dark/light and locomotor behavior were performed over 4 days as described (Blundell et al., 2008). Student’s t-test was used for two-group comparisons while two-way ANOVA with repeated measures was used to analyze locomotor activity.
2.5. Experiment 4
We trained 24 male C57BL/6 J mice in a classical fear conditioning paradigm in which a novel environment was paired with foot-shock. One day later, mice were injected subcutaneously with metyrapone (50 mg/kg, n = 12) or vehicle (n = 12) and 90 min later, re-exposed to the training environment for 5 min. This procedure was repeated for the following 2 days. On the fourth day, mice were re-exposed to the training environment but they did not receive an injection of metyrapone or vehicle. Three days later (day 7), all mice were re-exposed to the training environment and fear memory was assessed. Four days later (day 11), all mice were re-exposed to the training environment and then 4 h later, given a reminder shock (0.2 mA × 1) in the training environment. The reminder shock of 0.2 mA × 1 with a 4 h delay was empirically determined and does not result in significant contextual fear conditioning in naïve mice and can robustly reverse an established extinguished contextual fear memory (Blundell et al., 2008; Cai et al., 2006). One day later, all mice were re-exposed to the training environment (day 12).
2.6. Experiment 5
We trained 24 male C57BL/6J mice in a classical fear conditioning paradigm in which a novel environment was paired with foot-shock. One day later, mice were injected subcutaneously with metyrapone (50 mg/kg, n = 10) or vehicle (n = 10). Unlike experiment 4, mice were not re-exposed to the training environment. In this experiment, mice were simply returned to their home cages. This procedure was repeated for the following 3 days. On the fourth day, all mice were re-exposed to the training environment and fear memory assessed.
2.7. Experiment 6
We trained 24 male C57BL/6 J mice in a classical fear conditioning paradigm in which a novel environment was paired with foot-shock. One day later, all mice were re-exposed to the training environment for 5 min. Ninety minutes prior to re-exposure, all mice were injected subcuetaneously with metyrapone (50 mg/kg). Two to 5 min after re-activation, mice were injected with corticosterone (10.0 mg/kg, n = 12) or vehicle (n = 12). This procedure was repeated for the following 2 days. On the fourth day, mice were re-exposed to the training environment but they did not receive an injection and fear memory was assessed.
2.8. Experiment 7
Corticosterone measurements were performed in the manner described by (Lagace et al., 2010). Sixteen C57BL/6J mice at 8 weeks of age were trained in fear conditioning as described above and then re-exposed to the fear conditioning chamber 24 h later for 5 min. Ninety minutes prior to re-exposure to the context, mice received vehicle or metyrapone. Three or 30 min after reexposure to the context, trunk blood was collected, plasma was isolated via centrifugation at 3000 rpm for 10 min, and samples were stored on ice until freezing at −20 °C for subsequent corticosterone measurement (n = 4/group). Corticosterone measurements were performed using the mouse OCTEIA Corticosterone Enzyme Immuno-Assay (EIA) (AC-15F1, Immunodiagnostic Systems, Scottsdale, AZ). Appropriate dilutions of serum samples were employed, all samples were assayed in triplicate, and samples had an absorbance values inside the linear portion of standard curve. Student’s t-test was used for two-group comparisons.
2.9. Drug preparation/administration
The morning of the experiment, corticosterone and metyrapone stock solutions were prepared for each dose in normal saline with 5% ethanol and kept at 4 °C in light-tight boxes until use. Mice received i.p. (corticosterone and vehicle) or s.c. (metyrapone and vehicle) injections of volumes ranging from 0.3 to 0.5 ml based on their daily weight. Both the dose of corticosterone (10 mg/kg) and the dose of metyrapone (50 mg/kg) were based on previous data (Barrett & Gonzalez-Lima, 2004; Cai et al., 2006). In addition, the dose of 50 mk/kg of metyrapone suppressed increases in plasma concentrations of corticosterone in stressed rats, but did not affect plasma corticosterone levels in non-stressed rats (Roozendaal et al., 1996).
3. Results
3.1. Corticosterone augments multiple-trial extinction in a lasting manner, and this augmentation is rescued by a reminder shock (Exp. 1)
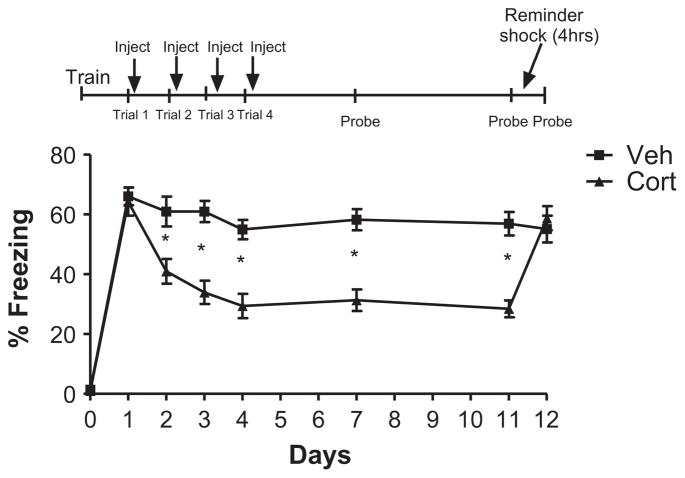
To test whether corticosterone’s facilitation of fear memory extinction was long-lasting, mice were first trained in contextual fear conditioning. As expected, 24 h after training, re-exposure to the training environment elicited significant fear responses (increased freezing behavior) indicating an intact memory of the learned association (Fig. 1). Four days of repeated corticosterone or vehicle administration following re-activation (context exposure) trials led to significantly enhanced extinction as shown by decreased fear memory expression (Fig. 1; mixed ANOVA: main effect of drug F(1, 22) = 13.53, p < 0.0013, main effect of trial F(5110) = 24.00, p < 0.0001, trial × drug interaction, F(5110) = 14.74, p < 0.0001). Student’s t-test of days 1–4 (p = 0.75, p = 0.0054, p < 0.0001, p < 0.0001, respectively) indicated that corticosterone- treated animals exhibited significantly less freezing than vehicle-treated animals.
Fig. 1.
Corticosterone augments multiple trial extinction in a lasting manner, and this effect is rescued by reminder shock. Repeated injection of corticosterone 2– 5 min after extinction trials for 4 days led to significantly enhanced extinction as shown by decreased fear memory expression [Mixed ANOVA: main effect of drug F(1, 22) = 13.53, p < 0.0013, main effect of trial F(5110) = 24.00, p < 0.0001, trial × - drug interaction, F(5110) = 14.74, p < 0.0001]. Student’s t-test of days 1–4 (p = 0.75, p = 0.0054, p < 0.0001, p < 0.0001, respectively) indicate enhanced extinction in the corticosterone-treated mice. When the underlying memory trace was examined 1 week later in the absence of corticosterone, extinction of fear expression remained enhanced (probe 11 days, student’s t-test, p < 0.0001). As expected for an effect on extinction, the effect of corticosterone was completely reversed by interposing a reminder shock 4 h after reactivation (student’s t-test, p = 0.56). No significant differences were observed during training or following reactivation of the fear memory (Trial 1) across groups (p > 0.05). N = 12 in all groups. Error bars represent SEM in all figures.
To test whether corticosterone’s facilitation of fear memory extinction was long-lasting, we tested the memory trace 1 week later in the absence of corticosterone. Importantly, extinction of fear expression remained enhanced (Fig. 1: probe 7 days, student’s t-test, p < 0.0001) in corticosterone-treated mice. To ensure that corticosterone was facilitating extinction and not blocking reconsolidation, a subthreshold reminder shock was presented 4 h after memory re-activation (given 4 h after re-activation on probe day 7). The reminder shock resulted in no significant contextual fear conditioning in naive mice as demonstrated previously (Blundell et al., 2008). As expected for an effect on extinction, the effect of corticosterone was completely reversed by interposing a reminder shock 4 h after re-activation (student’s t-test, p = 0.56). Thus, repeated re-exposure of a contextual fear memory in the presence of corticosterone facilitates extinction. These data suggest that endogenous corticosterone release during fear memory re-activation may be a natural mechanism to reduce emotional strength of traumatic memories.
3.2. Metyrapone paired with a single extinction trial enhances memory retrieval (Exp. 2)
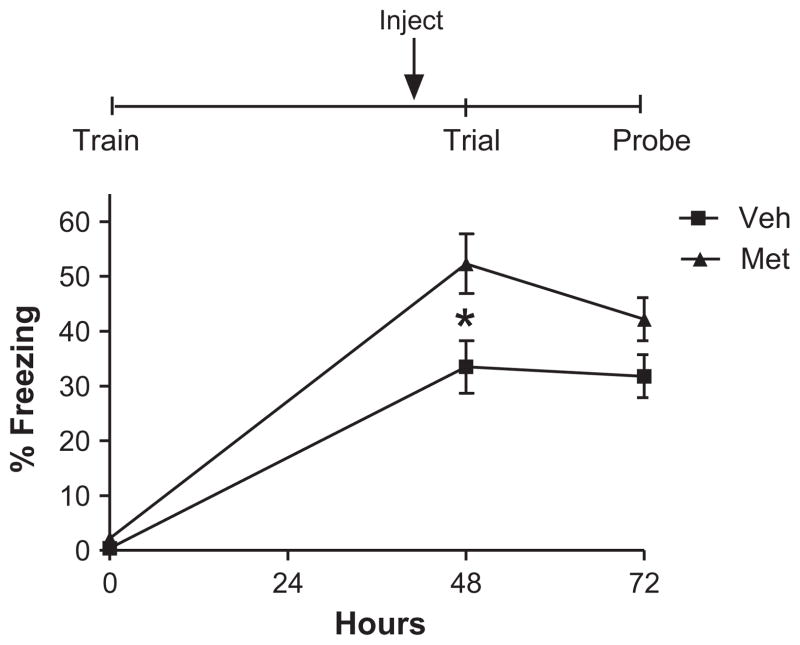
We previously demonstrated that corticosterone given prior to memory re-activation reduces memory recall (Cai et al., 2006). Thus, we asked whether endogenous corticosterone release augments memory retrieval. To block the corticosterone release in this experiment we injected the mice with the corticosterone or cortisol synthesis inhibitor, metyrapone prior to re-activation. A single injection of metyrapone enhanced immediate fear memory retrieval compared to vehicle-treated controls (Fig. 2, Mixed ANOVA, main effect of group F(1, 18) = 7.29, p = 0.015; main effect of day F(2, 36) = 99.83, p < 0.000001, group × day interaction F(2, 36) = 3.59, p = 0.038), post hoc comparisons (Fisher LSD) of days 0 (training) and 1 (Veh. vs. Met., p = 0.79, p < 0.0000001, respectively). However, one injection of metyrapone was not sufficient to sustain this enhancement in memory retrieval 24 h later (post hoc comparisons, Fisher LSD; probe day 2, p = 0.133; Fig. 2).
Fig. 2.
Metyrapone paired with a single extinction trial enhances memory retrieval. Mice injected with metyrapone 90 min prior to reactivation show enhanced fear memory retrieval, compared to controls [mixed ANOVA, main effect of group F(1, 18) = 7.29, p = 0.015; main effect of day F(2, 36) = 99.83, p < 0.000001, group × - day interaction F(2, 36) = 3.59, p = 0.038], post hoc comparisons (Fisher LSD) of days 0 (training) and 1 (Veh. vs Met.., p = 0.79, p < 0.0000001, respectively). However, one injection of metyrapone was not sufficient to sustain this enhancement in memory retrieval 24 h later (post hoc comparisons, Fisher LSD; probe day 2, p = 0.133). N = 10 for all groups.
3.3. Administration of metyrapone does not change anxiety-like behavior or locomotor activity (Exp. 3)
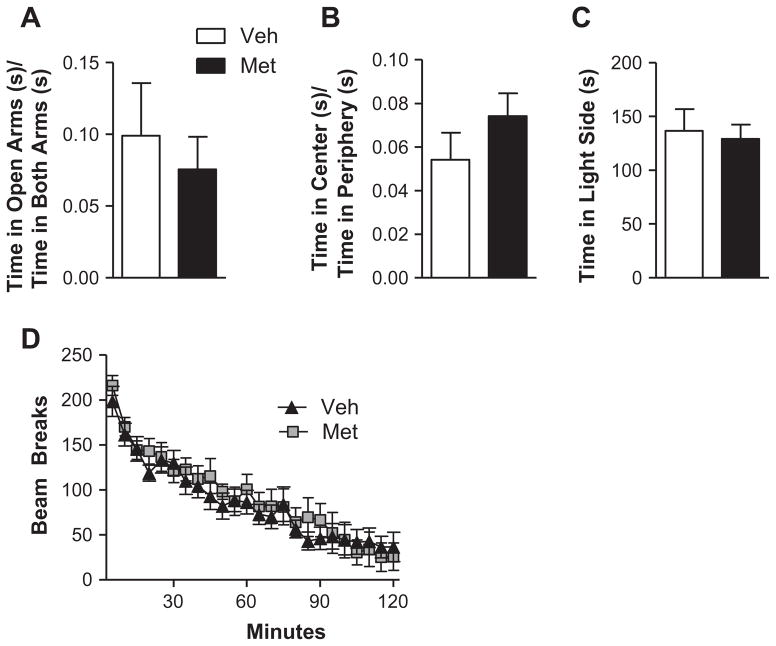
The effects of corticosterone or metyrapone on traumatic memory recall and extinction are not caused by non-specific alterations in behavior but are rather a selective effect of corticosterone (as shown in (Cai et al., 2006)) or meytrapone (Fig. 3) on fear memory processes. Acute administration of 50 mg/kg metyrapone did not result in altered anxiety-like behavior, as measured in three different behavioral tasks. In the elevated plus maze, the ratio of time spent in the open arms to time spent in both arms was similar for mice that received vehicle and metyrapone (Fig. 3A, student’s t-test, t(22) = 0.54, p = 0.60]. In the open field task, there was no difference between groups in the ratio of time spent in the center of the field to time spent in the periphery of the field (Fig. 3B, student’s t-test, t(22) = −1.22, p = 0.23), and in the dark/light box, mice that received vehicle and metyrapone both spent comparable amounts of time in the light side of the chamber [Fig. 3C, student’s t-test, t(22) = 0.31, p = 0.76). Mice were then placed in a novel home cage, and their locomotor activity was measured across a 2 h period. Metyrapone administration did not affect locomotor activity during the session, and all mice regardless of drug treatment showed a decrease in locomotor activity across the 2 h session, suggesting that they all exhibited locomotor habituation (Fig. 3D, 2-way repeated measures ANOVA; Main effect of Treatment: F(1, 22) = 0.12, p = 0.74; main effect of time: F(23,506) = 62.19, p < 0.000001; treatment × time interaction: F(23,506) = 1.03, p = 0.43). Therefore, systemic administration of metyrapone did not have a generalized effect on anxiety-like behavior or locomotor activity. These control data further support a selective effect of metyrapone on fear memory retrieval and extinction.
Fig. 3.
Administration of metyrapone does not affect anxiety-like behavior or locomotor activity. Acute administration of 50 mg/kg metyrapone did not result in altered anxiety-like behavior, as measured in three different behavioral tasks. (A) In the elevated plus maze, the ratio of time spent in the open arms to time spent in both arms was similar for mice that received vehicle and metyrapone [student’s t-test, t(22) = 0.54, p = 0.60]. Legend applies to panels A–C. (B) In the open field task, there was no difference between groups in the ratio of time spent in the center of the field to time spent in the periphery of the field [student’s t-test, t(22) = −1.22, p = 0.23]. (C) In the dark/light box, mice that received vehicle and metyrapone both spent comparable amounts of time in the light side of the chamber [student’s t-test, t(22) = 0.31, p = 0.76]. (D) In a novel home cage, metyrapone administration did not affect locomotor activity and all mice showed a decrease in locomotor activity across the 2 h session [2-way repeated measures ANOVA; main effect of treatment: F(1, 22) = 0.12, p = 0.74; main effect of time: F(23,506) = 62.19, p < 0.000001; treatment × time interaction: F(23,506) = 1.03, p = 0.43]. N = 12 for all groups.
3.4. Metyrapone inhibits multiple-trial extinction in a lasting manner and is dependent on re-activation of the fear memory (Exps. 4 and 5)
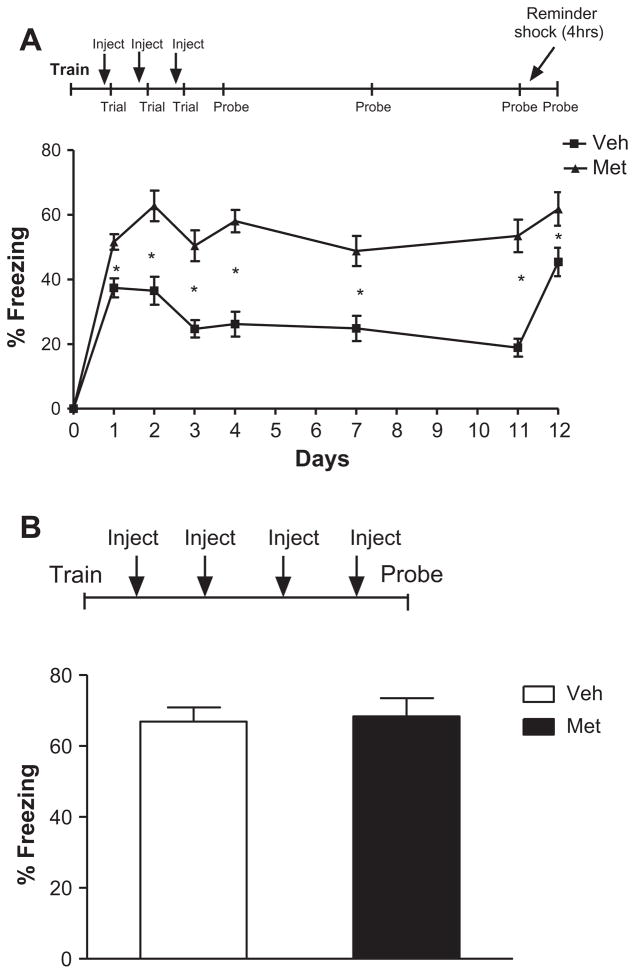
Although the effects of metyrapone given prior to a single reactivation trial did not significantly affect subsequent memory recall, we did observe a trend toward an increase in subsequent memory recall (Fig. 2, probe). Based on this result, we hypothesized that re-activation of fear memory during glucocorticoid block might prevent the extinction process after multiple re-activations. Thus, we tested the effects of repeated (three) pairings of metyrapone and re-activation on subsequent recall. Although single-trial pre-re-activation metyrapone effects were not long-lasting (Fig. 2), multiple-trial pre-re-activation metyrapone effects lasted at least 1 week (Mixed ANOVA, main effect of group F(1, 22) = 35.38, p = 0.000006; main effect of day F(6, 32) = 10.13, p < 0.000001, group × day interaction F(6132) = 3.08, p = 0.0074; Fig. 4A). Student’s t-test of days 1–3 (p = 0.0011, p = 0.00048, p = 0.00011, respectively) indicating enhanced memory in the metyrapone-treated animals. It is important to note that during the probe tests (on days 4, 7, and 11, and 12); memory was assessed in the absence of metyrapone. Student’s t-test of days 4, 7 and 11 (p < 0.000001, p < .00070, p < 0.000001) indicate sustained enhanced fear memory, even in the absence of metyrapone. To ensure that the vehicle group had extinguished, a subthreshold reminder shock (as described above) was employed 4 h following re-activation on day 11. As expected, reminder shock reversed the effects of repeatedly exposing mice to the context in the absence of the shock (student’s t-test, Veh.: day 11 vs. day 12, p < 0.000001). Reminder shock had no effect on the metyrapone-treated animals (student’s t-test, Met.: day 11 vs. day 12, p = 0.26). However, metyrapone- and vehicle-treated animals did differ significantly on day 12 (day 12: Met. vs. Veh., p < 0.025). In addition, pre-retrieval metyrapone effects with multiple trials were dependent on re-activation of the memory and were not simply an effect of repeated metyrapone administration (1-way ANOVA, no main effect of group, F(1, 22) = 0.053, p = 0.82; Fig. 4B). It should be noted that freezing levels of the vehicle-treated mice in Fig. 4A differ from freezing levels in vehicle-treated mice in Fig. 1. The difference between these two control group freezing levels is likely a direct result of the difference in experimental manipulation prior to the experiment. For the data described in Fig. 1 and Fig. 4, there are two major differences between the two groups of vehicle-treated mice: (1) the time point when they received their injection in comparison to re-activation and (2) the route of injection (i.p. or s.c.). For Fig. 1, controls were injected i.p. 2–5 min after re-activation, in order to match the corticosterone group. In Fig. 4, controls received subcutaneous injections 90 min before re-activation. While we strive to minimize the stress of injections for the mice, it is still very likely that the general experience and stress of injections and injection-related handling has an effect on behavior.
Fig. 4.
Metyrapone inhibits multiple trial extinction in a lasting manner and is dependent on reactivation of the fear memory. (A) Repeated injection of meytrapone 90 min prior to extinction trials for 3 days led to significantly enhanced fear memory lasting at least 1 week [mixed ANOVA, main effect of group F(1, 22) = 35.38, p = 0.000006; main effect of day F(6, 32) = 10.13, p < 0.000001, group × day interaction F(6132) = 3.08, p = 0.0074]. Student’s t-test of days 1–3 (p = 0.0011, p = 0.00048, p = 0.00011, respectively) indicate enhanced memory in the metyrapone-treated animals. Student’s t-test of days 4, 7 and 11 (p < 0.000001, p < .00070, p < 0.000001) indicate sustained enhanced fear memory, even in the absence of metyrapone. Reminder shock reversed the effects of repeatedly exposing mice to the context in the absence of the shock (student’s t-test, Veh.: day 11 vs. day 12, p < 0.000001). Reminder shock had no effect on the metyrapone-treated animals (student’s t-test, Met.: day 11 vs. day 12, p = 0.26). Metyrapone- and vehicle-treated animals did differ significantly on day 12 (day 12: Met. vs. Veh., p < 0.025). No significant differences were observed during training across groups (p > 0.05). (B) Pre-retrieval metyrapone effects with multiple trials were dependent on reactivation of the memory and were not simply an effect of repeated metyrapone administration (1-way ANOVA, no main effect of group, F(1, 22) = 0.053, p = 0.82). No significant differences were observed during training across groups (p > 0.05). N = 12 for all groups.
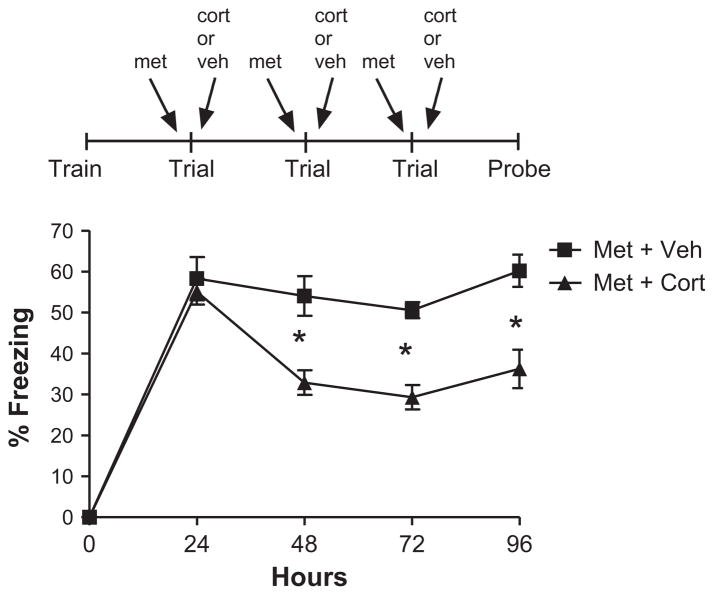
3.5. Reversal of metyrapone’s effect on fear memory extinction with corticosterone (Exp. 6)
To ensure that metyrapone’s effect on fear memory extinction was acting via blockade of endogenous corticosterone synthesis, we tested whether corticosterone administration could rescue metyrapone’s block of fear memory extinction. As expected, metyrapone- treated animals (Met. + Veh. and Met. + Cort. groups) elicited significant fear responses (as measured by freezing behavior) indicating re-activation of a learned association between this environment and the aversive foot-shock stimulus (Fig. 5). As shown in Fig. 5, metyrapone-treated animals given corticosterone following re-activation of a fear memory show significantly less fear memory compared to metryapone-treated mice given vehicle (Mixed ANOVA, main effect of group F(1, 22) = 26.30, p = 0.00004; main effect of day F(3, 66) = 9.47, p = 0.000028; group × day interaction F(3, 66) = 3.83, p = 0.014), post hoc comparisons for days 1–3 (Fisher LSD, p = 0.63, p = 0.0044, p < 0.000001, respectively). Importantly, when the underlying memory trace was examined 1 day later in the absence of corticosterone or metyrapone, inhibition of fear expression remained enhanced in the corticosterone-treated group (post hoc comparisons, Fisher LSD, day 4: Met.–Veh. vs. Met.–Cort., p = 0.0025). Furthermore, extinction was exhibited in the corticosterone-treated group (main effect of day F(3, 33) = 21.96, p < 0.000001) but not seen in vehicle-treated animals (no main effect of day F(3, 33) = 1.096, p = 0.365).
Fig. 5.
Reversal of metyrapone’s effect on fear memory extinction with corticosterone. Metyrapone-treated animals given corticosterone following reactivation of a fear memory show significantly less fear memory compared to metryapone-treated mice given vehicle [mixed ANOVA, main effect of group F(1, 22) = 26.30, p = 0.00004; main effect of day F(3, 66) = 9.47, p = 0.000028; group × day interaction F(3, 66) = 3.83, p = 0.014), post hoc comparisons for days 1–3 [Fisher LSD, p = 0.63, p = 0.0044, p < 0.000001, respectively]. When the underlying memory trace was examined 1 day later in the absence of corticosterone or metyrapone, inhibition of fear expression remained enhanced in the corticosterone-treated group (post hoc comparisons, Fisher LSD, day 4: Met.–Veh. vs. Met.–Cort., p = 0.0025). Furthermore, extinction was exhibited in the corticosterone-treated group (main effect of day F(3, 33) = 21.96, p < 0.000001) but not seen in vehicle-treated animals (no main effect of day F(3, 33) = 1.096, p = 0.365). No significant differences were observed during training across groups (p > 0.05). N = 12 for all groups.
3.6. Metyrapone blocks the rise in endogenous corticosterone during re-activation (Exp. 7)
To ensure that meytrapone was blocking the endogenous corticosterone rise during re-activation of the fear memory, we measured corticosterone levels in metyrapone- or vehicle-treated mice after re-activation of a contextual fear memory. At 3 min after re-exposure to the training environment, corticosterone levels were significantly reduced in the metyrapone treated mice compared to vehicles controls (M 69.9 ± 20.0 vs. V 216.8 ± 27.2, student’s t-test, t(6) = 4.4, p < 0.005) This effect was also observed at 30 min after re-exposure to the training environment (M 68.5 ± 6.7 vs. V 206.6 ± 20.9, student’s t-test, t(6) = 6.3, p < 0.001). Endogenous corticosterone levels were reduced after re-activation of a fear memory in the presence of metyrapone suggesting that metyrapone’s effect on retrieval and extinction acts through corticosterone. It is important to note that baseline corticosterone levels were not affected by metyrapone.
4. Discussion
The inability to extinguish intense fear memories is an important clinical problem in psychiatric disorders such as PTSD (Fyer, 1998; Morgan, Grillon, Southwick, Davis, & Charney, 1995; Yang et al., 2006). Indeed, patients with anxiety disorders have deficits in extinction learning (Blechert et al., 2007; Michael et al., 2007). Thus, the goal of our experiments was to identify factors that modulate the progressive extinction of fear memories. Current and previous data (Abrari et al., 2008; Cai et al., 2006; Yang et al., 2006) highlight the key role of glucocorticoids in the extinction of fear memory. Unlike previous experiments, however, here we showed that repeated corticosterone administration during re-activation of a contextual fear memory facilitated extinction for at least 1 week (Fig. 1). In this experiment, repeated corticosterone injection immediately following re-activation of the contextual fear memory resulted in enhanced extinction. Importantly, when the underlying memory trace was examined 1 week later in the absence of corticosterone, extinction of fear expression remained enhanced. Persistent inhibition of the fear memory in the absence of the drug is critical if glucocorticoid treatment is to be used as an effective treatment for PTSD, or other anxiety disorders. To ensure that corticosterone was facilitating extinction and not blocking reconsolidation, a subthreshold reminder shock was presented 4 h after memory re-activation. Consistent with previous data (Cai et al., 2006), the effect of corticosterone was completely reversed by the reminder shock. Thus, repeated re-exposure of a contextual fear memory in the presence of corticosterone facilitates extinction. In contrast to our findings, recent data suggest that glucocorticoids may also act by inhibiting reconsolidation (Schwabe and Wolf, 2010; Wang, Zhao, Ghitza, Li, & Lu, 2008). While our data strongly suggest that glucocorticoids facilitate extinction, perhaps in specific situations, glucocorticoids may also inhibit reconsolidation. Overall, these data suggest that endogenous corticosterone release during re-activation is a natural mechanism to reduce emotional strength of traumatic memories.
Our working hypothesis was that stress hormone release during fear memory recall is necessary for the extinction of fear memories, and without it, fear memories persist. Consistent with this hypothesis, blockade of the stress hormones during re-activation of the fear memory prevents extinction. While others have shown that blocking corticosterone attenuates fear memory extinction in the short-term (Barrett & Gonzalez-Lima, 2004; Yang et al., 2006), we show that these effects are long-lasting. Indeed, repeated injections of metyrapone prior to re-activation of the contextual fear memory prevented extinction of the fear memory. Importantly, when the underlying memory trace was examined 1 week later in the absence of metyrapone, fear expression remained enhanced (Fig. 4A). In addition, the effects of metyrapone on extinction required re-activation of the fear memory. Injection of metyrapone alone, without re-activation of the fear memory, did not alter subsequent memory (Fig. 4B). Furthermore, the inhibition of extinction by metyrapone was rescued by exogenous administration of corticosterone immediately following extinction trials (Fig. 5), confirming that metyrapone’s effect on extinction is mediated by inhibition of corticosterone synthesis. This is consistent with the fact that corticosterone levels during re-activation of a contextual fear memory were blocked by metyrapone.
Unlike previous studies (Barrett & Gonzalez-Lima, 2004; Yang et al., 2006) we show that a single injection of metyrapone prior to re-activation enhances retrieval of a fear memory without affecting the underlying memory trace (Fig. 2). This is not surprising as corticosterone reduces retrieval of a conditioned fear memory (Cai et al., 2006). Multiple pairings of metyrapone with re-activation of the fear memory however, are sufficient to produce a long-lasting block of extinction, either by preventing the consolidation of extinction or as a result of increasing contextual fear memory retrieval. Given that we now show effects lasting at least 1 week following the last metyrapone injection, metyrapone is likely acting by preventing corticosterone’s enhancing effect on consolidation of extinction. However, we cannot completely rule out that metyrapone’s lasting effect on reducing extinction is somehow mediated via its augmentation of retrieval, though this would be a novel finding. Also, our data suggest that metyrapone’s effect on extinction can be blocked by administration of corticosterone following the re-activation. Unlike the previous studies that examined extinction of a cued fear memory (Barrett & Gonzalez- Lima, 2004; Yang et al., 2006), we examined contextual fear conditioning and thus demonstrate that blocking glucocortoicoid synthesis appears to have a generalized effect on inhibition of extinction. Furthermore, we now provide evidence of systemic efficacy at a dose that does not appear to cause noticeable effects on anxiety-like behavior or locomotor activity (Fig. 3A–D).
While our data suggest that glucocorticoids play a critical role in extinction of contextual fear memory, the locus of action and receptor sub-type are not known. Extinction memory appears to be distributed across the amygdala (de Kloet, Joels, & Holsboer, 2005; McEwen, Weiss, & Schwartz, 1968), hippocampus (Andreasen & Lambert, 1991), and mPFC (Milad et al., 2006; Quirk, Garcia, & Gonzalez-Lima, 2006). Glucocorticoids are known to play a key role in the consolidation of fear memory in these brain areas. Indeed, extensive evidence indicates that the enhancing effects of glucocorticoids on consolidation of a fear memory involve the amygdala (Donley et al., 2005; McGaugh, 2004; Roozendaal & McGaugh, 1996; Roozendaal and McGaugh, 1997). Glucocorticoids infused into the hippocampus also enhance memory of inhibitory avoidance training (Roozendaal & McGaugh, 1997). Importantly, lesions of the amygdala (in particular, the BLA) block the glucocorticoid memory enhancing effects when administered in the hippocampus (Roozendaal & McGaugh, 1997). Glucocorticoids infused in the mPFC also enhance fear memory consolidation (Roozendaal & McGaugh, 1997). It appears that glucocorticoid activation within the mPFC may enhance fear memory consolidation by a stimulatory influence on BLA activity (de Quervain et al., 2009). In order to further delineate the contribution of the mPFA, BLA, or hippocampus in mediating glucocorticoids effects on extinction as observed in this experiment, future experiments are reproducing this work and specifically targeting these regions with an infusion of a glucocortoicoid receptor agonist or antagonist. These future experiments would also test the hypothesis that metyrapone may affect extinction by reducing the binding of glucocorticoids to low affinity glucocortoicoid receptors (McEwen, De Kloet, & Rostene, 1986).
Our data suggest that repeated re-activation of fear memories followed by normal corticosterone surges may serve to extinguish the ability to recall traumatic memories over time. Clinically, this finding suggests that PTSD patients may have abnormal recall of traumatic memories due to abnormalities in glucocorticoids. Indeed, patients with PTSD have lower circulating levels of corticosterone and altered hypothalamic-pituitary axis activity (Heim et al., 1998; Kellner et al., 2000; Mason et al., 1986; Yehuda, 2002; Yehuda, 2009; Yehuda et al., 1990; Yehuda et al., 1998). Blunted corticosterone responses might actually lead to persistent, pathologically strong traumatic memories in affected individuals due to an inhibition of the normal biological mechanisms promoting extinction of those memories (de Quervain et al., 2009). Repeated re-activation of memories in unaffected individuals, followed by normal corticosterone surges, may serve to extinguish their ability to recall traumatic memories over time. In contrast, a blunted corticosterone response may result in dampened extinction processes with pathologically persistent fear responses to contextual cues. If this is true, then our data (see Fig. 5) suggest that PTSD patients who exhibit blunted cortisol may benefit from supplemental cortisol treatment during re-activation of their traumatic event(s). Indeed, glucocorticoid administration in humans has been shown to be a relevant pharmacologic treatment of established disorders of emotional memories such as PTSD or phobia (Aerni et al., 2004; Schelling et al., 2006; Soravia et al., 2006).
In conclusion, the endogenous glucocorticoid surge during reactivation of fear memories contributes to their natural extinction over time. In the absence of the surge, fear memories persist. This may have important implications for the role of the HPA axis in PTSD.
Acknowledgments
This work was supported by 1R01MH081164 and 1R21HD065290 (C.M.P.), National Alliance for Research on Schizophrenia and Depression Young Investigator Award the (J.B. and D.C.L.), Anxiety Disorder Association of America Young Investigator Award (J.B.), National Science and Engineering Research Council Discovery Grant (J.B.), K02 DA023555 (A.J.E.). J.B. and C.M.P. conceived and designed the experiments; J.B., C.A.B., and D.C.L. carried them out. J.B., C.A.B. and D.L. performed statistical analysis and created figures; J.B. wrote the manuscript with input from C.M.P., C.A.B., D.C.L, and A.J.E.
Footnotes
5. Conflict of intrest
None declared.
References
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. Cortisol variation in humans affects memory for emotionally laden and neutral information. Behavioral Neuroscience. 2003;117:505–516. doi: 10.1037/0735-7044.117.3.505. [DOI] [PubMed] [Google Scholar]
- Abrari K, Rashidy-Pour A, Semnanian S, Fathollahi Y. Administration of corticosterone after memory reactivation disrupts subsequent retrieval of a contextual conditioned fear memory: Dependence upon training intensity. Neurobiology of Learning and Memory. 2008;89:178–184. doi: 10.1016/j.nlm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. American Journal of Psychiatry. 2004;161:1488–1490. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- Andreasen M, Lambert JD. Noradrenaline receptors participate in the regulation of GABAergic inhibition in area CA1 of the rat hippocampus. Journal of Physiology. 1991;439:649–669. doi: 10.1113/jphysiol.1991.sp018686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett D, Gonzalez-Lima F. Behavioral effects of metyrapone on Pavlovian extinction. Neuroscience Letters. 2004;371:91–96. doi: 10.1016/j.neulet.2004.08.046. [DOI] [PubMed] [Google Scholar]
- Barsegyan A, Mackenzie SM, Kurose BD, McGaugh JL, Roozendaal B. Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. Proceedings of the National Academy Science USA. 2010;107:16655–16660. doi: 10.1073/pnas.1011975107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosomatic Medicine. 2007;69:935–943. doi: 10.1097/PSY.0b013e31815a8f6b. [DOI] [PubMed] [Google Scholar]
- Blundell J, Kouser M, Powell CM. Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiology of Learning and Memory. 2008;90:28–35. doi: 10.1016/j.nlm.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai WH, Blundell J, Han J, Greene RW, Powell CM. Postreactivation glucocorticoids impair recall of established fear memory. Journal of Neuroscience. 2006;26:9560–9566. doi: 10.1523/JNEUROSCI.2397-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. Journal of Neuroscience. 2005;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, MacMillan DD, 2nd, Tsekhanov S, Wright RL, Baran SE, Fuchs RA. Influence of chronic corticosterone and glucocorticoid receptor antagonism in the amygdala on fear conditioning. Neurobiology of Learning and Memory. 2004;81:185–199. doi: 10.1016/j.nlm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Frontiers in Neuroendocrinology. 2009;30:358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Donley MP, Schulkin J, Rosen JB. Glucocorticoid receptor antagonism in the basolateral amygdala and ventral hippocampus interferes with long-term memory of contextual fear. Behavioural Brain Research. 2005;164:197–205. doi: 10.1016/j.bbr.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Fyer AJ. Current approaches to etiology and pathophysiology of specific phobia. Biological Psychiatry. 1998;44:1295–1304. doi: 10.1016/s0006-3223(98)00274-1. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hanker JP, Hellhammer DH. Abuse-related posttraumatic stress disorder and alterations of the hypothalamic-pituitary-adrenal axis in women with chronic pelvic pain. Psychosomatic Medicine. 1998;60:309–318. doi: 10.1097/00006842-199805000-00017. [DOI] [PubMed] [Google Scholar]
- Kellner M, Wiedemann K, Yassouridis A, Levengood R, Guo LS, Holsboer F, et al. Behavioral and endocrine response to cholecystokinin tetrapeptide in patients with posttraumatic stress disorder. Biological Psychiatry. 2000;47:107–111. doi: 10.1016/s0006-3223(99)00118-3. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, et al. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proceedings of the National Academy Science USA. 2010;107:4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheu FS, Joober R, Beaulieu S, Lupien SJ. Differential effects of adrenergic and corticosteroid hormonal systems on human short- and long-term declarative memory for emotionally arousing material. Behavioral Neuroscience. 2004;118:420–428. doi: 10.1037/0735-7044.118.2.420. [DOI] [PubMed] [Google Scholar]
- Markram K, Lopez Fernandez MA, Abrous DN, Sandi C. Amygdala upregulation of NCAM polysialylation induced by auditory fear conditioning is not required for memory formation, but plays a role in fear extinction. Neurobiology of Learning and Memory. 2007;87:573–582. doi: 10.1016/j.nlm.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Mason JW, Giller EL, Kosten TR, Ostroff RB, Podd L. Urinary free-cortisol levels in posttraumatic stress disorder patients. Journal of Nervous and Mental Disease. 1986;174:145–149. doi: 10.1097/00005053-198603000-00003. [DOI] [PubMed] [Google Scholar]
- McEwen BS, De Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiological Reviews. 1986;66:1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss JM, Schwartz LS. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Current Opinion in Neurobiology. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- Michael T, Blechert J, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in panic disorder: Enhanced resistance to extinction. Journal of Abnormal Psychology. 2007;116:612–617. doi: 10.1037/0021-843X.116.3.612. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biological Psychology. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychologica (Amst) 2008;127:567–580. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Morgan CA, III, Grillon C, Southwick SM, Davis M, Charney DS. Fear-potentiated startle in posttraumatic stress disorder. Biological Psychiatry. 1995;38:378–385. doi: 10.1016/0006-3223(94)00321-S. [DOI] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proceedings of the National Academy Science USA. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pitman RK. Post-traumatic stress disorder, hormones, and memory. Biological Psychiatry. 1989;26:221–223. doi: 10.1016/0006-3223(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biological Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiology of Learning and Memory. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Bohus B, McGaugh JL. Dose-dependent suppression of adrenocortical activity with metyrapone: Effects on emotion and memory. Psychoneuroendocrinology. 1996;21:681–693. doi: 10.1016/s0306-4530(96)00028-5. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews Neuroscience. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Amygdaloid nuclei lesions differentially affect glucocorticoid-induced memory enhancement in an inhibitory avoidance task. Neurobiology of Learning and Memory. 1996;65:1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. European Journal of Neuroscience. 1997;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Sandi C, Rose SP. Corticosteroid receptor antagonists are amnestic for passive avoidance learning in day-old chicks. European Journal of Neuroscience. 1994;6:1292–1297. doi: 10.1111/j.1460-9568.1994.tb00319.x. [DOI] [PubMed] [Google Scholar]
- Schelling G, Roozendaal B, Krauseneck T, Schmoelz MD, DEQ, Briegel J. Efficacy of hydrocortisone in preventing posttraumatic stress disorder following critical illness and major surgery. Annals of the New York Academy of Sciences. 2006;1071:46–53. doi: 10.1196/annals.1364.005. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Stress impairs the reconsolidation of autobiographical memories. Neurobiology of Learning and Memory. 2010;94:153–157. doi: 10.1016/j.nlm.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, et al. Glucocorticoids reduce phobic fear in humans. Proceedings of the National Academy Sciences USA. 2006;103:5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XY, Zhao M, Ghitza UE, Li YQ, Lu L. Stress impairs reconsolidation of drug memory via glucocorticoid receptors in the basolateral amygdala. Journal of Neuroscience. 2008;28:5602–5610. doi: 10.1523/JNEUROSCI.0750-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology. 2006;31:912–924. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Current status of cortisol findings in post-traumatic stress disorder. Psychiatric Clinics of North America. 2002;25:341–368. doi: 10.1016/s0193-953x(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Annals of the New York Academy of Sciences. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: A translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Yehuda R, McFarlane AC, Shalev AY. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biological Psychiatry. 1998;44:1305–1313. doi: 10.1016/s0006-3223(98)00276-5. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Nussbaum G, Wahby V, Giller EL, Jr, Mason JW. Low urinary cortisol excretion in patients with posttraumatic stress disorder. Journal of Nervous and Mental Disease. 1990;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Zimmerman JM, Maren S. NMDA receptor antagonism in the basolateral but not central amygdala blocks the extinction of Pavlovian fear conditioning in rats. European Journal of Neuroscience. 2010;31:1664–1670. doi: 10.1111/j.1460-9568.2010.07223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]