Sir,
The liver is the second largest organ in the body, and is often seen as the most important one. Toxins, infectious agents, medications, and serum inflammatory mediators may result in a diverse range of disease processes, leading to loss of normal histological architecture, reduced cell mass, and loss of blood flow. Consequently, functional liver capacity may be lost. Efforts have been made to search for effective hepatoprotective agents. Therefore, the prevention of liver diseases has a great significance both in theory and in practice.[1] Butea monosperma (lam.) Taub-(Fabiaceae) is a medium size deciduous tree, found throughout India and traditionally used for the treatment of hepatopathy, ulcers, tumors, and diabetes[2]. B. monosperma has been scientifically investigated for anthelmintic[3] anticonvulsive[4], antidiarrheal[5], antifertility[6], antimicrobial[7] and antistress[8] activities. This study was performed to evaluate the hepatoprotective potential of ethanolic extract of B. monosperma in carbon tetrachloride(CCl4) intoxicated rats.
Plant material was collected from Dharmapuri district, Tamilnadu, in the month of July and was authenticated by a Botanist, Plant Anatomy Research Center, Chennai. The powdered bark material was extracted using ethanol in a soxhelet apparatus. The solvent was completely removed by using a rotary flash evaporator.
Wistar albino rats of either sex, weighing 200–250 gm, maintained under standard husbandry conditions (temperature 23±2°C, relative humidity 55±10% and 12 hr light:12 hr dark cycle) were used for all experiments. Animals were allowed to take standard laboratory feed and tap water. The experiments were performed after the experimental protocols were approved by the institutional animal ethics committee of Periyar College of Pharmaceutical Sciences for girls, Tirchy, Tamil Nadu.
An acute toxicity study was performed for ethanolic bark extract of B. monosperma according to the acute toxic classic method as per OECD guidelines using female albino rats. Rats were divided into five groups of six each that is normal control, CCl4 control, two test groups, and standard group.
Normal control group : 5% CMC (10 ml/kg; oral),
CCl4 control group : CCl4 in 50% v/v, olive oil (3 ml/kg; i.p.)
Test groups : Ethanolic extract (100 mg/kg and 200 mg/kg; oral).
Standard drug treated group : Silymarin (25 mg/kg; oral)
All the doses were administered daily once for 7 days after CCl4 administration.[9] On eight day, the blood sample was collected from all the groups and allowed to clot for the separation of serum.
The serum was analyzed for the estimation of biochemical parameters such as glutamic oxaloacetic transaminase (SGOT) and glutamic pyruvic transaminase (SGPT), alkaline phosphatase (ALP), total bilirubin (TBL), and acid phosphatase (ACP). All the determinations were carried out using standard kits.
One animal from each of the treated groups showing maximum activity as indicated by improved biochemical parameters was used for this purpose. The animals were killed, liver was removed, and fixed in a mixture of 75 ml of saturated picric acid, 25 ml of 40% formaldehyde, and 5 ml of glacial acetic acid for 12 h, then embedded in paraffin using conventional methods[10] and cut into sections and stained using the hematoxylin–eosin dye. The sections were observed under a microscope for histopathological changes in the liver architecture and their photomicrographs were taken.
The results are expressed as mean ± SD of six animals from each group. The data were analysed by one-way analysis of variance (ANOVA) followed by Dunnett's test. A P value < 0.05 was considered significant.
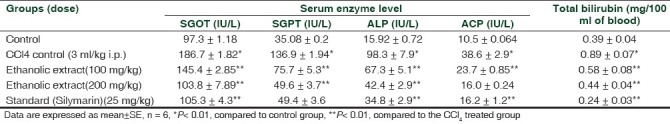
The ethanolic extract did not cause any transience up to 2000 mg/kg and were considered safe (OECD, 1996). CCl4 intoxication in normal rats significantly elevated the levels of SGOT, SGPT, ALP, ACP, and TBL, indicative of acute hepatocellular damage and biliary obstruction. The rats treated with ethanolic extract (100 and 200 mg/kg) and silymarin showed a significant decline in all the elevated SGOT, SGPT, ALP, ACP, and TBL levels [Table 1].
Table 1.
Effect of the ethanolic bark extract of Butea monosperma on CCl4 induced hepatotoxicity in rats
Histopathological examination of liver sections of the normal control group showed normal cellular architecture with separate hepatic cells, sinusoidal spaces, and a central vein. Disarrangement of normal hepatic cellswith intense necrosis and vacuolization of periportal vein are observed in CCl4 intoxicated liver. The liver tissue of CCl4 control treated animals showed hydropic changes and mild portal chronic inflammatory cell infiltrate. The liver sections of the rat intoxicated with CCl4 and treated with ethanolic extract (100 and 200 mg/kg) showed the absence of necrosis and overall no visible changes were observed as compared to the standard group.
This study was performed to assess the hepatoprotective activity of B. monosperma in rats against CCl4 as hepatotoxin to prove its claims in folklore practice in liver disorders. The ethanolic extract of B. monosperma shows significant (P < 0.01) hepatoprotective effects in the CCl4 intoxication in rats. Phytochemical analysis on the extracts showed the presence of flavonoids in the ethanolic extract. According to these results, it may be hypothesized that flavonoids present in the ethanolic extract could be considered responsible for the hepatoprotective activity of plants.
The hepatotoxicity of CCl4 has been reported to be due to the formation of the highly reactive trichloro free radical (CCl3) formed by the hemolytic cleavage of CCl4 or by an even more reactive species, trichloromethylperoxy free radical (CCl3COO·) formed by the reaction of ·CCl3with O2, which attacks polyunsaturated fatty acids. It produces hepatotoxicity through altering liver microsomal membranes in experimental animals.[11,12] From Table 1, it is discernible that the ethanolic extract of the plant was able to decrease all the elevated biochemical parameters due to the hepatotoxin.
Normalization in the levels of SGOT and SGPT is an indication of stabilization of plasma membrane in addition to repair of hepatic tissue damages caused by CCl4. Reduction of ALP and ACP levels with parallel depletion of the raised bilirubin level indicative of the stability of the biliary function in injury with CCl4. The protective effects exhibited by the ethanolic extract are similar to that observed with standard drug silymarin. Histological examination of the liver sections reveals that the normal liver architecture was commoved by CCl4 intoxication. The sections from the rats intoxicated with CCl4 and treated with ethanolic extracts, the normal cellular architecture was retained as compared to silymarin, confirming the protective effect of the ethanolic extract at 100 and 200 mg/ kg dose level. It was observed from the liver sections that the protective effect of 200 mg/kg dose of the ethanolic extract was more than that of 100 mg/kg dose. At 200 mg/kg dose of the extract, CCl4 intoxicated liver cells showed good protective effects as compared to the 100 mg/kg dose. On the basis of our results, it can be concluded that the ethanolic extract of B.utea monosperma possesses hepatoprotective activity against CCl4 intoxication in rats.
REFERENCES
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirtikar K R, Basu B D. Indian Medicinal Plant. (2nd ed) 1:314. [Google Scholar]
- 3.Prashanth D, Asha A.K, Amit A, Padmaja R. Anthelmintic activity of Butea monosperma. Fitoterapia. 2001;72:421–2. doi: 10.1016/s0367-326x(00)00333-6. [DOI] [PubMed] [Google Scholar]
- 4.Kasture VS, Kasture SB, Chopde CT. Anticonvulsive activity of Butea monosperma flowers in laboratory animals. Pharmacol. Biochem. Behav. 2002;72:965–72. doi: 10.1016/s0091-3057(02)00815-8. [DOI] [PubMed] [Google Scholar]
- 5.Gunakkunru A, Padmanaban K, Thirumal P, Pritila J, Parimala G, Vengatesan N. Anti-diarrhoeal activity of Butea monosperma in experimental animals. J Ethnopharmacol. 2005;98:241–4. doi: 10.1016/j.jep.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Johri RK, Pahwa GS, Sharma SC, Zutshi U. Determination of Estrogenic/antiestrogenic potential of antifertility substances using rat uterine peroxidase assay. Contraception. 1991;44:549–5. doi: 10.1016/0010-7824(91)90157-b. [DOI] [PubMed] [Google Scholar]
- 7.Rani P, Khullar N. Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multi-drug resistant Salmonella typhi. Phytotherapy Res. 2004;18:670–3. doi: 10.1002/ptr.1522. [DOI] [PubMed] [Google Scholar]
- 8.Bhatwadekar AD, Chintawar SD, Logade NA, Somani RS, Veena, Kasture S, Kasture SB. Antistress activity of Butea monosperma flowers. Indian J. Pharmacol. 1999;31:153–5. [Google Scholar]
- 9.Rao KS, Mishra SH. Screening of anti-inflammatory and hepatoprotective activitiy of alantolactone isolated from the roots of Inula racemosa. Indian Drugs. 1997;34:571–5. [Google Scholar]
- 10.Galighor A. E, Kozloff E. N. Essentials of practical micro technique. 2nd ed. New York: Lea and Febiger; 1976. p. 210. [Google Scholar]
- 11.Ashok SK, Somayaji SN, Bairy KL. Hepatoprotective effects of Ginkgo biloba against carbon tetrachloride induced hepatic injury in rats. Indian J. Pharmacol. 2006;33:260–6. [Google Scholar]
- 12.Sureshkumar S.V, Mishra S. Hepatoprotective effect of extracts from Pergularia daemia Forsk. J. Ethnopharmacol. 2006;107:164–8. doi: 10.1016/j.jep.2006.02.019. [DOI] [PubMed] [Google Scholar]


