Abstract
Given the lethality of H5N1 avian influenza viruses (AIV) and the recurring spread from poultry to humans, an effective vaccine against H5N1 viruses may be needed to prevent a pandemic. We generated experimental vaccine vectors based on recombinant vesicular stomatitis virus (VSV) expressing the H5 hemagglutinin from an H5N1 virus isolated in 1997. The HA gene was expressed either from an attenuated wild-type VSV vector or from a single-cycle vector containing a deletion of the VSV G gene. We found that all of the vectors induced potent neutralizing antibody titers against the homologous and antigenically heterologous H5N1 viruses isolated in 2004 and 2005. Vaccination of mice with any combination of prime or prime/boost vectors provided long-lasting protection (> 7 months) against challenge with AIV, even in animals receiving a single dose of single-cycle vaccine. Our data indicate that these recombinants are promising vaccine candidates for pandemic influenza.
Keywords: VSV, AIV, vaccine, H5N1, cross neutralizing antibody
INTRODUCTION
The influenza pandemics that occurred in 1918, 1957 and 1968 killed over 40 million people worldwide. Pandemics arise when novel influenza A subtypes emerge and spread to humans, generally from avian reservoirs (Wright and Webster, 2001). Viruses in the influenza A genus are subdivided on the basis of the antigenicity of the envelope glycoproteins, hemagglutinin (HA) and neuraminidase (NA). Currently, there are 16 known types of HA (H1–16) and 9 of NA (N1–9) (Fouchier et al., 2005; Wright and Webster, 2001).
In 1997, outbreaks of the H5N1 subtype of avian influenza virus (AIV) occurred in Hong Kong, in both poultry and humans. This was the first time that a H5 subtype was known to cause disease in humans. H5N1 viruses emerged again in 2003 and continue to cause disease in poultry and humans to the present day (Gillim-Ross and Subbarao, 2006). Since 2003, at least 261 people have been infected by H5N1 viruses with an associated mortality rate of over 60% (http://www.who.int/csr/disease/avian_influenza). Despite the unusually high mortality rate, the human outbreaks probably have been contained because the current H5N1 strains do not spread efficiently from person to person (Katz et al., 1999; Ungchusak et al., 2005). This high mortality rate and continued spread triggered the World Health Organization and the U.S. Centers for Disease Control and Prevention to issue warnings about a possible H5N1 pandemic. The H5N1 strains that have emerged since 1997 have already split into numerous sublineages or clades (2005; Chen et al., 2006), illustrating the rapid evolution of this virus. The ability of H5N1 subtypes of AIVs to infect humans has emphasized the need for vaccines protecting against AIV infection.
Compared to human influenza vaccines targeting epidemic influenza, development of AIV vaccines is more problematic. Traditional influenza vaccines are prepared by growing attenuated reassortant viruses in embryonated chicken eggs. These attenuated viruses encode the HA and NA proteins predicted to be present in the upcoming seasonal epidemic viruses. However, the AIVs with the greatest potential public health threat are highly pathogenic avian influenza (HPAI) viruses that can be too pathogenic to grow efficiently in eggs and require costly levels of biocontainment. Because a newly emerged H5N1 virus might spread rapidly in the population, the long lead-time required for traditional vaccine production is also a major concern.
In an effort to create an H5N1 AIV vaccine, many groups are exploring new approaches to generate a vaccine that will protect against H5N1 strains (reviewed in (Gillim-Ross and Subbarao, 2006; Horimoto and Kawaoka, 2006)). Both inactivated and live attenuated AIV vaccines are under development. Other strategies such as inactivated subvirion, recombinant subunit and DNA vaccines are being investigated. Virus vectors, such as adenovirus and Newcastle disease virus vectors, encoding influenza antigens are also being explored as potential AIV vaccines for both avian and human use (Ge et al., 2007; Horimoto and Kawaoka, 2006; Park et al., 2006; Veits et al., 2006).
Experimental virus vaccine vectors based on attenuated vesicular stomatitis viruses (VSV) that express foreign viral antigens have been developed (Brandsma et al., 2007; Daddario-DiCaprio et al., 2006; Egan et al., 2004; Garbutt et al., 2004; Jones et al., 2005; Kahn et al., 2001; Kapadia et al., 2005; Publicover, Ramsburg, and Rose, 2005; Ramsburg et al., 2004; Reuter et al., 2002; Roberts et al., 1999; Roberts et al., 1998; Roberts et al., 2004; Schlereth et al., 2000; Schnell et al., 1997). These vectors protect animals against numerous viral challenges. The initial studies on VSV as a vaccine vector showed that a VSV expressing the HA protein of the WSN strain of influenza elicited high neutralizing antibody titers in mice and protected mice against challenge with a lethal dose of the WSN virus (Roberts et al., 1999; Roberts et al., 1998).
VSV is a versatile vaccine vector for many reasons including the fact that exposure and seropositivity to VSV is negligible in the human population. Additionally, it replicates solely in the cytoplasm, does not undergo recombination, and generates no DNA intermediates that might integrate into host genomes (Rose and Whitt, 2001). VSV’s relatively simple genome can accommodate at least 4.5 kilobases of foreign gene(s) that are highly expressed in infected cells. Moreover, VSV is effective when administered intranasally and is easily manufactured on a large scale in cell lines already approved for vaccine production. There are also highly attenuated mutants of VSV as well as replication defective, single-cycle VSV vectors that still generate potent immune responses (Publicover, Ramsburg, and Rose, 2004; Publicover, Ramsburg, and Rose, 2005; Roberts et al., 1999).
In the present study, we have generated replicating and single-cycle VSV vectors that express the H5 HA gene derived from a prototypic H5N1 AIV, A/Hong Kong/156/97 (HK/156), originally isolated from a lethal human infection (Subbarao et al., 1998). To assess the efficacy of these vectors, we examined the ability of the VSVs expressing H5 HA to induce a neutralizing antibody response against the homologous virus. Additionally, we determined if these vaccines could elicit cross-neutralizing antibody titers against distantly related H5N1 viruses. All of our vectors, with and without boosting, were able to induce a neutralizing antibody response against all H5N1 viruses tested. Furthermore, the response was 100% protective in a mouse model of AIV challenge. This protection was achieved with a single dose of vaccine and was long-lasting.
RESULTS
Construction of replication competent and single-cycle VSV vectors expressing an avian influenza H5 HA protein
In order to generate VSV-based vaccine vectors for H5N1 avian influenza, we incorporated the HA gene from the H5N1 AIV strain, HK/156, into the three different recombinant VSV (rVSV) vectors shown in Fig. 1A. The two replication competent vectors had the HA gene inserted into the fifth genome position downstream of the VSV G gene. One of these vectors (VSV-H5 HA, Fig. 1A) contained the VSV G from the Indiana serotype. To allow for effective boosting after priming with the vector containing the Indiana G protein, the boosting vector substituted this gene with the VSV G gene from the New Jersey serotype (VSV-NJG-H5 HA, Fig. 1A). Priming with VSV vectors precludes effective boosting with the same vector because of the high level of neutralizing antibody generated against the VSV G protein (Rose et al., 2000). Additionally, we generated a single-cycle vector where the G gene was deleted (VSVΔG-H5 HA, Fig. 1A). This vector was propagated in a complementing cell line expressing VSV G (Schnell et al., 1997). However, it cannot spread in animals beyond initially infected cells because it does not encode the VSV G protein. Such single-cycle vectors eliminate concerns about pathogenesis of VSV recombinants.
Fig. 1. Recombinant VSV vectors expressing the H5 HA from the A/HK/156/97.
(A) Diagram of the recombinant VSV vector genomes showing the insertion site of the H5 HA gene, the replacement of the G gene (Indiana serotype) with that from the New Jersey (NJ) serotype in the VSV-NJG-H5 HA vector, and the deletion of the G gene in the VSV- G-H5ΔHA vector. (B, C) Whole cell extracts (WCE; left panels) prepared from BHK-21 cells infected with the indicated viruses or virions purified from infected cell supernatants (right panels) were subjected to analysis by SDS-PAGE. Western blot analyses were performed using antibodies specific for (B) H5 HA or (C) VSV. The anti-VSV blot (C) is the same blot from (B) that was stripped and re-probed with anti-VSV (Indiana) antibody. The full-length (HA0) and cleaved isoforms (HA1, HA2) of H5 HA as well as the VSV proteins (G, N, P and M) are indicated by the arrows.
Expression of the HA gene from the recombinant VSV vectors
To determine if the H5 HA gene was expressed from these recombinant vectors, western blot analysis was performed on whole cell extracts of infected cells. Proteins with the mobilities expected of the H5 HA protein (HA0) and its cleaved forms (HA1 and HA2) accumulated in cells infected with all three vectors, but not in cells infected with the parent wild-type (WT) virus (Fig. 1B, left panel). When the same blot was stripped and re-probed with anti-VSV (Indiana) antibodies, VSV proteins were detected in the infected cells, except for the G protein in cells infected with the single-cycle vector, VSVΔG-H5 HA, which does not encode VSV G, or in cells infected with VSV-NJG-H5 HA because the NJ G protein is not detected well by the anti-VSV (Indiana) serum (Fig. 1C, left panel). A trace of cross reactivity to the NJ G protein is detected in the blot of virion proteins (Fig. 1C, right panel).
H5 HA is incorporated into the recombinant VSV virions
To determine if the H5 HA protein was incorporated into recombinant VSV virions, we purified virions by ultracentrifugation and then performed a western blot analysis of the virion proteins with anti-HA antibodies. Figure 1B (right panel) shows that the cleaved forms, HA1 and HA2, were incorporated into virions of rVSVs expressing HA, but not into WT virions. The presence of little or no HA0 in the recombinant virions is expected because HA0 should be cleaved before reaching the cell surface (Wright and Webster, 2001) where VSV budding occurs (Rose and Whitt, 2001). Re-probing of the same blot with anti-VSV serum showed that the expected VSV proteins were also present in the virions (Fig. 1C, right panel).
Immunization with the VSV recombinants elicits a potent neutralizing antibody response to H5N1 AIV
To evaluate the ability of the rVSV vaccine vectors to generate neutralizing antibodies against avian influenza, mice were primed intramuscularly (i.m.) or intranasally (i.n.) with VSV-H5 HA, or i.m only with VSVΔG-H5 HA. Half of the mice in each group were boosted one month later by the respective route with the G serotype switch vector, VSV-NJG-H5 HA. An rVSV vector that expresses an unrelated protein was used as a negative control vector.
Serum antibody responses against the homologous AIV, HK/156, were analyzed in individual mice at various times post-prime (Fig. 2) using an AIV microneutralization assay that measures 100% neutralization of infectivity (Suguitan et al., 2006). A single dose of either VSV-H5 HA or the single-cycle vector, VSVΔG-H5 HA, elicited neutralizing antibody titers against the homologous virus as early as 1 and 2 months post-prime (data not shown), but peaked at 3 months post-prime (Fig. 2, gray bars). These antibodies persisted at 5.5 months post-prime (Fig. 2, black bars). Neutralizing titers against HK/156 were enhanced substantially after boosting in the animals vaccinated i.m. After the prime, the animals vaccinated i.n. had higher titers than the animals vaccinated i.m. (Fig. 2). Boosting of the i.n. vaccinees was less effective (Fig. 2) perhaps because of the presence of a higher initial level of neutralizing antibodies directed against H5 HA after the prime.
Fig. 2. Neutralization of the homologous AIV strain by sera from VSV vaccinated mice.
Mice were vaccinated i.m. or i.n. with VSV-H5 HA (H5; n=6), VSV-ΔG-H5 HA (ΔG H5; n=6) or the negative control vector, VSV-Gag (Gag; n=3). At 1 month post-prime, animals were boosted with VSV-NJG-H5 HA (n=3) where indicated. At various times post-prime, sera from individual mice were analyzed for a neutralizing antibody response against the homologous HK/156 AIV strain (3 or 5.5 mo post-prime, gray and black bars, respectively). Each bar represents the neutralizing antibody titer from an individual mouse as determined by 100% inhibition of CPE in a microneutralization assay.
Vaccination elicits a broad cross-neutralizing antibody response against heterologous AIV strains
Because of the frequency of antigenic change (drift) in influenza viruses, it is important to determine if vaccine candidates can generate neutralizing antibodies against AIVs from other clades within the same subtype. We therefore examined the ability of our rVSVs to generate a cross-neutralizing antibody response against the heterologous AIVs, A/Vietnam/1203/04 (VN/1203) and A/Indonesia/5/05 (INA/5). These are viruses from other clades that emerged 7–8 years after isolation of the HK/156 strain and were isolated in geographically distinct areas. We found that a single i.m. or i.n. dose of either VSV-H5 HA or VSVΔG-H5 HA elicited detectable cross-neutralizing antibodies against the clade 1 virus, VN/1203 (Fig. 3A, gray bars), and the clade 2 virus, INA/5, (Fig. 3A, black bars) in some of the animals. In general, boosting of the animals substantially increased the cross-neutralizing antibody titers against both AIVs (Fig. 3A).
Fig. 3. Cross-neutralization of heterologous AIV strains by sera from VSV vaccinated mice.
Mice were vaccinated i.m. or i.n. with VSV-H5 HA (H5; n=6), VSV-ΔG-H5 HA (ΔG H5; n=6) or the negative control vector, VSV-Gag (Gag; n=3). At 1 month post-prime, animals were boosted with VSV-NJG-H5 HA (n=3) where indicated. At various times post-prime, sera from individual mice were analyzed for a neutralizing antibody response against the heterologous strains (A) VN/1203 (gray bars), INA/5 (black bars) (3 mo post-prime) or (B) HK/483 (5.5 mo post-prime). Each bar represents the neutralizing antibody titer from an individual mouse as determined by 100% inhibition of CPE in a microneutralization assay. The dotted horizontal line at a value of 10 represents the limit of detect for this assay. A value of 10 is considered negative. Asterisks (*) indicate mice that were not challenged with HK/483 (Fig. 4) because they died of unrelated causes (e.g. during anesthesia) prior to challenge.
We also examined whether vaccination with these vectors could induce a cross-neutralizing antibody response against another more closely related clade 3 virus, A/Hong Kong/483/97 (HK/483), a highly pathogenic avian influenza (HPAI) virus. HK/483 is much more pathogenic in mice than the homologous HK/156 strain (Lu et al., 1999), and was therefore more suitable for use in the subsequent challenge studies. A single vaccination elicited substantial cross-neutralizing antibody titers against HK/483 in individual mice that were enhanced by boosting (Fig. 3B, Table 1). The response generated against HK/483 (Fig. 3B) was greater than that seen against VN/1203 and INA/5 (Fig. 3A), but was lower than that raised against the homologous clade 3 strain, HK/156 (Fig. 2).
Table 1.
Serologic response to and protection from challenge with HK/483.
| Antigen* | nAb Titer† | % Wt Loss‡ | % Survival§ | P-value¶ | TTD (d)|| |
|---|---|---|---|---|---|
| H5** | 104 (16–640) | 2.9 (1.5–6.6) | 100 (13/13) | < 0.0001 | NA |
| H5 (Prime only) | 54 (16–254) | 2.4 (1.6–4.8) | 100 (6/6) | 0.0005 | NA |
| H5 (Prime + Boost) | 185 (101–640) | 3.4 (1.5–6.6) | 100 (7/7) | 0.0002 | NA |
| Gag | 10 (10) | 31.6 (25–34.3) | 0 (0/6) | NA | 8–9 |
| Naive | ND | 32.4 (30.5–34.5) | 0 (0/2) | NA | 8 |
Each row represents the combined data for both i.n. and i.m. routes and for all vaccine vectors expressing the indicated antigen.
Summary of individual mouse geometric mean neutralizing antibody (nAb) titers from Fig. 3B, 5.5 months post-prime; range of titers in parentheses.
Summary of Fig. 4 data as the geometric mean of maximum percent weight (Wt) loss; percent weight loss range in parentheses.
In parentheses, number of survivors over total number of animals per group.
When compared to the Gag control group (Logrank test).
TTD, time to death.
Sum of rows 2 [H5 (Prime only)] and 3 [H5 (Prime + Boost)].
Vaccination confers long-term protection against AIV challenge
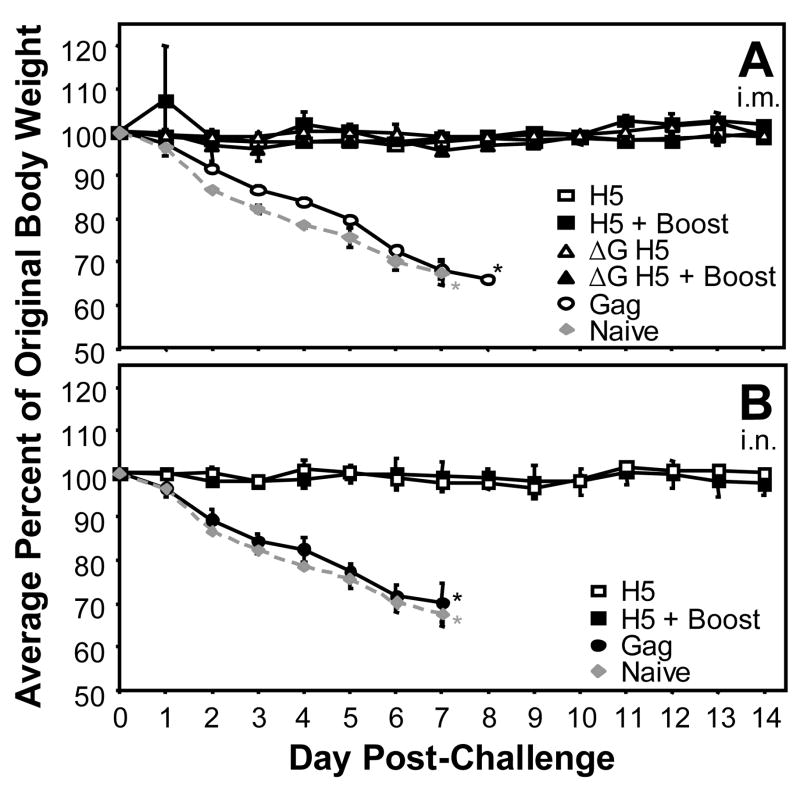
While several experimental vaccine vectors expressing the H5 HA show promise, to our knowledge no study has examined the long-term efficacy of vaccination in a mammalian model. Mice vaccinated with the rVSVs expressing the HK/156 H5 HA were challenged i.n. with the HPAI HK/483 at 7.5 months post-prime or 6.5 months post-boost in those animals that were boosted. The mice that were challenged were the same mice for which the immunogenicity of the vaccine vectors was determined (Figs. 2 and 3) except for 5 mice that died prior to challenge. Mice vaccinated with the negative control virus, VSV-Gag, lost approximately 30% of their body weight within the first week post-challenge (Fig. 4) and these mice died by 8–9 days post-challenge as did the naïve control mice (Fig. 4, Table 1). Mice that were vaccinated with any combination of the prime and boost vectors, including all mice receiving a single vaccine dose of either the replicating VSV-H5 HA or the single-cycle vector, VSVΔG-H5 HA, remained healthy throughout the duration of the experiment and did not exhibit any weight loss or other signs of disease (Fig. 4, Table 1). Survival was highly significant when vaccinated mice were compared to the VSV-Gag vaccinated control mice with P-values ranging from <0.0001 to 0.0005 (Table 1). The mean geometric titer against the challenge virus in these vaccinated animals was 83, ranging from 16 to 640 (Table 1). Even the animals with the lowest titers at 5.5 months post-prime (Fig. 3B, Table 1) were completely protected from challenge at 7.5 months post-prime (Fig. 4, Table 1). Complete protection was achieved by both i.m (Fig. 4A) and i.n. vaccination (Fig. 4B).
Fig. 4. Vaccination with VSV vectors expressing a H5 HA protects mice against a HPAI virus.
Balb/c mice were vaccinated (A) i.m. or (B) i.n. with VSV-H5 HA (squares; n=4 and n=5, respectively), VSVΔG-H5 HA (triangles; n=4) or VSV-Gag (circles; n=3). At 1 month post-prime, some mice were boosted with VSV-NJG-H5 HA [solid squares (A, n=2; B, n=3) and solid triangles (A, n=2; B, n=3)]. At 7.5 months post-prime, mice were challenged i.n. with 100 LD50 of HK/483. Naive mice (n=2) were also challenged (gray diamonds, dashed line). For comparison the same group of naive mice is depicted in both panels. Body weights and survival were assessed daily. *Termination of analysis due to death of animals.
DISCUSSION
The highly pathogenic nature of recently emerged H5N1 viruses, the continued spread through poultry populations, and the high mortality they cause in humans has generated major concern about a possible AIV pandemic. This concern has led to new efforts to develop AIV vaccines including development of non-traditional methods. VSV vaccine vectors expressing foreign antigens are capable of providing protection against a number of respiratory viral diseases, such as SARS (Kapadia et al., 2005), influenza (Roberts et al., 1999; Roberts et al., 1998), and RSV (Kahn et al., 2001). VSV vectors have shown promise as AIDS vaccines in a monkey model (Egan et al., 2004; Ramsburg et al., 2004; Rose et al., 2001) and highly attenuated VSVs expressing HIV antigens will soon be entering clinical trials as experimental AIDS vaccines.
We generated experimental VSV-based H5N1 AIV vaccines by inserting the H5 HA gene from the HK/156 strain into recombinant WT and single-cycle VSV vectors. We chose the H5 HA protein because the only major correlate of protection against influenza is antibody to its surface glycoproteins, and primarily to HA (Wright and Webster, 2001). Recovered rVSVs expressed full-length and cleaved HA in infected cells and the cleaved HA1 and HA2 were incorporated into the rVSV virions (Fig. 1). We assessed the serological response to vaccination in mice after i.n. and i.m. inoculation. Some mice received a single dose of vaccine while others received a boost with a serotype switch vector. We found that high neutralizing antibody titers were present in all mice receiving any combination of the H5-expressing rVSV vectors by either route. The titers peaked at about 3 months post-prime, but were still high after 5.5 months post-prime (Fig. 2).
Because influenza viruses undergo frequent mutation, it is important that any vaccine intended for pandemic influenza be able to cross-neutralize antigenically distinguishable AIVs of the same subtype. We found that our rVSV vectors elicited cross-neutralizing antibodies against three distinct H5N1 viruses, VN/1203, INA/5 and HK/483 (Fig. 3, Table 1) at levels suggestive of protection against these heterologous strains. The vaccinated mice were challenged at 7.5 months post-prime with a lethal dose of the HPAI HK/483. All vaccinees, including those that received a single dose of the single-cycle vector, survived challenge and were protected from AIV-associated disease (Fig. 4, Table 1), while all control mice died within 8–9 days post-challenge.
Our serologic data correlate with complete protection from pulmonary replication as seen in a previous study after administration of two doses of a cold-adapted (ca) H5N1 vaccine (Suguitan et al., 2006). The titers induced by the rVSV vaccines are higher than were achieved after two doses of H5N1 ca vaccine (Suguitan et al., 2006). Moreover, vaccination with rVSV expressing HA from the WSN strain shows that rVSV vaccination against influenza can completely prevent influenza replication in the lungs following challenge (Roberts et al., 1999). The prevention of AIV-associated weight loss and the high neutralizing antibody titers after rVSV vaccination in this study is suggestive of protection against replication. Direct assay of virus replication was not possible in the BSL-4 facility where the experiments were carried out and regulations prevented removal of samples for assay outside of the facility. Replication will therefore be addressed in future studies.
VSV-based AIV vaccine candidates have potential advantages over other vaccine candidates including ease of delivery by the intranasal route, negligible VSV seropositivity in humans, and production in cell lines already approved for manufacture of human vaccines rather than in eggs. A VSV-based vaccine also eliminates safety concerns about growing AIV-based vaccines, such as risk of exposure or accidental spread during production. Furthermore, VSV vectors are effective at relatively low doses and are typically effective, as seen here, after a single vaccination not requiring the use of an adjuvant.
Although WT VSV does not cause neurological disease in natural host animals, it can be neuroinvasive following intranasal inoculation of mice (Sabin and Olitsky, 1937). Safety concerns about use of replication competent VSV vectors have therefore been addressed. The prototype attenuated VSV vector (Clarke et al., 2006) is not neuroinvasive in non-human primates after intranasal administration (Johnson et al., 2006). Furthermore, more highly attenuated live VSV vectors have been generated that lack neurovirulence in non-human primates following direct intracranial (thalamic) inoculation (Johnson et al., 2006). Also, the vector we described here lacking the VSV G gene eliminates concerns about neurovirulence because it replicates for only a single cycle.
Another potential safety concern arises from the fact that the H5 HA protein is incorporated into VSV virions. However, we have been unable to demonstrate any infectivity of the viruses due to the H5 HA protein in the vectors expressing HA. Because influenza virus requires the receptor-destroying activity of NA for propagation (Lamb and Krug, 2001), the absence of the influenza NA gene in our constructs also predicts lack of pathogenicity due to HA incorporation into virions. We are currently generating VSV recombinants expressing soluble, secreted forms of H5 HA that could not be incorporated into virion membranes. Such recombinants would provide an additional safety factor, and could potentially be just as effective at generating neutralizing antibody to influenza. VSV-based vectors have excellent potential as vaccines protecting against pandemic AIV and warrant further development.
MATERIALS AND METHODS
Cells and Viruses
BHK-21 and BHK-G (Schnell et al., 1997) cells were grown in Dulbecco’s Modified Essential Medium (DMEM) containing 5% fetal bovine serum (FBS) and 1X Pen-Strep. The BHK-G cell maintenance media was supplemented with 75 μg/ml G418 and 5 ng/ml tetracycline (Tet). MDCK cells were propagated in MEM/BSA [minimal essential medium (MEM) supplemented with 0.3% bovine serum albumin (BSA) and 1X Pen-Strep].
Recombinant VSVs were grown and titrated by plaque assay on BHK-21 or BHK-G cells as previously described (Lawson et al., 1995; Schnell et al., 1997). The VSVΔG-H5 HA stock used for vaccination was resuspended in PBS after centrifugation of the infected cell media at 25,000 rpm in a Beckman SW28 rotor (112,398 × g) for 1 h at 4°C. The negative control rVSV vector, VSV-Gag, containing the SIV Gag gene in the fifth VSV genome position has been described previously (Rose et al., 2001).
The AIVs, A/Hong Kong/156/1997 (HK/156), A/Hong Kong/483/1997 (HK/483), A/Vietnam/1203/2004 (VN/1203), and A/Indonesia/5/2005 (INA/5), were kindly provided by Drs. Nancy Cox and Alexander Klimov, Influenza Branch, Centers for Disease Control and Prevention, Atlanta, GA, and were propagated in the allantoic cavity of specific pathogen-free eggs as described previously (Suguitan et al., 2006). HK/483 used for mouse challenge experiments was grown and titrated by plaque assay on MDCK cells in media supplemented with 1.0 μg/ml TPCK-treated trypsin.
Construction and recovery of recombinant VSV vectors
The H5 HA gene (Accession #AF046088; kindly provided by Y. Kawaoka, University of Wisconsin, Madison, WI) was amplified by PCR with the primers 5′-GGACCCGGGAAAATGGAGAAAACAGTGCTTCTTC-3′ and 5′-GGACTCGAGATCGATCTCTGTTAGTTTTTTCATACCTTAAATGCAAATTCTGCATTG-3′ that introduce 5′ XmaI and 3′ XhoI sites (underlined), respectively, and then cloned into XmaI-XhoI digested pBlueScript II SK to generate pBS-H5. The H5 HA gene was amplified by PCR from pBS-H5 using the primers, 5′-GATCGATCCTCGAGATCATGGAGAAAACAGTGCTTCTTCTTGC-3′ and 5′-GAAATACTACGCTAGCTTAAATGCAAATTCTGCATTGTAAAC-3′ that introduce 5′ XhoI and 3′ NheI sites (underlined), respectively. The XhoI-NheI digested PCR product was cloned into the XhoI and NheI sites of the pVSV-XN2 (Schnell et al., 1996) and pVSV-NJG-XN (Rose et al., 2000) vectors to generate pVSV-H5 HA and pVSV-NJG-H5 HA, respectively. The pVSVΔG-H5 vector was generated by replacing the HA gene (A/WSN strain) from pVSVΔG-HA (Roberts et al., 1999) with the H5 HA PCR product.
The recombinant VSVs, VSV-H5 HA, and VSV-NJG-H5 HA, were recovered using the above plasmids as previously described (Lawson et al., 1995). Briefly, BHK-21 cells were infected with the T7 polymerase expressing vaccinia virus, vTF7-3 (Fuerst et al., 1986), at a MOI of 10. At 1 h post-infection (hpi), the cells were transfected with the appropriate H5 HA plasmids plus the support plasmids, pBS-N, pBS-P, pBS-G and pBS-L. The cell culture media was collected, filtered through a 0.2 μm filter and passaged onto BHK-21 cells 48 h post-transfection. Once CPE was observed the culture media was filtered through a 0.1 μm filter, plaque purified and used to grow up virus stocks. Growth and recovery of VSVΔG-H5 HA was achieved by complementation from BHK-G cells expressing VSV G (Schnell et al., 1997). In order to optimize titers, either BHK-G cells or BHK-21 cells transiently expressing G from pCAGGS-G (Publicover, Ramsburg, and Rose, 2005) were used.
Western blot analysis
(i) Cell extracts
BHK-21 cells were infected with the indicated virus. At 4.5 hpi, cells were washed twice with ice-cold PBS and incubated with lysis buffer (1% NP-40, 0.4% deoxycholate, 62.5 mM EDTA, 50 mM Tris-HCl pH 8) for 5 min on ice.
(ii) VSV virions
The culture media of infected cells exhibiting 100% CPE was clarified by low speed centrifugation, layered on top of 20% sucrose in TE (pH 7.4) and subjected to ultracentrifugation in a Beckman SW50.1 rotor at 38,000 rpm (173,069 × g) for 1 h at 4°C. The pellet was then resuspended in lysis buffer.
(iii) SDS-PAGE/Western blot
Infected cell or virion lysates were combined with SDS-sample buffer [250 mM Tris-HCl pH 6.8, 30% glycerol (v/v), 8% SDS, 0.02% bromophenol blue, 10% 2-mercaptoethanol(v/v)] and subjected to SDS-PAGE on a 10% acrylamide gel. The proteins were transferred to nitrocellulose with a semi-dry transfer apparatus (Bio-Rad Laboratories, Inc., Hercules, CA) according to the manufacturer’s instructions. The blots were incubated overnight at 4°C in PBST-milk [PBS containing 0.5% Tween-20 (PBST) and 4% non-fat milk] and then in a 1:1,000 dilution of NR-665 polyclonal anti-influenza virus H5 HA A/Hong Kong/156(483)/97 sheep antiserum in PBST-milk for 1 h at room temperature. The NR-665 antibody was obtained through the NIH Biodefense and Emerging Infections Research Repository, NIAID, NIH. After washing 3 times with PBST, the blots were incubated with donkey anti-sheep IgG conjugated to HRP (Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:10,000 dilution in PBST-milk for 1 h. The blots were washed as before and incubated with a 1:1 mixture of ECL western blotting detection reagents (GE Healthcare, Piscataway, NJ). Chemiluminescence was detected using a LAS-3000 imaging system (Fujifilm). The blots were then incubated in stripping buffer (62.5 mM Tris-HCl pH 6.8, 2% SDS, 100 mM 2-mercaptoethanol) for 30 min at 50°C, washed several times with PBS, and probed for VSV proteins as described above using a rabbit polyclonal anti-VSV antibody at a 1:1,000 dilution and mouse anti-rabbit IgG conjugated to HRP (Pierce Biotechnology, Rockford, IL) at a 1:10,000 dilution.
Vaccinations
The Yale University Institutional Animal Care and Use Committee approved all vaccinations. Six to eight week old Balb/c female mice (Charles River Laboratories, Wilmington, MA) were primed intramuscularly (i.m.) with 50 μl or intranasally (i.n.) with 25 μl containing 106 plaque forming units (pfu) of VSV-H5 HA in DMEM or approximately 106 pfus of VSVΔG-H5 HA in PBS. Mice vaccinated i.n. were lightly anesthetized with 20% isoflurane (v/v; Baxter, Deerfield, IL) in propylene glycol prior to vaccination. At 1 month post-prime, half of the mice were boosted i.m. or i.n. with 106 pfus of VSV-NJG-H5 HA in DMEM as described above.
Microneutralization assay
Anti-H5 HA neutralizing antibody titers were determined as previously described (Suguitan et al., 2006). Briefly, serum obtained from vaccinated mice was heat inactivated at 56°C for 30–60 min and subjected to serial 2-fold dilutions starting at a 1:10 dilution. Equal volumes of 100 TCID50 of the indicated virus and diluted serum were combined, incubated at room temperature for 1 h, and added to MDCK cells in quadruplicate. Neutralizing titers are represented as the reciprocal dilution at 100% neutralization as defined by the absence of CPE at 4 days. Samples that did not display any neutralizing activity were arbitrarily given a value of 10. These assays were conducted using enhanced BSL-3 containment procedures in laboratories approved for use by the U.S. Department of Agriculture and Centers for Disease Control and Prevention.
H5N1 challenge
At 7.5 months post-prime, vaccinated mice were i.n. challenged with 100 LD50 (4.5 pfu) of HK/483 in 50 μl MEM/BSA. The LD50 was determined by the Reed and Muench method (Reed and Muench, 1938). Mice were weighed daily and analyzed for disease for 14 days. Mice exhibiting severe morbidity were humanely euthanized. Data are shown as the average percent of original pre-challenge weight. The challenge was performed using BSL-4 containment procedures under an approved animal use protocol and according to the guidelines of the Canadian Council on Animal Care.
Acknowledgments
We thank Kimberly L. Mills, NIAID, for technical support for microneutralization assays performed at the NIH. This study was supported in part by the following: NIH grant AI057158 (J.K.R.); Cancer Research Institute Postdoctoral Fellowship (A.R.); the Public Health Agency of Canada; and the Intramural Research Program of the NIH, NIAID.
Abbreviations
- AIV
avian influenza virus
- HA
hemagglutinin
- HK/156
AIV strain A/Hong Kong/156/1997
- HK/483
AIV strain A/Hong Kong/483/1997
- HPAI
highly pathogenic influenza virus
- i.m
intramuscular
- i.n
intranasal
- INA/5
AIV strain A/Indonesia/5/2005
- NA
neuraminidase
- VSV
vesicular stomatitis virus
- rVSV
recombinant VSV
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Evolution of H5N1 avian influenza viruses in Asia. Emerg Infect Dis. 2005;11(10):1515–21. doi: 10.3201/eid1110.050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandsma JL, Shlyankevich M, Buonocore L, Roberts A, Becker SM, Rose JK. Therapeutic efficacy of vesicular stomatitis virus-based E6 vaccination in rabbits. Vaccine. 2007;25(4):751–62. doi: 10.1016/j.vaccine.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Chen H, Smith GJ, Li KS, Wang J, Fan XH, Rayner JM, Vijaykrishna D, Zhang JX, Zhang LJ, Guo CT, Cheung CL, Xu KM, Duan L, Huang K, Qin K, Leung YH, Wu WL, Lu HR, Chen Y, Xia NS, Naipospos TS, Yuen KY, Hassan SS, Bahri S, Nguyen TD, Webster RG, Peiris JS, Guan Y. Establishment of multiple sublineages of H5N1 influenza virus in Asia: implications for pandemic control. Proc Natl Acad Sci U S A. 2006;103(8):2845–50. doi: 10.1073/pnas.0511120103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DK, Nasar F, Lee M, Johnson JE, Wright K, Calderon P, Guo M, Cooper D, Hendry RM, Udem SA. Synergistic Attenuation of Vesicular Stomatitis Virus by Combination of Specific G Gene Truncations and N Gene Translocations. J Virol. 2006 doi: 10.1128/JVI.01911-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daddario-DiCaprio KM, Geisbert TW, Geisbert JB, Stroher U, Hensley LE, Grolla A, Fritz EA, Feldmann F, Feldmann H, Jones SM. Cross-protection against Marburg virus strains by using a live, attenuated recombinant vaccine. J Virol. 2006;80 (19):9659–66. doi: 10.1128/JVI.00959-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MA, Chong SY, Rose NF, Megati S, Lopez KJ, Schadeck EB, Johnson JE, Masood A, Piacente P, Druilhet RE, Barras PW, Hasselschwert DL, Reilly P, Mishkin EM, Montefiori DC, Lewis MG, Clarke DK, Hendry RM, Marx PA, Eldridge JH, Udem SA, Israel ZR, Rose JK. Immunogenicity of attenuated vesicular stomatitis virus vectors expressing HIV type 1 Env and SIV Gag proteins: comparison of intranasal and intramuscular vaccination routes. AIDS Res Hum Retroviruses. 2004;20(9):989–1004. doi: 10.1089/aid.2004.20.989. [DOI] [PubMed] [Google Scholar]
- Fouchier RA, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, Rimmelzwaan GF, Olsen B, Osterhaus AD. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol. 2005;79 (5):2814–22. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst TR, Niles EG, Studier FW, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986;83(21):8122–6. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt M, Liebscher R, Wahl-Jensen V, Jones S, Moller P, Wagner R, Volchkov V, Klenk HD, Feldmann H, Stroher U. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol. 2004;78(10):5458–65. doi: 10.1128/JVI.78.10.5458-5465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, Deng G, Wen Z, Tian G, Wang Y, Shi J, Wang X, Li Y, Hu S, Jiang Y, Yang C, Yu K, Bu Z, Chen H. Newcastle disease virus-based live attenuated vaccine completely protects chickens and mice from lethal challenge of homologous and heterologous H5N1 avian influenza viruses. J Virol. 2007;81(1):150–8. doi: 10.1128/JVI.01514-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillim-Ross L, Subbarao K. Emerging respiratory viruses: challenges and vaccine strategies. Clin Microbiol Rev. 2006;19(4):614–36. doi: 10.1128/CMR.00005-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horimoto T, Kawaoka Y. Strategies for developing vaccines against H5N1 influenza A viruses. Trends Mol Med. 2006;12(11):506–14. doi: 10.1016/j.molmed.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Nasar F, Coleman JW, Price RE, Javadian A, Draper K, Lee M, Reilly PA, Clarke DK, Hendry RM, Udem SA. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology. 2006 doi: 10.1016/j.virol.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Feldmann H, Stroher U, Geisbert JB, Fernando L, Grolla A, Klenk HD, Sullivan NJ, Volchkov VE, Fritz EA, Daddario KM, Hensley LE, Jahrling PB, Geisbert TW. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11(7):786–90. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- Kahn JS, Roberts A, Weibel C, Buonocore L, Rose JK. Replication-competent or attenuated, nonpropagating vesicular stomatitis viruses expressing respiratory syncytial virus (RSV) antigens protect mice against RSV challenge. J Virol. 2001;75 (22):11079–87. doi: 10.1128/JVI.75.22.11079-11087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia SU, Rose JK, Lamirande E, Vogel L, Subbarao K, Roberts A. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology. 2005;340(2):174–82. doi: 10.1016/j.virol.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JM, Lim W, Bridges CB, Rowe T, Hu-Primmer J, Lu X, Abernathy RA, Clarke M, Conn L, Kwong H, Lee M, Au G, Ho YY, Mak KH, Cox NJ, Fukuda K. Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti-H5 antibody among household and social contacts. J Infect Dis. 1999;180(6):1763–70. doi: 10.1086/315137. [DOI] [PubMed] [Google Scholar]
- Lamb R, Krug R. Orthomyxoviridae: The Viruses and Their Replication. In: Knipe D, Howley P, editors. Fields’ Virology. Lippencott-Raven; Philadelphia: 2001. pp. 1487–1524. [Google Scholar]
- Lawson ND, Stillman EA, Whitt MA, Rose JK. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci U S A. 1995;92(10):4477–81. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Tumpey TM, Morken T, Zaki SR, Cox NJ, Katz JM. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J Virol. 1999;73(7):5903–11. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MS, Steel J, Garcia-Sastre A, Swayne D, Palese P. Engineered viral vaccine constructs with dual specificity: avian influenza and Newcastle disease. Proc Natl Acad Sci U S A. 2006;103(21):8203–8. doi: 10.1073/pnas.0602566103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Publicover J, Ramsburg E, Rose JK. Characterization of nonpathogenic, live, viral vaccine vectors inducing potent cellular immune responses. J Virol. 2004;78(17):9317–24. doi: 10.1128/JVI.78.17.9317-9324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Publicover J, Ramsburg E, Rose JK. A single-cycle vaccine vector based on vesicular stomatitis virus can induce immune responses comparable to those generated by a replication-competent vector. J Virol. 2005;79(21):13231–8. doi: 10.1128/JVI.79.21.13231-13238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsburg E, Rose NF, Marx PA, Mefford M, Nixon DF, Moretto WJ, Montefiori D, Earl P, Moss B, Rose JK. Highly effective control of an AIDS virus challenge in macaques by using vesicular stomatitis virus and modified vaccinia virus Ankara vaccine vectors in a single-boost protocol. J Virol. 2004;78(8):3930–40. doi: 10.1128/JVI.78.8.3930-3940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L, Muench H. A Simple Method of Estimating Fifty Percent Endpoints. Amer J Hyg. 1938;27:493–7. [Google Scholar]
- Reuter JD, Vivas-Gonzalez BE, Gomez D, Wilson JH, Brandsma JL, Greenstone HL, Rose JK, Roberts A. Intranasal vaccination with a recombinant vesicular stomatitis virus expressing cottontail rabbit papillomavirus L1 protein provides complete protection against papillomavirus-induced disease. J Virol. 2002;76(17):8900–9. doi: 10.1128/JVI.76.17.8900-8909.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Buonocore L, Price R, Forman J, Rose JK. Attenuated vesicular stomatitis viruses as vaccine vectors. J Virol. 1999;73(5):3723–32. doi: 10.1128/jvi.73.5.3723-3732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Kretzschmar E, Perkins AS, Forman J, Price R, Buonocore L, Kawaoka Y, Rose JK. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J Virol. 1998;72(6):4704–11. doi: 10.1128/jvi.72.6.4704-4711.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Reuter JD, Wilson JH, Baldwin S, Rose JK. Complete protection from papillomavirus challenge after a single vaccination with a vesicular stomatitis virus vector expressing high levels of L1 protein. J Virol. 2004;78(6):3196–9. doi: 10.1128/JVI.78.6.3196-3199.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J, Whitt M. Rhabdoviridae: The Viruses and Their Replication. In: Knipe D, Howley P, editors. Fields’ Virology. Lippencott-Raven; Philadelphia: 2001. pp. 1221–40. [Google Scholar]
- Rose NF, Marx PA, Luckay A, Nixon DF, Moretto WJ, Donahoe SM, Montefiori D, Roberts A, Buonocore L, Rose JK. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell. 2001;106(5):539–49. doi: 10.1016/s0092-8674(01)00482-2. [DOI] [PubMed] [Google Scholar]
- Rose NF, Roberts A, Buonocore L, Rose JK. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. J Virol. 2000;74(23):10903–10. doi: 10.1128/jvi.74.23.10903-10910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin A, Olitsky P. Influence of host factors on neuroinvasiveness of vesicular stomatitis virus. J Exp Med. 1937;66:15–34. doi: 10.1084/jem.66.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlereth B, Rose JK, Buonocore L, ter Meulen V, Niewiesk S. Successful vaccine-induced seroconversion by single-dose immunization in the presence of measles virus-specific maternal antibodies. J Virol. 2000;74(10):4652–7. doi: 10.1128/jvi.74.10.4652-4657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell MJ, Buonocore L, Whitt MA, Rose JK. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J Virol. 1996;70(4):2318–23. doi: 10.1128/jvi.70.4.2318-2323.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell MJ, Johnson JE, Buonocore L, Rose JK. Construction of a novel virus that targets HIV-1-infected cells and controls HIV-1 infection. Cell. 1997;90(5):849–57. doi: 10.1016/s0092-8674(00)80350-5. [DOI] [PubMed] [Google Scholar]
- Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279(5349):393–6. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- Suguitan AL, Jr, McAuliffe J, Mills KL, Jin H, Duke G, Lu B, Luke CJ, Murphy B, Swayne DE, Kemble G, Subbarao K. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006;3(9):e360. doi: 10.1371/journal.pmed.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungchusak K, Auewarakul P, Dowell SF, Kitphati R, Auwanit W, Puthavathana P, Uiprasertkul M, Boonnak K, Pittayawonganon C, Cox NJ, Zaki SR, Thawatsupha P, Chittaganpitch M, Khontong R, Simmerman JM, Chunsutthiwat S. Probable person-to-person transmission of avian influenza A (H5N1) N Engl J Med. 2005;352(4):333–40. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- Veits J, Wiesner D, Fuchs W, Hoffmann B, Granzow H, Starick E, Mundt E, Schirrmeier H, Mebatsion T, Mettenleiter TC, Romer-Oberdorfer A. Newcastle disease virus expressing H5 hemagglutinin gene protects chickens against Newcastle disease and avian influenza. Proc Natl Acad Sci U S A. 2006;103(21):8197–202. doi: 10.1073/pnas.0602461103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P, Webster R. Orthomyxoviruses. In: Knipe D, Howley P, editors. Fields’ Virology. Lippencott-Raven; Philadelphia: 2001. pp. 1533–79. [Google Scholar]