Abstract
Therapeutic cancer vaccines are a unique treatment modality in that they initiate a dynamic process of activating the host immune system, which can then be exploited by concurrent or subsequent therapies. The addition of immunotherapy to standard-of-care cancer therapies has shown evidence of efficacy in preclinical models and in the clinical setting. This review examines the preclinical and clinical interactions between vaccine-mediated tumor-specific immune responses and local radiation, systemic chemotherapy, or select small-molecule inhibitors, as well as the potential synergy between these modalities.
INTRODUCTION
While there have been remarkable advancements in cancer treatment over the past few years, with the advent of new therapies, the goals of reducing disease burden and improving quality of life are only sometime achieved. As some cancer vaccines demonstrate clinical activity, they will likely be used earlier in the disease process. This will require the development of strategies to employ cancer vaccines with standard-of-care therapies that modulate the immune response. There is growing evidence that a multimodality approach targeting different aspects of the immune system may yield the greatest clinical benefit.
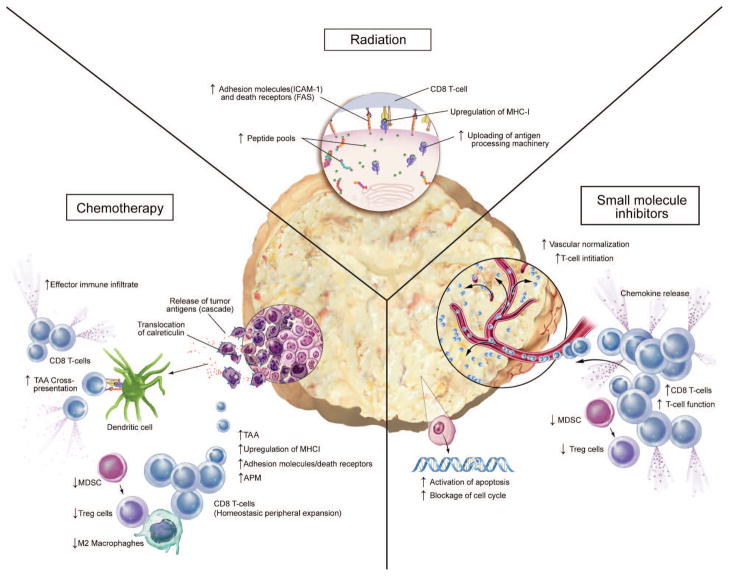
This review focuses on the use of therapeutic cancer vaccines with conventional therapies such as radiation, chemotherapy, and small molecule inhibitors (SMIs). Various immunomodulatory effects of conventional therapies can be exploited to enhance the antitumor activity induced by vaccines (Fig. 1). For radiation therapy, these include a) upregulation of tumor antigens, costimulatory molecules, Fas, and major histocompatibility complex (MHC) moieties, which makes tumors more susceptible to immune-mediated attack; b) upregulation of cytokines, chemokines, and adhesion molecules, which enhances T-cell trafficking to the tumor site and prolongs T-cell/tumor contact; and c) downregulation of regulatory T cells (Tregs), which facilitates generation of antigen-specific T cells. Chemotherapy’s immunomodulatory effects include a) induction of immunogenic tumor-cell death, leading to activation of dendritic cells (DCs) and facilitating cross-priming and tumor-specific T-cell generation; b) upregulation of tumor antigens, adhesion molecules, antigen-processing machinery (APM) and MHC, which increases T-cell recognition and triggers T-cell killing; and c) induction of leukopenia followed by differential homeostatic peripheral expansion (HPE) that favors tumor-specific T cells. Finally, select, targeted SMIs can a) increase the number and function of tumor antigen-specific T cells and decrease the number and function of myeloid-derived suppressor cells (MDSCs) and Tregs; b) block the tumor-cell cycle and induce apoptosis; and c) inhibit neoangiogenesis, modulate hypoxia, and normalize tumor vasculature.
Figure 1.
Multiple mechanisms of synergy between radiation therapy, chemotherapy, or small molecule inhibitors and immunotherapy.
Given the potential immunomodulatory effects of these established cancer therapies, combining them with cancer vaccines provides an opportunity to improve patient survival and quality of life.
COMBINING RADIATION THERAPY AND IMMUNOTHERAPY
Radiation is the standard treatment for many cancer types, traditionally employed to locally eradicate tumor cells or alter tumor and/or tumor stroma architecture with either curative or palliative intent. Although local control of the primary tumor is necessary and can usually prevent metastasis, radiation generally fails to control pre-existing systemic disease, which may be present as undetectable micrometastases. Although radiation has generally been considered immunosuppressive, several recent studies have shown that radiation actually has the potential to be immunomodulatory. Radiation-induced cell death is an immunologically active process wherein dying tumor cells release tumor-associated antigens (TAAs) that can potentially be exploited to stimulate robust tumor-specific immune responses (Fig. 1). Cells undergoing radiation-induced cell death also develop distinctive changes on their plasma membranes. These changes act as danger signals to promote phagocytosis by antigen-presenting cells (APCs) such as macrophages and DCs. Certain proteins, including heat-shock proteins, calreticulin, and high-mobility group box 1 (HMGB1), have been shown to be critical danger signals.1–3 Plasma membrane expression of heat-shock proteins, which occurs following radiation, helps mark damaged cells for elimination by the immune system and facilitates antigen cross-presentation, DC maturation, and natural killer (NK) cell activation.4–7 Calreticulin is a crucial determinant of whether dying tumor cells are phagocytosed by APCs.8, 9 The nuclear nonhistone protein HMGB1 binds to toll-like receptor 4 (TLR4), thereby providing a signal to DCs to initiate TLR4-dependent antigen processing. Friedman has previously described a “danger model” of immunity wherein ionizing radiation generates an inflammatory microenvironment filled with apoptotic and necrotic cells, cytokines, chemokines, inflammatory mediators, and acute-phase reactant proteins.10 This milieu of immune modulators can activate APCs and support their processing of newly exposed TAAs. Activated APCs then migrate to the location of radiation-induced cell death, undergo maturation, and present post-radiation cellular debris and antigens to T cells.
Radiation also modulates tumor-cell phenotype and consequently increases immune recognition. Local tumor radiation induces upregulation of MHC-I, Fas/CD95, and the costimulatory molecules B7.1, intercellular adhesion molecule 1 (ICAM-1), and lymphocyte function-associated antigen 3 (LFA-3).11–14 MHC-I is responsible for direct presentation of tumor-antigen peptides to cytotoxic T lymphocytes (CTLs), while increases in adhesion molecules improve cell-to-cell attachment and thus enhance T cells’ ability to kill target cells.14–16 Fas, a member of the tumor necrosis factor receptor family, is a death receptor that induces apoptosis upon binding to Fas-ligand. Fas-ligand displays a complex pattern of inducible and constitutive expression associated with a number of functions as a death factor and costimulatory molecule in lymphocyte activation. Activated CTLs express cell-surface Fas ligand, which binds to Fas molecules on the target cell surface, giving the target cell the signal to undergo apoptosis.14, 17, 18 Fas-mediated apoptosis has been shown to play an important role in CTL-mediated tumor cell destruction in addition to granzyme-dependent killing.
Garnett et al. demonstrated that radiation is able to alter the cell-surface expression of a variety of immunomodulatory molecules such as Fas, ICAM-1, MHC-I, and TAAs such as carcinoembryonic antigen (CEA) and mucin-1 (MUC-1).18 They examined 23 human carcinoma cell lines (12 colon, 7 lung, 4 prostate) for responses to nonlethal doses of radiation (10 or 20 Gy) and found that at least one of the above-named surface molecules increased in 21 of 23 (91%) cell lines studied. Furthermore, all irradiated cell lines demonstrated significantly enhanced killing compared to nonirradiated cell lines, suggesting that nonlethal doses of radiation render human tumor cells more amenable to immune recognition and attack. Microarray analysis revealed that many additional genes had been modulated by radiation. These upregulated gene products may make tumor cells even more susceptible to T cell-mediated immune attack or serve as additional targets for immunotherapy. Additionally, recent studies have indicated that radiation can affect the tumor microenvironment and tumor vasculature.19 These effects can facilitate homing of both APCs and effector T cells through changes in extracellular matrix proteins and adhesion molecules on endothelial cells and radiation-induced inflammatory signals.20–25
Interest in combining radiation and immune-based therapies for the treatment of cancer is growing in proportion to our understanding of the immunomodulatory effects of radiation and radiation’s effect on tissues. A great deal of preclinical research into combining radiation plus active therapeutic cancer vaccines has been translated into clinical studies of this combination as a multimodal therapy for cancer (Table 1 & 2).
Table 1.
Preclinical studies of combination therapy.
| Preclinical studies of combination therapy
| ||||
|---|---|---|---|---|
| Agent | Vaccine | Model | Results | Refs |
| Radiation | ||||
| EBRT | rV/F-CEA/TRICOM | MC38-CEA colon carcinoma model | Improved cure rate | 17 |
| 153-Samarium (Quadramet®) | Various human/murine tumor cell lines in vitro | Increased immune-mediated killing | 34 | |
| Yttrium-90 | rV/F-CEA/TRICOM | MC38-CEA colon carcinoma model | Increased survival | 31 |
| Chemotherapy | ||||
| Cisplatin, vinorelbine | Yeast-CEA | mLLC-CEA metastatic Lewis lung carcinoma model | Increased survival | 46 |
| Cyclophosphamide, doxorubicin, paclitaxel | GM-CSF/HER2/neu whole-tumor cell | NT mammary tumor | Delayed tumor growth | 45 |
| Cyclophosphamide | STn-KLH modified (Theratope®) | 410.4 mammary tumor model expressing MUC1 and STn | Delayed tumor growth | 105 |
| Docetaxel | rV/F-CEA/TRICOM | MC38-CEA colon carcinoma model | Reduced tumor burden | 49 |
| Small molecules | ||||
| GX15-070 | rV/F-CEA/TRICOM | mLLC-CEA metastatic Lewis lung carcinoma model | Improved tumor shrinkage | 93 |
| Sunitinib | IL-12 & 4-1 BB activation | MCA26 colon carcinoma model | Improved tumor shrinkage/survival | 106 |
| Sunitinib | rMVA/F-CEA/TRICOM | MC38-CEA colon carcinoma model | Improved tumor shrinkage/survival | 93 |
Table 2.
Clinical studies of combination therapy.
| Clinical trials of combination therapy | ||||
|---|---|---|---|---|
| Agent | Vaccine | Carcinoma | Phase | Refs |
| Radiation | ||||
| EBRT | rV/F-PSA/B7.1 | Localized prostate cancer | II | 26 |
| EBRT | rV/F-CEA/TRICOM | CEA+ solid tumors with liver metastases | II | 27 |
| 153-Samarium (Quadramet®) | PROSTVAC | Metastatic CRPC | II | 30 |
| Chemotherapy | ||||
| Cisplatin, vinorelbine | TG4010 | NSCLC | II | 66 |
| Cyclophosphamide | GVAX | Pancreatic cancer | II | 107 |
| Cyclophosphamide | Stimuvax | CRPC | I | 108 |
| Cyclophosphamide | Theratope | Metastatic breast cancer | III | 73 |
| Cyclophosphamide, doxorubicin | GM-CSF/HER2+/neu whole-tumor cell | Metastatic breast cancer | I | 74 |
| Docetaxel | PANVAC | Metastatic breast cancer | II | 109 |
| Docetaxel | Provenge | Metastatic CRPC | III | 110 |
| Docetaxel | rV/F-PSA/B7.1 | Metastatic CRPC | II | 111 |
| Docetaxel | PROSTVAC | Metastatic CRPC | II | 112 |
| Docetaxel | TroVax | Metastatic CRPC | II | 113 |
| FOLFIRI | ALVAC-CEA/B7.1 | Metastatic colorectal cancer | II | 114 |
| FOLFIRI | TroVax | Metastatic colorectal cancer | II | 115 |
| FOLFOX | TroVax | Metastatic colorectal cancer | II | 116 |
| Small molecule inhibitor | ||||
| Sunitinib | TroVax | Metastatic renal cell carcinoma | III | 117 |
EBRT = external beam radiation therapy; CRPC = castration-resistant prostate cancer; NSCLC = non-small cell lung cancer
External Beam Radiation
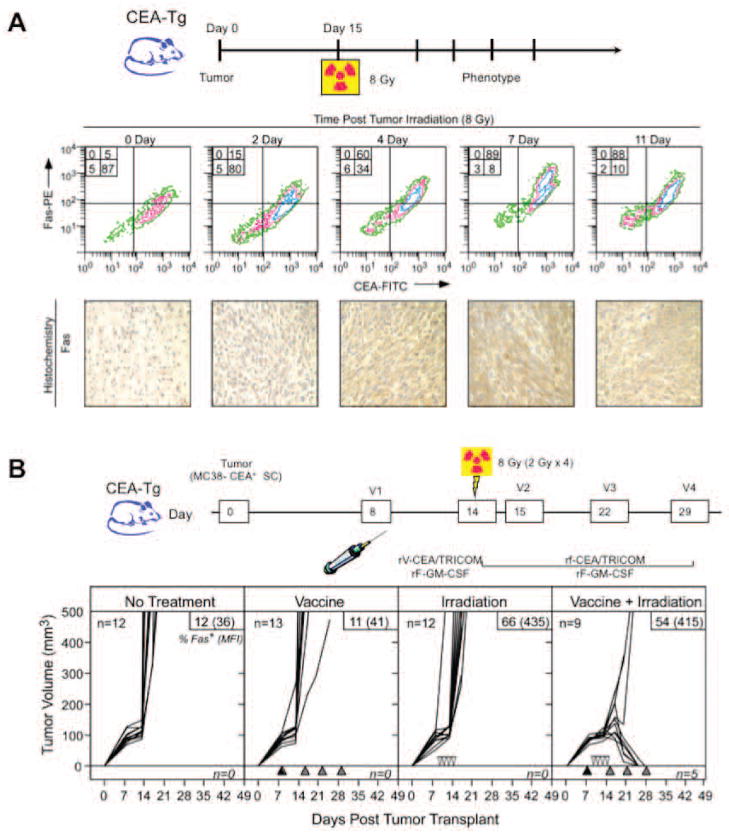
Chakraborty et al. examined the combination of low-dose external beam radiation therapy (EBRT) and active therapeutic vaccination for the treatment of subcutaneous (s.c.) tumors in a mouse model.17 After radiation with 8 Gy, CEA+ tumor cells demonstrated an upregulation of Fas that was maintained for > 11 days (Fig. 2A). A vaccine consisting of recombinant vaccinia (rV) and recombinant fowlpox (rF) vectors expressing CEA and a triad of costimulatory molecules (B7.1, ICAM-1, and LFA-3), designated rV/F-CEA/TRICOM, was used in this study. CEA+ murine colon carcinoma cells (MC38-CEA+) were implanted s.c. into mice transgenic for human CEA (CEA-Tg). After 8 days, mice were randomized to receive no treatment, radiation alone, vaccine alone, or a combination of radiation and vaccine (Fig. 2B). All untreated mice succumbed to progressive tumor growth by day 30. Neither radiation alone nor vaccine alone improved survival. However, the combination was curative in > 50% of mice while also imparting protection from subsequent tumor challenge. Interestingly, mice cured of their tumors demonstrated “antigen cascade,” a term that describes the development of CD4+ and CD8+ T-cell responses to tumor antigens not encoded in the vaccine (in this case, p53 and gp70).
Figure 2.
External beam radiation altered the phenotype of tumor cells and enhanced tumor-cell lysis when combined with vaccine. (A) Radiation-induced upregulation of Fas on tumor cells from s.c. MC38-CEA+ tumor-bearing mice was maintained for > 11 days post-irradiation. To confirm Fas expression, tumors were immunostained with anti-Fas mAb. (B) Used alone, neither vaccine nor radiation effectively reduced tumor volume, while the combination was curative in a majority of tumor-bearing mice. The combination also conferred protection from subsequent tumor challenge (data not shown). Adapted from.17
Results from these preclinical studies provided the rationale for clinical evaluation of the combination of EBRT and therapeutic cancer vaccines. A recent clinical trial assessed the use of a recombinant poxviral-based vaccine expressing prostate specific antigen (PSA) combined with standard definitive radiotherapy in patients with localized prostate cancer.26 Results from this clinical trial indicated that the combination was safe, well tolerated, and, more importantly, effective at generating a PSA-specific immune response. Approximately 76.5% of patients in the combination therapy arm showed a ≥ 3-fold increase in PSA-specific T cells vs. 0% in the radiation-alone arm (P < 0.0005).26 Another ongoing clinical trial is combining the rV/F-CEA/TRICOM vaccine with low-dose EBRT delivered directly to liver metastases in patients with CEA+ solid tumors.27
Bone-Seeking Radionuclide
In advanced stages, many primary human carcinomas such as thyroid, breast, kidney, prostate, and multiple myeloma typically involve painful bone metastases that require palliative therapy. Strontium-89 and Samarium-153 (153Sm-EDTMP; Quadramet®, Cytogen) are bone-seeking radiopharmaceuticals used to relieve the pain of bone metastases. Even though both agents are approved by the U.S. Food and Drug Administration (FDA), 153Sm-EDTMP is a superior choice for combination therapy with cancer vaccines due to its shorter half-life, which allows for repeated administration and faster recovery from treatment-induced pancytopenia. The dose of palliative radiation delivered to bone metastases by 153Sm-EDTMP is calculated to be between 18 and 80 Gy.28, 29 As noted, these doses are associated with phenotypic modulation of human tumor cells. One study demonstrated that of 10 human tumor-cell lines representing tumors that metastasize to bone (4 prostate, 2 breast, and 4 lung), 100% upregulated Fas and CEA, 70% upregulated MUC-1, 40% upregulated MHC-I, and 30% upregulated ICAM-1 when exposed to clinically relevant palliative levels of 153Sm-EDTMP for 4 days. Exposure of LNCaP cells (prostate cancer) to 153Sm- EDTMP also resulted in upregulation of tumor antigens such as PSA, prostate specific membrane antigen, prostatic acid phosphatase, CEA, and MUC-1. Upregulation of these tumor antigens rendered the cells more susceptible to lysis by CTLs specific for PSA, CEA, and MUC-1, suggesting that 153Sm- EDTMP works synergistically with immunotherapy.
The combination of 153Sm- EDTMP and vaccine is currently being studied in a randomized phase II study at the National Cancer Institute, designed to determine if 153Sm- EDTMP combined with vaccine can improve time to progression over 153Sm- EDTMP alone in patients with castration-resistant prostate cancer (CRPC) metastatic to bone.30 Patients will receive 153Sm- EDTMP alone or in combination with an rV/rF-PSA/TRICOM vaccine (PROSTVAC®, Bavarian Nordic) administered in a diversified prime/boost regimen.
Radiolabeled Monoclonal Antibodies
Therapeutic radionuclides can be delivered systemically to cancer cells via monoclonal antibodies (mAb). This method precisely and preferentially targets tumor cells and seeks out micrometastases unobservable by current imaging technology and thus insusceptible to EBRT. A recent report cited radiolabeled mAb’s ability to alter tumor-cell phenotype and enhance immunologic targeting of tumor cells, as well as a therapeutic synergy between radiolabeled mAb and cancer immunotherapy.31 This study employed an yttrium-90 (Y-90)-labeled anti-CEA mAb either alone or in combination with a CEA-targeted vaccine to treat mice implanted with CEA+ murine carcinoma cells. The combination of vaccine and a single dose of Y-90-labeled anti-CEA mAb resulted in a statistically significant increase in survival of tumor-bearing mice over either modality alone. Additionally, the combination group exhibited a significant increase in the percentage of viable tumor-infiltrating CEA-specific CD8+ T cells compared to the vaccine alone group. Surprisingly, the tumor-infiltrating T cells were unaffected by the residential radiation being emitted by the radiolabeled mAb. This finding was consistent with a preclinical study by Grayson et al. which found that murine memory T cells are more resistant to apoptosis than naïve T cells after whole-body irradiation. Mice cured of tumors also demonstrated an antigen cascade, as seen with EBRT.32
Brachytherapy
Brachytherapy involves implanting a radiation source into or near the site of a malignant tumor to target tumor cells with continuous high-dose radiation. A single study reported the ability of iodine-125 and a recombinant poxviral vaccine to modulate tumor cell phenotype and enhance antigen-specific killing of tumor cells.33 While more in-depth studies are needed to validate these results, they do suggest a clinical role for the combination of brachytherapy and cancer vaccines.
In summary, a growing body of evidence suggests that an appropriate dose of radiation can have immunomodulatory effects capable of activating the immune system and subsequently enhancing immune-mediated attack on tumor cells. Many preclinical studies have demonstrated that radiotherapy and cancer vaccines combined work synergistically to generate more robust antitumor effects.1, 13, 17, 18, 31, 34 Promising results from these preclinical studies have led to several clinical trials (Table 2). As the field of cancer therapy advances, monotherapies may fall into disfavor. In fact, many preclinical and clinical studies have combined more than 2 therapeutic modalities. One murine study combined vaccine, local radiation, and reduction of immune suppressor cells,35 while an in vitro study reported the combination of systemic multiagent chemotherapy with 5-fluorouracil (5-FU) and cisplatin with tumor irradiation for the treatment of head/neck squamous cell carcinoma (HNSCC).36
COMBINING CHEMOTHERAPY AND IMMUNOTHERAPY
The clinical efficacy of standard-of-care chemotherapy regimens relies mostly on direct cytotoxicity to cancer cells. Until recently, it was generally believed that when used in combination with a cancer vaccine, chemotherapy would invariably have a negative effect on vaccine-mediated immune responses and antitumor activity.37 However, mounting evidence suggests that certain chemotherapeutic agents have immunomodulatory properties that can be exploited to enhance vaccine-mediated antitumor effects (Fig. 1). This synergy can be mediated by multiple mechanisms, depending on the type of cytotoxic agent and the specific vaccine employed, as well as the dosing schedule of each modality.
Chemotherapeutic agents can modulate the phenotype of tumor cells by altering the expression of TAAs, MHC-I, ICAM-1, and APM, making them more susceptible to immune-mediated attack.36, 38, 39 These agents can also induce “immunogenic death” of tumor cells, leading to IL-12-mediated activation of DCs, followed by antigen presentation and cross-presentation to T cells, resulting in CTLs with greater and more efficient cytotoxic potential.40–42 In addition, cytotoxic agents can have direct effects on the host immune system, including a) modulation of immune regulatory elements such as Tregs and MDSCs,43–45 b) induction of leukopenia followed by differential HPE of regulatory and effector immune subsets,46–48 and c) synergy with vaccine to increase effector immune responses to multiple TAAs.49
Recent evidence also suggests that specific chemotherapeutic regimens can reduce the tumor growth rate in cancer patients when combined with certain cancer vaccines.50 Detailed reviews of the synergistic effects of cancer chemotherapy and immunotherapy regimens have previously been published.40, 51 Many preclinical studies have explored combinations of mature vaccine platforms with chemotherapy, some of which have been translated into the clinic (Table 1 & 2).
Platinum Alkylating Agents: Oxaliplatin, Cisplatin, Cisplatin/5-FU, and Cisplatin Plus Vinorelbine
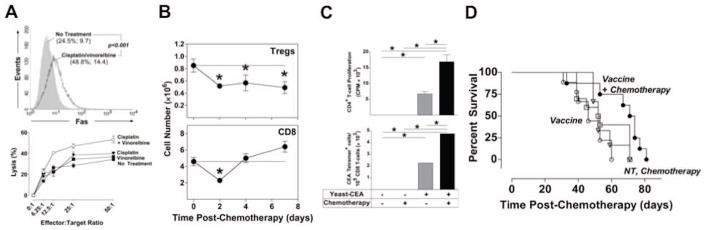
Platinum alkylating agents such as oxaliplatin and cisplatin are commonly used to treat a variety of malignancies, including non-small cell lung cancer (NSCLC) and HNSCC. The cytotoxicity of these agents is rendered through DNA crosslinking. However, accumulating evidence suggests that nontoxic concentrations of these agents can induce immune-relevant changes in tumor cells and several components of the immune system. These alterations could be exploited in a combined chemotherapy/vaccine regimen to achieve potent antitumor immunity. In one study, tumor cells exposed to oxaliplatin expressed higher levels of MHC-I proteins and secreted cytokines able to augment DC maturation, resulting in the generation of CTLs with increased cytotoxic potential.52 Cisplatin has also been shown to modulate tumor cell characteristics toward a more immunogenic phenotype. Exposure to nontoxic levels of cisplatin increased expression of functional Fas receptor on murine tumor cells, leading to augmented CTL-mediated lysis.53–55. Increased sensitivity to antigen-specific CTLs was also observed in human colon carcinoma cell lines treated with cisplatin, an effect associated with enhanced expression of ICAM-1 and Fas.56 Similar results have been reported with chemotherapy combinations including cisplatin. In one study, exposure of HNSCC cell lines to cisplatin plus 5-FU resulted in a synergistic increase of ICAM-1.57 Concurrent exposure of Lewis lung tumor cells to sublethal concentrations of cisplatin plus vinorelbine was shown to modulate expression of survival genes and increase expression of Fas and MHC-I molecules, resulting in increased sensitivity to CTL-mediated lysis (Fig. 3A).46 These immune-relevant changes translated into decreased resistance to Fas-dependent lysis by CTLs. In other preclinical studies, the addition of cisplatin augmented antitumor immunity elicited by viral, DNA, and subunit vaccine platforms.58–60
Figure 3.
When combined with vaccine, chemotherapy altered tumor-cell phenotype, enhanced vaccine-mediated T-cell responses, and improved survival of tumor-bearing mice. (A) Treatment with cisplatin/vinorelbine increased Fas cell-surface expression and sensitivity to CTL-mediated killing of Lewis lung carcinoma cells 48 h post-exposure in vitro. (B) Cisplatin/vinorelbine reduced the homeostatic peripheral expansion of Tregs for > 8 days in C57BL/6 mice, while CD8+ T cell expansion recovered by day 4 post-chemotherapy. (C) Combining cisplatin/vinorelbine with a yeast-CEA vaccine improved CD4+ T cell proliferation and CEA-specific T-cell responses. (D) The combination of cisplatin/vinorelbine with yeast-CEA increased survival in a murine model of established NSCLC. Adapted from.46
Chemotherapeutic regimens often result in moderate to severe lymphopenia, an adverse event considered detrimental to the generation of effective antitumor immunity.61–63 However, recent reports focusing on the mechanisms of HPE of immune subsets following iatrogenic leukopenia suggest that this period of T cell reconstitution presents a unique opportunity to expand vaccine-mediated antitumor immunity.46, 49, 64, 65 A recent study combining a yeast-CEA vaccine with chemotherapy demonstrated that cisplatin plus vinorelbine differentially modulates the HPE of effector and regulatory T cell subsets (Fig. 3B) and synergizes with vaccine, resulting in enhanced CEA-specific immune responses (Fig. 3C). Moreover, in a preclinical murine model of established NSCLC, the combination of this chemotherapeutic doublet with vaccine increased survival of tumor-bearing mice, an effect mediated by both CD4+ and CD8+ T cell subsets (Fig. 3D). Cisplatin plus vinorelbine has been clinically evaluated in combination with a recombinant modified vaccinia Ankara (MVA) vaccine expressing MUC-1 and IL-2 (TG4010).66
Taxanes: Paclitaxel and Docetaxel
Taxanes are among the most widely used cancer chemotherapeutic agents and have been employed to treat a variety of malignancies, such as breast, prostate, and lung carcinomas. In addition to the well-described cytotoxic effects of taxanes, elicited through microtubule disruption, nontoxic concentrations can induce an immunogenic phenotype in tumor cells that is more amenable to immune-mediated lysis. For example, exposure of human colon carcinoma cells to nontoxic concentrations of paclitaxel has been shown to upregulate the expression of APM proteins, including calmodulin, low molecular mass polypeptide 2 and 7, transporter 1, and tapasin, suggesting the potential for increased recognition by CTLs.38 Similarly, exposure of human ovarian carcinoma cells to sublethal concentrations of paclitaxel induced enhanced NK cell-mediated lysis mediated by increased ICAM-1 expression.67
In addition to their direct effects on tumor immunogenicity, taxanes can modulate various elements of the host immune system, including increasing macrophage activation and inducing intratumoral release of inflammatory cytokines, resulting in augmented tumor lysis.68 Sublethal concentrations of paclitaxel have been shown to enhance IL-12-dependent antigen presentation by DCs, an effect associated with increased expression of APM components and enhanced costimulation.41 Further, it has been reported that exposing DCs to paclitaxel-pretreated tumor cells generates CD8+ T cells with higher lytic potential.38
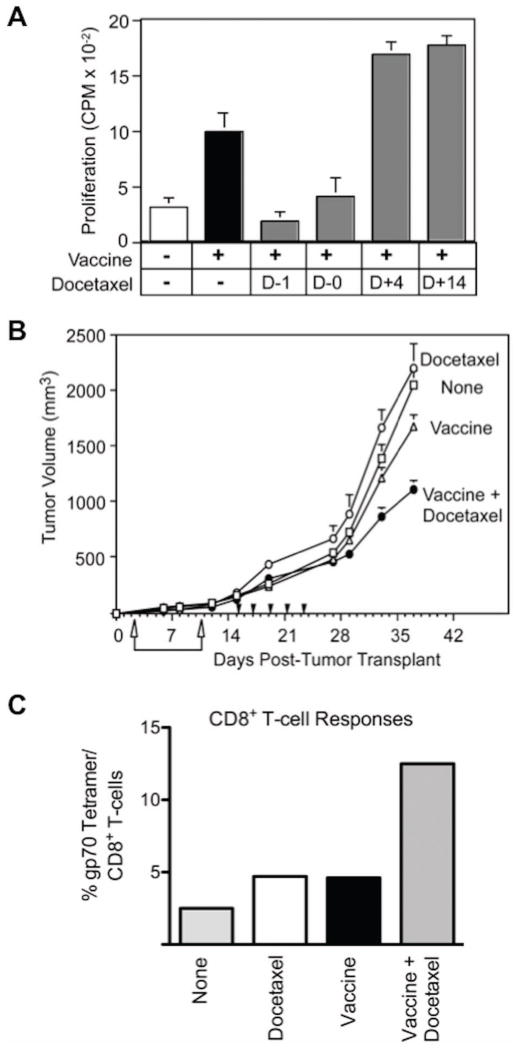
Accumulating evidence suggests that the immediate period of T cell reconstitution following chemotherapy-associated lymphopenia offers a unique opportunity to expand effective antitumor immunotherapy.46, 49, 64, 65 For example, docetaxel has been reported to modulate T cell, B cell, and NK cell subsets and to enhance CD8+ function while deleting Tregs.49 In preclinical studies in CEA-Tg mice transplanted with CEA+ tumor cells, antitumor responses were enhanced by a combination of docetaxel and an rV/F-CEA/TRICOM vaccine regimen, compared to responses induced by vaccine or docetaxel alone (Fig. 4A and B). Docetaxel administered after vaccination optimally enhanced immune responses to the recombinant viral vaccines, including antigen-specific T cell responses to the TAA delivered by the vaccine, as well as to cascade antigens derived by the tumor (Fig. 4C).49 Docetaxel has been or is currently being evaluated in combination with a) an rV vaccine expressing PSA and B7.1 (rV-PSA/B7.1; PROSTVAC®) b) a diversified prime/boost vaccine using vaccinia and fowlpox viruses expressing MUC-1, CEA, and TRICOM (PANVAC®), and c) the DC vaccine Provenge® (Dendreon Corp.), among other cancer vaccine platforms.
Figure 4.
Docetaxel treatment improved vaccine-mediated immune and antitumor responses. (A) CEA-specific murine CD4+ T-cell responses improved when docetaxel was administered after rV/F-CEA/TRICOM vaccine. (B) Docetaxel significantly increased the antitumor activity of vaccine in CEA-Tg mice bearing s.c. MC38-CEA+ tumors. (C) The CD8+ cascade response to gp70 improved following vaccine plus docetaxel treatment in MC38-CEA+ tumor-bearing mice. Adapted from.49
Alkylating Agents: Cyclophosphamide and Combinations
Alkylating agents like cyclophosphamide are included in chemotherapy regimens for a wide range of malignancies. In addition to their direct cytotoxic effect on tumor cells through DNA alkylation, these agents have immunomodulatory properties that can be exploited in a therapeutic regimen that includes a cancer vaccine. Cyclophosphamide has been shown to increase human leukocyte antigen expression and cytokine secretion in tumor cells, leading to increased maturation of DCs and augmented CTL killing 52. Direct effects of cyclophosphamide on DCs and other elements of the host immune system are well documented.42, 44 For instance, CD8+ T cells exposed to cyclophosphamide have increased lytic function.69
Accumulating evidence indicates that Tregs play a crucial role in T cell tolerance of tumors, constituting a major barrier to the generation of effective antitumor immunity in carcinoma patients.70, 71 Cyclophosphamide has been shown to abrogate the suppressive influence of Tregs, allowing the activation of potent, vaccine-mediated immunity.43–45 In an experimental melanoma model, systemic cyclophosphamide combined with a DC vaccine led to improved antitumor effects.72 Metronomic doses of cyclophosphamide have been evaluated clinically in combination with a sialyl-Tn-keyhole limpet hemocyanin vaccine (THERATOPE®, Biomira) for the treatment of metastatic breast cancer.73
In another example, proper timing of cyclophosphamide, doxorubicin, and paclitaxel treatment enhanced the antitumor immune response to a whole tumor cell vaccine in a preclinical model of breast carcinoma.45 In this case, the augmented antitumor effects of the combination of vaccine plus chemotherapy were due to enhanced vaccine efficacy rather than the direct cytotoxic effect of chemotherapy on cancer cells. The combination of cyclophosphamide and doxorubicin was recently evaluated in combination with a GM-CSF-secreting HER2/neu-expressing whole tumor cell vaccine in patients with metastatic breast cancer.74
Anthracyclines: Doxorubicin
Anthracyclines such as doxorubicin are DNA-intercalating agents used to treat a wide range of malignancies, including carcinomas of the breast, ovary, bladder, and lung. Cancer cells exposed to cytotoxic concentrations of doxorubicin undergo rapid translocation of ERp57 and calreticulin to the cell surface, triggering caspase-dependent immunogenic cell death, an effect not observed with other DNA-damaging agents.40, 75 Noncytotoxic concentrations of doxorubicin enhance IL-12-dependent antigen presentation by DCs, an effect associated with modulation of APM components, leading to increased effector T cell function.41
Antimetabolites: Gemcitabine, Methotrexate, and 5-FU
Antimetabolites are indicated for the treatment of several malignancies, including carcinomas of the pancreas and colon, and HNSCC. Various immunomodulatory properties of these agents have been revealed. For example, gemcitabine can upregulate MHC-I on tumor cells, resulting in enhanced sensitivity to CTL-mediated lysis.52 Similarly, treatment of human colon carcinoma cell lines with 5-FU can enhance their sensitivity to the cytotoxic effects of CD8+ T cells by inducing expression of ICAM-1 and Fas.56 Direct effects of antineoplastic agents such as methotrexate on T cell cytotoxicity have also been reported 69.
Antimetabolites can enhance DC function by direct and indirect mechanisms. In one report, direct exposure of DCs to methotrexate resulted in increased antigen presentation to T cells.41 Stimulation of DC function has been associated with upregulation of IL-12 and APM elements, and augmented DC function has been seen after exposure to tumor cells treated with gemcitabine.52 It has also been demonstrated that gemcitabine can selectively reduce MDSCs in tumor-bearing mice without affecting other immune cell populations.76
Chemotherapy Plus Radiation
Cisplatin plus 5-FU chemotherapy, combined with tumor irradiation, is the standard of care for HNSCC. The combination of chemotherapy and radiation has been shown to significantly decrease Bcl-2 and increase the sensitivity of human HNSCC target cells to perforin-mediated, MHC-restricted CTL killing, compared to target cells exposed to either modality alone.36 The studies described in this review highlight the rational basis for the clinical combination of immunotherapy and the current standard of care for HNSCC.
Other Potential Combinations
The immunomodulatory functions of many other chemotherapeutic agents are currently being investigated, for potential use in combination with therapeutic cancer vaccines. For example, lenalidomide, a chemotherapeutic agent FDA-approved for the treatment of multiple myeloma, has been shown to have several immunomodulatory effects, including augmentation of T cell function, stimulation of NK cell cytotoxicity, and suppression of Treg function and proliferation.77, 78 Like other agents of its class, lenalidomide is also antiangiogenic and antiapoptotic, and can reduce the metastatic capacity of tumors.79, 80
There is increasing interest in the potential therapeutic benefits of regimens combining cancer vaccines plus standard-of-care chemotherapy. However, there are several important considerations. First, employing vaccine and chemotherapy early in the disease process can have significantly different clinical outcomes than administering vaccine after multiple chemotherapeutic regimens in advanced-stage disease, when the immune system is most likely impaired. Second, not all chemotherapeutic agents are compatible with vaccine. And third, when used with chemotherapy, the timing of vaccine administration may be extremely important. Accumulating preclinical evidence of the immunomodulatory effects of chemotherapy presents new options for combining chemotherapy with vaccine to generate effective antitumor immunity in the clinical setting. Several mature platforms are already in use clinically (Table 1). Further clinical studies will be required to optimize the use of these and other combination regimens.
COMBINING SMALL MOLECULE INHIBITORS AND IMMUNOTHERAPY
In the last decade, use of targeted SMIs for the treatment of many tumor types has increased.81 The major difference between standard chemotherapeutic agents and SMIs is that the former suppress rapidly proliferating cells while the latter target specific protein-protein interactions, such as growth factors and their receptors.82 Compared to standard chemotherapy, targeted therapy with SMIs has the advantage of modulating specific cellular pathways that are crucial for tumor biology, along with the benefits of decreased toxicity and increased effectiveness.
There are also many potential benefits of combining SMIs with immunotherapy. Some SMIs can selectively increase immune activation (Fig. 1) by inhibiting immune suppressor cells such as Tregs and MDSCs and/or by activating immune effector cells such as CTLs and DCs. SMIs can make tumor cells more susceptible to immune-mediated killing by improving tumor-specific antigen presentation and/or FAS-mediated killing. Also, the synergistic effect of combining SMIs with vaccine can justify the administration of SMIs at a lower dose, further decreasing the potential for toxicity. Achieving an optimal outcome when combining immunotherapy and SMIs requires determining the appropriate timing of SMI treatment and vaccine administration. The best combination schedule should result in robust immune stimulation against TAAs, with little or no toxicity against immune effector cells.
Bcl-2 Inhibitors
One class of SMIs inhibits Bcl-2 molecules. SMIs that alter the balance between pro- and antiapoptotic Bcl-2 family members have shown potential benefit in preclinical cancer models.83 The Bcl-2 inhibitors ABT-737 and GX15-070, currently being tested as cancer therapeutics, act by mimicking the proapoptotic BH3 domain in order to induce apoptosis in cancer cells.84 ABT-737 targets Bcl-2 and Bcl-2-related proteins such as Bcl-xL and Bcl-w, but not A1 or Mcl-1, which may prove valuable in treating lymphoma and other blood cancers as well as solid tumors.85, 86 When peptide-pulsed DC vaccination was given both prior to and after tumor implantation, ABT-737 administration increased the antitumor activity of vaccination in a CT26 colon carcinoma model (Table 1). ABT-737 is currently being evaluated in advanced phase clinical trials.84
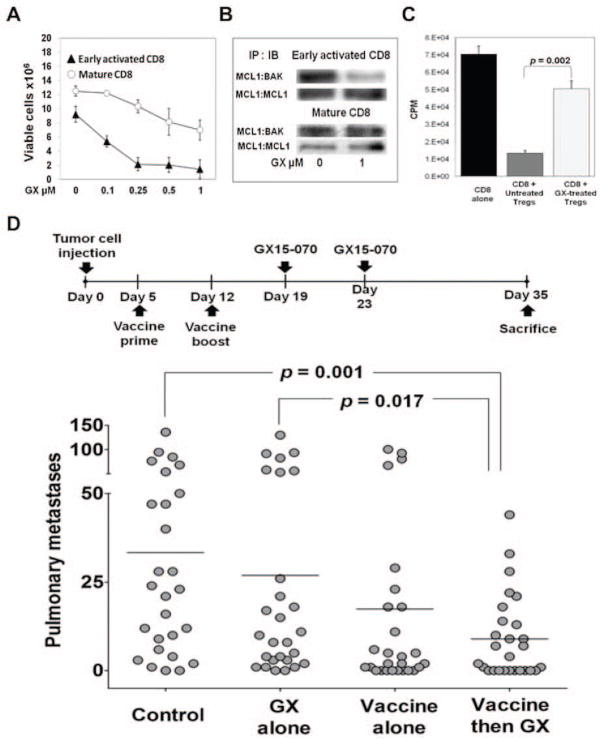
GX15-070, a pan-Bcl-2 inhibitor, is a synthetic derivative of bacterial prodiginines.87 GX15-070, which has the ability to bind all antiapoptotic Bcl-2 family members, including Bcl-2, Bcl-xL, Bcl-w, Mcl-1, and BAK,88 induces apoptosis in hematologic and solid tumor cells in vitro and in vivo and is currently being investigated in clinical trials.89–91 The effect of GX15-070 on CD8+ T cells is dependent on their activation status (Fig. 5A). Upregulation of the Mcl-1 gene has been reported within 10 h of T cell receptor ligation, indicating that Mcl-1 is involved in early T cell activation.92 The fact that GX15-070 inhibits Mcl-1 ligation to the proapoptotic BAK could explain why early-activated lymphocytes are more sensitive to the inhibitor (Fig. 5B). Mature CD8+ lymphocytes, which are resistant to GX15-070, display increased binding of the proapoptotic BAK to the antiapoptotic Mcl-1 (Fig. 5B). These data suggest that if vaccination were to precede GX15-070 treatment by an interval sufficient to overcome early activation, vaccine-induced T cells would not be negatively affected by the inhibitor.93 Furthermore, the proliferation of CD8+ T cells was significantly higher when they were cocultured with Tregs from GX15-070-treated mice than when they were cocultured with Tregs from untreated mice, indicating that GX15-070 inhibits Treg function (Fig. 5C). This suggests that GX15-070 can mediate an increase in immune-mediated antitumor activity by decreasing Treg-dependent immune suppression. This effect, along with an increased intratumoral activated CD8:Treg ratio in mice first vaccinated with rV/F-CEA/TRICOM then treated with the inhibitor, suggests that such a combination can produce a favorable milieu for immune activity against tumor cells.93, 94 Sequential therapy with this vaccine followed by GX15-070 effectively reduced orthotopic pulmonary tumors in immunocompetent mice (Fig. 5D), suggesting a rationale for the design of similar combination protocols for clinical studies.93
Figure 5.
Vaccinating prior to treatment with a Bcl-2 inhibitor (GX15-070) increased CD8+ resistance to apoptosis, diminished Treg suppression, and decreased pulmonary tumors. (A) Mature CD8+ T lymphocytes were more resistant to increasing concentrations of GX15-070 than early-activated lymphocytes after 72 h of treatment in vitro. (B) Mature CD8+ T lymphocytes exhibited increased binding of the proapoptotic BAK to the antiapoptotic Mcl-1 after GX15-070 treatment. (C) In C57BL/6 mice, treatment with GX15-070 reduced the suppressive activity of splenic Tregs. (D) Vaccination followed by GX15-070 treatment led to significantly fewer pulmonary metastases in a model of Lewis lung carcinoma. Adapted from.93
The studies described above indicate that when combining SMIs with immunotherapy, the appropriate interval between administration of each agent is important. Vaccine-induced immunity may be reduced when the Bcl-2 inhibitor is administered concurrently with or shortly after vaccine, since early-activated lymphocytes are extremely sensitive to GX15-070. Thus, in a combination setting, it is important that vaccine be administered long enough before GX15-070 to allow activated lymphocytes to mature.
Tyrosine Kinase Inhibitors
Another promising and intensely studied class of SMIs that could be used in combination with immunotherapy is tyrosine kinase inhibitors (TKIs). Approximately 30 kinase targets are being developed to the level of clinical trial, the vast majority of which are being investigated for the treatment of cancer. To date, approximately 80 TKIs have advanced to some stage of clinical evaluation and 11 have received FDA approval for cancer treatment,81 possibly because many tyrosine kinases have been found to be integral to the processes leading to tumor cell proliferation and survival.
Sunitinib and sorafenib are members of a class of TKIs that inhibit tumor vasculature. Sunitinib, an orally available inhibitor of multiple TKIs, was approved by the FDA in 2006 for the treatment of advanced renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumors.95, 96 Sunitinib is currently being evaluated as a treatment for many other solid and hematologic malignancies in numerous clinical trials, including nearly 150 studies sponsored by the National Cancer Institute.
Tyrosine kinase receptors targeted by sunitinib, such as receptors for vascular endothelial growth factor (VEGF) and platelet-derived growth factor, are widely expressed in many tumor cell types and tumor vasculature, allowing sunitinib to act directly against tumor cells and tumor stroma.97–99 Sunitinib also targets tyrosine kinase receptors expressed on MDSCs, such as c-KIT and VEGFR-1, making it a promising immunomodulatory.100 In fact, sunitinib exerts powerful immunomodulatory effects in cancer patients, such as shifting Th2 immune responses to Th1 and inhibiting immune suppressor cells, making this TKI an attractive candidate for combination with immunotherapies.101, 102
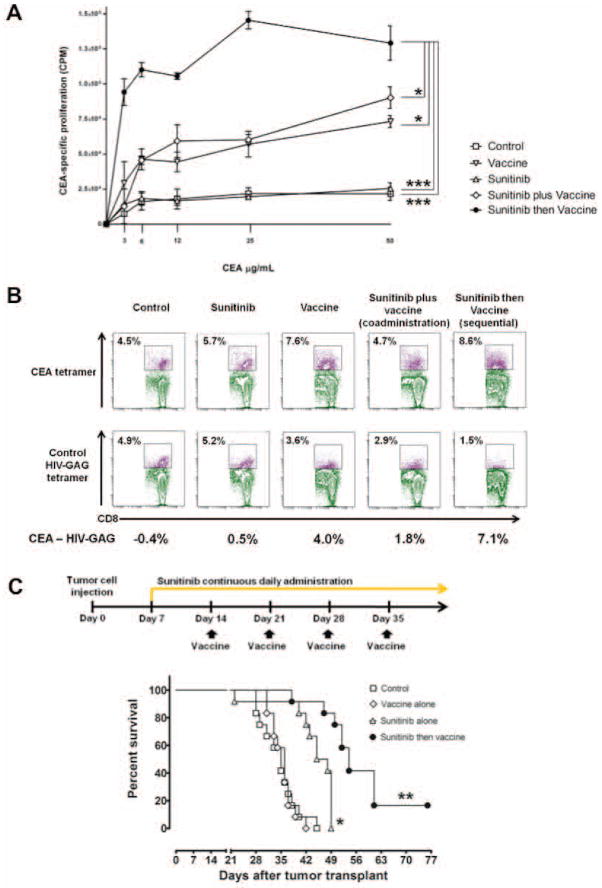
A recent preclinical study investigated the immunomodulatory effects of sunitinib in order to support the rational design of clinical trials combining sunitinib with immunotherapeutic platforms for the treatment of solid tumors.103 Using a mouse model, this study investigated the effects of sunitinib given for 4 weeks at concentrations comparable to 37.5 to 50 mg/day in humans, followed by 2 weeks off (sunitinib 4/2). In vivo, one cycle of sunitinib 4/2 caused bimodal immune effects: a decrease in regulatory cells during the 4 weeks of treatment, followed by an immune-suppression rebound during the 2 weeks of treatment interruption. A regimen of sunitinib followed by vaccine caused increased proliferation of antigen-specific CD4+ T cells (Fig. 6A) and increased numbers of antigen-specific CD8+ T cells (Fig. 6B). In contrast, coadministration resulted in a transient decrease of T lymphocytes at day 2 following sunitinib treatment, suggesting that giving vaccine at the initiation of sunitinib treatment could compromise the vaccine-induced immune response. In CEA-Tg mice bearing CEA+ tumors, continuous sunitinib treatment followed by vaccine increased intratumoral infiltration of antigen-specific T cells, decreased Tregs and MDSCs, reduced tumor volume, and increased survival (Fig. 6C). These data indicate that a) the immunomodulatory activity of continuous sunitinib can create a more immune-permissive environment, and b) in combination with immunotherapy, sunitinib should precede vaccine in order to precondition the immune system and maximize the response to vaccine-mediated immune enhancement. A recent randomized phase III clinical study combining MVA encoding the TAA 5T4 (MVA-5T4; TroVax®, Oxford BioMedica) with sunitinib in RCC showed no difference in survival between patients receiving sunitinib alone and patients receiving sunitinib with vaccine.104 However, in this trial patients were vaccinated prior to receiving sunitinib, which, as indicated above, may not be the most appropriate regimen.
Figure 6.
Vaccinating after sunitinib treatment increased tumor-specific immunity and improved survival in tumor-bearing mice. (A) In CEA-Tg mice, sunitinib followed by vaccine increased proliferation of CEA-specific CD4+ T cells. (B) The number of CEA tetramer+ CD8+ T cells also increased with this combination. (C) In mice bearing s.c. MC38-CEA+ tumors, the sequential combination improved survival compared to single control, single therapies, or different combination timings (data not shown). * = P < 0.05; *** = P < 0.001. Adapted from.103
Clinical translation of combinatorial therapies involving SMIs and vaccines must take into consideration the particular effects of the SMI on immune cells. Studies have indicated that an SMI that selectively inhibits immune suppressor cells (i.e., sunitinib) should be administered prior to vaccine in order to enhance the vaccine-mediated immune response to TAAs. If, on the other hand, the SMI alters lymphocyte activation (i.e., GX15-070), vaccinating before SMI treatment and allowing sufficient time for the activated lymphocytes to mature should result in more resistance to toxicity. Finally, if the SMI does not affect activation of effector lymphocytes and does not inhibit immune suppressors, it can be coadministered with immunotherapy.
SYNERGY
Taken together, the results from the preclinical and clinical studies described herein demonstrate the rationale for, and potential advantages of, combining therapeutic cancer vaccines with radiation, chemotherapy, or SMIs therapy. Each modality affects a different part of the immune system and tumor biology, potentially enhancing the action of the other modalities.
Cancer chemotherapy began in the 1940s with only nitrogen mustards and evolved to include combinations of multiple classes of chemotherapy agents targeting distinct factors of tumor growth (i.e., FOLFOX, FOLFIRI). Currently the same evolution is occurring in the field of small molecule inhibitors with the approval of Gleavec, bevicizumab, vandetanib, and gefitinib just to name a few. We envision combination immunotherapy evolving in a similar way, from vaccines as monotherapy, to vaccines combined with standard-of-care radiation, chemotherapy, and small-molecule therapeutics, to novel experimental therapies. As each standard modality has unique features that can enhance vaccine efficacy, it is conceivable that a multimodal approach encompassing several therapy platforms in combination with vaccines could result in even greater synergistic antitumor effects.
Acknowledgments
The authors thank Bonnie L. Casey for editorial assistance in the preparation of this review.
Footnotes
Financial Disclosures: The authors have no financial relationships to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
James W. Hodge, Senior Scientist, Recombinant Vaccine Group, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892.
Andressa Ardiani, Recombinant Vaccine Group, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892.
Benedetto Farsaci, Recombinant Vaccine Group, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892.
Anna R. Kwilas, Recombinant Vaccine Group, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892.
Sofia Gameiro, Recombinant Vaccine Group, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892.
References
- 1.Kamrava M, Bernstein MB, Camphausen K, Hodge JW. Combining radiation, immunotherapy, and antiangiogenesis agents in the management of cancer: the Three Musketeers or just another quixotic combination? Mol Biosyst. 2009 Nov;5(11):1262–70. doi: 10.1039/b911313b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007 Sep;13(9):1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi ME, Beltrame M, Paonessa G. Specific recognition of cruciform DNA by nuclear protein HMG1. Science. 1989 Feb 24;243(4894 Pt 1):1056–9. doi: 10.1126/science.2922595. [DOI] [PubMed] [Google Scholar]
- 4.Binder RJ, Blachere NE, Srivastava PK. Heat shock protein-chaperoned peptides but not free peptides introduced into the cytosol are presented efficiently by major histocompatibility complex I molecules. J Biol Chem. 2001 May 18;276(20):17163–71. doi: 10.1074/jbc.M011547200. [DOI] [PubMed] [Google Scholar]
- 5.Gehrmann M, Marienhagen J, Eichholtz-Wirth H, Fritz E, Ellwart J, Jaattela M, et al. Dual function of membrane-bound heat shock protein 70 (Hsp70), Bag-4, and Hsp40: protection against radiation-induced effects and target structure for natural killer cells. Cell Death Differ. 2005 Jan;12(1):38–51. doi: 10.1038/sj.cdd.4401510. [DOI] [PubMed] [Google Scholar]
- 6.Hickman-Miller HD, Hildebrand WH. The immune response under stress: the role of HSP-derived peptides. Trends Immunol. 2004 Aug;25(8):427–33. doi: 10.1016/j.it.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Singh-Jasuja H, Toes RE, Spee P, Munz C, Hilf N, Schoenberger SP, et al. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med. 2000 Jun 5;191(11):1965–74. doi: 10.1084/jem.191.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007 Jan;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 9.Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L, et al. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008 Jan;15(1):3–12. doi: 10.1038/sj.cdd.4402269. [DOI] [PubMed] [Google Scholar]
- 10.Friedman EJ. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr Pharm Des. 2002;8(19):1765–80. doi: 10.2174/1381612023394089. [DOI] [PubMed] [Google Scholar]
- 11.Sheard MA. Ionizing radiation as a response-enhancing agent for CD95-mediated apoptosis. Int J Cancer. 2001 Aug 20;96(4):213–20. doi: 10.1002/ijc.1020. [DOI] [PubMed] [Google Scholar]
- 12.Vereecque R, Buffenoir G, Gonzalez R, Cambier N, Hetuin D, Bauters F, et al. gamma-ray irradiation induces B7.1 expression in myeloid leukaemic cells. Br J Haematol. 2000 Mar;108(4):825–31. doi: 10.1046/j.1365-2141.2000.01967.x. [DOI] [PubMed] [Google Scholar]
- 13.Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003 Jun 15;170(12):6338–47. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 14.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006 May 15;203(5):1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baluna RG, Eng TY, Thomas CR. Adhesion molecules in radiotherapy. Radiat Res. 2006 Dec;166(6):819–31. doi: 10.1667/RR0380.1. [DOI] [PubMed] [Google Scholar]
- 16.Zamai L, Rana R, Mazzotti G, Centurione L, Di Pietro R, Vitale M. Lymphocyte binding to K562 cells: effect of target cell irradiation and correlation with ICAM-1 and LFA-3 expression. Eur J Histochem. 1994;38( Suppl 1):53–60. [PubMed] [Google Scholar]
- 17.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004 Jun 15;64(12):4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 18.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004 Nov 1;64(21):7985–94. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 19.Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005 Nov 1;63(3):655–66. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganss R, Ryschich E, Klar E, Arnold B, Hammerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002 Mar 1;62(5):1462–70. [PubMed] [Google Scholar]
- 21.Brooks PC, Roth JM, Lymberis SC, DeWyngaert K, Broek D, Formenti SC. Ionizing radiation modulates the exposure of the HUIV26 cryptic epitope within collagen type IV during angiogenesis. Int J Radiat Oncol Biol Phys. 2002 Nov 15;54(4):1194–201. doi: 10.1016/s0360-3016(02)03748-3. [DOI] [PubMed] [Google Scholar]
- 22.Hallahan D, Kuchibhotla J, Wyble C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res. 1996 Nov 15;56(22):5150–5. [PubMed] [Google Scholar]
- 23.Hallahan DE, Virudachalam S. Accumulation of P-selectin in the lumen of irradiated blood vessels. Radiat Res. 1999 Jul;152(1):6–13. [PubMed] [Google Scholar]
- 24.Ryschich E, Harms W, Loeffler T, Eble M, Klar E, Schmidt J. Radiation-induced leukocyte adhesion to endothelium in normal pancreas and in pancreatic carcinoma of the rat. Int J Cancer. 2003 Jul 1;105(4):506–11. doi: 10.1002/ijc.11073. [DOI] [PubMed] [Google Scholar]
- 25.Hallahan D, Geng L, Qu S, Scarfone C, Giorgio T, Donnelly E, et al. Integrin-mediated targeting of drug delivery to irradiated tumor blood vessels. Cancer Cell. 2003 Jan;3(1):63–74. doi: 10.1016/s1535-6108(02)00238-6. [DOI] [PubMed] [Google Scholar]
- 26.Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005 May 1;11(9):3353–62. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 27.Vaccine Therapy and Radiation Therapy in Treating Patients With Carcinoembryonic Antigen-Positive Solid Tumors That Have Metastasized to the Liver. 2004 [updated 2004 June 12, 2010; cited 2011]; Available from: http://www.clinicaltrials.gov/ct2/show/NCT00085241.
- 28.Eary JF, Collins C, Stabin M, Vernon C, Petersdorf S, Baker M, et al. Samarium-153-EDTMP biodistribution and dosimetry estimation. J Nucl Med. 1993 Jul;34(7):1031–6. [PubMed] [Google Scholar]
- 29.Maini CL, Bergomi S, Romano L, Sciuto R. 153Sm-EDTMP for bone pain palliation in skeletal metastases. Eur J Nucl Med Mol Imaging. 2004 Jun;31( Suppl 1):S171–8. doi: 10.1007/s00259-004-1540-y. [DOI] [PubMed] [Google Scholar]
- 30.153Sm-EDTMP With or Without a PSA/TRICOM Vaccine To Treat Men With Androgen-Insensitive Prostate Cancer. 2007 [updated 2007 January 11, 2011; cited 2011]; Available from: http://clinicaltrials.gov/ct2/show/NCT00450619.
- 31.Chakraborty M, Gelbard A, Carrasquillo JA, Yu S, Mamede M, Paik CH, et al. Use of radiolabeled monoclonal antibody to enhance vaccine-mediated antitumor effects. Cancer Immunol Immunother. 2008 Aug;57(8):1173–83. doi: 10.1007/s00262-008-0449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002 Oct 1;169(7):3760–70. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- 33.Sharp HJ, Wansley EK, Garnett CT, Chakraborty M, Camphausen K, Schlom J, et al. Synergistic antitumor activity of immune strategies combined with radiation. Front Biosci. 2007;12:4900–10. doi: 10.2741/2436. [DOI] [PubMed] [Google Scholar]
- 34.Chakraborty M, Wansley EK, Carrasquillo JA, Yu S, Paik CH, Camphausen K, et al. The use of chelated radionuclide (samarium-153-ethylenediaminetetramethylenephosphonate) to modulate phenotype of tumor cells and enhance T cell-mediated killing. Clin Cancer Res. 2008 Jul 1;14(13):4241–9. doi: 10.1158/1078-0432.CCR-08-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kudo-Saito C, Schlom J, Camphausen K, Coleman CN, Hodge JW. The requirement of multimodal therapy (vaccine, local tumor radiation, and reduction of suppressor cells) to eliminate established tumors. Clin Cancer Res. 2005 Jun 15;11(12):4533–44. doi: 10.1158/1078-0432.CCR-04-2237. [DOI] [PubMed] [Google Scholar]
- 36.Gelbard A, Garnett CT, Abrams SI, Patel V, Gutkind JS, Palena C, et al. Combination chemotherapy and radiation of human squamous cell carcinoma of the head and neck augments CTL-mediated lysis. Clin Cancer Res. 2006 Mar 15;12(6):1897–905. doi: 10.1158/1078-0432.CCR-05-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Mehren M, Arlen P, Gulley J, Rogatko A, Cooper HS, Meropol NJ, et al. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin Cancer Res. 2001 May;7(5):1181–91. [PubMed] [Google Scholar]
- 38.Kaneno R, Shurin GV, Kaneno FM, Naiditch H, Luo J, Shurin MR. Chemotherapeutic agents in low noncytotoxic concentrations increase immunogenicity of human colon cancer cells. Cell Oncol (Dordr) 2011 Apr;34(2):97–106. doi: 10.1007/s13402-010-0005-5. [DOI] [PubMed] [Google Scholar]
- 39.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010 Apr 1;120(4):1111–24. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011 Mar;8(3):151–60. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 41.Shurin GV, Tourkova IL, Kaneno R, Shurin MR. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol. 2009 Jul 1;183(1):137–44. doi: 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfannenstiel LW, Lam SS, Emens LA, Jaffee EM, Armstrong TD. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell Immunol. 2010;263(1):79–87. doi: 10.1016/j.cellimm.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leao IC, Ganesan P, Armstrong TD, Jaffee EM. Effective depletion of regulatory T cells allows the recruitment of mesothelin-specific CD8 T cells to the antitumor immune response against a mesothelin-expressing mouse pancreatic adenocarcinoma. Clin Transl Sci. 2008 Dec;1(3):228–39. doi: 10.1111/j.1752-8062.2008.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005 Apr 1;105(7):2862–8. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 45.Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001 May 1;61(9):3689–97. [PubMed] [Google Scholar]
- 46.Gameiro SR, Caballero JA, Higgins JP, Apelian D, Hodge JW. Exploitation of differential homeostatic proliferation of T-cell subsets following chemotherapy to enhance the efficacy of vaccine-mediated antitumor responses. Cancer Immunol Immunother. 2011 May 5; doi: 10.1007/s00262-011-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kochenderfer JN, Simpson JL, Chien CD, Gress RE. Vaccination regimens incorporating CpG-containing oligodeoxynucleotides and IL-2 generate antigen-specific antitumor immunity from T-cell populations undergoing homeostatic peripheral expansion after BMT. Blood. 2007 Jul 1;110(1):450–60. doi: 10.1182/blood-2006-11-057935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mackall CL, Hakim FT, Gress RE. Restoration of T-cell homeostasis after T-cell depletion. Semin Immunol. 1997 Dec;9(6):339–46. doi: 10.1006/smim.1997.0091. [DOI] [PubMed] [Google Scholar]
- 49.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008 Jun 1;14(11):3536–44. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stein WD, Gulley JL, Schlom J, Madan RA, Dahut W, Figg WD, et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011 Feb 15;17(4):907–17. doi: 10.1158/1078-0432.CCR-10-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramakrishnan R, Gabrilovich DI. Mechanism of synergistic effect of chemotherapy and immunotherapy of cancer. Cancer Immunol Immunother. 2011 Mar;60(3):419–23. doi: 10.1007/s00262-010-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newcomb EW, Lukyanov Y, Kawashima N, Alonso-Basanta M, Wang SC, Liu M, et al. Radiotherapy enhances antitumor effect of anti-CD137 therapy in a mouse Glioma model. Radiat Res. 2010 Apr;173(4):426–32. doi: 10.1667/RR1904.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merritt RE, Mahtabifard A, Yamada RE, Crystal RG, Korst RJ. Cisplatin augments cytotoxic T-lymphocyte-mediated antitumor immunity in poorly immunogenic murine lung cancer. J Thorac Cardiovasc Surg. 2003 Nov;126(5):1609–17. doi: 10.1016/s0022-5223(03)00707-4. [DOI] [PubMed] [Google Scholar]
- 54.Sundelin K, Roberg K, Grenman R, Hakansson L. Effects of cisplatin, alpha-interferon, and 13-cis retinoic acid on the expression of Fas (CD95), intercellular adhesion molecule-1 (ICAM-1), and epidermal growth factor receptor (EGFR) in oral cancer cell lines. J Oral Pathol Med. 2007 Mar;36(3):177–83. doi: 10.1111/j.1600-0714.2006.00503.x. [DOI] [PubMed] [Google Scholar]
- 55.Matsuzaki I, Suzuki H, Kitamura M, Minamiya Y, Kawai H, Ogawa J. Cisplatin induces fas expression in esophageal cancer cell lines and enhanced cytotoxicity in combination with LAK cells. Oncology. 2000 Nov;59(4):336–43. doi: 10.1159/000012192. [DOI] [PubMed] [Google Scholar]
- 56.Bergmann-Leitner ES, Abrams SI. Treatment of human colon carcinoma cell lines with anti-neoplastic agents enhances their lytic sensitivity to antigen-specific CD8+ cytotoxic T lymphocytes. Cancer Immunol Immunother. 2001 Nov;50(9):445–55. doi: 10.1007/s002620100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takizawa K, Kamijo R, Ito D, Hatori M, Sumitani K, Nagumo M. Synergistic induction of ICAM-1 expression by cisplatin and 5-fluorouracil in a cancer cell line via a NF-kappaB independent pathway. Br J Cancer. 1999 Jun;80(7):954–63. doi: 10.1038/sj.bjc.6690449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song CK, Han HD, Noh KH, Kang TH, Park YS, Kim JH, et al. Chemotherapy enhances CD8(+) T cell-mediated antitumor immunity induced by vaccination with vaccinia virus. Mol Ther. 2007 Aug;15(8):1558–63. doi: 10.1038/sj.mt.6300221. [DOI] [PubMed] [Google Scholar]
- 59.Tseng CW, Hung CF, Alvarez RD, Trimble C, Huh WK, Kim D, et al. Pretreatment with cisplatin enhances E7-specific CD8+ T-Cell-mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res. 2008 May 15;14(10):3185–92. doi: 10.1158/1078-0432.CCR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bae SH, Park YJ, Park JB, Choi YS, Kim MS, Sin JI. Therapeutic synergy of human papillomavirus E7 subunit vaccines plus cisplatin in an animal tumor model: causal involvement of increased sensitivity of cisplatin-treated tumors to CTL-mediated killing in therapeutic synergy. Clin Cancer Res. 2007 Jan 1;13(1):341–9. doi: 10.1158/1078-0432.CCR-06-1838. [DOI] [PubMed] [Google Scholar]
- 61.Bretti S, Berruti A, Gorzegno G, La Ciura P, Paze E, Celano A, et al. Multicenter Phase II trial of intermediate dose cisplatin and vinorelbine in inoperable non-small cell lung cancer patients. Lung Cancer. 1996 Jun;14(2–3):353–60. doi: 10.1016/0169-5002(96)00559-4. [DOI] [PubMed] [Google Scholar]
- 62.Gebbia V, Galetta D, Lorusso V, Caruso M, Verderame F, Pezzella G, et al. Cisplatin plus weekly vinorelbine versus cisplatin plus vinorelbine on days 1 and 8 in advanced non-small cell lung cancer: a prospective randomized phase III trial of the G.O.I.M (Gruppo Oncologico Italia Meridionale) Lung Cancer. 2008 Sep;61(3):369–77. doi: 10.1016/j.lungcan.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 63.Hakim FT, Cepeda R, Kaimei S, Mackall CL, McAtee N, Zujewski J, et al. Constraints on CD4 recovery postchemotherapy in adults: thymic insufficiency and apoptotic decline of expanded peripheral CD4 cells. Blood. 1997 Nov 1;90(9):3789–98. [PubMed] [Google Scholar]
- 64.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002 Oct 25;298(5594):850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007 Oct;19(5):318–30. doi: 10.1016/j.smim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramlau R, Quoix E, Rolski J, Pless M, Lena H, Levy E, et al. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. J Thorac Oncol. 2008 Jul;3(7):735–44. doi: 10.1097/JTO.0b013e31817c6b4f. [DOI] [PubMed] [Google Scholar]
- 67.Law KS, Chen HC, Liao SK. Non-cytotoxic and sublethal paclitaxel treatment potentiates the sensitivity of cultured ovarian tumor SKOV-3 cells to lysis by lymphokine-activated killer cells. Anticancer Res. 2007 Mar-Apr;27(2):841–50. [PubMed] [Google Scholar]
- 68.Chan OT, Yang LX. The immunological effects of taxanes. Cancer Immunol Immunother. 2000 Jul;49(4–5):181–5. doi: 10.1007/s002620000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Markasz L, Skribek H, Uhlin M, Otvos R, Flaberg E, Eksborg S, et al. Effect of frequently used chemotherapeutic drugs on cytotoxic activity of human cytotoxic T-lymphocytes. J Immunother. 2008 Apr;31(3):283–93. doi: 10.1097/CJI.0b013e3181628b76. [DOI] [PubMed] [Google Scholar]
- 70.Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004 Jul 15;173(2):1444–53. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 71.Nizar S, Meyer B, Galustian C, Kumar D, Dalgleish A. T regulatory cells, the evolution of targeted immunotherapy. Biochim Biophys Acta. 2010 Aug;1806(1):7–17. doi: 10.1016/j.bbcan.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 72.Liu JY, Wu Y, Zhang XS, Yang JL, Li HL, Mao YQ, et al. Single administration of low dose cyclophosphamide augments the antitumor effect of dendritic cell vaccine. Cancer Immunol Immunother. 2007 Oct;56(10):1597–604. doi: 10.1007/s00262-007-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miles D, Roche H, Martin M, Perren TJ, Cameron DA, Glaspy J, et al. Phase III Multicenter Clinical Trial of the Sialyl-TN (STn)-Keyhole Limpet Hemocyanin (KLH) Vaccine for Metastatic Breast Cancer. Oncologist. 2011 May 14; doi: 10.1634/theoncologist.2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009 Dec 10;27(35):5911–8. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005 Dec 19;202(12):1691–701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005 Sep 15;11(18):6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 77.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004 Apr;4(4):314–22. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 78.Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009 Jul;58(7):1033–45. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu WM, Henry JY, Meyer B, Bartlett JB, Dalgleish AG, Galustian C. Inhibition of metastatic potential in colorectal carcinoma in vivo and in vitro using immunomodulatory drugs (IMiDs) Br J Cancer. 2009 Sep 1;101(5):803–12. doi: 10.1038/sj.bjc.6605206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dredge K, Horsfall R, Robinson SP, Zhang LH, Lu L, Tang Y, et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005 Jan;69(1–2):56–63. doi: 10.1016/j.mvr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 81.Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009 Jan;9(1):28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
- 82.Arkin MR, Wells JA. Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat Rev Drug Discov. 2004 Apr;3(4):301–17. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- 83.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009 Feb 15;15(4):1126–32. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chonghaile TN, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene. 2008 Dec;27( Suppl 1):S149–57. doi: 10.1038/onc.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005 Jun 2;435(7042):677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 86.Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005 Jul 15;106(2):408–18. doi: 10.1182/blood-2004-07-2761. [DOI] [PubMed] [Google Scholar]
- 87.Williamson NR, Fineran PC, Gristwood T, Chawrai SR, Leeper FJ, Salmond GP. Anticancer and immunosuppressive properties of bacterial prodiginines. Future Microbiol. 2007 Dec;2:605–18. doi: 10.2217/17460913.2.6.605. [DOI] [PubMed] [Google Scholar]
- 88.Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007 Dec 4;104(49):19512–7. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang S, Okumura K, Sinicrope FA. BH3 mimetic obatoclax enhances TRAIL-mediated apoptosis in human pancreatic cancer cells. Clin Cancer Res. 2009 Jan 1;15(1):150–9. doi: 10.1158/1078-0432.CCR-08-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Parikh SA, Kantarjian H, Schimmer A, Walsh W, Asatiani E, El-Shami K, et al. Phase II study of obatoclax mesylate (GX15-070), a small-molecule BCL-2 family antagonist, for patients with myelofibrosis. Clin Lymphoma Myeloma Leuk. 2010 Aug 1;10(4):285–9. doi: 10.3816/CLML.2010.n.059. [DOI] [PubMed] [Google Scholar]
- 91.Perez-Galan P, Roue G, Lopez-Guerra M, Nguyen M, Villamor N, Montserrat E, et al. BCL-2 phosphorylation modulates sensitivity to the BH3 mimetic GX15-070 (Obatoclax) and reduces its synergistic interaction with bortezomib in chronic lymphocytic leukemia cells. Leukemia. 2008 Jul 3; doi: 10.1038/leu.2008.175. [DOI] [PubMed] [Google Scholar]
- 92.Wang M, Windgassen D, Papoutsakis ET. A global transcriptional view of apoptosis in human T-cell activation. BMC Med Genomics. 2008 Oct 23;1(1):53. doi: 10.1186/1755-8794-1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Farsaci B, Sabzevari H, Higgins JP, Di Bari MG, Takai S, Schlom J, et al. Effect of a small molecule BCL-2 inhibitor on immune function and use with a recombinant vaccine. Int J Cancer. 2010 Oct 1;127(7):1603–13. doi: 10.1002/ijc.25177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hodge JW, Grosenbach DW, Aarts WM, Poole DJ, Schlom J. Vaccine therapy of established tumors in the absence of autoimmunity. Clin Cancer Res. 2003 May;9(5):1837–49. [PubMed] [Google Scholar]
- 95.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006 Oct 14;368(9544):1329–38. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 96.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009 Aug 1;27(22):3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fletcher JA, Rubin BP. KIT mutations in GIST. Curr Opin Genet Dev. 2007 Feb;17(1):3–7. doi: 10.1016/j.gde.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 98.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003 Jan;9(1):327–37. [PubMed] [Google Scholar]
- 99.Osusky KL, Hallahan DE, Fu A, Ye F, Shyr Y, Geng L. The receptor tyrosine kinase inhibitor SU11248 impedes endothelial cell migration, tubule formation, and blood vessel formation in vivo, but has little effect on existing tumor vessels. Angiogenesis. 2004;7(3):225–33. doi: 10.1007/s10456-004-3149-y. [DOI] [PubMed] [Google Scholar]
- 100.Kao J, Ko EC, Eisenstein S, Sikora AG, Fu S, Chen SH. Targeting immune suppressing myeloid-derived suppressor cells in oncology. Crit Rev Oncol Hematol. 2011 Jan;77(1):12–9. doi: 10.1016/j.critrevonc.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Asemissen AM, Brossart P. Vaccination strategies in patients with renal cell carcinoma. Cancer Immunol Immunother. 2009 Jul;58(7):1169–74. doi: 10.1007/s00262-009-0706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008 Oct 15;14(20):6674–82. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 103.Farsaci B, Higgins J, Hodge J. Conseqence of Dose Scheduling of Sunitinib on Host Immune Response Elements and Vaccine Combiantion Therapy. International Journal of Cancer. 2011 doi: 10.1002/ijc.26219. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Amato RJ, Hawkins RE, Kaufman HL, Thompson JA, Tomczak P, Szczylik C, et al. Vaccination of metastatic renal cancer patients with MVA-5T4: a randomized, double-blind, placebo-controlled phase III study. Clin Cancer Res. 2010 Nov 15;16(22):5539–47. doi: 10.1158/1078-0432.CCR-10-2082. [DOI] [PubMed] [Google Scholar]
- 105.Julien S, Picco G, Sewell R, Vercoutter-Edouart AS, Tarp M, Miles D, et al. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br J Cancer. 2009 Jun 2;100(11):1746–54. doi: 10.1038/sj.bjc.6605083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer research [Research Support, NIH, Extramural Research Support, Non-US Gov’t] 2009 Mar 15;69(6):2514–22. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vaccine Therapy With or Without Cyclophosphamide in Treating Patients Undergoing Chemotherapy and Radiation Therapy for Stage I or Stage II Pancreatic Cancer That Can Be Removed by Surgery. 2008 [updated 2008 August 5, 2010; cited 2011]; Available from: http://clinicaltrials.gov/ct2/show/NCT00727441.
- 108.North SA, Graham K, Bodnar D, Venner P. A pilot study of the liposomal MUC1 vaccine BLP25 in prostate specific antigen failures after radical prostatectomy. J Urol. 2006 Jul;176(1):91–5. doi: 10.1016/S0022-5347(06)00494-0. [DOI] [PubMed] [Google Scholar]
- 109.Arlen PM, Pazdur M, Skarupa L, Rauckhorst M, Gulley JL. A randomized phase II study of docetaxel alone or in combination with PANVAC-V (vaccinia) and PANVAC-F (fowlpox) in patients with metastatic breast cancer (NCI 05-C-0229) Clin Breast Cancer. 2006 Jun;7(2):176–9. doi: 10.3816/CBC.2006.n.032. [DOI] [PubMed] [Google Scholar]
- 110.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. Journal of Clinical Oncology [Clinical Trial, Phase III Multicenter Study Randomized Controlled Trial Research Support, Non-US Gov’t] 2006 Jul 1;24(19):3089–94. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 111.Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006 Feb 15;12(4):1260–9. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Docetaxel and Prednisone With or Without Vaccine Therapy in Treating Patients With Metastatic Hormone-Resistant Prostate Cancer. 2010 [updated 2010 March 23, 2011; cited 2011]; Available from: http://clinicaltrials.gov/ct/show/NCT01145508.
- 113.TroVax® In Subjects With Hormone Refractory Prostate Cancer (HRPC) 2010 [updated 2010 May 5, 2011; cited 2011]; Available from: http://clinicaltrials.gov/ct2/show/NCT01194960.
- 114.Kaufman HL, Lenz HJ, Marshall J, Singh D, Garett C, Cripps C, et al. Combination chemotherapy and ALVAC-CEA/B7.1 vaccine in patients with metastatic colorectal cancer. Clin Cancer Res. 2008 Aug 1;14(15):4843–9. doi: 10.1158/1078-0432.CCR-08-0276. [DOI] [PubMed] [Google Scholar]
- 115.Harrop R, Drury N, Shingler W, Chikoti P, Redchenko I, Carroll MW, et al. Vaccination of colorectal cancer patients with TroVax given alongside chemotherapy (5-fluorouracil, leukovorin and irinotecan) is safe and induces potent immune responses. Cancer Immunol Immunother. 2008 Jul;57(7):977–86. doi: 10.1007/s00262-007-0428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harrop R, Drury N, Shingler W, Chikoti P, Redchenko I, Carroll MW, et al. Vaccination of colorectal cancer patients with modified vaccinia ankara encoding the tumor antigen 5T4 (TroVax) given alongside chemotherapy induces potent immune responses. Clin Cancer Res. 2007 Aug 1;13(15 Pt 1):4487–94. doi: 10.1158/1078-0432.CCR-07-0704. [DOI] [PubMed] [Google Scholar]
- 117.Amato RJ, Hawkins RE, Kaufman HL, Thompson JA, Tomczak P, Szczylik C, et al. Vaccination of metastatic renal cancer patients with MVA-5T4: a randomized, double-blind, placebo-controlled phase III study. Clinical Cancer Research. 2010 Nov 15;16(22):5539–47. doi: 10.1158/1078-0432.CCR-10-2082. [DOI] [PubMed] [Google Scholar]