Abstract
Introduction
Registries are becoming increasingly important for rare diseases as experimental therapies develop. This report describes the methodology behind the National Registry of Myotonic Dystrophy (DM) and Facioscapulohumeral Muscular Dystrophy (FSHD) Patients and Family Members to facilitate the development of other rare disease registries. We also highlight data about the pathophysiology and select burdens of DM and FSHD reported at baseline and longitudinally.
Methods
The Registry consists of de-identified, patient reported information collected at baseline and annually and information from review of medical records. Investigators can use the Registry to analyze de-identified data and to facilitate recruitment into clinical studies.
Results
To date, the Registry has enrolled 1611 members, facilitated 24 studies, and collected data annually for up to 8 years. Genetic test results were obtained in 56.2% of enrollees. Approximately one-third of members used assistive devices and another one-third reported psychological problems at baseline. Wheelchair use was reported for both short and long distances by 7.0% of DM and 18.1% of FSHD members. Approximately 60% of members reported their employment was affected by their disease.
Conclusions
Strengths of the Registry include large sample sizes, stringent review of clinical and molecular data, annually updated information, and regular interactions between patients and investigators. Registry data provide new insights into the burdens of DM and FSHD, such as, psychological problems and reduced employment. Opportunities abound for investigators to utilize Registry resources to assess the impact of these and other burdens on health care costs, progression of symptoms, and quality of life.
Keywords: Myotonic dystrophy, Facioscapulohumeral muscular dystrophy, Registry, Disease progression, Burdens of disease
1. Introduction
Myotonic dystrophy (DM) and facioscapulohumeral muscular dystrophy (FSHD) are the most common adult muscular dystrophies [1–3]. These diseases are inherited, cause slowly progressive muscle weakness, and are often disabling. DM consists of two forms classified as myotonic dystrophy type 1 (DM1) and myotonic dystrophy type 2 (DM2). Symptoms of both subtypes include delayed muscle relaxation (myotonia), muscle weakness, and complications involving the eyes, the brain, smooth muscles, and cardiovascular and endocrine systems [1,4–6]. Distinguishing clinical features are greater distal muscle weakness and earlier onset of symptoms in DM1 and greater proximal weakness with less muscle wasting in DM2 [7,8]. In addition, a congenital form occurs in DM1 but not DM2 [1].
Both subtypes are genetic repeat disorders, in which repeats of DNA in a certain gene are above the normal threshold. DM1 results from a repetitive expansion of the nucleotides cytosine (C), thymine (T), and guanine (G) [9–11]. Healthy cells have 5–37 sequences of these CTG repeats, whereas DM1 patients have 50 to more than 2000 of these repeats. Myotonic dystrophy type 2 (DM2) results from a repetitive expansion of four nucleotides, CCTG [12,13]. Information on how these genetic repeats cause the symptoms of DM is discussed elsewhere [14–16]. The estimated prevalence of DM1 is 1 in 8000, and recent evidence suggests DM2 is similar to or more prevalent than DM1 in certain countries [1,17].
The second most common adult muscular dystrophy is FSHD with an estimated prevalence of 1 in 15,000 to 20,000 [2,18]. FSHD in almost all cases is caused by a genetic mutation that leads to shortened fragments of DNA on chromosome 4. Recent data suggest a unified pathogenic model of FSHD [19]. The symptoms of FSHD include a distinctive pattern of muscle weakness that affects facial and scapular fixator muscles [3,20,21]. Muscle wasting often progresses slowly and typically in a descending pattern to include distal anterior leg and hip girdle muscles. Patients may have hearing loss and retinal vascular pathology [22].
Despite published studies relevant to the natural history of FSHD [23] and DM [24–27], precise information about the long-term rate of progression of symptoms remains limited. In addition to the rarity of these diseases, there is a lack of agreement on the most appropriate endpoint measures to assess long term progression and which biomarkers are informative, cost-effective, and correlate best with specific disease manifestations and patient-reported outcomes of well-being and burdens of disease [28].
To meet these needs in DM, FSHD, and other rare diseases, the Office of Rare Diseases at the National Institutes of Health (NIH) has emphasized the importance of developing registries to accelerate translational research and to connect patients with investigators [29–31]. The primary goals of this paper are to: 1.) describe the methods of the National Registry to facilitate the development of other rare disease registries and guide the creation of international registries in DM and FSHD; and 2.) present the demographic and genetic characteristics of current members as well as baseline and longitudinal data on selected burdens of disease.
2. Materials and methods
2.1. Purpose of Registry
The goals of the Registry are to assist researchers in the recruitment of patients into clinical studies, to develop an extensive database of de-identified patient information, and to allow for the analysis of data related to the pathophysiology and clinical spectrum of disease manifestations in DM and FSHD.
2.2. Organization and leadership
The Registry is funded by the National Institute of Arthritis, Musculoskeletal, and Skin Diseases and the National Institute of Neurological Disorders and Stroke. Registry leadership consists of two NIH Project Officers, an Internal Steering Committee (principal and co-investigators based at the University of Rochester), and an External Steering Committee. The Internal and External Steering Committees comprise the Scientific Advisory Committee, and its members include scientists and clinicians who are recognized experts in neuro-muscular medicine as well as patient advocates. Principal and co-investigators have presented and shared information about the Registry with colleagues in Australia, Peru, Canada, Germany, Italy, Netherlands, and the United Kingdom [32,33]. In addition, the principal investigator of the Registry currently serves as an advisory member for the Office of Rare Diseases Research initiatives to establish a Global Rare Diseases Patient Registry and Data Repository, and most recently served as one of the key-note speakers at a workshop about model informed consents. Proceedings from this meeting will be published as guidelines for obtaining informed consents from subjects enrolled in registries (http://rarediseases.info.nih.gov/PatientRegistry.aspx).
2.3. Patients and data collection
The Research Subjects Review Board at the University of Rochester Medical Center reviews and approves all Registry materials and activities. Patients in the United States are recruited to join the Registry through a variety of methods including a public website (www.dystrophyregistry.org). Patients, investigators, and physicians also learn about the Registry through recruitment letters sent annually to neuromuscular disease specialists, advertisements through patient advocacy groups, advertisements on NIH-affiliated and patient support group websites, and presentations at national and international research and medical conferences. In accordance with NIH and World Health Organization guidelines, the Registry is listed on ClinicalTrials.gov (http://clinicaltrials.gov/ct2/show/NCT00082108), which is a website that “describes federally and privately supported clinical trials conducted in the United States and around the world.”
Patients can obtain Registry forms from neuromuscular clinics, by printing forms from the Registry website, or by contacting the Registry staff (e.g., email, toll-free phone call) and requesting that an application packet be mailed to them. The application packet to join the Registry includes:
Patient Information Form, a disease-specific questionnaire that captures patient-reported information such as demographics, education and employment, use of assistive devices, physical limitations, medical history, diagnostic tests, rehabilitation therapies received, and other medical problems (http://www.urmc.rochester.edu/neurology/nih-registry/join/index.cfm).
Release of Medical Information Form, which allows the Registry to obtain medical records to confirm applicants’ diagnoses.
Consent Form, which describes the purpose of the Registry and the benefits and risks of participation.
All the forms associated with the Registry are paper-based. Patients complete each of the forms listed above and send the application packet to Registry staff in a postage paid envelope. Submitted applications are reviewed by Registry staff to ensure that all forms have been completed adequately. Once an application is complete, medical records for the patient are requested from the patient’s care providers and reviewed by Registry staff and investigators.
2.4. Eligibility and classification of enrollees
Eligibility is determined when the Principal Investigator or Co-investigators have confirmed an enrollee’s consent, reviewed their Patient Information Form, and verified a diagnosis through medical record review. In order to increase recruitment opportunities, genetic confirmation of the disease is not required for enrollment in the Registry. The clinical and genetic criteria for enrollment are outlined in a disease-specific Physician Checklist Form to facilitate determination of eligibility (www.urmc.rochester.edu/neurology/nih-registry/research/investigators.cfm). Members of the Registry are classified as:
Definite DM or FSHD: Individuals with diagnostic genetic testing according to current guidelines for DM and FSHD [32,34,35] are classified as “definite” DM or FSHD.
Probable DM or FSHD: Individuals without genetic testing but who meet the defined clinical criteria [1,2,23,35–37] are classified as “probable” DM or FSHD.
Possible DM or FSHD: Individuals with symptoms suggesting DM or FSHD but without detailed clinical and genetic testing information are classified as possible DM or FSHD.
Unaffected Family: Unaffected blood relatives and spouses complete an abbreviated Patient Information Form that collects demographic information, family history, and documents if any diagnostic tests have been completed to rule out DM or FSHD.
The Physician Checklist Forms were developed by the Scientific Advisory Committee and capture information on the patient’s pattern of muscle weakness, diagnostic muscle tests (e.g., electromyography and muscle biopsy results), and diagnostic genetic testing. Several categories are used to classify enrollees because of the variety and complexity of symptoms, onset and subtypes of DM and FSHD, variability in clinical and diagnostic procedures, regional health care differences, the quality of medical records available for review, and the inclusion of unaffected family members. A member’s classification is updated if additional diagnostic information becomes available through follow-up data collection.
Benefits to patients who join the Registry include receiving notification of research studies, learning about advances in the knowledge of their disease, and connecting with experts in neuromuscular disease.
2.5. Data entry, annually updated forms, and security
Data from the two disease groups are stored in separate Microsoft Access databases. These databases are very similar in structure, yet contain some differences due to the capture of disease-specific information. After initial contact is made with applicants, Registry staff enter demographic data and the dates the application and medical records were received. After the Principal and Co-investigators have reviewed the medical records and completed the Physician Checklist Form to determine eligibility, Registry staff enter information from both the Patient Information Form and the Physician Checklist Form into the Registry database.
Annual update forms are sent to members and include information reported in the member’s baseline Patient Information Form and changes from any previously returned forms. Each year members can record changes in their symptoms and any significant milestones in their lives on these forms, which are mailed back to the Registry in a postage paid envelope. These follow-up data allow investigators to track disease-related changes from year to year and enable investigators to have the most up-to-date information available to target recruitment.
The database of the Registry is stored on file servers maintained by the University of Rochester Information Systems Division. This group is responsible for the security of all University network servers, including firewalls, virus checking, network and workstation access passwords, and backup and disaster recovery. In addition to these security layers, the University of Rochester Neuromuscular Center further safeguards data privacy by requiring individual application passwords and restricting access of confidential data to only those staff members with a direct need. Individual identifiers are limited to a single data table. All other files are linked by a non-informative, random identifier.
The underlying source documents (consent, medical records, patient forms) are similarly protected. They are locked in file cabinets in secured areas. File folders are stored in ID number order (rather than by name) to further protect them from unauthorized access.
2.6. Review of research applications and release of information
Investigators are required to submit a brief research protocol and application form to analyze de-identified data from the Registry database or to recruit members of the Registry to participate in clinical studies. These application forms are available on the Registry website or by contacting Registry leaders or staff. Investigators must also submit confirmation of necessary approvals from their Institutional Review Board. Submitted applications are then reviewed anonymously by members of the Scientific Advisory Committee to determine the scientific merit, feasibility, and safety of the study.
Once an application is approved, Registry staff and leadership work closely with the investigator to facilitate their research plans. To assist recruitment for an approved project, a list of Registry members potentially eligible to participate is compiled based upon the inclusion and exclusion criteria submitted by the researcher. The Registry then sends a letter to each potentially eligible member of the Registry to describe and provide contact information for the study. Registry members are invited to contact the investigator if they have questions or if they are interested in participating in the study. The Registry does not provide any identifiable patient data directly to investigators. At the conclusion of the study, researchers are required to report the number of participants that were recruited through the Registry and to acknowledge the Registry if results are published. Results may not be published for studies or surveys that are not research (e.g., surveys to improve clinic experience or educational outreach) or if manuscripts are rejected.
To provide investigators with de-identified data for an approved research application, Registry staff review the inclusion and exclusion criteria in the protocol and develop data reports based upon all eligible members in the Registry. Registry staff can provide assistance with clarifying the data and highlighting variables that may correspond to the specific objectives in the research protocol, but the statistical analysis and summaries are performed by the investigator. Researchers are required to acknowledge the Registry if the results are published.
2.7. Analyses of data at time of enrollment and after follow-up period
We analyzed data from all members of the Registry who have been classified with the following diagnostic categories from the Physician Checklist Form: definite, probable, congenital, and juvenile DM1; definite and probable DM2; and definite and probable FSHD. Congenital DM1 was defined as onset of DM symptoms at age<4 weeks based on retrospective chart review [1]. Juvenile or childhood DM1 was defined as onset of DM symptoms at age<10 years and an uneventful prenatal and neonatal history, and normal physical development in the first year based on retrospective chart review [38].
Select burdens of disease were analyzed at enrollment and at follow-up for members who met the following criteria: a.) 18 yrs and older, b.) returned at least one annual update form; and c.) met the inclusion criteria noted above from the Physician Checklist Form. Juvenile and congenital DM members were excluded from these analyses. Variables analyzed were use of assistive devices (cane, leg brace, and wheelchair/scooter); use of therapies (occupational, physical, and psychological counseling); and psychosocial burdens (reduced employment, disability due to disease manifestations, and psychological problems).
Short-term progression of these burdens of disease was determined by comparing information collected at enrollment to information reported by patients on their most recently completed annual update form. We report the mean length of time and the range for the follow-up period (in years). Data are summarized as the percentage of patients who reported: 1.) no change; and 2.) increased values (for example, requiring a new assistive device).
Data are reported as means±standard deviations, frequencies, and percentages. We used T-tests and chi-square tests to compare results among disease groups. Comparisons were made between DM1 and DM2 patients, DM1 and FSHD patients, and DM2 and FSHD patients. Pearson correlations were used to examine the associations between genetic testing results and clinical characteristics. Significance was set at α = 0.05.
3. Results
3.1. Subject characteristics
3.1.1. Recruitment
Overall, 2373 applications have been mailed to interested individuals or downloaded from the Registry website; 1851 (78.0%) applications have been returned. Of the people who returned applications, 1611 (87.0%) were eligible for enrollment. The remaining 13.0% of people who submitted applications were not enrolled due to alternative diagnoses or a lack of response to requests for missing application materials. Patients and unaffected family members learned about the Registry most often through the Registry’s website (26.6%), their physician (16.7%), and friends and family (18.5%).
3.1.2. Enrollment
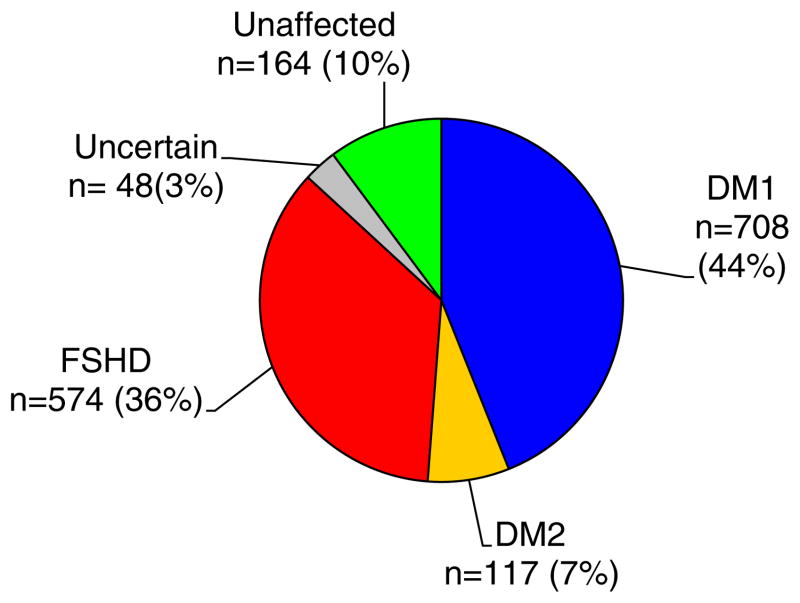
The Registry has enrolled 1611 members since enrollment began in 2002 (Fig. 1). The demographic results indicate that women accounted for a slightly greater percentage of enrollees in all disease groups (Table 1). Patients from every state have enrolled in the Registry with highest representation in the South (29.4%), followed by the Northeast (25.0%), Midwest (24.7%), and West (20.9%).
Fig. 1.
Data summarizes the classification of the 1611 affected members of the Registry (frequency and percent of total enrollment).
Table 1.
Data summarizes the classification of the 1,611 affected members of the Registry (frequency and percent of total enrollment).
| DM1 (n=622) | Congenital and childhood DM1 (n=86) | DM2 (n=117) | FSHD (n=574) | |
|---|---|---|---|---|
| Mean (±standard deviation) | ||||
| Enrollment age (yrs) | 43.5 (12.1) | 12.8 (9.9) | 54.3 (12.4) | 46.1 (15.9) |
| Age at onset (yrs) | 25.9 (13.0) | 3.3 (4.9) | 33.5 (13.9) | 21.5 (15.0) |
| Body Mass Index (kg/m2) | 25.2 (5.6) | 19.6 (6.0) | 26.9 (5.2) | 24.9 (5.6) |
| % (n) | ||||
| Female | 51.1% (318) | 43.0% (37) | 66.7% (78) | 52.3% (300) |
| Genetically confirmed | 51.4% (320) | 68.6% (59) | 58.1% (68) | 59.1% (339) |
| Racial categories | ||||
| Asian | 0.5% (3) | 3.5% (3) | 0.9% (1) | 2.4% (14) |
| American Indian/Alaska Native | 0.6% (4) | 0% (0) | 0.9% (1) | 1.1% (6) |
| Black or African American | 0.6% (4) | 1.2% (1) | 0 (0%) | 0.7% (4) |
| Native Hawaiian or Other Pacific Islander | 0.2% (1) | 0% (0) | 0% (0) | 0% (0) |
| White | 95.2% (592) | 89.5% (77) | 97.4% (114) | 93.4% (536) |
| Not reported | 2.9% (18) | 5.8% (5) | 0.9% (1) | 2.4% (14) |
| % (n) | ||||
| Ethnicity | ||||
| Hispanic | 4.5% (28) | 4.7% (4) | 0.9% (1) | 3.3% (19) |
Ages of onset of symptoms were similar between the Registry and other data. For example, DM2 members reported having their symptoms first appear on average at 33 years compared to findings from investigators at the University of Minnesota (mean=37 years [4]). Symptoms for DM1 and FSHD patients commonly develop in the second decade as also seen in the Registry [1,3].
3.1.3. Genetic test results
Genetic test results have been obtained in 56.2% of enrolled DM1, DM2, and FSHD members (n=786/1399; Table 1). The average (±standard deviation) CTG repeat size in DM1 was 419.0±305.0 (n=298) and the average repeat size in congenital and childhood DM1 was 1040.0±544.6 (n=55). The overall range of CTG repeats was 50 to 2307. Twenty-six members had a positive genetic test indicated in their medical record, but for various reasons, the exact repeat size was not provided. There was a significant negative correlation between the size of the CTG repeat and age of onset of the disease in congenital and childhood DM1 (r=−0.58; p<0.0001) and in adult DM1 (r=−0.35; p<0.0001).
The median size of the DM2 mutant allele was 14,000 base pairs (n=47; range: 300 to 15,600) with no significant association observed with age of onset. Mutant allele sizes were not available for 21 members who had a positive genetic test recorded in their medical record. Of note, 40.4% of DM2 members had DNA analyses indicating DM2 mutant alleles of “greater than 15,600 base pairs” (n=19/47).
The average fragment size of the contracted DNA fragment in FSHD patients was 26.7±7.2 kb (n=316; range: 11 to 48). Deletion sizes were not available in 23 members with a reported positive FSHD genetic test in their medical record. Smaller DNA fragments were significantly correlated with earlier onset of disease (r=0.31; p<0.0001).
3.2. Selected burdens of disease at baseline
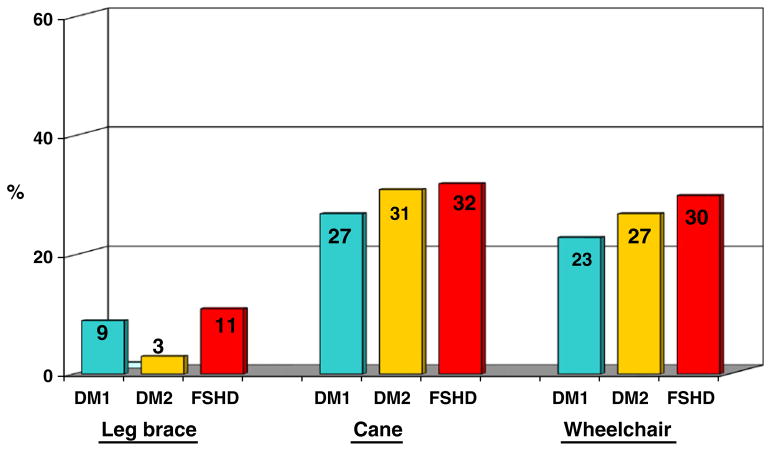
Approximately one-third of all members used assistive devices for ambulation (Fig. 2). FSHD members reported significantly more use of wheelchairs for short or long distances (29.6%) compared to DM1 (23.3%; p=0.02) and similar use compared to DM2 at enrollment (26.7%; p=0.46). Specifically, wheelchair use was reported as “always” (for both short and long distances) by 2.4% of DM1, 2.8% of DM2, and 8.3% of FSHD members. DM2 members reported significantly less use of leg braces (2.9%) compared to DM1 (9.4%; p=0.03) and FSHD (11.3%; p=0.008) subjects at enrollment. No other significant differences were reported in use of assistive devices at enrollment.
Fig. 2.
Data summarize percentages of Registry members who reported use of assistive devices for ambulation at baseline. Wheelchair use is shown for either short or long distances. A significantly higher percentage of FSHD members reported use of wheelchairs at baseline compared to DM1 (p=0.02) and similar use compared to DM2 (p=0.55). A significantly lower percentage of DM2 members reported use of leg braces compared to DM1 members (p=0.03) and FSHD members (p=0.008) at baseline. No other significant differences were apparent at baseline. After follow-up, wheelchair use was reported as “always” (for both short and long distances) by 6.3% of DM1, 8.3% of DM2, and 18.1% of FSHD members.
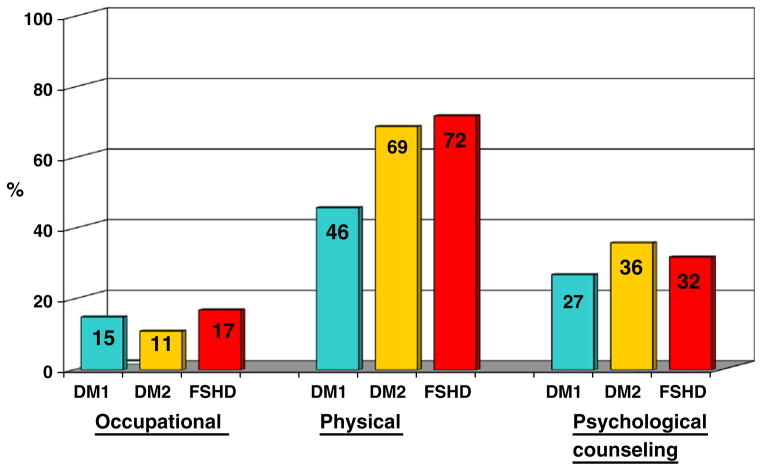
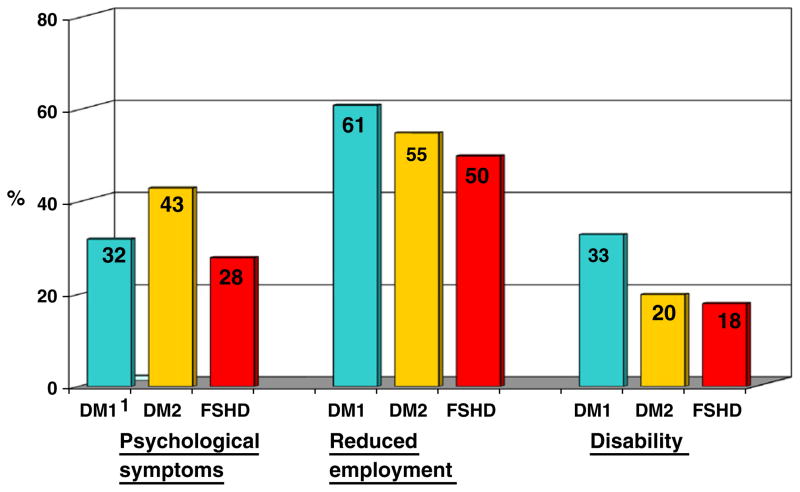
Physical therapy use was significantly less in DM1 (46.0%) compared to DM2 (68.6%; p<0.0001) and FSHD members (71.9%, p<0.0001) (Fig. 3). More than one-third of members reported having received psychological counseling (Fig. 3). Psychological problems occurred in all disease groups, with significantly more problems reported in DM2 (42.9%) compared to DM1 (31.7%; p=0.02) and FSHD members (28.1%; p=0.003). More than 50% of all adult members reported that symptoms of their disease adversely affected their employment (Fig. 4) by forced disability, early retirement, or job loss, or that their job had been modified to accommodate physical limitations.
Fig. 3.
Data summarize percentages of Registry members who reported use of various therapies at baseline. A significantly lower percentage of DM1 members reported use of physical therapy compared to DM2 (p<0.0001) and FSHD (p<0.0001) members at baseline. No other significant differences were apparent at baseline.
Fig. 4.
Data summarize percentages of Registry members who reported psycho-social burdens at baseline. A significantly higher percentage of DM2 members reported psychological problems compared to DM1 (p=0.03) and FSHD (p=0.003) members at baseline. Disease manifestations reduced employment opportunities in a significantly higher percentage of DM1 members compared to FSHD members at baseline (p=0.0008). No other significant differences were apparent at baseline.
3.3. Selected burdens of disease at follow-up
Of all enrollees, the return rate of annual update forms ranged from 80.2% in Year 1 (n=1201 returned of 1497 mailed) to 63.5% in Year 8 (n=209/329). Of those who returned at least one annual update form, the average length of follow-up was 3.9 years in DM1 (n=511), 3.8 years in DM2 (n=105), and 4.7 years in FSHD (n=487) with a range of 1–8 years. Approximately 5% of members in each disease group reported new use of canes and less than 2% of each disease group reported new use of leg braces after follow-up. The greatest increase of wheelchair use for any distance was reported by FSHD members (28.6%) compared to both DM subtypes (~20% increased use in wheelchairs). The majority of FSHD members who reported increased wheelchair use reported a change from no wheelchair use at enrollment to wheelchair use only for long distance at follow-up (13.9%). Wheelchair use was reported as “always” (for both short and long distances) by 6.3% of DM1, 8.3% of DM2, and 18.1% of FSHD members at follow-up. In addition to more members using assistive devices, approximately 10% of members reported new use of occupational, physical, and psychological therapies. At follow-up, receipt of disability insurance was reported by 43.3% in DM1 (10.6% increase from baseline), 39.1% of DM2 (19.1% increase), and 27.6% in FSHD (9.5% increase).
Death occurred in 12.7% of DM1 members (n=65/511) after follow-up, which was significantly more frequent than in FSHD (2.9%; p<0.001) and DM2 (3.8%; p=0.008). Average age at death was reported as 55.7±11.0 years in DM1; 70.8± 6.5 years in DM2; and 64.0±11.3 years in FSHD. Death was reported by family members through annual update forms, telephone, email, or postal letters. The Registry database currently does not capture information about cause of death because it is not a main outcome of the Registry and because of potential challenges in acquiring death certificates (sensitivity of families and various state laws regarding consent of spouses and blood relatives).
3.4. Investigator studies using the resources of the Registry
Investigators have used the resources of the Registry to facilitate 24 research studies on topics such as excessive sleepiness, pain, pregnancy, and molecular pathology. A description of each study and how the Registry was utilized (facilitation of recruitment or supplying anonymous data) are listed on the web (www.urmc.rochester.edu/neurology/nih-registry/research/protocols.cfm). These approved studies have included 12 clinical based studies (e.g., involving a drug intervention), 9 questionnaire based studies (e.g., completing a survey on quality of life through the mail), and 3 studies involving analysis of de-identified data in the Registry. To date, the Registry has been acknowledged in 10 published manuscripts, 10 poster presentations, and 4 oral presentations at national and international meetings (www.urmc.rochester.edu/neurology/nih-registry/publications.cfm).
An example of a published study is “Pregnancy and Birth Outcomes in Women with FSHD.” Investigators recruited subjects from the Registry and from other resources to complete a questionnaire on pregnancy and childbirth [39]. These investigators reported that 70% (34/48) of the subjects enrolled in the study were recruited from the Registry.
Another study measured the prevalence of pain in DM and FSHD patients [40]. The investigators of this study reported that 296 Registry members contacted them to participate in the study, of which 79.4% (235 of 296) completed and returned the investigators’ questionnaires. Overall, 91% of participants in this study were members of the Registry (235 of 257 total enrollees).
Anonymous data from members of the Registry have been reported in several poster and platform presentations, including information such as neoplasms in DM1, infantile and childhood onset FSHD, and burdens of disease. A recent presentation included information on gastrointestinal symptoms in DM1 and DM2 members of the Registry [41].
4. Discussion
4.1. Effectiveness of the Registry and its use to facilitate recruitment of patients into clinical studies
Members of the Registry have diverse characteristics including broad age ranges, equal genders, and broad spectrum of disease symptoms, onsets, and severities. These characteristics are consistent with those described in previous studies. For example, Registry members have similar ages of symptom onset, a wide range of mild to severe symptoms, and similar psychological problems and use of assistive devices compared to other clinical studies [2,4,42]. Opportunities exist to further compare our results by analyzing data in international registries and by developing epidemiological studies similar to research being conducted by the Centers for Disease Control in other muscular dystrophies [43]. The methods of the National Registry can serve as a model for international databases in DM and FSHD and guide the development of other rare disease registries [30,31]. The transferable strengths of the Registry include stringent review of medical records, annually updated data, model consent forms, and succinct yet comprehensive collection of data sufficient to facilitate the recruitment of subjects into clinical studies. Investigators can tailor their recruitment based on the diverse characteristics and severities of disease manifestations of members of the Registry.
Recruitment of members for clinical studies has yielded high enrollment rates. For example, investigators in the Fields Center for FSHD and Neuromuscular Research at the University of Rochester and University of Leiden Medical Center were approved to use the resources of the Registry in 2009. The goal of their study is to examine genotypic variations in a large patient sample and to collect biological samples for analysis of expression profiles of mRNA and protein products of several candidate genes. Use of the Registry has facilitated the recruitment of patients with diverse genotypes, inheritance patterns, and phenotypes. Members of the Registry have expressed a high level of interest in this study. Within one month after the Registry mailed 221 recruitment letters to eligible members, investigators received approximately 125 phone calls from patients interested in participating in the study. To maintain active participation of patients and interest in the Registry, opportunities exist to provide more information about clinical studies to members and to encourage investigators to send follow-up letters to patients to describe important information derived from their research, such as correlates of genetic testing results, natural history of these diseases, or risks and benefits of various interventions.
4.2. Diagnostic genetic testing results
To increase the sample size and diversity of patients enrolled in the Registry, genetic confirmation of disease is not required for enrollment. Patients often refrain from genetic testing because of a documented family history of the disease and privacy concerns. Patients may also not have adequate health insurance to receive genetic testing. Even if diagnostic exams have been performed, obtaining medical records is often difficult because there is no centralized electronic health information system in the United States, diagnostic results can be 10–15 years old, and patients have seen multiple primary care and neuromuscular physicians [44]. If additional medical records are obtained, a member’s classification is updated based on any new genetic testing results and muscle biopsy reports.
4.2.1. Myotonic dystrophy
The Registry has enrolled approximately equal percentages of genetically confirmed DM1 members with mild symptoms based on small DM1 mutations (less than 100 repeats) and more severely affected members with larger DM1 mutations (above 1000 repeats). Our data confirm previous research indicating a correlation between larger DM1 mutations and earlier onset of the disease [1,16]. Anticipation (earlier onset and greater severity of symptoms across generations) is prominent in DM1 and most evident from maternal transmission resulting in congenital DM1 [1]. Opportunities exist to conduct additional studies on how the genetic instability of DM1 may contribute to disease progression over time and across generations. Such information may help lead to therapeutic interventions that counter the genetic instability [45].
Associations between DM2 genetic size and severity of disease manifestations are not well understood or may be lacking compared to DM1 [16]. Data from the Registry correspond to previous research that has found no correlation between DM2 genetics and age of onset [4]. About 40% of genetically confirmed DM2 members in the Registry had a genetic report indicating DM2 mutant alleles to be “greater than 15,600 base pairs” as measured by traditional diagnostic assays [46–48]. Additional research is needed to determine if alleles greater than 15,600 base pairs can be more accurately quantified and if such information is clinically relevant and is associated with more severe disease symptoms.
4.2.2. FSHD
Genetic test results of FSHD members enrolled in the Registry indicate a broad spectrum of genetic mutations. Shorter DNA fragments in FSHD have been shown to be associated with more severe symptoms [49,50]. Data from the Registry concur and also indicate that smaller DNA fragments are associated with earlier onset of symptoms. Additional data from the Registry support the hypothesis that the size of the FSHD mutation is associated with infantile FSHD and more severe symptoms [51]. Classification of infantile FSHD was defined as facial weakness before 5 years of age and proximal weakness before 10 years of age [52]. The Registry contains one of the largest samples of patients with infantile FSHD symptoms (n=53), of which a subset had smaller FSHD mutations, more severe symptoms requiring greater wheelchair use by the age of 18 years, and greater hearing loss compared to adult onset FSHD [51]. The mechanisms of these more severe symptoms remain unknown and require further investigation. Opportunities exist to recruit members of the Registry with various genetic mutation sizes and to develop studies to correlate various biomarkers with disease symptoms.
4.3. Burdens of disease
Better understanding of the pathophysiology of DM and FSHD and earlier detection may help clinicians better manage the burdens of disease most often reported by patients, such as psychological disorders, pain, and loss of independence [40,53,54]. Nearly one-third of DM and FSHD members of the Registry reported psychological symptoms at baseline (Fig. 4). These data concur with the results of a recent study from the Netherlands, in which 32% of each group of DM, FSHD, and neuropathy patients reported experiencing a psychological disorder in their lifetime, the most common being depression and phobias [42]. Approximately 25% of adults in the general population report a mental illness [55]. Studies are necessary to further measure the psychological symptoms reported in DM and FSHD members in the Registry and to determine how these results compare to those in the general population and to assess the overall severity of these problems.
Other psychosocial problems were reported by members of the Registry; most prominently, about 60% of members indicated that symptoms of their disease have adversely affected their employment (Fig. 4). In addition, approximately 25% of members reported receiving disability insurance due to their disease. Studies are needed to assess the effects of lost employment on health related quality of life, caregiver burden, and financial constraints in patients and their family members.
In addition to psychosocial stressors, pain is a common symptom in DM and FSHD patients. Approximately 80% of FSHD members reported pain on the Registry Patient Information Form (data not shown). Data from the Registry compare to results reported by investigators from the University of California at Davis and University of Washington, who recruited members of the Registry to complete questionnaires on the effects of pain in DM and FSHD [40]. Results indicated that 60% of DM (n=78 of130 surveyed) and 82% of FSHD (n=104 of 127 surveyed) subjects reported pain, with approximately 25% of these DM and FSHD respondents reporting that their pain was severe. Opportunities exist to recruit members of the Registry to continue to study pain and biopsychosocial factors and to develop better treatments for chronic pain, such as medications or assistive devices [56].
A high percentage of members in the Registry report use of assistive devices (canes, braces, and wheelchairs) but the overall benefit of such treatments and their potential impact on pain are unknown (Fig. 2). Pain has been reported to be more severe and prominent in patients using assistive devices [40], but data are limited on the progression of muscle weakness and use of assistive devices over time in DM and FSHD patients. The greatest change in use of assistive devices occurred in FSHD members, of whom 18% required a wheelchair for short and long distances after an average of 4.6 years of follow-up. These results are similar to those of a previous study in FSHD, in which symptoms progressed such that 20% of subjects required the use of wheelchairs [2]. The effects of muscle wasting on use of assistive devices, pain, economic burden, and use of medication and other therapies require further investigation.
The majority of Registry members reported using occupational and physical therapies at enrollment and continued to use these therapies after follow-up. Reasons for the small change in use are unclear, but it may indicate that patients are receiving ongoing benefits of such treatment. The efficacies of various therapies in the treatment of DM and FSHD have not been adequately studied and require further research. Additional efforts are needed to study the effects of such therapies in DM1 members, who were 20% less likely to use physical therapy at enrollment compared to the FSHD and DM2 members enrolled in the Registry.
5. Summary
The Registry provides an invaluable resource to track the progression of DM and FSHD manifestations and to facilitate additional research. Opportunities exist to use the resources of the Registry to develop collaborative investigations in a number of areas largely unexplored in DM and FSHD, including physical therapy, medical economics, psychology, epidemiology, and therapeutic trials.
Acknowledgments
The Registry is supported through the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute of Neurological Disorders and Stroke (grant #U54-NS048843 and contracts #N01-AR-5-2274 and #NO1-AR-0-2250). We appreciate the continued guidance and thoughtful leadership of our NIH Program Directors, Drs. Glen H. Nuckolls and John D. Porter. We also wish to give our sincere thanks and appreciation to Dr. Richard W. Lymn, former NIH Program Director, who guided the creation of the Registry and who continues to provide ongoing advice and encouragement to enhance its success.
Abbreviations
- CCTG
cytosine (C), thymine (T), and guanine (G), nucleotides that are repetitive and cause myotonic dystrophy type 2
- CTG
cytosine (C), thymine (T), and guanine (G), nucleotides that are repetitive and cause myotonic dystrophy type 1
- FSHD
facioscapulohumeral muscular dystrophy
- DM
myotonic dystrophy
- DM1
myotonic dystrophy type 1
- DM2
myotonic dystrophy type 2
- NIH
National Institutes of Health
References
- 1.Harper PS. Myotonic Dystrophy. London: W.B. Saunders Company; 2001. [Google Scholar]
- 2.Padberg GW. Facioscapulohumeral disease University of Leiden. Leiden: The Netherlands; 1982. [Google Scholar]
- 3.Tawil R, van der Maarel SM. Facioscapulohumeral muscular dystrophy. Muscle Nerve. 2006;34(1):1–15. doi: 10.1002/mus.20522. [DOI] [PubMed] [Google Scholar]
- 4.Day JW, Ricker K, Jacobsen JF, Rasmussen LJ, Dick KA, Kress W, et al. Myotonic dystrophy type 2: molecular, diagnostic and clinical spectrum. Neurology. 2003;60(4):657–64. doi: 10.1212/01.wnl.0000054481.84978.f9. [DOI] [PubMed] [Google Scholar]
- 5.Machuca-Tzili L, Brook D, Hilton-Jones D. Clinical and molecular aspects of the myotonic dystrophies: a review. Muscle Nerve. 2005;32(1):1–18. doi: 10.1002/mus.20301. [DOI] [PubMed] [Google Scholar]
- 6.Ranum LP, Day JW. Myotonic dystrophy: clinical and molecular parallels between myotonic dystrophy type 1 and type 2. Curr Neurol Neurosci Rep. 2002;2(5):465–70. doi: 10.1007/s11910-002-0074-6. [DOI] [PubMed] [Google Scholar]
- 7.Ricker K, Koch MC, Lehmann-Horn F, Pongratz D, Otto M, Heine R, et al. Proximal myotonic myopathy: a new dominant disorder with myotonia, muscle weakness, and cataracts. Neurology. 1994;44(8):1448–52. doi: 10.1212/wnl.44.8.1448. [DOI] [PubMed] [Google Scholar]
- 8.Thornton CA, Griggs RC, Moxley RT. Myotonic dystrophy with no trinucleotide repeat expansion. Ann Neurol. 1994;35(3):269–72. doi: 10.1002/ana.410350305. [DOI] [PubMed] [Google Scholar]
- 9.Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992;68(4):799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- 10.Fu YH, Pizzuti A, Fenwick RG, Jr, King J, Rajnarayan S, Dunne PW, et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255(5049):1256–8. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- 11.Mahadevan M, Tsilfidis C, Sabourin L, Shutler G, Amemiya C, Jansen G, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science. 1992;255(5049):1253–5. doi: 10.1126/science.1546325. [DOI] [PubMed] [Google Scholar]
- 12.Liquori CL, Ricker K, Moseley ML, Jacobsen JF, Kress W, Naylor SL, et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. 2001;293(5531):864–7. doi: 10.1126/science.1062125. [DOI] [PubMed] [Google Scholar]
- 13.Ranum LP, Rasmussen PF, Benzow KA, Koob MD, Day JW. Genetic mapping of a second myotonic dystrophy locus. Nat Genet. 1998;19(2):196–8. doi: 10.1038/570. [DOI] [PubMed] [Google Scholar]
- 14.Miller JW, Urbinati CR, Tengumnuay P, Stenberg MG, Byrne BJ, Thornton CA, et al. Recruitment of human muscleblind proteins to (CUG)(n) expansions associated with myotonic dystrophy. EMBO J. 2000;19(17):4439–48. doi: 10.1093/emboj/19.17.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheeler TM, Thornton CA. Myotonic dystrophy: RNA-mediated muscle disease. Curr Opin Neurol. 2007;20(5):572–6. doi: 10.1097/WCO.0b013e3282ef6064. [DOI] [PubMed] [Google Scholar]
- 16.Cho DH, Tapscott SJ. Myotonic dystrophy: emerging mechanisms for DM1 and DM2. Biochim Biophys Acta. 2007;1772(2):195–204. doi: 10.1016/j.bbadis.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Suominen T, Bachinski LL, Auvinen S, Hackman P, Baggerly KA, Angelini C, et al. Population frequency of myotonic dystrophy: higher than expected frequency of myotonic dystrophy type 2 (DM2) mutation in Finland. Eur J Hum Genet. 2011;19(7):776–82. doi: 10.1038/ejhg.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flanigan KM, Coffeen CM, Sexton L, Stauffer D, Brunner S, Leppert MF. Genetic characterization of a large, historically significant Utah kindred with facioscapulohumeral dystrophy. Neuromuscul Disord. 2001;11(6–7):525–9. doi: 10.1016/s0960-8966(01)00201-2. [DOI] [PubMed] [Google Scholar]
- 19.Lemmers RJ, van der Vliet PJ, Klooster R, Sacconi S, Camano P, Dauwerse JG, et al. A unifying genetic model for facioscapulohumeral muscular dystrophy. Science. 2010;329(5999):1650–3. doi: 10.1126/science.1189044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Greef JC, Lemmers RJ, Camano P, Day JW, Sacconi S, Dunand M, et al. Clinical features of facioscapulohumeral muscular dystrophy 2. Neurology. 2010;75(17):1548–54. doi: 10.1212/WNL.0b013e3181f96175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Statland JM, Tawil R. Facioscapulohumeral muscular dystrophy: molecular pathological advances and future directions. Curr Opin Neurol. 2011;24(5):423–8. doi: 10.1097/WCO.0b013e32834959af. [DOI] [PubMed] [Google Scholar]
- 22.Padberg GW, Brouwer OF, de Keizer RJ, Dijkman G, Wijmenga C, Grote JJ, et al. On the significance of retinal vascular disease and hearing loss in facioscapulohumeral muscular dystrophy. Muscle Nerve. 1995;2:S73–80. [PubMed] [Google Scholar]
- 23.The FSH-DY Group. A prospective, quantitative study of the natural history of facioscapulohumeral muscular dystrophy (FSHD): implications for therapeutic trials. Neurology. 1997;48(1):38–46. doi: 10.1212/wnl.48.1.38. [DOI] [PubMed] [Google Scholar]
- 24.de Die-Smulders CE, Howeler CJ, Thijs C, Mirandolle JF, Anten HB, Smeets HJ, et al. Age and causes of death in adult-onset myotonic dystrophy. Brain. 1998;121(Pt 8):1557–63. doi: 10.1093/brain/121.8.1557. [DOI] [PubMed] [Google Scholar]
- 25.Echenne B, Rideau A, Roubertie A, Sebire G, Rivier F, Lemieux B. Myotonic dystrophy type I in childhood: Long-term evolution in patients surviving the neonatal period. Eur J Paediatr Neurol. 2008;12(3):210–23. doi: 10.1016/j.ejpn.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Mathieu J, Allard P, Potvin L, Prevost C, Begin P. A 10-year study of mortality in a cohort of patients with myotonic dystrophy. Neurology. 1999;52(8):1658–62. doi: 10.1212/wnl.52.8.1658. [DOI] [PubMed] [Google Scholar]
- 27.Reardon W, Newcombe R, Fenton I, Sibert J, Harper PS. The natural history of congenital myotonic dystrophy: mortality and long term clinical aspects. Arch Dis Child. 1993;68(2):177–81. doi: 10.1136/adc.68.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendell JR, Csimma C, McDonald CM, Escolar DM, Janis S, Porter JD, et al. Challenges in drug development for muscle disease: a stakeholders’ meeting. Muscle Nerve. 2007;35(1):8–16. doi: 10.1002/mus.20686. [DOI] [PubMed] [Google Scholar]
- 29.Forrest CB, Bartek RJ, Rubinstein Y, Groft SC. The case for a global rare-diseases registry. Lancet. 2011;377(9771):1057–9. doi: 10.1016/S0140-6736(10)60680-0. [DOI] [PubMed] [Google Scholar]
- 30.Heger M. A registry of registries? The US backs the idea for patients. Nat Med. 2011;17(1):4. doi: 10.1038/nm0111-4a. [DOI] [PubMed] [Google Scholar]
- 31.Rubinstein YR, Groft SC, Bartek R, Brown K, Christensen RA, Collier E, et al. Creating a global rare disease patient registry linked to a rare diseases biorepository database: Rare Disease-HUB (RD-HUB) Contemp Clin Trials. 2010;31(5):394–404. doi: 10.1016/j.cct.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tawil R, van der MS, Padberg GW, van Engelen BG. 171st ENMC international workshop: Standards of care and management of facioscapulohumeral muscular dystrophy. Neuromuscul Disord. 2010;20(7):471–5. doi: 10.1016/j.nmd.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Thompson R, Schoser B, Monckton DG, Blonsky K, Lochmuller H. Patient Registries and Trial Readiness in Myotonic Dystrophy–TREAT-NMD/Marigold International Workshop Report. Neuromuscul Disord. 2009;19(12):860–6. doi: 10.1016/j.nmd.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Udd B, Meola G, Krahe R, Wansink DG, Bassez G, Kress W, et al. Myotonic dystrophy type 2 (DM2) and related disorders Report of the 180th ENMC Workshop including guidelines on diagnostics and management 3–5 December 2010, Naarden, The Netherlands. Neuromuscul Disord. 2011;21(6):443–50. doi: 10.1016/j.nmd.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 35.New nomenclature and DNA testing guidelines for myotonic dystrophy type 1 (DM1) The International Myotonic Dystrophy Consortium (IDMC) Neurology. 2000;54(6):1218–21. doi: 10.1212/wnl.54.6.1218. [DOI] [PubMed] [Google Scholar]
- 36.Tawil R, McDermott MP, Mendell JR, Kissel J, Griggs RC. Facioscapulohumeral muscular dystrophy (FSHD): design of natural history study and results of baseline testing. FSH-DY Group. Neurology. 1994;44(3 Pt 1):442–6. doi: 10.1212/wnl.44.3_part_1.442. [DOI] [PubMed] [Google Scholar]
- 37.Udd B, Meola G, Krahe R, Thornton C, Ranum LP, Bassez G, et al. 140th ENMC International Workshop: Myotonic Dystrophy DM2/PROMM and other myotonic dystrophies with guidelines on management. Neuromuscul Disord. 2006;16(6):403–13. doi: 10.1016/j.nmd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Koch MC, Grimm T, Harley HG, Harper PS. Genetic risks for children of women with myotonic dystrophy. Am J Hum Genet. 1991;48(6):1084–91. [PMC free article] [PubMed] [Google Scholar]
- 39.Ciafaloni E, Pressman EK, Loi AM, Smirnow AM, Guntrum DJ, Dilek N, et al. Pregnancy and birth outcomes in women with facioscapulohumeral muscular dystrophy. Neurology. 2006;67(10):1887–9. doi: 10.1212/01.wnl.0000244471.05316.19. [DOI] [PubMed] [Google Scholar]
- 40.Jensen MP, Hoffman AJ, Stoelb BL, Abresch RT, Carter GT, McDonald CM. Chronic pain in persons with myotonic dystrophy and facioscapulohumeral dystrophy. Arch Phys Med Rehabil. 2008;89(2):320–8. doi: 10.1016/j.apmr.2007.08.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hilbert JE, Martens WB, Smirnow AM, Moxley RT, III Registry Scientific Advisory Committee. Gastrointestinal (GI) Symptoms in Myotonic Dystrophy Type 1 and Type 2 (DM1 and DM2) Ann Neurol. 2008 Aug 26;64(S12):S7. Poster (M-14) 2008. [Google Scholar]
- 42.Kalkman JS, Schillings ML, Zwarts MJ, van Engelen BG, Bleijenberg G. Psychiatric disorders appear equally in patients with myotonic dystrophy, facioscapulohumeral dystrophy, and hereditary motor and sensory neuropathy type I. Acta Neurol Scand. 2007;115(4):265–70. doi: 10.1111/j.1600-0404.2006.00737.x. [DOI] [PubMed] [Google Scholar]
- 43.Miller LA, Romitti PA, Cunniff C, Druschel C, Mathews KD, Meaney FJ, et al. The muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet): surveillance methodology. Birth Defects Res A Clin Mol Teratol. 2006;76(11):793–7. doi: 10.1002/bdra.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haux R. Health information systems – past, present, future. Int J Med Inform. 2006;75(3–4):268–81. doi: 10.1016/j.ijmedinf.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Wheeler TM. Myotonic dystrophy: therapeutic strategies for the future. Neurotherapeutics. 2008;5(4):592–600. doi: 10.1016/j.nurt.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bachinski LL, Czernuszewicz T, Ramagli LS, Suominen T, Shriver MD, Udd B, et al. Premutation allele pool in myotonic dystrophy type 2. Neurology. 2009;72(6):490–7. doi: 10.1212/01.wnl.0000333665.01888.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Day JW, Roelofs R, Leroy B, Pech I, Benzow K, Ranum LP. Clinical and genetic characteristics of a five-generation family with a novel form of myotonic dystrophy (DM2) Neuromuscul Disord. 1999 Jan;9(1):19–27. doi: 10.1016/s0960-8966(98)00094-7. [DOI] [PubMed] [Google Scholar]
- 48.Schoser B, Ashizawa T. How much expansion to be diseased? : toward repeat size and myotonic dystrophy type 2. Neurology. 2009;72(6):484–5. doi: 10.1212/01.wnl.0000341937.70150.64. [DOI] [PubMed] [Google Scholar]
- 49.Tawil R, Forrester J, Griggs RC, Mendell J, Kissel J, McDermott M, et al. Evidence for anticipation and association of deletion size with severity in facioscapulohumeral muscular dystrophy. The FSH-DY Group. Ann Neurol. 1996;39(6):744–8. doi: 10.1002/ana.410390610. [DOI] [PubMed] [Google Scholar]
- 50.Lunt PW, Jardine PE, Koch MC, Maynard J, Osborn M, Williams M, et al. Correlation between fragment size at D4F104S1 and age at onset or at wheelchair use, with a possible generational effect, accounts for much phenotypic variation in 4q35-facioscapulohumeral muscular dystrophy (FSHD) Hum Mol Genet. 1995;4(5):951–8. doi: 10.1093/hmg/4.5.951. [DOI] [PubMed] [Google Scholar]
- 51.Hilbert JE, Martens WB, Smirnow AM, Tawil R, Moxley RT, III Registry Scientific Advisory Committee. Clinical description of infantile Facioscapulohumeral Muscular Dystrophy (FSHD) Neurology. 2007;68 [Number 12, Supplement 1], Muscular Dystrophy Integrated Neuroscience Session, Poster IN11-2.006. [Google Scholar]
- 52.Brouwer OF, Padberg GW, Wijmenga C, Frants RR. Facioscapulohumeral muscular dystrophy in early childhood. Arch Neurol. 1994;51(4):387–94. doi: 10.1001/archneur.1994.00540160085011. [DOI] [PubMed] [Google Scholar]
- 53.Abresch RT, Seyden NK, Wineinger MA. Quality of life. Issues for persons with neuromuscular diseases. Phys Med Rehabil Clin N Am. 1998;9(1):233–48. [PubMed] [Google Scholar]
- 54.Perez L, Huang J, Jansky L, Nowinski C, Victorson D, Peterman A, et al. Using focus groups to inform the Neuro-QOL measurement tool: exploring patient-centered, health-related quality of life concepts across neurological conditions. J Neurosci Nurs. 2007;39(6):342–53. doi: 10.1097/01376517-200712000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Kessler RC, Wang PS. The descriptive epidemiology of commonly occurring mental disorders in the United States. Annu Rev Public Health. 2008;29:115–29. doi: 10.1146/annurev.publhealth.29.020907.090847. [DOI] [PubMed] [Google Scholar]
- 56.Miro J, Raichle KA, Carter GT, O’Brien SA, Abresch RT, McDonald CM, et al. Impact of biopsychosocial factors on chronic pain in persons with myotonic and facioscapulohumeral muscular dystrophy. Am J Hosp Palliat Care. 2009;26(4):308–19. doi: 10.1177/1049909109335146. [DOI] [PMC free article] [PubMed] [Google Scholar]