Abstract
Background:
A serious, albeit rare, sequel of therapeutic ionizing radiotherapy is delayed development of a new, histologically distinct neoplasm within the radiation field.
Methods:
We identified 27 cases, from a 10-year period, of intracranial tumors arising after cranial irradiation. The original lesions for which cranial radiation was used for treatment included: tinea capitis (1), acute lymphoblastic leukemia (ALL; 5), sarcoma (1), scalp hemangioma (1), cranial nerve schwannoma (1) and primary (13) and metastatic (1) brain tumors, pituitary tumor (1), germinoma (1), pinealoma (1), and unknown histology (1). Dose of cranial irradiation ranged from 1800 to 6500 cGy, with a mean of 4596 cGy. Age at cranial irradiation ranged from 1 month to 43 years, with a mean of 13.4 years.
Results:
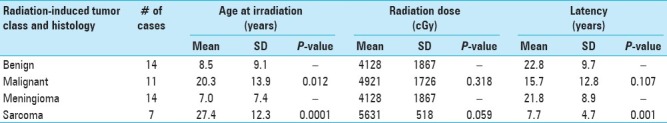
Latency between radiotherapy and diagnosis of a radiation-induced neoplasm ranged from 4 to 47 years (mean 18.8 years). Radiation-induced tumors included: meningiomas (14), sarcomas (7), malignant astrocytomas (4), and medulloblastomas (2). Data were analyzed to evaluate possible correlations between gender, age at irradiation, dose of irradiation, latency, use of chemotherapy, and radiation-induced neoplasm histology. Significant correlations existed between age at cranial irradiation and development of either a benign neoplasm (mean age 8.5 years) versus a malignant neoplasm (mean age 20.3; P = 0.012), and development of either a meningioma (mean age 7.0 years) or a sarcoma (mean age 27.4 years; P = 0.0001). There was also a significant positive correlation between latency and development of either a meningioma (mean latency 21.8 years) or a sarcoma (mean latency 7.7 years; P = 0.001). The correlation between dose of cranial irradiation and development of either a meningioma (mean dose 4128 cGy) or a sarcoma (mean dose 5631 cGy) approached significance (P = 0.059).
Conclusions:
Our study is the first to show that younger patients had a longer latency period and were more likely to have lower-grade lesions (e.g. meningiomas) as a secondary neoplasm, while older patients had a shorter latency period and were more likely to have higher-grade lesions (e.g. sarcomas).
Keywords: Radiation effects, radiotherapy, secondary brain tumors
INTRODUCTION
Ionizing radiation is widely used to treat a variety of both intracranial and extracranial neoplastic and non-neoplastic processes. The long-term undesirable side effects of radiation therapy on the central nervous system (CNS) include tissue necrosis, vasculopathy, and radiation-induced malignancy, according to the criteria proposed by Cahan et al.[10] in 1948 and modified by Schrantz and Araoz in 1972.[57] Although considered rare long-term sequelae, there are many reports in the literature of radiation-induced tumors following cranial irradiation.[12–14,22,24,55,62,63] The oncogenic risk of radiation exposure has been examined in several populations, including atomic bomb survivors,[27,34,47,48,59,69] high levels of radiation from extensive diagnostic radiographic procedures,[7,18,19,28,32,38,46,64,70] environmental or work-related exposure,[3,9,19,26,31,39,44] or radiotherapy to treat benign and malignant disease.[5,8,10,16,17,20,29,36,40,41,53,54,56,57,61,65,67,68]
Cranial irradiation, in the form of radiotherapy or radiosurgery, is widely used as a primary or adjuvant treatment modality for a variety of primary CNS lesions, including gliomas, ependymomas, medulloblastomas, meningiomas, pituitary lesions, and vascular malformations. Development of a radiation-induced neoplasm is a relatively rare event compared to other long-term complications of radiation therapy. The first report of a radiation-induced tumor of the CNS was by Mann et al.[43] in 1953. Since 1960, over 280 radiation-induced tumors have been reported in the literature. The most commonly described intracranial radiation-induced malignancies include meningioma, sarcoma, and glioma. Ependymoma, schwannoma, primitive neuroectodermal tumor (PNET), and pituitary adenoma have been rarely described.
We performed an institutional review of our experience in treating radiation-associated tumors to aid in the further study and prevention of these lesions. We were able to identify 27 cases of radiation-induced intracranial tumors, including two rare radiation-induced medulloblastomas. Patient data were analyzed to evaluate possible correlations between gender, age at irradiation, dose of irradiation, latency, use of chemotherapy, and radiation-induced neoplasm histology.
MATERIALS AND METHODS
After obtaining Institutional Review Board (IRB) approval for the collection of this data and the submission of this manuscript, we queried a comprehensive institutional database to identify patients with intracranial tumors treated at our institution between 1990 and 2000. The charts of those patients with new brain tumors who had received therapeutic cranial irradiation were reviewed to identify patients who developed secondary neoplasms that met the Cahan[10] criteria for radiation-induced malignancy: 1) occurrence in the field of irradiation used to treat the primary disease; 2) absence prior to irradiation and appearance after a period greater than 1 year following irradiation; 3) histological distinction from the primary neoplasm; and 4) absence of known genetic or familial predisposition to multiple or secondary malignancy.
Individual case data [Table 1] including the medical record, diagnostic and portal images, and histology specimens were analyzed for gender, original diagnosis, age at radiation treatment, port location and total radiotherapy dose, use of adjuvant chemotherapy, age at presentation of secondary tumor, location and histological diagnosis of radiation-induced tumor, treatment for radiation-induced tumor, and survival.
Table 1.
Patient population characteristics
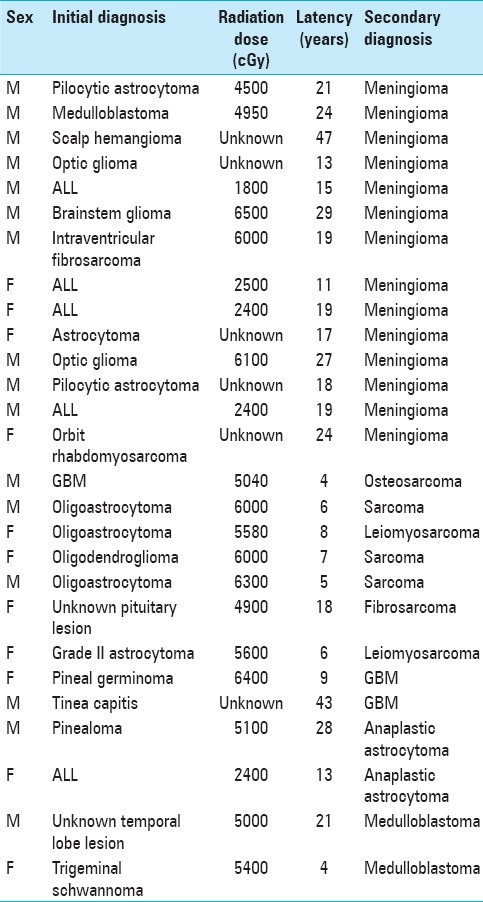
Given the amount of time elapsed between radiation and the development of a second malignancy and the fact that the majority of patients did not receive their initial radiation at the University of Washington, no treatment plans were available for the initial treatment. Of the 27 patients, 21 had received a known dose of radiation, and based on the medical record and general knowledge of treatment techniques available, 15 patients could have radiation treatment plans recreated to simulate their previous radiation. This provided an estimation of whether the radiation-induced tumors occurred in a high-dose region or low-dose region. Further, more refined analysis was not possible due to lack of detailed treatment plans.
Statistical analyses were performed using SPSS 8.0 for Windows. Correlations for age at irradiation, radiation dose, latency, secondary tumor histology, secondary tumor malignancy, and influence of chemotherapy were calculated using Mann-Whitney U, Wilcox on Rank Sum Test, and Fisher's Exact Test.
RESULTS
Latency between radiotherapy and diagnosis of a radiation-induced neoplasm ranged from 4 to 47 years (mean 18.8 years). Radiation-induced tumors included: meningiomas (14), sarcomas (7), malignant astrocytomas (4), and medulloblastomas (2). Data were analyzed to evaluate possible correlations between gender, age at irradiation, dose of irradiation, latency, use of chemotherapy, and radiation-induced neoplasm histology. Significant correlations [Table 2] existed between age at cranial irradiation and development of either a benign neoplasm (mean age 8.5 years) versus a malignant neoplasm (mean age 20.3 years; P = 0.012), and development of either a meningioma (mean age 7.0 years) or a sarcoma (mean age 27.4 years; P = 0.0001). There was also a significant positive correlation between latency and development of either a meningioma (mean latency 21.8 years) or a sarcoma (mean latency 7.7 years; P = 0.001). The correlation between dose of cranial irradiation and development of either a meningioma (mean dose 4128 cGy) or a sarcoma (mean dose 5631 cGy) approached significance (P = 0.059).
Table 2.
Younger age and shorter latency period both predict meningioma as the radiation-induced tumor. Older age and shorter latency predict sarcoma
Based on the recreated plans and medical record, it appears that 22 of the 27 radiation-induced tumors occurred in a high-dose radiation region (14 of 15 from the recreated plans and 8 of 12 based on the medical record). Of the remaining cases, 3 out of 27 radiation-induced neoplasms most likely developed in a low-dose radiation region (1 of 15 from the recreated plans and 2 of 12 based on the medical record). In two cases, it was not possible to assign whether the tumor occurred in a relatively high-dose or relatively low-dose radiation area.
DISCUSSION
Cranial irradiation has been used as prophylactic treatment in acute lymphoblastic leukemia (ALL), as principle treatment for tinea capitis and some vascular malformations, and in multimodality therapy for certain primary and metastatic tumors. Since the early 1970s, successful treatment of ALL includes the addition of cranial irradiation to prevent CNS relapse due to meningeal seeding. The use of radiation therapy reduced the incidence of CNS relapse from 70% to 10% and improved the ALL cure rate to greater than 50% of patients.[15,45,49] The improved survival has allowed time for the development of late adverse effects, including new neoplastic disease. Case reports and follow-up studies in patients in remission of ALL in the USA[4,6,14,21,23,30,35,42,50,51,52,55,66] and Europe[37] suggested an increased risk of a new malignancy, particularly CNS malignancy, associated with cranial irradiation.
Before the introduction in 1959 of the antifungal griseofulvin, X-ray epilation of the scalp was the standard treatment for tinea capitis. For decades, the Adamson-Keinbock technique was used, resulting in brain parenchyma exposure ranges of 175 cGy at the surface to 70 cGy at the base.[1] Two long-term studies examined the relation between radiotherapy in childhood for tinea capitis and the subsequent development of tumors within the field of radiation. A total of 13,049 patients were included and maximum follow-up periods were 29 years in a study of patients at New York University[2] and 33 years in a study of patients in Israel.[53] Data from 2215 patients at New York University Medical Center showed a higher incidence of tumors of brain, parotid, skin, bone, and thyroid tissue.[60] Data from 10,834 patients in Israel showed a relative risk for all tumors of 6.9 and a relative risk of 8.4 when only tumors of neural origin of the head and neck were considered.[54]
The risk of developing a secondary malignancy within the original field of irradiation increases with radiation doses as low as 1–2 Gy.[54] A growing number of cases, including those induced by stereotactic radiosurgery,[58,71] are being presented in the literature. Carlson et al. identified 13 histologically verified intracranial malignancies occurring in the stereotactic radiation fields within the literature.[11] One case involved a 25-year-old female with history of neurofibromatosis who had undergone stereotactic radiosurgery to a right and then a left vestibular schwannoma at the age of 16 and 18 years, respectively. She then developed a rhabdomyosarcoma of the right medulla 10 years later within her prior stereotactic radiation fields.[11] Another case in the literature documented a 70-year-old female with prior history of left occipital region meningioma treated with stereotactic radiosurgery who then developed a GBM 7 years following the procedure in the radiation treatment fields.[71]
Our study is the first to show that younger patients had a longer latency period and were more likely to have lower-grade lesions (e.g. meningiomas) as a secondary neoplasm, while older patients had a shorter latency period and were more likely to have higher-grade lesions (e.g. sarcomas).
No threshold radiation dose has been identified for malignant transformation; however, a majority of the secondary malignancies that developed from cranial radiation in this study appeared to fall within the high dose region of radiation, which ranged from 1800 to 6500 cGy. Although the volume of tissue irradiated could not be assessed in this analysis, it would seem intuitive that the more tissue irradiated, the increased likelihood of developing a secondary malignancy.
An important caveat to point out from this study is that all of the patients included in this analysis received radiation at least two or more decades ago when radiation treatment planning was cruder with larger, less conformal volumes and less accurate tumor targeting. CT planning had yet to be developed, making it more difficult to precisely delineate the tumor and thereby requiring more generous margins to prevent tumor misses. Additionally, treatment machines were much less sophisticated and consisted of Cobalt 60, orthovoltage machines, and low-energy linear accelerators. Variable energies, complex beam shaping, and multi-beam arrangements were unavailable during this era. Tumor imaging was also limited as MRI had yet to gain widespread use. Based on these limitations, patients treated with cranial irradiation several decades ago required large, simple fields, where a large volume of the cranium was included in the treatment portals.
Significant advances in radiation oncology have led to more conformal and targeted therapy, but it is unclear if these new techniques and technological advancements will decrease the risk of radiation-induced malignancies, particularly given the fact that conformal treatment planning and use of alternate radiation sources can expose more normal tissue to lower doses of radiation.[25,33] Careful monitoring and close follow-up of patients treated within cranial radiation in this more advanced era will help to answer this question.
CONCLUSION
Younger age at the time of radiation and longer latency period prior to diagnosis of a radiation-induced neoplasm increase the likelihood of benign tumor development. Conversely, older aged patients and shorter latency periods increase the risk of more malignant secondary neoplasms. As survival time increases for patients undergoing cranial radiation therapy with the development of more efficacious radiotherapy technologies and chemotherapeutic agents, the clinician must keep in mind the risk of secondary neoplasms.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/1/48/96068
Contributor Information
Abhineet Chowdhary, Email: minkuc@gmail.com.
Alex M. Spence, Email: aspence@uw.edu.
Lindsay Sales, Email: Lbarclay@uw.edu.
Robert C. Rostomily, Email: rosto@uw.edu.
Jason K. Rockhill, Email: jkrock@uw.edu.
Daniel L. Silbergeld, Email: dls@uw.edu.
REFERENCES
- 1.Adamson H. A simplified method of x-ray application for the cure of ringworm of the scalp: Keinbock's method. Lancet. 1909:1378–80. [Google Scholar]
- 2.Albert RE, Omran AR, Brauer EW, Dove DC, Cohen NC, Schmidt H, et al. Follow-up study of patients treated by x-ray for tinea capitis. Am J Public Health Nations Health. 1966;56:2114–20. doi: 10.2105/ajph.56.12.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambach W. Occupational low-dose exposure to ionising radiation. Lancet. 1994;344:1037. doi: 10.1016/s0140-6736(94)91704-3. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JR, Treip CS. Radiation-induced intracranial neoplasms. A report of three possible cases. Cancer. 1984;53:426–9. doi: 10.1002/1097-0142(19840201)53:3<426::aid-cncr2820530310>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Beaty O, 3rd, Hudson MM, Greenwald C, Luo X, Fang L, Wilimas JA, et al. Subsequent malignancies in children and adolescents after treatment for Hodgkin's disease. J Clin Oncol. 1995;13:603–9. doi: 10.1200/JCO.1995.13.3.603. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia S, Sather HN, Pabustan OB, Trigg ME, Gaynon PS, Robison LL. Low incidence of second neoplasms among children diagnosed with acute lymphoblastic leukemia after 1983. Blood. 2002;99:4257–64. doi: 10.1182/blood.v99.12.4257. [DOI] [PubMed] [Google Scholar]
- 7.Boice JD. Cancer following medical irradiation. Cancer. 1981;47(Suppl 5):1081–90. doi: 10.1002/1097-0142(19810301)47:5+<1081::aid-cncr2820471305>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Boice JD, Jr, Blettner M, Kleinerman RA, Stovall M, Moloney WC, Engholm G, et al. Radiation dose and leukemia risk in patients treated for cancer of the cervix. J Natl Cancer Inst. 1987;79:1295–311. [PubMed] [Google Scholar]
- 9.Brown RA. Public health effects of occupational and environmental radiation exposure. JAMA. 1991;266:652–5. [PubMed] [Google Scholar]
- 10.Cahan WG, Woodard HQ. Sarcoma arising in irradiated bone: Report of 11 cases. Cancer. 1948;1:3–29. doi: 10.1002/1097-0142(194805)1:1<3::aid-cncr2820010103>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Carlson ML, Babovic-Vuksanovic D, Messiaen L, Scheithauer BW, Neff BA, Link MJ. Radiation-induced rhabdomyosarcoma of the brainstem in a patient with neurofibromatosis Type 2. J Neurosurg. 2010;112:81–7. doi: 10.3171/2009.6.JNS09105. [DOI] [PubMed] [Google Scholar]
- 12.Carpentier AF, Chantelard JV, Henin D, Poisson M. Osteosarcoma following radiation treatment for meningioma: Report of a case and effective treatment with chemotherapy. J Neurooncol. 1994;21:249–53. doi: 10.1007/BF01063774. [DOI] [PubMed] [Google Scholar]
- 13.Choudhary A, Pradhan S, Huda MF, Mohanty S, Kumar M. Radiation induced meningioma with a short latent period following high dose cranial irradiation - Case report and literature review. J Neurooncol. 2006;77:73–7. doi: 10.1007/s11060-005-9009-9. [DOI] [PubMed] [Google Scholar]
- 14.Chung CK, Stryker JA, Cruse R, Vannuci R, Towfighi J. Glioblastoma multiforme following prophylactic cranial irradiation and intrathecal methotrexate in a child with acute lymphocytic leukemia. Cancer. 1981;47:2563–6. doi: 10.1002/1097-0142(19810601)47:11<2563::aid-cncr2820471108>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 15.Cortes J, O’Brien SM, Pierce S, Keating MJ, Freireich EJ, Kantarjian HM. The value of high-dose systemic chemotherapy and intrathecal therapy for central nervous system prophylaxis in different risk groups of adult acute lymphoblastic leukemia. Blood. 1995;86:2091–7. [PubMed] [Google Scholar]
- 16.Curtis RE, Boice JD, Jr, Stovall M, Bernstein L, Greenberg RS, Flannery JT, et al. Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N Engl J Med. 1992;326:1745–51. doi: 10.1056/NEJM199206253262605. [DOI] [PubMed] [Google Scholar]
- 17.Darby SC, Reeves G, Key T, Doll R, Stovall M. Mortality in a cohort of women given X-ray therapy for metropathia haemorrhagica. Int J Cancer. 1994;56:793–801. doi: 10.1002/ijc.2910560606. [DOI] [PubMed] [Google Scholar]
- 18.Desmond AN, O’Regan K, Curran C, McWilliams S, Fitzgerald T, Maher MM, et al. Crohn's disease: Factors associated with exposure to high levels of diagnostic radiation. Gut. 2008;57:1524–9. doi: 10.1136/gut.2008.151415. [DOI] [PubMed] [Google Scholar]
- 19.Duggan MJ, Greenslade E, Jones BE. External radiation doses from occupational exposure. Nature. 1969;221:831–3. doi: 10.1038/221831a0. [DOI] [PubMed] [Google Scholar]
- 20.Favus MJ, Schneider AB, Stachura ME, Arnold JE, Ryo UY, Pinsky SM, et al. Thyroid cancer occurring as a late consequence of head-and-neck irradiation. Evaluation of 1056 patients. N Engl J Med. 1976;294:1019–25. doi: 10.1056/NEJM197605062941901. [DOI] [PubMed] [Google Scholar]
- 21.Fontana M, Stanton C, Pompili A, Amadori S, Mandelli F, Meloni G, et al. Late multifocal gliomas in adolescents previously treated for acute lymphoblastic leukemia. Cancer. 1987;60:1510–8. doi: 10.1002/1097-0142(19871001)60:7<1510::aid-cncr2820600718>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 22.Gnanalingham KK, Chakraborty A, Galloway M, Revesz T, Powell M. Osteosarcoma and fibrosarcoma caused by postoperative radiotherapy for a pituitary adenoma. Case report. J Neurosurg. 2002;96:960–3. doi: 10.3171/jns.2002.96.5.0960. [DOI] [PubMed] [Google Scholar]
- 23.Gold DG, Neglia JP, Dusenbery KE. Second neoplasms after megavoltage radiation for pediatric tumors. Cancer. 2003;97:2588–96. doi: 10.1002/cncr.11356. [DOI] [PubMed] [Google Scholar]
- 24.Hader WJ, Drovini-Zis K, Maguire JA. Primitive neuroectodermal tumors in the central nervous system following cranial irradiation: A report of four cases. Cancer. 2003;97:1072–6. doi: 10.1002/cncr.11121. [DOI] [PubMed] [Google Scholar]
- 25.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65:1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 26.Hicks N, Zack M, Caldwell GG, Fernbach DJ, Falletta JM. Childhood cancer and occupational radiation exposure in parents. Cancer. 1984;53:1637–43. doi: 10.1002/1097-0142(19840415)53:8<1637::aid-cncr2820530802>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 27.Hollingsworth JW. Radiation effects: Cancer in atomic bomb survivors. Science. 1987;236:99. doi: 10.1126/science.236.4797.99. [DOI] [PubMed] [Google Scholar]
- 28.Isherwood I, Young IM, Bowker KW, Bramall GK. Radiation dose to the eyes of the patient during neuroradiological investigations. Neuroradiology. 1975;10:137–41. doi: 10.1007/BF00341814. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson P, Holmberg E, Lundell M, Mattsson A, Holm LE, Wallgren A. Intracranial tumors after exposure to ionizing radiation during infancy: A pooled analysis of two Swedish cohorts of 28,008 infants with skin hemangioma. Radiat Res. 1998;150:357–64. [PubMed] [Google Scholar]
- 30.Kaschten B, Flandroy P, Reznik M, Hainaut H, Stevenaert A. Radiation-induced gliosarcoma. Case report and review of the literature. J Neurosurg. 1995;83:154–62. doi: 10.3171/jns.1995.83.1.0154. [DOI] [PubMed] [Google Scholar]
- 31.Khalil MF, Thomas A, Aassad A, Rubin M, Taub RN. Epithelioid angiosarcoma of the small intestine after occupational exposure to radiation and polyvinyl chloride: A case report and review of literature. Sarcoma. 2005;9:161–4. doi: 10.1080/13577140500389069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, van der Wieken R. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: The access study. J Am Coll Cardiol. 1997;29:1269–75. doi: 10.1016/s0735-1097(97)00064-8. [DOI] [PubMed] [Google Scholar]
- 33.Kry SF, Salehpour M, Followill DS, Stovall M, Kuban DA, White RA, et al. The calculated risk of fatal secondary malignancies from intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62:1195–203. doi: 10.1016/j.ijrobp.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 34.Land CE. Studies of cancer and radiation dose among atomic bomb survivors. The example of breast cancer. JAMA. 1995;274:402–7. [PubMed] [Google Scholar]
- 35.Latz D, Alfrink M, Nassar N, Beyerle C. Breast cancer in a male patient after treatment of acute lymphoblastic leukemia including total body irradiation and bone marrow transplantation. Onkologie. 2004;27:477–9. doi: 10.1159/000080369. [DOI] [PubMed] [Google Scholar]
- 36.Little MP. Cancer after exposure to radiation in the course of treatment for benign and malignant disease. Lancet Oncol. 2001;2:212–20. doi: 10.1016/S1470-2045(00)00291-6. [DOI] [PubMed] [Google Scholar]
- 37.Löning L, Zimmermann M, Reiter A, Kaatsch P, Henze G, Riehm H, et al. Secondary neoplasms subsequent to Berlin-Frankfurt-Munster therapy of acute lymphoblastic leukemia in childhood: Significantly lower risk without cranial radiotherapy. Blood. 2000;95:2770–5. [PubMed] [Google Scholar]
- 38.Louvard Y, Lefevre T, Allain A, Morice M. Coronary angiography through the radial or the femoral approach: The CARAFE study. Catheter Cardiovasc Interv. 2001;52:181–7. doi: 10.1002/1522-726x(200102)52:2<181::aid-ccd1044>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 39.Lowder WM, Condon WJ. Measurement of the exposure of human populations to environmental radiation. Nature. 1965;206:658–62. doi: 10.1038/206658a0. [DOI] [PubMed] [Google Scholar]
- 40.Lundell M, Hakulinen T, Holm LE. Thyroid cancer after radiotherapy for skin hemangioma in infancy. Radiat Res. 1994;140:334–9. [PubMed] [Google Scholar]
- 41.Lundell M, Mattsson A, Karlsson P, Holmberg E, Gustafsson A, Holm LE. Breast cancer risk after radiotherapy in infancy: A pooled analysis of two Swedish cohorts of 17,202 infants. Radiat Res. 1999;151:626–32. [PubMed] [Google Scholar]
- 42.Malone M, Lumley H, Erdohazi M. Astrocytoma as a second malignancy in patients with acute lymphoblastic leukemia. Cancer. 1986;57:1979–85. doi: 10.1002/1097-0142(19860515)57:10<1979::aid-cncr2820571016>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 43.Mann I, Yates PC, Ainslie JP. Unusual case of double primary orbital tumour. Br J Ophthalmol. 1953;37:758–62. doi: 10.1136/bjo.37.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muirhead CR, O’Hagan JA, Haylock RG, Phillipson MA, Willcock T, Berridge GL, et al. Mortality and cancer incidence following occupational radiation exposure: Third analysis of the National Registry for Radiation Workers. Br J Cancer. 2009;100:206–12. doi: 10.1038/sj.bjc.6604825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muriel FS, Svarch E, Pavlovsky S, Eppinger-Helft M, Braier J, Vergara B, et al. Comparison of central nervous system prophylaxis with cranial radiation and intrathecal methotrexate versus intrathecal methotrexate alone in acute lymphoblastic leukemia. Blood. 1983;62:241–50. [PubMed] [Google Scholar]
- 46.Parker L, Pearce MS, Dickinson HO, Aitkin M, Craft AW. Stillbirths among offspring of male radiation workers at Sellafield nuclear reprocessing plant. Lancet. 1999;354:1407–14. doi: 10.1016/S0140-6736(99)04138-0. [DOI] [PubMed] [Google Scholar]
- 47.Pinkston JA, Wakabayashi T, Yamamoto T, Asano M, Harada Y, Kumagami H, et al. Cancer of the head and neck in atomic bomb survivors: Hiroshima and Nagasaki, 1957-1976. Cancer. 1981;48:2172–8. doi: 10.1002/1097-0142(19811115)48:10<2172::aid-cncr2820481010>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 48.Preston DL, Cullings H, Suyama A, Funamoto S, Nishi N, Soda M, et al. Solid cancer incidence in atomic bomb survivors exposed in utero or as young children. J Natl Cancer Inst. 2008;100:428–36. doi: 10.1093/jnci/djn045. [DOI] [PubMed] [Google Scholar]
- 49.Pui CH. Acute lymphoblastic leukemia in children. Curr Opin Oncol. 2000;12:3–12. doi: 10.1097/00001622-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Pui CH, Cheng C, Leung W, Rai SN, Rivera GK, Sandlund JT, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349:640–9. doi: 10.1056/NEJMoa035091. [DOI] [PubMed] [Google Scholar]
- 51.Regelson W, Bross ID, Hananian J, Nigogosyan G. Incidence of second primary tumors in children with cancer and leukemia: A seven-year survey of 150 consecutive autopsied cases. Cancer. 1965;18:58–72. doi: 10.1002/1097-0142(196501)18:1<58::aid-cncr2820180110>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 52.Relling MV, Rubnitz JE, Rivera GK, Boyett JM, Hancock ML, Felix CA, et al. High incidence of secondary brain tumours after radiotherapy and antimetabolites. Lancet. 1999;354:34–9. doi: 10.1016/S0140-6736(98)11079-6. [DOI] [PubMed] [Google Scholar]
- 53.Ron E, Modan B. Benign and malignant thyroid neoplasms after childhood irradiation for tinea capitis. J Natl Cancer Inst. 1980;65:7–11. [PubMed] [Google Scholar]
- 54.Ron E, Modan B, Boice JD, Jr, Alfandary E, Stovall M, Chetrit A, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319:1033–9. doi: 10.1056/NEJM198810203191601. [DOI] [PubMed] [Google Scholar]
- 55.Salvati M, Cervoni L, Caruso R, Delfini R, Gagliardi FM. High-dose radiation-induced meningiomas in elderly. Neurosurg Rev. 1996;19:81–3. doi: 10.1007/BF00418073. [DOI] [PubMed] [Google Scholar]
- 56.Sanders J, Sale GE, Ramberg R, Clift R, Buckner CD, Thomas ED. Glioblastoma multiforme in a patient with acute lymphoblastic leukemia who received a marrow transplant. Transplant Proc. 1982;14:770–4. [PubMed] [Google Scholar]
- 57.Schrantz JL, Araoz CA. Radiation induced meningeal fibrosarcoma. Arch Pathol. 1972;93:26–31. [PubMed] [Google Scholar]
- 58.Shamisa A, Bance M, Nag S, Tator C, Wong S, Norén G, et al. Glioblastoma multiforme occurring in a patient treated with gamma knife surgery. Case report and review of the literature. J Neurosurg. 2001;94:816–21. doi: 10.3171/jns.2001.94.5.0816. [DOI] [PubMed] [Google Scholar]
- 59.Shimizu Y, Schull WJ, Kato H. Cancer risk among atomic bomb survivors. The RERF Life Span Study. Radiation Effects Research Foundation. JAMA. 1990;264:601–4. [PubMed] [Google Scholar]
- 60.Shore RE, Albert RE, Pasternack BS. Follow-up study of patients treated by X-ray epilation for Tinea capitis: Resurvey of post-treatment illness and mortality experience. Arch Environ Health. 1976;31:21–8. doi: 10.1080/00039896.1976.10667184. [DOI] [PubMed] [Google Scholar]
- 61.Smith PG, Doll R. Late effects of x irradiation in patients treated for metropathia haemorrhagica. Br J Radiol. 1976;49:224–32. doi: 10.1259/0007-1285-49-579-224. [DOI] [PubMed] [Google Scholar]
- 62.Spallone A, Gagliardi FM, Vagnozzi R. Intracranial meningiomas related to external cranial irradiation. Surg Neurol. 1979;12:153–9. [PubMed] [Google Scholar]
- 63.Stein ME, Drumea K, Guilbord JN, Ben-Itzhak O, Kuten A. Case report: Late aggressive meningioma following prophylactic cranial irradiation for acute lymphoblastic leukaemia. Br J Radiol. 1995;68:1123–5. doi: 10.1259/0007-1285-68-814-1123. [DOI] [PubMed] [Google Scholar]
- 64.Tien HC, Tremblay LN, Rizoli SB, Gelberg J, Spencer F, Caldwell C, et al. Radiation exposure from diagnostic imaging in severely injured trauma patients. J Trauma. 2007;62:151–6. doi: 10.1097/TA.0b013e31802d9700. [DOI] [PubMed] [Google Scholar]
- 65.Tucker MA, D’Angio GJ, Boice JD, Jr, Strong LC, Li FP, Stovall M, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317:588–93. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 66.Walter AW, Hancock ML, Pui CH, Hudson MM, Ochs JS, Rivera GK, et al. Secondary brain tumors in children treated for acute lymphoblastic leukemia at St Jude Children's Research Hospital. J Clin Oncol. 1998;16:3761–7. doi: 10.1200/JCO.1998.16.12.3761. [DOI] [PubMed] [Google Scholar]
- 67.Weiss HA, Darby SC, Doll R. Cancer mortality following X-ray treatment for ankylosing spondylitis. Int J Cancer. 1994;59:327–38. doi: 10.1002/ijc.2910590307. [DOI] [PubMed] [Google Scholar]
- 68.Wong FL, Boice JD, Jr, Abramson DH, Tarone RE, Kleinerman RA, Stovall M, et al. Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk. JAMA. 1997;278:1262–7. doi: 10.1001/jama.278.15.1262. [DOI] [PubMed] [Google Scholar]
- 69.Yoshimoto Y, Delongchamp R, Mabuchi K. In-utero exposed atomic bomb survivors: Cancer risk update. Lancet. 1994;344:345–6. doi: 10.1016/s0140-6736(94)91389-7. [DOI] [PubMed] [Google Scholar]
- 70.Yoshinaga S, Hauptmann M, Sigurdson AJ, Doody MM, Freedman DM, Alexander BH, et al. Nonmelanoma skin cancer in relation to ionizing radiation exposure among U.S. radiologic technologists. Int J Cancer. 2005;115:828–34. doi: 10.1002/ijc.20939. [DOI] [PubMed] [Google Scholar]
- 71.Yu JS, Yong WH, Wilson D, Black KL. Glioblastoma induction after radiosurgery for meningioma. Lancet. 2000;356:1576–7. doi: 10.1016/S0140-6736(00)03134-2. [DOI] [PubMed] [Google Scholar]


