Abstract
Vitamin D insufficiency affects almost 50% of the population worldwide. An estimated 1 billion people worldwide, across all ethnicities and age groups, have a vitamin D deficiency (VDD). This pandemic of hypovitaminosis D can mainly be attributed to lifestyle (for example, reduced outdoor activities) and environmental (for example, air pollution) factors that reduce exposure to sunlight, which is required for ultraviolet-B (UVB)-induced vitamin D production in the skin. High prevalence of vitamin D insufficiency is a particularly important public health issue because hypovitaminosis D is an independent risk factor for total mortality in the general population. Current studies suggest that we may need more vitamin D than presently recommended to prevent chronic disease. As the number of people with VDD continues to increase, the importance of this hormone in overall health and the prevention of chronic diseases are at the forefront of research. VDD is very common in all age groups. As few foods contain vitamin D, guidelines recommended supplementation at suggested daily intake and tolerable upper limit levels. It is also suggested to measure the serum 25-hydroxyvitamin D level as the initial diagnostic test in patients at risk for deficiency. Treatment with either vitamin D2 or vitamin D3 is recommended for deficient patients. A meta-analysis published in 2007 showed that vitamin D supplementation was associated with significantly reduced mortality. In this review, we will summarize the mechanisms that are presumed to underlie the relationship between vitamin D and understand its biology and clinical implications.
Keywords: Cancer, fat soluble vitamin, hypertension, obesity, vitamin D analogs
INTRODUCTION
Vitamin D insufficiency affects almost 50% of the population worldwide.[1] An estimated 1 billion people worldwide, across all ethnicities and age groups, have a vitamin D deficiency (VDD).[1–3] This pandemic of hypovitaminosis D can mainly be attributed to lifestyle and environmental factors that reduce exposure to sunlight, which is required for ultraviolet-B (UVB)-induced vitamin D production in the skin. Black people absorb more UVB in the melanin of their skin than do white people and, therefore, require more sun exposure to produce the same amount of vitamin D.[4]
The high prevalence of vitamin D insufficiency is a particularly important public health issue because hypovitaminosis D is an independent risk factor for total mortality in the general population.[5] Emerging research supports the possible role of vitamin D against cancer, heart disease, fractures and falls, autoimmune diseases, influenza, type-2 diabetes, and depression. Many health care providers have increased their recommendations for vitamin D supplementation to at least 1000 IU.[6] A meta-analysis published in 2007 showed that vitamin D supplementation was associated with significantly reduced mortality.[7] In this review, we will focus on the biology of vitamin D and summarize the mechanisms that are presumed to underlie the relationship between vitamin D and its clinical implications.
Biology of the sunshine vitamin
Vitamin D is unique because it can be made in the skin from exposure to sunlight.[3,8–10] Vitamin D exists in two forms. Vitamin D2 is obtained from the UV irradiation of the yeast sterol ergosterol and is found naturally in sun-exposed mushrooms. UVB light from the sun strikes the skin, and humans synthesize vitamin D3, so it is the most “natural” form. Human beings do not make vitamin D2, and most oil-rich fish such as salmon, mackerel, and herring contain vitamin D3. Vitamin D (D represents D2, or D3, or both) that is ingested is incorporated into chylomicrons, which are absorbed into the lymphatic system and enter the venous blood. Vitamin D that comes from the skin or diet is biologically inert and requires its first hydroxylation in the liver by the vitamin D-25-hydroxylase (25-OHase) to 25(OH)D.[3,11] However, 25(OH)D requires a further hydroxylation in the kidneys by the 25(OH)D-1-OHase (CYP27B1) to form the biologically active form of vitamin D 1,25(OH)2D.[3,11] 1,25(OH)2D stimulates intestinal calcium absorption.[12] Without vitamin D, only 10–15% of dietary calcium and about 60% of phosphorus are absorbed. Vitamin D sufficiency enhances calcium and phosphorus absorption by 30–40% and 80%, respectively.[3,13]
Vitamin D receptor (VDR) is present in most tissues and cells in the body.[6,14] 1,25(OH)2D has a wide range of biological actions, such as inhibition of cellular proliferation and inducing terminal differentiation, inhibiting angiogenesis, stimulating insulin production, inhibiting renin production, and stimulating macrophage cathelicidin production.[6,14–16] The local production of 1,25(OH)2D may be responsible for regulating up to 200 genes[17] that may facilitate many of the pleiotropic health benefits that have been reported for vitamin D.[3,8,9,14]
Vitamin D deficiency: Prevalence
VDD has been historically defined and recently recommended by the Institute of Medicine (IOM) as a 25(OH)D of less than 0.8 IU. Vitamin D insufficiency has been defined as a 25(OH)D of 21–29 ng/mL.[1,18–23] Children and young- and middle-aged adults are at equally high risk for VDD and insufficiency worldwide. VDD is common in Australia, the Middle East, India, Africa, and South America.[1,24,25] Pregnant and lactating women who take a prenatal vitamin and a calcium supplement with vitamin D remain at high risk for VDD.[26–28]
Vitamin D deficiency, why it happens?
The major source of vitamin D for children and adults is exposure to natural sunlight.[1,29–32] Thus, the major cause of VDD is inadequate exposure to sunlight.[29,33–35] Wearing a sunscreen with a sun protection factor of 30 reduces vitamin D synthesis in the skin by more than 95%.[36] People with a naturally dark skin tone have natural sun protection and require at least three to five times longer exposure to make the same amount of vitamin D as a person with a white skin tone.[37,38] There is an inverse association of serum 25(OH)D and body mass index (BMI) greater than 30 kg/m2, and thus, obesity is associated with VDD.[39]
Patients with one of the fat malabsorption syndromes and bariatric patients are often unable to absorb the fat-soluble vitamin D, and patients with nephritic syndrome lose 25(OH)D bound to the vitamin D-binding protein in the urine.[1] Patients on a wide variety of medications, including anticonvulsants and medications to treat AIDS/HIV, are at risk because these drugs enhance the catabolism of 25(OH)D and 1,25(OH)2D.[40] Patients with chronic granuloma-forming disorders (sarcoidosis, tuberculosis, and chronic fungal infections), some lymphomas, and primary hyperparathyroidism who have increased metabolism of 25(OH)D to 1,25(OH)2D are also at high risk for VDD.[41,42]
Vitamin D deficiency: Consequences
VDD results in abnormalities in calcium, phosphorus, and bone metabolism. VDD causes a decrease in the absorption of dietary calcium and phosphorus, resulting in an increase in PTH levels.[1,3,18,43] The PTH-mediated increase in osteoclastic activity creates local foci of bone weakness and causes a generalized decrease in bone mineral density (BMD), resulting in osteopenia and osteoporosis. An inadequate calcium–phosphorus product causes a mineralization defect in the skeleton.[1,44] In young children who have little mineral in their skeleton, this defect results in a variety of skeletal deformities classically known as rickets.[45,46] VDD also causes muscle weakness; affected children have difficulty in standing and walking,[46,47] whereas the elderly have increasing sway and more frequent falls,[48,49] thereby increasing their risk of fracture.
Groups at risk of vitamin-D inadequacy
Obtaining sufficient vitamin D from natural food sources alone is difficult. Consumption of vitamin D-fortified foods and exposure to some sunlight are essential for maintaining a healthy vitamin D status. Dietary supplements might be required to meet the daily need for vitamin D in some group of people.[50]
Breastfed infants
Vitamin D requirements cannot ordinarily be met by human milk alone,[23,51] which provides <25 IU/L to 78 IU/L.[52] Vitamin D content of human milk is related to the mother's vitamin D status; therefore mothers who supplement with high doses of vitamin D may have high levels of vitamin D in their milk.[52] American Association of Paediatricians (AAP) recommends that exclusively and partially breastfed infants must be supplemented with 400 IU of vitamin D per day,[52,53] the recommended daily allowance for this nutrient during infancy.
Older adults
Older adults are at high risk of developing vitamin D insufficiency because of aging. Their skin cannot synthesize vitamin D as efficiently, they are likely to spend more time indoors, and they may have inadequate intakes of the vitamin.[23]
People with limited sun exposure
Homebound individuals, women who wear long robes and head coverings for religious reasons, and people with occupations that limit sun exposure are unlikely to obtain adequate vitamin D from sunlight.[54,55] The significance of the role that sunscreen may play in reducing vitamin D synthesis is still unclear.[23] Intake of RDA levels of vitamin D from foods and/or supplements will provide adequate amounts of this nutrient to these individuals.
People with dark skin
Larger amounts of the pigment melanin in the epidermal layer result in darker skin and reduce the skin's ability to produce vitamin D from sunlight.[23] It is not sure that lower levels of 25(OH)D for persons with dark skin have significant health consequences. Intake of RDA levels of vitamin D from foods and/or supplements will provide adequate amounts of this nutrient to these individuals.
People with fat malabsorption
Vitamin D is fat soluble, therefore it requires some dietary fat in the gut for absorption. Individuals with reduced ability to absorb dietary fat might require vitamin D supplements.[56] Fat malabsorption is associated with a variety of medical conditions including some forms of liver disease, cystic fibrosis, and Crohn's disease.[57]
People who are obese or who have undergone gastric bypass surgery
A BMI value of ≥30 is associated with lower serum 25(OH)D levels compared with nonobese individuals. Obese people may need larger than usual intakes of vitamin D to achieve 25(OH)D levels comparable to those of normal weight.[23] Greater amounts of subcutaneous fat sequester (captivate) more of the vitamin and alter its release into the circulation. Individuals who have undergone gastric bypass surgery may become vitamin D deficient over time without a sufficient intake of vitamin D from food or supplements; moreover part of the upper small intestine where vitamin D is absorbed is bypassed.[58,59]
Sources of vitamin D
A major source of vitamin D for most humans is synthesized from the exposure of the skin to sunlight typically between 1000 h and 1500 h in the spring, summer, and fall.[1,29,33,60] Vitamin D produced in the skin may last at least twice as long in the blood compared with ingested vitamin D.[61] When an adult wearing a bathing suit is exposed to one minimal erythemal dose of UV radiation (a slight pinkness to the skin 24 h after exposure), the amount of vitamin D produced is equivalent to ingesting between 10,000 and 25,000 IU.[33] A variety of factors reduce the skin's production of vitamin D3, including increased skin pigmentation, aging, and the topical application of a sunscreen.[1,36,37] An alteration in the zenith angle of the sun caused by a change in latitude, season of the year, or time of day dramatically influences the skin's production of vitamin D3.[1,33]
Physiological actions of vitamin D
Vitamin D is a fat-soluble vitamin that acts as a steroid hormone. In humans, the primary source of vitamin D is UVB-induced conversion of 7-dehydrocholesterol to vitamin D in the skin [Figure 1].[1,62] Vitamin D influences the bones, intestines, immune and cardiovascular systems, pancreas, muscles, brain, and the control of cell cycles.[63]
Figure 1.
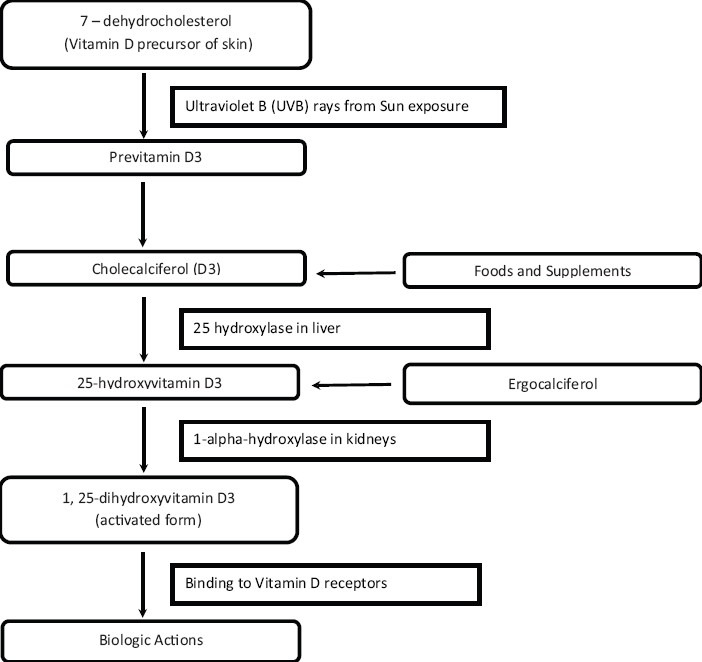
Vitamin D synthesis
Vitamin D undergoes two hydroxylations in the body for activation. Calcitriol (1,25-dihydroxyvitamin D3), the active form of vitamin D, has a half-life of about 15 h, while calcidiol (25-hydroxyvitamin D3) has a half-life of about 15 days.[63] Vitamin D binds to receptors located throughout the body. 25(OH)D is transformed by renal or extrarenal 1α-hydroxylase into 1,25-dihydroxyvitamin D (1,25[OH]2D), which circulates at much lower serum concentrations than 25(OH)D, but has a much higher affinity to the VDR.[64] Studies have, however, shown that many other cell types, including those of the vascular wall, express 1α-hydroxylase with subsequent intracellular conversion of 25(OH)D to 1,25(OH)2D, which exerts its effects at the level of the individual cell or tissue before being catabolized to biologically inactive calcitroic acid.[1,65,66] Factors such as fibroblast growth factor 23 and Klotho, which suppress 1α-hydroxylase expression, have also been shown to regulate the renal conversion of 25(OH)D to 1,25(OH)2D.[67] Importantly, extrarenal 1α-hydroxylase expression also underlies various regulatory mechanisms. In this context, extrarenal 1,25(OH)2D productions in macrophages are stimulated by Toll-like receptor as part of the innate immune response against intracellular bacteria.[68] Another example of extrarenal regulation of 1α-hydroxylase is that the increased production of 1,25(OH)2D by keratinocytes in wounds[69] therefore provides a good estimate of vitamin D status, but regulation of 1α-hydroxylase activity should also be considered. Vitamin D crosses the blood–brain barrier and the receptors for vitamin D are found across the brain, but its precise role is still not known.
Drug interactions
Vitamin D supplements may interact with several types of medications. Corticosteroids can reduce calcium absorption, which results in impaired vitamin D metabolism.[9] Since vitamin D is fat soluble, Orlistat and Cholestyramine can reduce its absorption and should be taken several hours apart from it.[9] Phenobarbital and phenytoin increase the hepatic metabolism of vitamin D to inactive compounds and decrease calcium absorption, which also impairs vitamin D metabolism.[9]
Dosing
Only a few foods are a good source of vitamin D. The best way to get additional vitamin D is through supplementation. Traditional multivitamins contain about 400 IU of vitamin D, but many multivitamins now contain 800 to 1000 IU. A variety of options are available for individual vitamin D supplements, including capsules, chewable tablets, liquids, and drops. Cod liver oil is a good source of vitamin D, but in large doses there is a risk of vitamin A toxicity.[70]
Clinical benefits of vitamin D
Cancer
Vitamin D decreases cell proliferation and increases cell differentiation, stops the growth of new blood vessels, and has significant anti-inflammatory effects.[71,72] Many studies have suggested a link between low vitamin D levels and an increased risk of cancer, with the strongest evidence for colorectal cancer. In the Health Professionals Follow-up Study (HPFS), subjects with high vitamin D concentrations were half as likely to be diagnosed with colon cancer as those with low concentrations.[71] A definitive conclusion cannot yet be made about the association between vitamin D concentration and cancer risk, but results from many studies are promising. There is some evidence linking higher vitamin D intake to a lower risk for breast cancer.[72] The effect of menopausal status on this association is still unclear.
Heart disease
Several studies are providing evidence that the protective effect of vitamin D on the heart could be via the renin–angiotensin hormone system, through the suppression of inflammation, or directly on the cells of the heart and blood-vessel walls.[17] In the Framingham Heart Study, patients with low vitamin D concentrations (<15 ng/mL) had a 60% higher risk of heart disease than those with higher concentrations.[17] In another study, which followed men and women for 4 years, patients with low vitamin D concentrations (<15 ng/mL) were three times more likely to be diagnosed with hypertension than those with high concentrations (>30 ng/mL).[73]
Hypertension
The third National Health and Nutrition Examination Survey (NHANES-III),[74] which is representative of the noninstitutionalized US civilian population, showed that systolic blood pressure and pulse pressure were inversely and significantly correlated with 25(OH)D levels among 12,644 participants. Age-associated increase in systolic blood pressure was significantly lower in individuals with vitamin D sufficiency.[75,76] The prevalence of arterial hypertension was also associated with reduced serum 25(OH)D levels in 4030 participants of the German National Interview and Examination Survey,[77] in 6810 participants of the 1958 British Birth Cohort,[78] and in other study populations.[79–87] The antihypertensive effects of vitamin D are mediated by renoprotective effects, suppression of the RAAS, by beneficial effects on calcium homeostasis, including the prevention of secondary hyperparathyroidism, and by vasculoprotection.[85]
Obesity
Low concentrations of circulating vitamin D are common with obesity and may represent a potential mechanism explaining the elevated risk of certain cancers and cardiovascular outcomes. Levels of 25(OH)D are inversely associated with BMI, waist circumference, and body fat but are positively associated with age, lean body mass, and vitamin D intake.
The prevalence of VDD is higher in black versus white children regardless of season predictors of VDD in children include black race, female sex, pre-pubertal status, and winter/spring season.[88] Weight loss is associated with an increase in 25(OH)D levels among postmenopausal overweight or obese women.[89]
Type 2 diabetes
A trial of nondiabetic patients aged 65 years and older found that those who received 700 IU of vitamin D (plus calcium) had a smaller rise in fasting plasma glucose over 3 years versus those who received placebo.[90] A correlation between vitamin D and the risk diabetes can be ruled in from the results.
Depression
A Norwegian trial of overweight subjects showed that those receiving a high dose of vitamin D (20,000 or 40,000 IU weekly) had a significant improvement in depressive symptom scale scores after 1 year versus those receiving placebo.[91] The result determines a correlation between vitamin D and the risk of depression.
Cognitive impairment
In the Invecchiare in Chianti (InCHIANTI) Italian population-based study, low levels of vitamin D were associated with substantial cognitive decline in the elderly population studied during a 6-year period.[92] Low levels of 25(OH)D may be especially harmful to executive functions, whereas memory and other cognitive domains may be relatively preserved.
Parkinson's disease
Parkinson's disease is a major cause of disability in the elderly population. Unfortunately, risk factors for this disease are relatively unknown. Recently, it has been suggested that chronically inadequate vitamin D intake may play a significant role in the pathogenesis of Parkinson's disease. A cohort study based on the Mini-Finland Health Survey demonstrated that low vitamin D levels may predict the development of Parkinson's disease.[93]
Fractures and falls
Vitamin D is known to help the body absorb calcium, and it plays a role in bone health. In addition, VDRs are located on the fast-twitch muscle fibers, which are the first to respond in a fall.[94] It is theorized that vitamin D may increase muscle strength, thereby preventing falls.[6] Many studies have shown an association between low vitamin D concentrations and an increased risk of fractures and falls in older adults.
A combined analysis of 12 fracture-prevention trials found that supplementation with about 800 IU of vitamin D per day reduced hip and nonspinal fractures by about 20%, and that supplementation with about 400 IU per day showed no benefit.[95] Researchers at the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University have examined the best trials of vitamin D versus placebo for falls. Their conclusion is that “fall risk reduction begins at 700 IU and increases progressively with higher doses.”[94]
Autoimmune diseases
VDD can contribute to autoimmune diseases such as multiple sclerosis (MS), type 1 diabetes, rheumatoid arthritis, and autoimmune thyroid disease.[96]
A prospective study of white subjects found that those with the highest vitamin D concentrations had a 62% lower risk of developing MS versus those with the lowest concentrations.[97] A Finnish study that followed children from birth noted that those given vitamin D supplements during infancy had a nearly 90% lower risk of developing type 1 diabetes compared with children who did not receive supplements.[98]
Influenza
VDD in the winter months may be the seasonal stimulus that triggers influenza outbreaks in the winter.[96] In a Japanese randomized, controlled trial, children given a daily vitamin D supplement of 1200 IU had a 40% lower rate of influenza type A compared with those given placebo; there was no significant difference in rates of influenza type B.[99]
Bacterial vaginosis
An analysis of data from the National Health and Nutrition Examination Survey showed that in pregnant women, VDD was associated with nearly a 3-fold increased risk for Bacterial Vaginosis (BV).[100] In non-pregnant women, VDD modulated the association between smoking and BV.
Pelvic floor disorders
The frequency of Pelvic floor disorders, including urinary and fecal incontinence, is increasing with age. Pelvic floor disorders have been linked to osteoporosis and low BMD and remain one of the most common reasons for gynaecologic surgery, with a failure rate of 30%. Subnormal levels of 25(OH)D are common among women, and lower levels are associated with a higher likelihood of pelvic floor disorders.[101] Results from the National Health and Nutrition Examination Survey confirmed that lower 25(OH) D levels are associated with a greater risk for urinary incontinence in women older than 50 years.
Age-related macular regeneration
High vitamin D blood levels appear to be associated with a decreased risk for the development of early age-related macular degeneration (AMD) among women younger than 75 years.[102] Among women younger than 75 years, there is a lower risk for early AMD with higher vitamin D levels, with a threshold effect at 15.22 ng/L serum 25 (OH)D.
RECOMMENDATION GUIDELINES: ENDOCRINE SOCIETY OF CLINICAL PRACTICE
Diagnostic procedure
ESCP recommend screening for VDD in individuals at risk for deficiency and not for patients who are not at risk. Serum circulating 25-hydroxyvitamin D [25(OH) D] level should be measured to evaluate vitamin D status in patients who are at risk for VDD. VDD is defined as a 25(OH) D below 20 ng/mL (50 nmol/L).[103]
Recommended dietary intakes of vitamin D
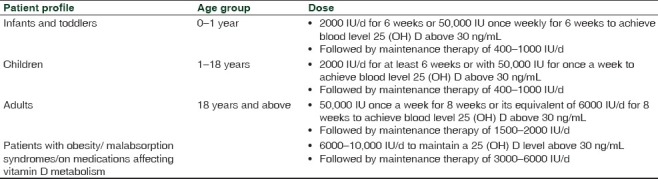
ESCP suggests that obese children and adults on anticonvulsant medications, glucocorticoids, antifungals such as ketoconazole, and medications for AIDS should be given at least two to three times more vitamin D for their age group to satisfy their body's vitamin D requirement[Table 1].
Table 1.
Recommended dietary intakes of vitamin D for patients at risk for vitamin D deficiency[103]

ESCP suggests that the maintenance tolerable upper limits (UL) of vitamin D, which is not to be exceeded without medical supervision, should be 1000 IU/d for infants up to 6 months, 1500 IU/d for infants from 6 months to 1 year, at least 2500 IU/d for children aged 1–3 years, 3000 IU/d for children aged 4–8 years, and 4000 IU/d for everyone over 8 years. Higher levels of 2000 IU/d for children 0–1 year, 4000 IU/d for children 1–18 years, and 10000 IU/d for children and adults 19 years and older may be needed to correct VDD.[103]
Treatment and prevention strategies
Vitamin D2 or vitamin D3 can be used for the treatment and prevention of VDD [Table 2]. In patients with extrarenal production of 1,25(OH)2D, serial monitoring of 25(OH)D levels and serum calcium levels during treatment with vitamin D to prevent hypercalcemia is suggested [Table 2]. Primary hyperparathyroidism and VDD need treatment with vitamin D.[103]
Table 2.
Treatment and prevention strategies[103]
Noncalcemic benefits of vitamin D
ESCP recommends prescribing vitamin D supplementation for fall prevention and do not recommend supplementation beyond recommended daily needs for the purpose of preventing cardiovascular disease or death or improving quality of life.[103]
Vitamin D analogs
Vitamin D has five natural analogs, called vitamers, and four synthetic analogs which are made synthetically. Vitamin D analogs are chemically classified as secosteroids, which are steroids with one broken bond.
Natural analogs of vitamin D
Vitamin D1 is a molecular compound of ergocalciferol (D2) with lumisterol in a 1:1 ratio.
Vitamin D2 (ergocalciferol) is produced by invertebrates, some plants, and fungi. Biological production of D2 is stimulated by ultraviolet light.
Vitamin D3 (cholecalciferol) is synthesized in the skin by the reaction of 7-dehydrocholesterol with UVB radiation, present in sunlight with an UV index of three or more.
Vitamin D4 is an analog scientifically known as 22-dihydroergocalciferol.
Vitamin D5 (sitocalciferol) is an analog created from 7-dehydrositosterol.
Synthetic analogs of vitamin D
Maxacalcitol (22-oxacalcitriol or OCT) is the first analog found to have a wider therapeutic window than 1,25(OH)2D3.[104]
Calcipotriol is derived from calcitriol was first discovered during trials involving the use of vitamin D for treating osteoporosis.
Dihydrotachysterol (DHT) is a synthetic form of vitamin D that many consider superior to natural D2 and D3. It becomes active by the liver without needing to go through hydroxylation in the kidneys.
Paricalcitol (19-norD2) is also derived from calcitriol. It is the first of the new vitamin D analogs to be approved for secondary hyperparathyroidism and differs from calcitriol in that it lacks the exocyclic carbon 19 and has a vitamin D2 side chain instead of a vitamin D3 side chain.[105]
Tacalcitol is a derivative of vitamin D3. It is known to hinder keratinocytes in the skin.
Doxercalciferol (1α(OH)D2) is a prodrug and must be activated in vivo. It is less toxic than 1α (OH)D3[106] when administered chronically.
Falecalcitriol (1,25(OH) 2-26, 27-F6-D3) is approved for secondary hyperparathyroidism in Japan.[105] It is more active than calcitriol because of its slower metabolism.[107]
CONCLUSION
Numbers of people with VDD are continuously increasing; the importance of this hormone in overall health and the prevention of chronic diseases are at the forefront of research. VDD is very common in all age groups. Very few foods contain vitamin D therefore guidelines recommended supplementation of vitamin D at tolerable UL levels. It is also suggested to measure the serum 25-hydroxyvitamin D level as the initial diagnostic test in patients at risk for deficiency. Treatment with either vitamin D2 or vitamin D3 is recommended for the deficient patients. More research is required to recommend screening individuals who are not at risk for deficiency or to prescribe vitamin D to attain the noncalcemic benefit for cardiovascular protection.
ACKNOWLEDGMENTS
We would like to acknowledge Mr. Anand Iyer, VP, Marketing and Sales, Torrent Pharmaceuticals Ltd., for providing us moral and infrastructural support for drafting this scientific review and Mr. Ramesh Jayswal, Executive—Information Science, Torrent Pharmaceuticals Ltd., who has enabled us with the required reference articles and scientific inputs to draft this review article.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158:531–7. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 3.Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: An international epidemiological investigation. J Intern Med. 2006;260:245–54. doi: 10.1111/j.1365-2796.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 4.Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30:150–6. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 5.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–37. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvard School of Public Health Nutrition Source. Vitamin D and health. [Last accessed on 2010 Aug 30]. Available from: http://www.hsph.harvard.edu/nutritionsource/what-shouldyou-eat/vitamin-d/index.html .
- 7.Autier P, Gandini S. Vitamin D supplementation and total mortality: A meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–7. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 8.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Chapter 7. Vitamin D. [Last accessed on 2010 Aug 02]. Available from: http://www.nal.usda.gov/fnic/DRI//DRI_Calcium/250–287.pdf .
- 9.NIH Office of Dietary Supplements. Dietary supplement fact sheet: Vitamin D. [Last accessed on 2010 Aug 04]. http://ods.od.nih.gov/factsheets/vitamind.asp .
- 10.Nair S. Symptoms of low vitamin D levels. [Last accessed on 2010 Sep 02]. Available from: http://www.buzzle.com/articles/symptoms-of-low-vitamin-d-levels.html .
- 11.MedlinePlus. 25-hydroxy vitamin D test. [Last accessed on 2010 Aug 04]. Available from: http://www.nlm.nih.gov/medlineplus/ency/article/003569.htm .
- 12.Moyad MA. Vitamin D: A rapid review: Side effects and toxicity. [Last accessed on 2010 Sep 02]. Available from: http://www.medscape.com/viewarticle/589256_10 .
- 13.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am J Clin Nutr. 2007;85:1586–91. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 14.Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, et al. J Natl Cancer Inst. 2008;100:1581–91. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stolzenberg-Solomon RZ, Vieth R, Azad A, Pietinen P, Taylor PR, Virtamo J, et al. A prospective nested case-control study of vitamin D status and pancreatic cancer risk in male smokers. Cancer Res. 2006;66:10213–9. doi: 10.1158/0008-5472.CAN-06-1876. [DOI] [PubMed] [Google Scholar]
- 16.Stolzenberg-Solomon RZ, Hayes RB, Horst RL, Anderson KE, Hollis BW, Silverman DT. Serum vitamin D and risk of pancreatic cancer in the Prostate, Lung, Colorectal, and Ovarian Screening Trial. Cancer Res. 2009;69:1439–47. doi: 10.1158/0008-5472.CAN-08-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr. 2004;80(6 Suppl):1706S–9S. doi: 10.1093/ajcn/80.6.1706S. [DOI] [PubMed] [Google Scholar]
- 19.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–6. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 20.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–6. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 21.Hansen KE, Jones AN, Lindstrom MJ, Davis LA, Engelke JA, Shafer MM. Vitamin D insufficiency: Disease or no disease? J Bone Miner Res. 2008;23:1052–60. doi: 10.1359/JBMR.080230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bischoff-Ferrari HA, Can U, Staehelin HB, Platz A, Henschkowski J, Michel BA, et al. Severe vitamin D deficiency in Swiss hip fracture patients. Bone. 2008;42:597–602. doi: 10.1016/j.bone.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Dietary reference intakes for calcium and vitamin D. Washington DC: The National Academies Press; 2011. IOM (Institute of Medicine) [PubMed] [Google Scholar]
- 24.Marwaha RK, Tandon N, Reddy DR, Aggarwal R, Singh R, Sawhney RC, et al. Vitamin D and bone mineral density status of healthy schoolchildren in northern India. Am J Clin Nutr. 2005;82:477–82. doi: 10.1093/ajcn.82.2.477. [DOI] [PubMed] [Google Scholar]
- 25.Thacher TD, Fischer PR, Strand MA, Pettifor JM. Nutritional rickets around the world: Causes and future directions. Ann Trop Paediatr. 2006;26:1–16. doi: 10.1179/146532806X90556. [DOI] [PubMed] [Google Scholar]
- 26.Hollis BW, Wagner CL. Vitamin D requirements during lactation: High-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr. 2004;80:1752S–8S. doi: 10.1093/ajcn/80.6.1752S. [DOI] [PubMed] [Google Scholar]
- 27.Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila) 2007;46:42–4. doi: 10.1177/0009922806289311. [DOI] [PubMed] [Google Scholar]
- 28.Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137:447–52. doi: 10.1093/jn/137.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moan J, Porojnicu AC, Dahlback A, Setlow RB. Addressing the health benefits and risks, involving vitamin D or skin cancer, of increased sun exposure. Proc Natl Acad Sci USA. 2008;105:668–73. doi: 10.1073/pnas.0710615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollis BW. Circulating 25-hydroxyvitaminDlevels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–22. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 31.Maeda SS, Kunii IS, Hayashi L, Lazaretti-Castro M. The effect of sun exposure on 25-hydroxyvitamin D concentrations in young healthy subjects living in the city of Sao Paulo, Brazil. Braz J Med Biol Res. 2007;40:1653–9. doi: 10.1590/s0100-879x2006005000162. [DOI] [PubMed] [Google Scholar]
- 32.Brot C, Vestergaard P, Kolthoff N, Gram J, Hermann AP, Sorensen OH. Vitamin D status and its adequacy in healthy Danish peri-menopausal women: Relationships to dietary intake, sun exposure and serum parathyroid hormone. Br J Nutr. 2001;86(Suppl 1):S97–103. doi: 10.1079/bjn2001345. [DOI] [PubMed] [Google Scholar]
- 33.Holick MF, Chen TC. Vitamin D deficiency: A worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 34.Holick MF, Chen TC, Sauter ER. Vitamin D and skin physiology: A D-lightful story. J Bone Miner Res. 2007;22(Suppl 2):V28–33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 35.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitaminDstatus of the US population: 1988–1994 compared to 2000–2004. Am J Clin Nutr. 2008;88:1519–27. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64:1165–8. doi: 10.1210/jcem-64-6-1165. [DOI] [PubMed] [Google Scholar]
- 37.Clemens TL, Henderson SL, Adams JS, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–6. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 38.Hintzpeter B, Scheidt-Nave C, Müller MJ, Schenk L, Mensink GB. Higher prevalence of vitamin D deficiency is associated with immigrant background among children and adolescents in Germany. J Nutr. 2008;138:1482–90. doi: 10.1093/jn/138.8.1482. [DOI] [PubMed] [Google Scholar]
- 39.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 40.Zhou C, Assem M, Tay JC, Watkins PB, Blumberg B, Schuetz EG, et al. Steroid and xenobiotic receptor and vitamin D receptor crosstalk mediates CYP24 expression and drug-induced osteomalacia. J Clin Invest. 2006;116:1703–12. doi: 10.1172/JCI27793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams JS, Hewison M. Hypercalcemia caused by granuloma forming disorders. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 6th ed. Washington, DC: American Society for Bone and Mineral Research; 2006. pp. 200–2. [Google Scholar]
- 42.Grey A, Lucas J, Horne A, Gamble G, Davidson JS, Reid IR. Vitamin D repletion in patients with primary hyperparathyroidism and coexistent vitamin D insufficiency. J Clin Endocrinol Metab. 2005;90:2122–6. doi: 10.1210/jc.2004-1772. [DOI] [PubMed] [Google Scholar]
- 43.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–24. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 44.Aaron JE, Gallagher JC, Anderson J, Stasiak L, Longton EB, Nordin BE, et al. Frequency of osteomalacia and osteoporosis in fractures of the proximal femur. Lancet. 1974;1:229–33. doi: 10.1016/s0140-6736(74)92545-8. [DOI] [PubMed] [Google Scholar]
- 45.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–73. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 46.Gordon CM, Williams AL, Feldman HA, May J, Sinclair L, Vasquez A, et al. Treatment of hypovitaminosis D in infants and toddlers. J Clin Endocrinol Metab. 2008;93:2716–21. doi: 10.1210/jc.2007-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–72. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: A meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–64. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 49.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: A meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dietary Supplement Fact Sheet: Vitamin D. Office of Dietary Supplements, National Institutes of Health. 2011 Jun 24; [Google Scholar]
- 51.Picciano MF. Nutrient composition of human milk. Pediatr Clin North Am. 2001;48:53–67. doi: 10.1016/s0031-3955(05)70285-6. [DOI] [PubMed] [Google Scholar]
- 52.Wagner CL, Greer FR. American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–52. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 53.American Academy of Pediatrics Committee on Environmental Health. Ultraviolet light: A hazard to children. Pediatrics. 1999;104:328–33. [PubMed] [Google Scholar]
- 54.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–8. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 55.Webb AR, Pilbeam C, Hanafin N, Holick MF. An evaluation of the relative contributions of exposure to sunlight and of diet to the circulating concentrations of 25-hydroxyvitamin D in an elderly nursing home population in Boston. Am J Clin Nutr. 1990;51:1075–81. doi: 10.1093/ajcn/51.6.1075. [DOI] [PubMed] [Google Scholar]
- 56.Lo CW, Paris PW, Clemens TL, Nolan J, Holick MF. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985;42:644–9. doi: 10.1093/ajcn/42.4.644. [DOI] [PubMed] [Google Scholar]
- 57.Holick MF. Vitamin D. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease. 10th ed. Philadelphia: Lippincott Williams and Wilkins; 2006. [Google Scholar]
- 58.Malone M. Recommended nutritional supplements for bariatric surgery patients. Ann Pharmacother. 2008;42:1851–8. doi: 10.1345/aph.1L321. [DOI] [PubMed] [Google Scholar]
- 59.Compher CW, Badellino KO, Boullata JI. Vitamin D and the bariatric surgical patient: A review. Obes Surg. 2008;18:220–4. doi: 10.1007/s11695-007-9289-6. [DOI] [PubMed] [Google Scholar]
- 60.Holick MF. Vitamin D: A D-lightful health perspective. Nutr Rev. 2008;66(10 Suppl 2):S182–94. doi: 10.1111/j.1753-4887.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 61.Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest. 1993;91:2552–5. doi: 10.1172/JCI116492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lau AH, How PP. The role of the pharmacist in the identification and management of secondary hyperparathyroidism. US Pharm. 2007;32:62–72. [Google Scholar]
- 63.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–83. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 64.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 65.Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest. 2005;35:290–304. doi: 10.1111/j.1365-2362.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 66.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–76. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Medici D, Razzaque MS, Deluca S, Rector TL, Hou B, Kang K, et al. FGF-23-Klotho signaling stimulates proliferation and prevents vitamin D-induced apoptosis. J Cell Biol. 2008;182:459–65. doi: 10.1083/jcb.200803024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 69.Schauber J, Dorschner RA, Coda AB, Büchau AS, Liu PT, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–11. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cannell JJ, Vieth R, Willett W, Zasloff M, Hathcock JN, White JH, et al. Cod liver oil, vitamin A toxicity, frequent respiratory infections, and the vitamin D deficiency epidemic. Ann Otol Rhinol Laryngol. 2008;117:864–70. doi: 10.1177/000348940811701112. [DOI] [PubMed] [Google Scholar]
- 71.Ahn J, Peters U, Albanes D, Purdue MP, Abnet CC, Chatterjee N, et al. Serum vitamin D concentration and prostate cancer risk: A nested case-control study. J Natl Cancer Inst. 2008;100:796–804. doi: 10.1093/jnci/djn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anderson LN, Cotterchio M, Vieth R, Knight JA. Vitamin D and calcium intakes and breast cancer risk in pre- and postmenopausal women. Am J Clin Nutr. 2010;91:1699–707. doi: 10.3945/ajcn.2009.28869. [DOI] [PubMed] [Google Scholar]
- 73.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–9. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 74.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20:713–9. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 75.Judd SE, Nanes MS, Ziegler TR, Wilson PW, Tangpricha V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: Results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2008;87:136–41. doi: 10.1093/ajcn/87.1.136. [DOI] [PubMed] [Google Scholar]
- 76.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: Data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–65. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 77.Hintzpeter B, Mensink GB, Thierfelder W, Muller MJ, Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2008;62:1079–89. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- 78.Hypponen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: A cross-sectional study in the 1958 British Birth Cohort. Diabetes. 2008;57:298–305. doi: 10.2337/db07-1122. [DOI] [PubMed] [Google Scholar]
- 79.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–83. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 80.Kokot F, Pietrek J, Srokowska S, Wartenberg W, Kuska J, Jedrychowska M, et al. 25-hydroxyvitamin D in patients with essential hypertension. Clin Nephrol. 1981;16:188–92. [PubMed] [Google Scholar]
- 81.Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxyvitamin D is predictive of future glycemic status and insulin resistance: The Medical Research Council Ely Prospective study 1990-2000. Diabetes. 2008;57:2619–25. doi: 10.2337/db08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Duprez D, de Buyzere M, de Backer T, Clement D. Relationship between vitamin D3 and the peripheral circulation in moderate arterial primary hypertension. Blood Prees Monit. 1994;3:389–93. doi: 10.3109/08037059409102292. [DOI] [PubMed] [Google Scholar]
- 83.Smotkin-Tangorra M, Purushothaman R, Gupta A, Nejati G, Anhalt H, Ten S. Prevalence of vitamin D insufficiency in obese children and adolescents. J Pediatr Endocrinol Metab. 2007;20:817–23. doi: 10.1515/jpem.2007.20.7.817. [DOI] [PubMed] [Google Scholar]
- 84.Gannagé-Yared MH, Chedid R, Khalife S, Azzi E, Zoghbi F, Halaby G. Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young Middle-Eastern population. Eur J Endocrinol. 2009;160:965–71. doi: 10.1530/EJE-08-0952. [DOI] [PubMed] [Google Scholar]
- 85.Pilz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: A systematic review. Nat Rev Cardiol. 2009;6:621–30. doi: 10.1038/nrcardio.2009.135. [DOI] [PubMed] [Google Scholar]
- 86.Kulah E, Dursun A, Aktunc E, Acikgoz S, Aydin M, Can M, et al. Effects of angiotensin-converting enzyme gene polymorphism and serum vitamin D levels on ambulatory blood pressure measurement and left ventricular mass in Turkish hypertensive population. Blood Press Monit. 2007;12:207–13. doi: 10.1097/MBP.0b013e32813fa371. [DOI] [PubMed] [Google Scholar]
- 87.Landin-Wilhelmsen K, Wilhelmsen L, Wilske J, Lappas G, Rosén T, Lindstedt G, et al. Sunlight increases serum 25(OH) vitamin D concentration whereas 1,25(OH)2D3 is unaffected. Results from a general population study in Goteborg, Sweden (the WHO MONICA project) Eur J Clin Nutr. 1995;49:400–7. [PubMed] [Google Scholar]
- 88.Kumaravel R, de las Heras J, Chen TC. Vitamin D Status, Adiposity, and Lipids in Black American and Caucasian Children. J Clin Endocrinol Metab. 2011;96:1560–7. doi: 10.1210/jc.2010-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mason C, Xiao L, Imayama I, Duggan CR, Bain C, Foster-Schubert KE, et al. Effects of weight loss on serum vitamin D in postmenopausal women. Am J Clin Nutr. 2011;94:95–103. doi: 10.3945/ajcn.111.015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–6. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 91.Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: Randomized double blind trial. J Intern Med. 2008;264:599–609. doi: 10.1111/j.1365-2796.2008.02008.x. [DOI] [PubMed] [Google Scholar]
- 92.Llewellyn DJ, Lang IA, Langa KM, Muniz-Terrera G, Phillips CL, Cherubini A, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170:1135–41. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knekt P, Kilkkinen A, Rissanen H, Marniemi J, Sääksjärvi K, Heliövaara M. Serum vitamin D and the risk of Parkinson disease. Arch Neurol. 2010;67:808–11. doi: 10.1001/archneurol.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liebman B. From sun and sea: New study puts vitamin D and omega-3s to the test. Nutrition Action Healthletter. 2009 Nov 3-7; [Google Scholar]
- 95.Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: A meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169:551–61. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- 96.Christine G. Vitamin D Supplementation: An Update. US Pharm. 2010;35:58–76. [Google Scholar]
- 97.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 98.Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet. 2001;358:1500–3. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 99.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–60. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 100.Hensel KJ, Randis TM, Gelber SE, Ratner AJ. Pregnancy-specific association of vitamin D deficiency and bacterial vaginosis. Am J Obstet Gynecol. 2011;204(41):e1–9. doi: 10.1016/j.ajog.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 101.Badalian SS, Rosenbaum PF. Vitamin D and pelvic floor disorders in women: Results from the National Health and Nutrition Examination Survey. Obstet Gynecol. 2010;115:795–803. doi: 10.1097/AOG.0b013e3181d34806. [DOI] [PubMed] [Google Scholar]
- 102.Millen AE, Voland R, Sondel SA, Parekh N, Horst RL, Wallace RB, et al. Vitamin D Status and Early Age-Related Macular Degeneration in Postmenopausal Women. Arch Ophthalmol. 2011;129:481–9. doi: 10.1001/archophthalmol.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Holick MF, Binkley NC, Heike A, Bischoff-Ferrari, Gordon CM, Hanley DA, et al. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 104.Murayama E, Miyamoto K, Kubodera N, Mori T, Matsunaga I. Synthetic studies of vitamin D3 analogues. VIII. Synthesis of 22-oxavitamin D analogues. Chem Pharm Bull. 1986;34:4410–3. doi: 10.1248/cpb.34.4410. [DOI] [PubMed] [Google Scholar]
- 105.Brown AJ, Slatopolsky E. Vitamin D analogs: Therapeutic applications and mechanisms for selectivity. Mol Aspects Med. 2008;29:433–52. doi: 10.1016/j.mam.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 106.Sjoden G, Smith C, Lindgren U, DeLuca HF. 1a-Hydroxyvitamin D2 is less toxic than 1a-hydroxyvitamin D3 in the rat. Proc Soc Exp Biol Med. 1985;178:432–6. doi: 10.3181/00379727-178-42028. [DOI] [PubMed] [Google Scholar]
- 107.Imanishi Y, Inaba M, Seki H, Koyama H, Nishizawa Y, Morii H, et al. Increased biological potency of hexafluorinated analogs of 1,25-dihydroxyvitamin D3 on bovine parathyroid cells. J Steroid Biochem Mol Biol. 1999;70:243–8. doi: 10.1016/s0960-0760(99)00112-0. [DOI] [PubMed] [Google Scholar]


