Abstract
Cancer vaccines have shown success in curing tumors in pre-clinical models. Accumulating evidence also supports their ability to induce immune responses in patients. In many cases, these responses correlate with improved clinical outcomes. However, cancer vaccines have not yet demonstrated their true potential in clinical trials. This is likely due to the difficulty in mounting a significant antitumor response in patients with advanced disease because of preexisting tolerance mechanisms that are actively turning off immune recognition in cancer patients. This review will examine the recent progress being made in the design and implementation of whole cell cancer vaccines, one vaccine approach that simultaneously targets multiple tumor antigens to activate the immune response. These vaccines have been shown to induce antigen specific T cell responses. Pre-clinical studies evaluating these vaccines given in sequence with other agents and cancer treatment modalities support the use of immunomodulating doses of chemotherapy and radiation, as well as immune modulating pathway targeted monoclonal antibodies, to enhance the efficacy of cancer vaccines. Based on emerging pre-clinical data, clinical trials are currently exploring the use of combinatorial immune based therapies for the treatment of cancer.
Despite progress made in the field of cancer therapeutics, cancer remains a leading cause of death of both men and women. Furthermore, many cancer treatment modalities such as chemotherapy, radiation, and surgery entail side effects which decrease the quality of life for patients with advanced cancer in exchange for modest survival benefits. Immunotherapy holds the promise of a treatment which is specific and long lasting, protecting patients from cancer recurrence due to memory immune responses, and with limited adverse effects to healthy tissue. Although animal models have demonstrated the potential for effective treatment of cancer, there has so far been limited success seen in the clinic with immunotherapy. This may be due to multiple factors, including poorly designed trials that compare immunotherapy as solo therapy versus the standard of care treatment as well as limiting the inclusion of patients to those with advanced cancer who have already failed or are not eligible for conventional treatments. Rarely are single agents of any class effective in the treatment of cancer. Pre-clinical studies suggest that this holds true for immunotherapy as well due to underlying tolerance and immunosuppression. Thus, in order to successfully prolong survival and avoid tumor recurrence, immunotherapy will need to be used in combination with multiple immune-based or traditional modalities. Specifically, combination therapy with chemotherapy, radiation, monoclonal antibodies, and immune checkpoint inhibitors may be useful in addition to whole cell vaccine vectors to stimulate the protective immune response and inhibit the suppressive immune response.
Whole cell cancer vaccines
Modified whole cell cancer vaccines represent one form of immunotherapy currently in development and clinical trials. The advantage of whole tumor cells used as a vaccine rather than a specific protein or peptide tumor antigen is that the cells provide a source of all potential antigens, eliminating the need to identify the most optimal antigen to target in a particular type of cancer (Figure 1). Importantly, multiple tumor antigens can be targeted at once, generating immune responses to more than one tumor antigen, thereby bypassing issues of tumor antigen loss. Furthermore, immunized lymphocyte and serologic responses can be exploited to identify novel tumor antigens or categorize the importance of a response to a particular tumor antigen through the comparison of immune responses pre- and post-vaccination and by correlating responses with prognosis.
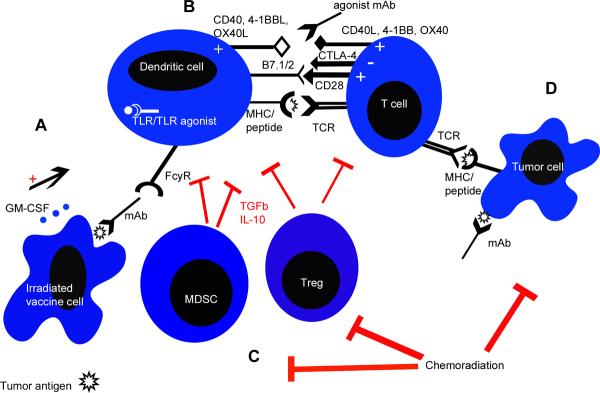
Figure 1.
Interactions of the immune system with a whole cell vaccine approach (GM-CSF-secreting tumor cell vaccine as one example) and other immune modulating therapies for the treatment of cancer. A) GM-CSF is secreted by irradiated vaccine cells, which attract dendritic cells (DCs) to the site of antigen. The antigen is then taken up by the DCs for processing and presentation. DCs can also be stimulated by monoclonal antibodies (mAb) that bind to specific tumor antigens on the vaccine cell surface via their Fc receptor recognizing the Fc portion of the mAb. Vaccine cell lines can also be modified to secrete other cytokines, express co-stimulatory molecules that further activate DCs (such as CD40L) or express molecules that block inhibitory signals such as TGFβ (not shown). Toll-like receptor (TLR) agonists or immunomodulatory chemotherapic agents such as paclitaxel can also stimulate DCs through TLRs to upregulate co-stimulatory molecules, increase cytokine production, and enhance antigen processing and presentation. B) DCs process and present tumor antigen derived from the vaccinating cells to CD4+ and CD8+ T cells. Tumor antigen is presented in the form of peptide/MHC complexes on APCs; T cells bind this complex with their T cell receptor (TCR). Additional signals are required for stimulation of T cells which can be provided by activated DCs or agonist antibodies to co-stimulatory molecule receptors such as anti-CD40, anti-4-1BB, and anti-OX40. The activation and proliferation of tumor antigen-specific T cells can also be increased with the use of blocking antibodies to immune checkpoint molecules such as CTLA-4 and PD-1. C) APCs and T cells can be suppressed by inhibitory cytokines and molecules such as TGFβ and IL-10 secreted by suppressive immune cell populations like MDSC and Tregs. Chemotherapy (such as cyclophosphamide and gemcitibine) and radiation, when used at immunomodulatory doses, can be used to inhibit these populations. D) Tumor cell killing occurs when the TCR expressed on effector CD8+ T cells recognizes tumor antigens presented by MHC molecules on the surface of tumor cells. If activated efficiently, CD8+ T cells can work synergistically with traditional treatments such as chemotherapy, radiation therapy, or monoclonal antibodies to kill or inhibit tumor cells.
Using autologous tumor cells in the generation of a whole cell vaccine ensures that patients are vaccinated with cells containing the same tumor antigens that their tumor expresses. However, this approach is technically limited because harvesting tumor cells and generating a vaccine line which expresses a standardized amount of cytokine is not always feasible and can be financially costly and time consuming.1 Vaccines made from allogeneic cells circumvent the issue of individualizing each patient's therapy and by using several cell lines derived from different tumors in the vaccine, there is an increased likelihood that the patient's tumor will share antigens expressed by the vaccine cells, including important tumor antigens overexpressed or mutated in a high percentage of that particular cancer. A concern of using allogeneic cells is that HLA mismatch between vaccinating cell lines and the patient will result in a response directed against foreign HLA molecules rather than tumor antigens. While anti-HLA responses do develop, they have not been shown to inhibit the tumor antigen response and have actually been associated with clinical response to whole cell vaccination.2,3 From an immunologic point of view, antigen presenting cells (APCs) from the patient are responsible for priming the CD8+ T cell response to tumor antigens contained in the irradiated vaccine cells and the presence of foreign MHC molecules may enhance this cross-priming.2,4
Whole cell vaccines have been genetically modified to express cytokines, chemokines or costimulatory molecules to stimulate the immune response to the injected irradiated tumor cells.1 Many phase I and II studies have shown this approach to be safe in patients with different types of cancer. Historically, vaccine induced immune responses have been assessed by measuring delayed-type hypersensitivity responses (DTH) to autologous tumor cells. While tumor cells alone do not elicit a DTH, DTH responses have been observed to correlate with a survival benefit in patients given gene modified vaccine cells.1 One cytokine, granulocyte macrophage colony-stimulating factor (GM-CSF) was found to be superior to other cytokines tested including IL-2, IL-4, IL-6, TNF-α, and IFN-γ when transduced into irradiated melanoma cells in pre-clinical studies.5 The addition of GM-CSF to a whole tumor cell vaccine resulted in a massive influx of dendritic cells (DCs), macrophages, eosinophils, and T cells at the vaccination site.1. The resection of lesions post-vaccination revealed necrotic tumors with many T cells and plasma cells whereas infiltrates were not seen in non-vaccinated patients' tumors.6 Many published studies now explain why GM-CSF stands out among cytokines for its ability to activate an effective tumor targeted T cell response.7 GM-CSF can recruit DCs to the site of the irradiated cells and stimulate antigen uptake, processing, and presentation.5 DCs facilitate the T cell response by cross-priming CD8+ T cells after uptake of GM-CSF-secreting whole tumor cells.4
Moving forward with GM-CSF-secreting whole cell tumor vaccines
The GM-CSF-secreting whole cell cancer vaccine approach has been studied in a number of different cancer types in animal models and in patients. In pancreatic cancer, both phase I and II trials have been completed with an irradiated, allogeneic GM-CSF–transduced vaccine derived from two pancreatic tumor lines. A phase I trial in 14 patients with stage 2/3 pancreatic adenocarcinoma to assess safety and the induction of systemic antitumor immune responses found no dose limiting toxicities and a DTH correlating with disease-free survival in 3 patients surviving greater than 25 months.8 All responders received the two highest dose levels and multiple vaccinations at the same dose.8 A phase II follow up study in which 60 patients received one vaccine injection 8 weeks following pancreaticoduodenectomy and prior to standard chemoradiation with up to 5 additional vaccines following chemoradiation, demonstrated that immunotherapy and chemoradiation can be safely combined as adjuvant therapy.9 Treatment with the vaccine resulted in survival consistent with that seen in the adjuvant setting with chemoradiation, with a significant improvement in survival over chemoradiation alone in the first two years following surgery.9 CD8+ T cell responses to mesothelin, a protein involved in pancreatic cancer metastasis, were enhanced following vaccination in patients with increased survival.9 Interestingly, patients with greater than three years disease-free survival also exhibited an expansion of the CD8+ T cell response to an increased number of mesothelin epitopes whereas the repertoire of anti-mesothelin T cells was limited for patients who survived less than 3 years, suggesting whole cell vaccination has the advantage inducing an immune response to multiple epitopes within an antigenic protein.9 These findings also suggest that patients receiving this vaccine might benefit clinically from repeated boosting beyond 2 years. Studies are on-going to test this question. Phase I trials have also been completed in other advanced cancers including renal cell carcinoma, metastatic melanoma, prostate cancer, lymphoma, leukemia, breast cancer, and non-small-cell lung cancer (NSCLC) and these have provided additional evidence for the safety and ability of GM-CSF-secreting whole cell vaccines to induce immune responses.7,10
Modification of GM-CSF-secreting whole cell vaccine vectors
Whole cell vaccines provide a source of tumor antigens, but a stimulus is required for antigens to be taken up by antigen-presenting cells in an immune stimulatory context and to recruit cells of both the innate and adaptive immune system. The genetic modification and irradiation of tumor cells generates vaccine cells that secrete cytokine but do not further proliferate in the host. Several new cancer vaccine developments are designed to stimulate the immune response by further building on the whole cell gene modified vaccine approach.
CpG oligodeoxynucleotides (ODN), a toll like receptor (TLR) 9 agonist, is one example of a molecule that stimulates TLRs expressed by antigen-presenting cells such as monocytes, macrophages and DCs to enhance the immune response to tumor antigens.11 When administered with a cancer vaccine, TLR ligands function to upregulate co-stimulatory molecules and cytokine expression by APCs, driving the adaptive response to antigens delivered in the vaccine. In a mouse model using transplantable neuroblastoma tumors, co-administration of CpG ODN served to enhance the effects of a GM-CSF-secreting whole cell vaccine.12 In a different vaccine approach involving dendritic cells fused with tumor cells, the addition of CpG ODN enhanced maturation of the fused cells, increasing the magnitude of the T cell response and resulting in tumor rejection after re-challenge.13 CpG ODN physically cross-linked to a tumor cell vaccine proved superior against tumor development or growth of established tumors in comparison to free CpG ODN given simultaneously with a whole cell vaccine, suggesting the co-localization of TLR agonist and tumor antigen is crucial for activation of DCs.11 Clinically, TLR agonists have been used to enhance the immunogenicity of peptide vaccines but have yet to be combined with whole cell vaccines in cancer patients.14
While GM-CSF can activate antigen-processing and presentation by DCs (Fig 1), the effects of immunosuppressive molecules found in the cytokine milieu of the tumor microenvironment can inhibit this maturation and activation of APCs and T cells induced by vaccination. TGFβ secreted by suppressive cell types inhibits CD8+ T cells directly through the transcriptional repression of proteins required for cytolytic effector function such as granzyme B and perforin, and indirectly through the suppression of DCs.15,16 TGFβ is also responsible for the induction of T regulatory cells (Tregs) which are inhibitory to the anti-tumor response.17 Therefore new efforts have been made to block TGFβ expression, starting with the vaccinating cell lines. A phase I trial of an autologous whole cell vaccine expressing both GM-CSF and a TGFβ antisense molecule represents the first combination of a whole cell vaccine with immunostimulatory and anti-immunosuppressive molecules, resulting in one complete response in metastatic melanoma and 17 out of 21 patients with stable disease for at least 2 months post-treatment.18 Another phase I trial of this approach in six grade IV astrocytoma patients showed a benefit in overall survival compared to historically treated patients.19 A phase II trial of belagenpumatucel-L (an allogeneic NSCLC whole cell vaccine containing antisense TGFβ) tested 3 different doses of cells, with the medium and high dose cohorts demonstrating increased median survival compared to the low dose cohort and historical rates of median survival in NSCLC.3
Combining other treatment modalities with whole cell vaccines
In the setting of developing cancers that have already escaped the protective immune response, it is unlikely that vaccination alone will be effective. A combination of chemotherapy, radiation and surgery, along with novel small molecule inhibitors and monoclonal antibodies, are currently administered to patients with the goal of eradicating cancer and eliminating recurrence due to resistant or mutated cancer cells. Phase I and II trials have shown promising results with whole cell vaccines through the induction of DTH and antigen-specific antibody and T cell responses. However, in phase III trials, it is difficult to show benefit beyond what is seen with conventional chemotherapy when vaccines are given as a single agent.
Costimulatory molecules
Agonist molecules have been studied as single agents and in combination with vaccines to further stimulate the immune response. A number of monoclonal antibodies have been shown to engage co-stimulatory receptors on T cells, including OX40, 4IBB, and CD40, mimicking the signals exchanged between activated antigen-presenting cells and T cells. As one example, OX40, a tumor necrosis factor receptor (TNFR) family molecule, enhanced proliferation and effector function of CD4+ T cells and natural killer (NK) cells via engagement by OX40L on DCs, resulting in increased anti-tumor immunity.20–22 In addition, treatment with this agonist antibody together with a GM-CSF-secreting whole cell vaccine augmented the immunodominant HER-2/neu-directed CD8+ T cell response in mice expressing the HER-2/neu tumor antigen.23 Whereas with vaccination alone, the anti-neu response became undetectable by day seven post-vaccine, an activated CD8+ T cell response persisted for weeks in 15–20% of mice treated with OX40 co-stimulation and vaccine.23 Furthermore, depletion of CD4+ T cells in these mice confirmed that co-stimulation acted directly on CD8+ T cells.23 Emerging data also suggest that combining two co-stimulatory agonist antibodies may be more effective than either alone. As an example, anti-OX40 and anti-4IBB agonist antibodies administered with DCs pulsed with apoptotic tumor cells also enhanced the immune response and tumor rejection in HER-2/neu transgenic mice.24 Several clinical trials are underway testing OX40 agonist antibodies in cancer patients.20
CD40 is another co-stimulatory molecule involved in bidirectional activation of APCs and T cells through signaling with its ligand, CD40L.25 Using transplantable melanomas in mice, CD40 agonist antibody worked in combination with CTLA-4 blockade to enhance the CD8+ T cell response to a tumor antigen delivered by an adenoviral vaccine vector.26 However, one clinical trial testing the human agonist CD40 antibody failed to show benefit.27 In addition, immunologic correlates demonstrated chronic B cell activation but depletion of T cells.27 Another clinical trial testing GM-CSF- and CD40L-transfected bystander cells plus irradiated autologous tumor cells in stage IV cancers demonstrated a correlation between stable disease in several melanoma patients and the induction of MART-1-specific T cells.28 More recently, CD40 agonist antibody was used in inoperable pancreatic cancer along with gemcitabine which induced a tumor regression in one patient while several other patients had stable disease.29 Further evaluation of this treatment regimen in a mouse model of pancreatic cancer demonstrated that tumor regression was the result of CD40-mediated macrophage activation rather than T cell activation.29 This was a surprising result that requires further follow up. It will also be interesting to evaluate agonist CD40 antibody activity with vaccination strategies in patients.
Monoclonal antibodies to tumor antigens and angiogenic molecules
The importance of the humoral component of the anti-tumor response has been demonstrated in both pre-clinical and clinical studies. In one study using a DNA vaccine with constructs for HER-2/neu and the extracellular domain of Flt-3 ligand, the induction of neu-specific antibodies was required along with the T cell-mediated response for complete protection to tumor challenge.30 As another example, non-transgenic mice develop an antibody and T cell response following GM-CSF-secreting neu-expressing whole cell vaccination and again, both components are required for elimination of neu-expressing tumors.31 Tolerant neu-N mice fail to mount a protective humoral response after vaccination, but administration of an anti-neu monoclonal antibody with a neu-targeted vaccine increases survival and tumor clearance compared to vaccination alone in these mice.32 Antibody therapy collaborates with vaccination to enhance the tumor antigen-specific T cell response through Fcγ receptor-mediated uptake of the antibody-coated vaccine cells in vaccine-draining lymph nodes and subsequent DC cross-priming of neu-specific CD8 T cells.32,33 Importantly, the timing and location of vaccine and antibody administration is such that vaccine cells continue to secrete GM-CSF for two days prior to antibody binding, allowing for optimal maturation of DCs that take up antibody-coated cells.33
The humoral response is also an important component of the antitumor response in patients. Both endogenous and administered antibodies have been shown to act via several mechanisms against tumors. As an example, endogenous antibodies to MICA (MHC class I chain-related molecule A, a ligand for the NKG2D receptors) were induced following treatment of metastatic melanoma patients with GM-CSF-secreting whole cell vaccination and the anti-CTLA-4 monoclonal antibody, ipilimumab.34 Mechanistically, anti-MICA antibodies inhibited soluble MICA from binding and downregulating NKG2D, a cytolysis-activating receptor on the surface of CD8+ T cells and NK cells.34 Anti-MICA antibodies also bound the antigen on the surface of tumor cells, enhancing cross-presentation to CD8+ T cells.34
A number of clinically approved monoclonal antibodies that target different tumor associated antigens including CD20 expressed by lymphomas, HER-2 expressed by breast cancers, and epidermal growth factor receptor (EGFR) expressed by head and neck and colorectal carcinomas, likely work in part through multiple immunologic mechanisms.35 These antibodies have been shown to have limited efficacy when given as monotherapy. 35 However, when given in combination with chemotherapy, monoclonal antibody therapy can reduce the risk of recurrence by up to 30%.35 While these antibodies can bind oncogenic proteins to block downstream signaling through these molecules or to clear necessary signaling molecules from the tumor cell surface, accumulating evidence suggests that therapeutically administered antibodies mediate antitumor activity through immune mechanisms as well.35 Antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) are two immune mechanisms that work through innate effector cells such as neutrophils, γδ T cells and NK cells that bind the antibody/tumor complex and lyse the tumor.35,36 Furthermore, certain Fcγ receptor FcγR) polymorphisms have been linked to outcomes in cancer patients treated with monoclonal antibodies and predict a protective or adverse outcome depending on the polymorphism.35 However, the link between FcγR polymorphisms and prognosis may not be entirely due to innate immune mechanisms such as ADCC and CDC. The enhanced humoral and T cell response to tumor antigens often observed after antibody therapy may represent an alternate mechanism by which these therapies add to traditional cancer treatment regimens. The kinetics of the response to monoclonal antibodies, which begin around one week post-treatment, are more consistent with DC cross-priming and development of T cell-mediated immune responses.33 Monoclonal antibodies bound to the surface of tumor cells can enhance Fc-mediated antigen uptake, processing and presentation by DCs, stimulating the CD8+ T cell response.33 Cytokines secreted by DCs and NK cells activated through their Fc receptors can contribute to the milieu that decreases Treg mediated suppression and activates T helper 1 type and cytotoxic T lymphocytes.36 Thus, the combination of a whole cell vaccine vector with monoclonal antibodies directed against a tumor antigen serves to stimulate the innate response to the vaccine cells, augmenting antigen presentation and the adaptive response to tumor antigens.
Antibodies targeting angiogenic molecules can inhibit immune-suppressive, pro-oncogenic pathways required for tumor cell survival, regardless of whether these antibodies are induced post-vaccination or administered therapeutically. GM-CSF-secreting whole cell vaccination and anti-CTLA-4 treatment was found to elicit tumor necrosis, infiltration of lymphocytes and granulocytes, destruction of tumor vessels, and induction of multiple antibodies including anti-vascular endothelial growth factor (VEGF).37 Several melanoma patients treated with the GM-CSF-secreting vaccine also had elevated angiopoietin-1 and angiopoietin-2 antibodies post-vaccination.37 These antibodies were capable of inhibiting Tie-2-dependent monocyte chemotaxis and angiopoietin-1/2 signaling in vitro, suggesting a potential role for the endogenous humoral response in controlling angiogenesis and oncogenic tumor-associated macrophage signaling.37
The survival benefit associated with treatment of patients with angiogenesis inhibitors such as the monoclonal antibody to VEGF, bevacizumab, is additive to regimens of chemotherapy.38 In addition, the overall benefit is modestly significant.38 Pre-clinical studies demonstrate that anti-angiogenic monoclonal antibodies may be useful in combination with immunotherapy. An anti-VEGF-R2 antibody (DC101) augments the tumor antigen-specific CD8+ T cell response in wild type mice, resulting in tumor cell death and inhibition of angiogenesis.39 DC101 antibody inhibited tumor growth when given with a neu-targeted vaccine in non-tolerant mice with neu-expressing tumors.39 However, in tolerant neu-N mice, immunomodulating doses of cyclophosphamide to decrease Treg activity were required in addition to antibody and vaccine for the induction of anti-neu CD8+ T cells and tumor regression.39 Despite concerns that the inhibition of neo-vascularization would impede trafficking of T cells to the site of the tumor, this was not observed and VEGF-R inhibition may have facilitated tumor infiltration by lymphocytes.39 VEGF signaling pathways promote immature myeloid cells in the tumor microenvironment; thus, anti-angiogenic monoclonal antibodies can decrease immunosuppressive signaling and improve the quality and function of APCs.40 These studies emphasize that angiogenic molecules are an important target of immunotherapy both when targeted directly with the use of monoclonal antibodies, and also following induction of vaccine induced anti-angiogenic responses that indirectly result in destruction of tumor vasculature by vaccine-induced effector cells.
Radiation and chemotherapy to stimulate innate and adaptive immunity
Accumulating evidence from pre-clinical and clinical studies supports the concept that radiation and chemotherapy can enhance the anticancer immunity induced by cancer vaccines. Although radiation and chemotherapy are commonly implemented and well established cancer treatments with which immune based therapies have been co-administered, the collaborative effects of these different treatment modalities can be missed, often with loss of evidence of immune activity, if the optimal timing and dose of these approaches are not considered when integrating them. Three general approaches for effectively integrating these therapeutic modalities have emerged from prior studies. First, high doses of some chemotherapeutic drugs and radiation eliminate rapidly proliferating cells such as those of the immune system. While this is seen as an adverse effect on an existing immune response, in some circumstances, wiping out the existing response and “rebooting” the immune system can reduce immunosuppressive cell populations, promoting the expansion of effector T cells, and increasing the generation and function of effector lymphocyte activating DCs post-lymphodepletion.41 As an example, mice receiving sublethal total body irradiation followed by reconstitution with syngeneic splenocytes and GM-CSF-secreting cellular vaccination experienced an enhanced anti-tumor CD8+ T cell response and greater protection against a tumor challenge when compared to mice that were not lymphodepleted and reconstituted.42 Chemotherapy and radiation-associated lymphodepleting regimens have been the most successful in terms of response rates in patients receiving adoptive T cell therapy in clinical trials, suggesting that more intense lymphodepletion might also enhance responses to vaccination if given along with T cell transfer.43
Second, therapeutic doses of chemotherapy and radiation therapy can enhance the immune response to tumor antigens by inducing immunogenic death of tumor cells and release of tumor antigens. As an example, dying cells treated with oxaliplatin or radiation release the TLR4 ligand HMGB1 which activates DCs.44 Furthermore, several chemotherapy agents including cisplatin, doxorubicin, mitomycin C, fluorouracil, and camptothecin are capable of upregulating Fas expression on the surface of tumor cells and sensitizing cells to Fas-FasL mediated killing by lymphocytes.45 In addition, the use of irradiated tumor cells as a source of tumor antigens in cancer vaccines has been shown to enhance the immunogenicity of whole cell vaccines presumably by inducing apoptotic bodies of the tumor cells that are more easily taken up by APCs for antigen processing and presentation.46 Thus, this immunogenic cell death generated by radiation or chemotherapy provides additional rationale for pairing chemotherapy and immunotherapy.
Third, low doses of chemotherapy and radiation have been shown to alter sub-populations of immune suppressive cells allowing for the induction of a more effective immune response while circumventing the majority of immune cells. This is in contrast to high dose chemotherapy and radiation that eliminate most immune cells, providing a space for the expansion of endogenous or transferred effector cells to expand preferentially. It is possible that lower dose chemotherapy and radiation may fulfill the same purpose as high dose but with fewer side effects by specifically depleting immunosuppressive populations that impair the protective antitumor response. Tregs are increased in the peripheral blood, lymph nodes and tumors of cancer patients and have been associated with poor prognosis and suppression of tumor antigen immune responses.47 Low-dose total body irradiation has been shown to have immunomodulating effects, enhancing antigen-specific responses to vaccination through a decrease in Treg numbers and an increase in effector memory T cells.48 Cyclophosphamide (Cy) has been shown to deplete Tregs and when administered one day prior to vaccination, allows for the activation and tumor trafficking of a previously tolerized high avidity CD8+ T cell population in neu-N mice.49 The benefits of Cy may lie in its ability to promote protective Th1 and Th17 responses in addition to depleting Tregs which suppress the tumor antigen-specific CD8+ effector cells.50,51 Clinically, a phase I trial of GM-CSF-secreting vaccine with or without Cy has found that the combination of both agents is safe and the addition of Cy improves median survival from 2.3 months to 4.3 months in advanced pancreatic cancer patients.52 This combination of Cy and GM-CSF-secreting irradiated pancreatic tumor cells also resulted in the induction of higher avidity mesothelin-specific CD8+ T cell responses which correlated with improved survival.52 Other studies have also demonstrated Cy-mediated enhanced immune responses when given with a vaccine to mice and patients.53
Myeloid derived suppressor cells (MDSC) constitute another population of cells capable of suppressing antigen-specific responses induced by cancer vaccines.54 Current therapies such as the previously discussed TGFβ antisense GM-CSF-secreting whole cell vaccine approach could be one method of indirectly targeting signaling molecules from suppressive cell populations. However, several chemotherapeutics have been reported to successfully decrease MDSC numbers in cancer patients. The FOLFIRI regimen, irinotecan plus infusional 5-fluorouracil and leucovorin, followed by DC vaccination is effective in promoting Th1 CD4+ cells and CD8+ cytotoxic T cells while decreasing suppressive Treg and MDSC populations which rebound with chemotherapy alone in a mouse model of colorectal cancer.55 Docetaxel, which has been shown to have beneficial effects on the activation of APCs, selectively reduced the suppressive myeloid population while skewing myeloid cells towards a M1-like antitumor phenotype.56 5-fluorouracil and gemcitabine also selectively deplete MDSC, allowing a CD8+ effector population to emerge and thus, may be useful in combination with vaccination.54
Studies have also shown that additional immune stimulatory effects result when chemotherapeutic drugs are used at subclinical doses. Paclitaxel, given at low dose (20 mg/kg in mice), stimulates DCs through TLR signaling, resulting in accelerated DC antigen processing and presentation, upregulation of costimulatory markers, and IL-12 production.57 The combination of paclitaxel with a GM-CSF-secreting irradiated tumor cell vaccine and another immunomodulatory drug, Cy, resulted in the highest level of antigen-specific CD8+ T cell response in neu-N mice.57 Docetaxel, a paclitaxel analog, has been shown to enhance the effects of a GM-CSF-secreting melanoma vaccine in mice; combining the two agents at a dose where neither had impact on tumor growth or survival alone resulted in significant prolongation of survival.58
A number of clinical trials have incorporated immune modulating doses of these chemotherapeutic agents with vaccine approaches. However, extrapolating mouse doses to human doses presents a challenge. One reported clinical trial used an innovative combinatorial study design to identify the optimal doses of the most active combination of two drugs, Cy and doxorubicin (Dox) when combined with the GM-CSF-secreting whole cell vaccine in patients with metastatic breast cancer.59 The trial was based on pre-clinical studies that demonstrated that Cy given one day before and Dox given 7 days after vaccination was the optimal sequence for inducing the most effective anti-tumor immune response when combined with a HER-2/neu-targeted GM-CSF-secreting tumor vaccine in neu-N mice.50 In the clinical trial, doses of Cy greater than 200 mg/m2 negatively affected the DTH response to HER-2/neu peptides when compared to vaccine alone whereas vaccine given with 200 mg/m2 Cy augmented both DTH and antibody responses to HER-2/neu.59 The optimal dose of Dox was determined to be 35 mg/m2 for development of HER-2/neu-specific antibodies, whereas all doses of Dox allowed for DTH responses to HER-2/neu peptides.59 Interestingly, patients receiving the GM-CSF-secreting whole cell vaccine alone were observed to have progressively diminishing serum GM-CSF levels with each administration.59 However, dual chemotherapy and vaccine treatment sustained peak GM-CSF levels with each treatment, perhaps by inhibiting the anti-allogeneic response while maintaining the antitumor immune response.59 These studies represent alternative ways to administer chemotherapy at doses that result in fewer systemic side effects but enhanced anti-tumor immune responses.
Immune checkpoint inhibitors
Studies in patients have long demonstrated the existence of cancer antigen-targeted CD4+ and CD8+ T cells. Despite their existence, these cells are often dysfunctional with low cytokine production and increased expression of molecules associated with T cell exhaustion.60,61In vitro experiments have demonstrated that blockade of these “immune checkpoint” molecules can remove barriers to T cell activation and reverse exhaustion of tumor-antigen specific T cells.61 CTLA-4, an inhibitory molecule controlling the expansion and activation of CD4+ and CD8+ T cells, has become an important therapeutic target with the recent approval of a monoclonal antibody for use in metastatic melanoma.60 Pre-clinical studies demonstrate that CTLA-4 blockade may be useful in enhancing the immune response generated by cancer vaccines. Blockade of CTLA-4 paired with a GM-CSF-secreting whole cell vaccine in a B16 melanoma mouse model resulted in tumor clearance in the majority of mice whereas either treatment given as monotherapy had little effect on established tumors.62Anti-CTLA-4 antibody plus GM-CSF-secreting whole cell vaccination enhanced tumor rejection in a effector T cell intrinsic manner in mice and did not seem to work through Treg-mediated effects.63
Clinically, combination therapy with a cancer vaccine and blockade of CTLA-4 appears superior to vaccination alone as demonstrated in several trials. Ipilimumab with or without a gp100 peptide vaccine improved survival in metastatic melanoma (10.0 months) compared to a gp100 vaccine alone (6.4 months), with some treatment related grade 3 and 4 autoimmune events.64 Based on one study administering anti-CTLA-4 to previously vaccinated melanoma patients, the combination of GM-CSF-secreting whole cell-based vaccines with immune checkpoint blockade may be superior to the use of other vaccine vectors. In this study, patients who were treated with anti-CTLA-4 after previously receiving GM-CSF-secreting autologous tumor cells, demonstrated tumor necrosis and lymphocyte infiltration in their tumors.65 In contrast, similar results have not been observed in patients receiving vaccines that target a limited set of melanoma antigens.65 Additional clinical trials testing this monoclonal antibody in combination with other whole cell vaccines are ongoing.
Monoclonal antibodies to PD-1, an inhibitory co-receptor also found to be upregulated on exhausted T cells in cancer patients, are undergoing clinical testing in advanced cancers including melanoma, non-small cell lung cancer, and renal cell carcinoma.61 A phase I trial testing anti-PD-1 at different doses found it to be safe at all doses for patients with advanced cancers. In addition, treatment with this antibody resulted in one complete response and a number of partial responses.66 In contrast to anti-CTLA-4, which results in significant autoimmune effects, the adverse events in this study included only one grade 3 event and a few less serious autoimmune issues.66 To date, combination regimens with this antibody have only been tested experimentally in mouse models. However, PD-1 blockade given in combination with a GM-CSF-secreting tumor vaccine has been shown to enhance antitumor T cell responses and prolonged survival in mice when compared to either treatment alone.67
The possibilities for blockade of multiple immune checkpoints administered in combination with vaccines, chemotherapy, and radiation therapy to optimize anti-cancer immune responses are expanding as efforts to define the function of other molecules involved in the T cell response to tumor antigens continue. Antibody blockade of TIM3 signaling, another checkpoint molecule upregulated on tumor-infiltrating lymphocytes, demonstrated moderate results in both a tumor treatment model and in a model of suppression of carcinogen-induced tumorigenesis.68 This effect was even more significant in combination with antibodies to CTLA-4 and PD-1.68 LAG-3 is another inhibitory co-receptor found on CD4+ and CD8+ T cells, among other immune cell types.69 Deletion or blockade of LAG-3 has proven to be effective in enhancing CD8+ T cell function, increasing cytokine secretion, proliferation, and tumor infiltration in a CD8+ intrinsic manner, in response to a model tumor antigen in mice.69 Importantly, these pre-clinical findings are being rapidly translated into the clinical setting.
Conclusion and Future Directions
The important concepts learned from pre-clinical cancer vaccine studies have not yet translated into clinical successes in patients with cancer. However, a number of cancer vaccines have demonstrated immune activity in association with improved clinical outcomes in clinical trials. In addition, the first cancer vaccine was approved for the treatment of advanced prostate cancer. Like other cancer modalities, it is unlikely that vaccines as single agents will effectively treat existing cancers. Whole cell vaccine approaches have the advantage of targeting multiple tumor antigens at once, which should avoid antigen escape mechanisms and allow for effective targeting of the majority of tumor cells within a growing tumor. However, it has become clear that multiple inhibitory signals on T cells and the APCs responsible for activating the T cell response prevent effective immune activation and recognition of growing tumors in patients. This alone is enough to explain the lack of clinical activity of current vaccine approaches. Fortunately, these inhibitory signals are being rapidly defined and agents that modulate these signals are already demonstrating clinical responses. The future of cancer vaccines is directly linked to the success of these agents. These agents act by allowing effective induction of T cells. Most cancer patients do not have naturally existing endogenous tumor antigen-specific T cells. Thus, these agents will require cancer vaccines to generate antigen-targeted T cells. Together, these antagonist antibodies and cancer vaccines have the potential to induce optimally activated T cells in patients that will result in significant clinical outcomes.
Acknowledgments
Supported by NIH Grant No. R01CA122081, an NCI SPORE grant in breast cancer P50CA088843, an NCI SPORE grant in gastrointestional cancers P50CA62924, and the Lustgarten Foundation. Dr. Jaffee is the Dana and Albert “Cubby” Broccoli Professor of Oncology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: Through a licensing agreement to BioSante, Dr. Jaffee and the Johns Hopkins University have the potential to receive milestone payments and royalties for the GM-CSF whole cell vaccine (GVAX) in the future.
References
- 1.Simons J, Jaffee E, Weber C, Levitsky H, Nelson W, Carducci M, et al. Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte-macrophage colony-stimulating factor gene transfer. Cancer Res. 1997;57:1537–46. [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas MC, Greten TF, Pardoll DM, Jaffee EM. Enhanced tumor protection by granulocyte-macrophage colony-stimulating factor expression at the site of an allogeneic vaccine. Hum Gene Ther. 1998;9:835–43. doi: 10.1089/hum.1998.9.6-835. [DOI] [PubMed] [Google Scholar]
- 3.Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham C, Cutler J, et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol. 2006;24:4721–30. doi: 10.1200/JCO.2005.05.5335. [DOI] [PubMed] [Google Scholar]
- 4.Thomas A, Santarsiero L, Lutz E, Armstrong T, Chen Y, Huang L, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soiffer R, Lynch T, Mihm M, Jung K, Rhuda C, Schmollinger JC, et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 1998;95:13141–6. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dranoff G. GM-CSF-based cancer vaccines. Immunol Rev. 2002;188:147–54. doi: 10.1034/j.1600-065x.2002.18813.x. [DOI] [PubMed] [Google Scholar]
- 8.Jaffee E, Hruban R, Biedrzycki B, Laheru D, Schepers K, Sauter P, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 200;19:145–56. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 9.Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, et al. A Lethally Irradiated Allogeneic Granulocyte-Macrophage Colony Stimulating Factor-Secreting Tumor Vaccine for Pancreatic Adenocarcinoma: A Phase II Trial of Safety, Efficacy, and Immune Activation. Ann Surg. 2011;253:328–35. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le DT, Pardoll DM, Jaffee EM. Cellular vaccine approaches. Cancer J. 2010;16:304–10. doi: 10.1097/PPO.0b013e3181eb33d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirota H, Klinman DM. CpG-conjugated apoptotic tumor cells elicit potent tumor-specific immunity. Cancer Immunol Immunother. 2011;60:659–69. doi: 10.1007/s00262-011-0973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandler AD, Chihara H, Kobayashi G, Zhu X, Miller MA, Scott DL, et al. CpG oligonucleotides enhance the tumor antigen-specific immune response of a granulocyte macrophage colony-stimulating factor-based vaccine strategy in neuroblastoma. Cancer Res. 2003;63:394–9. [PubMed] [Google Scholar]
- 13.Hiraoka K, Yamamoto S, Otsuru S, Nakai S, Tamai K, Morishita R, et al. Enhanced tumor-specific long-term immunity of hemagglutinating virus of Japan-mediated dendritic cell-tumor fused cell vaccination by coadministration with CpG oligodeoxynucleotides. J Immunol. 2004;173:4297–307. doi: 10.4049/jimmunol.173.7.4297. [DOI] [PubMed] [Google Scholar]
- 14.Murad YM, Clay TM. CpG oligodeoxynucleotides as TLR9 agonists: therapeutic applications in cancer. BioDrugs. 2009;23:361–75. doi: 10.2165/11316930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Larmonier N, Marron M, Zeng Y, Cantrell J, Romanoski A, Sepassi M, et al. Tumor-derived CD4(+)CD25(+) regulatory T cell suppression of dendritic cell function involves TGF-beta and IL-10. Cancer Immunol Immunother. 2007;56:48–59. doi: 10.1007/s00262-006-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas DA, Massagué J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olivares J, Kumar P, Yu Y, Maples PB, Senzer N, Bedell C, et al. Phase I trial of TGF-{beta}2 antisense GM-CSF gene-modified autologous tumor cell (TAG) vaccine. Clin Cancer Res. 2011;17:183–92. doi: 10.1158/1078-0432.CCR-10-2195. [DOI] [PubMed] [Google Scholar]
- 19.Fakhrai H, Mantil JC, Liu L, Nicholson GL, Murphy-Satter CS, Ruppert J, et al. Phase I clinical trial of a TGF-beta antisense-modified tumor cell vaccine in patients with advanced glioma. Cancer Gene Ther. 2006;13:1052–60. doi: 10.1038/sj.cgt.7700975. [DOI] [PubMed] [Google Scholar]
- 20.Jensen SM, Maston LD, Gough MJ, Ruby CE, Redmond WL, Crittenden M, et al. Signaling through OX40 enhances antitumor immunity. Semin Oncol. 2010 Oct;37(5):524–32. doi: 10.1053/j.seminoncol.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, et al. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–16. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaini J, Andarini S, Tahara M, Saijo Y, Ishii N, Kawakami K, et al. OX40 ligand expressed by DCs costimulates NKT and CD4+ Th cell antitumor immunity in mice. J Clin Invest. 2007;117:3330–8. doi: 10.1172/JCI32693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murata S, Ladle BH, Kim PS, Lutz ER, Wolpoe ME, Ivie SE, et al. OX40 costimulation synergizes with GM-CSF whole-cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. J Immunol. 2006;176:974–83. doi: 10.4049/jimmunol.176.2.974. [DOI] [PubMed] [Google Scholar]
- 24.Cuadros C, Dominguez AL, Lollini PL, Croft M, Mittler RS, Borgström P, et al. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4-1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. Int J Cancer. 2005;116:934–43. doi: 10.1002/ijc.21098. [DOI] [PubMed] [Google Scholar]
- 25.Fonsatti E, Maio M, Altomonte M, Hersey P. Biology and clinical applications of CD40 in cancer treatment. Semin Oncol. 2010;37:517–23. doi: 10.1053/j.seminoncol.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Sorensen MR, Holst PJ, Steffensen MA, Christensen JP, Thomsen AR. Adenoviral vaccination combined with CD40 stimulation and CTLA-4 blockage can lead to complete tumor regression in a murine melanoma model. Vaccine. 2010;28:6757–64. doi: 10.1016/j.vaccine.2010.07.066. [DOI] [PubMed] [Google Scholar]
- 27.Rüter J, Antonia SJ, Burris HA, Huhn RD, Vonderheide RH. Immune modulation with weekly dosing of an agonist CD40 antibody in a phase I study of patients with advanced solid tumors. Cancer Biol Ther. 2010;10:983–93. doi: 10.4161/cbt.10.10.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dessureault S, Noyes D, Lee D, Dunn M, Janssen W, Cantor A, et al. A phase-I trial using a universal GM-CSF-producing and CD40L-expressing bystander cell line (GM.CD40L) in the formulation of autologous tumor cell-based vaccines for cancer patients with stage IV disease. Ann Surg Oncol. 2007;14:869–84. doi: 10.1245/s10434-006-9196-4. [DOI] [PubMed] [Google Scholar]
- 29.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orlandi F, Venanzi FM, Concetti A, Yamauchi H, Tiwari S, Norton L, et al. Antibody and CD8+ T cell responses against HER2/neu required for tumor eradication after DNA immunization with a Flt-3 ligand fusion vaccine. Clin Cancer Res. 2007;13:6195–203. doi: 10.1158/1078-0432.CCR-07-0258. [DOI] [PubMed] [Google Scholar]
- 31.Reilly RT, Machiels JP, Emens LA, Ercolini AM, Okoye FI, Lei RY, et al. The collaboration of both humoral and cellular HER-2/neu-targeted immune responses is required for the complete eradication of HER-2/neu-expressing tumors. Cancer Res. 2001;61:880–3. [PubMed] [Google Scholar]
- 32.Wolpoe ME, Lutz ER, Ercolini AM, Murata S, Ivie SE, Garrett ES, et al. HER-2/neu-specific monoclonal antibodies collaborate with HER-2/neu-targeted granulocyte macrophage colony-stimulating factor secreting whole cell vaccination to augment CD8+ T cell effector function and tumor-free survival in Her-2/neu-transgenic mice. J Immunol. 2003;171:2161–9. doi: 10.4049/jimmunol.171.4.2161. [DOI] [PubMed] [Google Scholar]
- 33.Kim P, Armstrong T, Song H, Wolpoe M, Weiss V, Manning E, et al. Antibody association with HER-2/neu-targeted vaccine enhances CD8 T cell responses in mice through Fc-mediated activation of DCs. J Clin Invest. 2008;118:1700–11. doi: 10.1172/JCI34333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jinushi M, Hodi FS, Dranoff G. Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc Natl Acad Sci U S A. 2006;103:9190–5. doi: 10.1073/pnas.0603503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28:4390–9. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roda JM, Parihar R, Magro C, Nuovo GJ, Tridandapani S, Carson WE., 3rd Natural killer cells produce T cell-recruiting chemokines in response to antibody-coated tumor cells. Cancer Res. 2006;66:517–26. doi: 10.1158/0008-5472.CAN-05-2429. [DOI] [PubMed] [Google Scholar]
- 37.Schoenfeld J, Jinushi M, Nakazaki Y, Wiener D, Park J, Soiffer R, et al. Active immunotherapy induces antibody responses that target tumor angiogenesis. Cancer Res. 2010;70:10150–60. doi: 10.1158/0008-5472.CAN-10-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 39.Manning EA, Ullman JG, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res. 2007;13:3951–9. doi: 10.1158/1078-0432.CCR-07-0374. [DOI] [PubMed] [Google Scholar]
- 40.Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res. 1999;5:2963–70. [PubMed] [Google Scholar]
- 41.Salem ML, Cole DJ. Dendritic cell recovery post-lymphodepletion: a potential mechanism for anti-cancer adoptive T cell therapy and vaccination. Cancer Immunol Immunother. 2010;59:341–53. doi: 10.1007/s00262-009-0792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma J, Urba WJ, Si L, Wang Y, Fox BA, Hu HM. Anti-tumor T cell response and protective immunity in mice that received sublethal irradiation and immune reconstitution. Eur J Immunol. 2003;33:2123–32. doi: 10.1002/eji.200324034. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable Complete Responses in Heavily Pretreated Patients with Metastatic Melanoma Using T Cell Transfer Immunotherapy. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-11-0116. Epub 2011 Apr 15, 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 45.Micheau O, Solary E, Hammann A, Martin F, Dimanche-Boitrel MT. Sensitization of cancer cells treated with cytotoxic drugs to fas-mediated cytotoxicity. J Natl Cancer Inst. 1997;89:783–9. doi: 10.1093/jnci/89.11.783. [DOI] [PubMed] [Google Scholar]
- 46.Li J, King AV, Stickel SL, Burgin KE, Zhang X, Wagner TE, et al. Whole tumor cell vaccine with irradiated S180 cells as adjuvant. Vaccine. 2009;27:558–64. doi: 10.1016/j.vaccine.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 47.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with breast or pancreas adenocarcinomas. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 48.Liu R, Xiong S, Zhang L, Chu Y. Enhancement of antitumor immunity by low-dose total body irradiation is associated with selectively decreasing the proportion and number of T regulatory cells. Cell Mol Immunol. 2010;7:157–62. doi: 10.1038/cmi.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, et al. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–1602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- 51.Viaud S, Flament C, Zoubir M, Pautier P, LeCesne A, Ribrag V, et al. Cyclophosphamide induces differentiation of Th17 cells in cancer patients. Cancer Res. 2011;71:661–5. doi: 10.1158/0008-5472.CAN-10-1259. [DOI] [PubMed] [Google Scholar]
- 52.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–63. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chong G, Morse MA. Combining cancer vaccines with chemotherapy. Expert Opin Pharmacother. 2005;6:2813–20. doi: 10.1517/14656566.6.16.2813. [DOI] [PubMed] [Google Scholar]
- 54.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 55.Kim HS, Park HM, Park JS, Sohn HJ, Kim SG, Kim HJ, et al. Dendritic cell vaccine in addition to FOLFIRI regimen improve antitumor effects through the inhibition of immunosuppressive cells in murine colorectal cancer model. Vaccine. 2010;28:7787–96. doi: 10.1016/j.vaccine.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 56.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583–94. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pfannenstiel LW, Lam SS, Emens LA, Jaffee EM, Armstrong TD. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell Immunol. 2010;263:79–87. doi: 10.1016/j.cellimm.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prell RA, Gearin L, Simmons A, Vanroey M, Jooss K. The anti-tumor efficacy of a GM-CSF-secreting tumor cell vaccine is not inhibited by docetaxel administration. Cancer Immunol Immunother. 2006;55:1285–93. doi: 10.1007/s00262-005-0116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–8. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Engelhardt JJ, Sullivan TJ, Allison JP. CTLA-4 overexpression inhibits T cell responses through a CD28-B7-dependent mechanism. J Immunol. 2006;177:1052–61. doi: 10.4049/jimmunol.177.2.1052. [DOI] [PubMed] [Google Scholar]
- 61.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–80. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–45. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–7. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li B, VanRoey M, Wang C, Chen TH, Korman A, Jooss K. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor-secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin Cancer Res. 2009;15:1623–34. doi: 10.1158/1078-0432.CCR-08-1825. [DOI] [PubMed] [Google Scholar]
- 68.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-{gamma}-mediated anti-tumor immunity and suppresses established tumors. Cancer Res. doi: 10.1158/0008-5472.CAN-11-0096. Epub 2011 Mar 23, 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 69.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–92. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]