Abstract
The basal amygdala (BA) plays a key role in mediating the facilitating effects of emotions on memory. Recent findings indicate that this function depends on the ability of BA neurons to generate coherent oscillatory activity, facilitating synaptic plasticity in target neurons. However, the mechanisms allowing BA neurons to synchronize their activity remain poorly understood. Here, we aimed to shed light on this question, focusing on a slow periodic inhibitory oscillation previously observed in the BA in vitro. Paired patch recordings showed that these large inhibitory postsynaptic potentials (IPSPs) occur almost synchronously in BA projection neurons, that they were typically not preceded by excitatory postsynaptic potentials (EPSPs), and that they had little or no correlate in neighboring amygdala nuclei or cortical fields. The initial phase of the IPSPs was associated with an increase in membrane potential fluctuations at 50–100 Hz. In keeping with this, the IPSPs seen in projection cells were correlated with repetitive firing at 50–100 Hz in presumed interneurons and they could be abolished by picrotoxin. However, the IPSPs were also sensitive to 6-cyano-7-nitroquinoxaline-2,3-dione, implying that they arose from the interplay between glutamatergic and GABAergic BA neurons. In support of this idea, we identified a small subset of projection cells (15%), positively identified as such by retrograde labeling from BA projection sites, that began firing shortly before the IPSP onset and presumably drove interneuronal firing. These results add to a rapidly growing body of data indicating that the BA contains at least two distinct types of projection cells that vary in their relation with interneurons and extra-amygdala targets.
INTRODUCTION
The basolateral complex of the amygdala (BLA) is a cortex-like structure that projects to subcortical structures, such as the striatum and mediodorsal thalamus, and forms reciprocal connections with various cortical regions, including the rhinal cortices, hippocampal formation, insula, and medial prefrontal cortex (mPFC) (Krettek and Price 1977a,b; Pitkanen 2000; Pitkanen et al. 2000). Except for the random orientation of neurons in the BLA, its cellular composition is reminiscent of that found in cortex (McDonald 1992). Indeed, the BLA contains glutamatergic projection cells (Carlsen 1988; Smith and Paré 1994) with a spiny pyramidal or stellate morphology and a low proportion of GABAergic local-circuit cells that are heterogeneous morphologically (McDonald 1992), neurochemically (McDonald and Mascagni 2001, 2002, 2004; Mueller et al. 2003), and physiologically (Jasnow et al. 2009; Rainnie et al. 2006; Sosulina et al. 2006; Woodruff and Sah 2007).
In recent years, it has become clear that the basal nuclei of the BLA [namely, the basolateral and basomedial nuclei (BA)] are involved in a variety of important functions including the acquisition, expression, and extinction of conditioned fear responses (Anglada-Figueroa and Quirk 2005; Goosens and Maren 2001; Herry et al. 2008), as well as the facilitation of memory by emotions (McGaugh 2000; Paré 2003). A recurrent observation in studies that examined the physiological substrates of these functions is that BA neurons generate oscillatory activity in various frequency bands (Pape and Driesang 1998; Paré et al. 1995a; Paré and Gaudreau 1996; Seidenbecher et al. 2003), entraining neurons in target structures (e.g., striatum, rhinal cortices) (Bauer et al. 2007; Popescu et al. 2009). Importantly, this oscillatory activity does not involve increases in the firing rates of BA projection cells, only a change in timing such that the spikes generated by different projection cells become more synchronized (Bauer et al. 2007; Paz et al. 2006; Popescu et al. 2009). However, the mechanisms supporting the ability of BA cells to synchronize their activity remain poorly understood. This study aimed to shed light on this question by focusing on the synchronizing mechanisms of a slow periodic oscillation (SPO) generated in the BA in vitro.
Indeed, it was reported that, in brain slices kept in vitro, periodic inhibitory postsynaptic potentials (IPSPs) of high-amplitude and duration coordinate the activity of BA projection cells (Chung and Moore 2009a,b; Rainnie 1999). These studies and a meeting abstract (Rainnie 1999) reported that SPOs are sensitive to bicuculline, occur almost simultaneously in different projection cells, and coincide with trains of action potentials in local circuit inhibitory BA neurons. The latter were triggered by repetitive excitatory postsynaptic potentials (EPSPs) that could be abolished by the non–N-methyl-d-aspartate (NMDA) glutamate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (Rainnie 1999). Although SPO-like events have not been reported in vivo, the study of SPOs is nevertheless of interest because it provides a unique opportunity to examine cellular interactions in the BLA. Indeed, in vivo studies are complicated by constantly fluctuating activity levels in the BLA and its inputs. In contrast, SPOs are a robust and stereotyped phenomenon that emerges from the specific connectivity of different BLA cells types. Consequently, the mechanisms underlying the genesis of SPOs can be analyzed in detail. Thus we used patch recordings in slices kept in vitro to study the mechanisms underlying the genesis of SPOs to further our understanding of intrinsic synaptic BA interactions.
METHODS
Slice preparation
All experiments were performed using coronal brain slices obtained from Sprague-Dawley rats (1 mo old, 75–100 g), in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the approval of the Institutional Animal Care and Use Committee of Rutgers University (Newark, NJ). The rats were anesthetized with ketamine, pentobarbital, and xylazine (80, 60, and 12 mg/kg, ip, respectively). After abolition of reflexes, they were perfused through the heart with one of three solutions. The brains were extracted and cut in 400-μm-thick slices with a vibrating microtome.
In some rats (hereafter “Control rats,” n = 6), we used ice-cold oxygenated artificial cerebrospinal fluid (ACSF) for perfusion and cutting. It contained (in mM) 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 26 NaHCO3, and 10 glucose (pH 7.3, 300 mOsm). In other rats, the same solution was used except that NaCl was omitted and either 242 mM sucrose (“Sucrose rats,” n = 5) or choline chloride (“Choline rats,” n = 19) was added. After cutting, slices were transferred to an incubating chamber where, regardless of group, they were allowed to recover for at least 1 h at room temperature in the control ACSF. They were transferred one at a time to a recording chamber perfused with the same solution (7 ml/min). Before the recordings began, the temperature of the chamber was gradually increased to 32°C.
Electrophysiology
Under visual guidance with differential interference contrast and infrared video microscopy, we obtained whole cell patch recordings of neurons in the BA nuclei, lateral (LA) nucleus, central amygdala (Ce), and adjacent cortical fields using pipettes (4–6 MΩ) pulled from borosilicate glass capillaries and filled with a solution containing (in mM) 130 K-gluconate, 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 10 KCl, 2 MgCl2, 2 ATP-Mg, and 0.2 GTP-tris(hydroxy-methyl)aminomethane (pH 7.2, 280 mOsm). The liquid junction potential was 10 mV with this solution, and the membrane potential was corrected accordingly. Current-clamp recordings were obtained with an Axoclamp 2B amplifier and digitized at 10 kHz with a Digidata 1200 interface (Axon Instruments, Foster City, CA).
Physiological identification of projection cells and interneurons
To characterize the electroresponsive properties of recorded cells, graded series of depolarizing and hyperpolarizing current pulses (increasing in 20-pA steps, 500 ms in duration) were applied from rest. The input resistance (Rin) of the cells was estimated in the linear portion of current–voltage plots. Using criteria derived from studies that correlated the physiological and morphological properties of BLA neurons (Faber and Sah 2002; Jasnow et al. 2009; Paré et al. 1995a; Rainnie et al. 1993; Sosulina et al. 2006; Washburn and Moises 1992a; Woodfruff and Sah 2007), we classified BA neurons as putative projection cells or interneurons based on their contrasting electroresponsive properties (reviewed in Pape and Paré 2010 and Sah et al. 2003).In particular, BA neurons were classified as principal cells when they displayed spike frequency adaptation during depolarizing current pulses and generated action potentials of comparatively long duration (≥0.8 ms at half-amplitude). Given the heterogeneous firing patterns of BLA interneurons reported in previous studies, we relied primarily on spike duration to identify these cells (≤0.6 ms at half-amplitude).
Detection of IPSP onset
The resting potential of principal BA cells was negative to the GABAA reversal potential (around −65 to −70 mV in these cells). To distinguish IPSPs from EPSPs and show the inhibitory nature of SPOs, the cells had to be depolarized to a membrane potential positive to the GABAA reversal potential by applying a low-amplitude steady depolarizing current. We selected a membrane potential of –60 mV because it allowed observation of IPSPs without current-evoked spiking. We used this potential consistently in all conditions to allow fair comparisons of SPO amplitudes and durations across the various conditions studied (control, sucrose, choline, knife cuts). To detect the initial sharp negative voltage change that characterized the SPOs recorded at this potential, we used custom software written in Matlab 2009a. Briefly, in each cell, we manually selected a slope threshold that distinguished SPOs from low-amplitude spontaneous IPSPs. The SPO onset was defined as the moment the slope of the membrane potential exceeded this threshold. Next we used supervised clustering of various parameters (shape similarity, amplitude, duration) to further insure that only SPOs were included in the analyses. In all cases, our criteria for classifying events as SPOs were set to allow a small number of false negatives, and absolutely no false positives.
We selected the slope method of SPO detection for two reasons. First, this was the most consistent feature of SPOs across BA cells. Second, this approach could be used to automatically and rapidly detect the time of occurrence of a high number of SPOs with little or no intervention on the part of the investigator. Initially, we tried to identify SPO onset manually but, because of spontaneous fluctuations in membrane potentials, this approach depended on subjective judgments on the part of the investigator. In any event, it is unlikely that the way we detected SPOs explains the variations in SPO correlates seen in other structures because we always used the same approach. Paired recordings were obtained with one recording in the BA, and another either at the same site, in the cortex, LA, or central nucleus (CE). SPO onset in the first BA cell was always used to align the activity of the second recorded cell in time. In other words, we used the times of occurrence of SPOs detected in the first BA cell to average the simultaneously occurring activity in the second cell. Because we always used the same approach, variations in SPO correlates between structures did not reflect how we detected SPOs but differences in what happened in the other cell at these times. Because the correlates of SPOs in the cortex, CE, or LA were very small or absent, it was impossible to measure the delay between the two on a case-by-case basis.
Computation of spectrograms
Following their initial negative slope, many SPOs showed an increase in the frequency of low-amplitude postsynaptic potentials. To characterize these events, we computed power spectrograms of voltage fluctuations for each SPO in each projection cell recorded. Spectrograms were computed for a period of 0.3 s before and 0.7 s after the SPO onset, using 80-ms windows sliding with 20-ms steps in the 1- to 100-Hz range (0.2-Hz steps). To eliminate the power artifacts induced by the general shape of the SPOs, we also calculated a spectrogram for the average shape of the SPOs recorded in each cell and subtracted it from the individual spectrograms of the corresponding cell. The resulting spectrograms were normalized to the 0.3-s baseline period preceding each SPO, averaged within each cell, and averaged across cells.
Retrograde tracing
In a subset of experiments (n = 17), we aimed to directly establish whether recorded cells were indeed projection neurons by performing retrograde tracer injections in known projection sites of the BA. In these cases, 3–5 days before the physiological experiments, a retrograde tracer (fluorescently labeled red latex microbeads, Lumafluor, www.lumafluor.com) was injected bilaterally in the mPFC or ventral striatum, two structures known to receive strong BA inputs (Carlsen 1988; Kita and Kitai 1990; Krettek and Price 1977b; McDonald 1987). To this end, rats (23–24 days old), were anesthetized with isoflurane, administered atropine (0.05 mg/kg, i.m.), and placed in a stereotaxic apparatus. A local anesthetic was injected subcutaneously in the regions of the scalp to be incised (bupivacaine, 0.2 ml). Ten minutes later, in aseptic conditions, the scalp was incised, the skin was retracted, and small holes were drilled above the injection target areas. Under stereotaxic guidance, Hamilton microsyringes were lowered to the target areas: the mPFC (in mm, relative to bregma: anterior 2.5, lateral 0.5 and ventral 5) or the ventral striatum (posterior 1, lateral 4.1 and ventral 6.9). Note that these coordinates were corrected for the smaller brains of the young animals we used. The retrobeads were pressure-injected at a rate of 0.2 μl/min, for a total volume of 0.5 μl per injection site. The wound was then sutured. Before returning the rats to their home cage, they were administered an analgesic with a long half-life (ketoprophen, 2 mg/kg, sc daily for 3 days) and 1 ml of lactated ringer (sc). To minimize the risk of infection, a local antibiotic was applied directly on the wound (Neosporin paste). A survival period of 3–5 days was allowed for the tracer to reach BA cell bodies, after which the rats were used for in vitro recordings, as described above.
To determine whether some BA projection cells are GABAergic, we combined retrograde tracing (in this case fluorescently labeled green latex microbeads, Lumafluor, www.lumafluor.com) with GAD-67 immunohistochemistry. The tracer was pressure injected in the mPFC or ventral striatum, as described above. After a 4-day survival period, the animals were deeply anesthetized with pentobarbital (80 mg/kg, ip) and perfused-fixed by transcardial perfusion with saline and then 4% paraformaldehyde. The brains were extracted from the skull and postfixed overnight in 4% paraformaldehyde. The region of interest was sectioned (60 μm) with a vibrating microtome, incubated in sodium borohydride (1% in 0.1 M PB for 30 min), washed repeatedly in PB followed by rinses in TBS, and placed in a blocking solution containing 3% normal goat serum, 1% BSA, and 0.3% Triton X-100 in 0.1 M TBS for 30 min. The sections were incubated for 48 h at 4°C with monoclonal mouse anti-GAD-67 (Millipore, Billerica, MA) diluted (1:1,000) in the same blocking solution. After several washes in TBS, sections were incubated with a cyanine 3 (Cy3)-conjugated donkey anti-mouse secondary antibody (Jackson ImmunoResearch, West Grove, PA) diluted (1:800) in the same blocking solution for 1 h. After several washes in TBS and PBS, the sections were mounted on glass slides and coverslipped with CC/Mount (Sigma, St. Louis, MO).
RESULTS
Database
A total of 129 BA neurons were recorded in this study. Figure 1A shows the location of a subset of these cells, all of which were recorded in pairs with another neuron in the BA nuclei, LA (n = 9), CE (n = 6), or surrounding cortical fields (n = 8). Of these 129 neurons, 24 were recorded in 9 Control rats, 16 in 5 Sucrose rats, and 89 in 19 Choline rats. The rationale for performing most experiments in Choline rats will become clear below, when we compare the incidence of SPOs in the three groups.
Fig. 1.
Slow periodic oscillations (SPOs) in basal amygdala (BA) neurons. A: scheme showing position of a subset of the BA neurons recorded in this study. These cells were recorded in pairs with another neuron in BA, central nucleus (CE), lateral nucleus of the amygdala (LA), or cortices surrounding the amygdala. B: simultaneous patch recording of BA neurons depolarized to a membrane potential of around –60 mV by current injection. Rest was −73 mV for cell 1 and −74 mV for cell 2. C: average of SPOs for the cells shown in B. D: reversal potential of SPOs. Same cell as shown at bottom of B. Inset between C and D plots SPO amplitude as a function of membrane potential at 2 different delays after SPO onset (black, 30 ms; red, 400 ms). CeL and CeM, lateral and medial sectors of the central nucleus of the amygdala; CTX, cerebral cortex; Str, striatum.
Incidence and properties of the SPOs
To study SPOs, recorded cells were first depolarized from rest to a membrane potential of –60 mV, as determined by depolarizing current injection. Consistent with previous findings (Rainnie 1999), SPOs typically appeared as long-lasting (0.5–1.2 s) IPSPs of large amplitude (3–10 mV; Fig. 1, B and C) that recurred at a low frequency (0.3–1 Hz) and were comprised of two phases: an early one reversing at around –65 mV (–66.7 ± 0.4 mV) and a late one reversing at around –85 mV (–84.2 ± 0.1 mV, n = 5; Fig. 1D). The differing reversal potentials of the early versus late phases of SPOs (Fig. 1D, inset) suggest that they are dominated by different types of GABA receptors (early, GABAA; late GABAB). In the vast majority of principal cells displaying SPOs (85%), these IPSPs were not preceded by EPSPs (Fig. 1C). It should be noted that the properties of SPOs recorded in principal neurons of the basolateral and basomedial nuclei were statistically indistinguishable (t-test, P ≥ 0.51; amplitude at −60 mV, 3.19 vs. 3.22 mV; duration, 0.35 vs. 0.37 s; frequency, 0.47 vs. 0.45 Hz, respectively). Thus for convenience, the data obtained in these two nuclei are pooled below.
We observed marked differences in the incidence of SPOs between the three groups. Indeed, SPOs were never observed in the Sucrose group, were present in 33% of Control rats (3 of 9 rats), and were present in most Choline subjects (90% or 17 of 19). In Control and Choline rats, if in a given preparation SPOs were seen in one BA cell, all BA cells recorded from that rat showed SPOs. The contrasting incidence of SPOs in the various groups led us to focus on the choline group, to increase the yield of the experiments.
Consistent with previous observations (Chung and Moore 2009a,b; Rainnie 1999), SPOs were abolished by addition of picrotoxin (100 μM, n = 8; Fig. 2A) or CNQX (20 μM, n = 6; Fig. 2B) to the ACSF. Interestingly, SPOs were nearly synchronous in pairs of simultaneously recorded BA projection cells (n = 30), regardless of the distance between them (≤1.55 mm; Fig. 2, C and D). This is consistent with the results of paired recordings described in a meeting abstract (Rainnie 1999).
Fig. 2.
Pharmacological sensitivity and synchronization of SPOs in the BA. A: addition of picrotoxin (100 μM) to the artificial cerebrospinal fluid (ACSF) abolishes SPOs. B: addition of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20 μM) to the ACSF abolishes SPOs. A and B are 2 different projection cells kept at around –60 mV with depolarizing current injection. C: simultaneous patch recordings of 2 different projection cells. C1: location of recorded cells. C2: activity of the cells shown in C1. Note simultaneous SPOs. D: evidence that SPOs occur almost simultaneously in projection cells. D1: cross-correlation of membrane voltage fluctuations recorded in 2 simultaneously recorded projection cells. Note that the highest cross-correlation index (0.87) occurs at 0 s offset. D2 and D3: the maximal downward slope of each SPO was detected, and these times were used to generate peri-SPO time histograms (D2) and calculate inter-SPO intervals (D3). All tests were conducted at a membrane potential of −60 mV obtained by depolarizing current injection. Rest (in mV) was −70 in A, −68 in B, and −71 for cell 1 and −75 for cell 2 of C2.
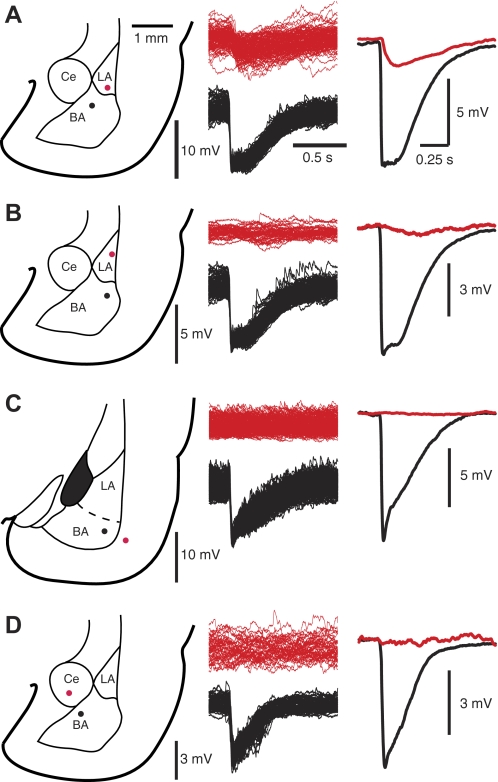
Origin and distribution of SPOs
To shed light on the origin and distribution of SPOs, we obtained paired recordings of neurons in different amygdala nuclei (LA, Ce) or neighboring cortical fields. In all cases, a principal BA neuron was recorded simultaneously with these other cells. To quantify the correlates of BA SPOs at these other sites, the maximal IPSP slope was detected for ≥100 SPO cycles seen in the BA cells and used as a temporal reference to average the membrane potential fluctuations that occurred in the other, simultaneously recorded neurons.
In paired BA-LA recordings (n = 9), contrasting results were obtained depending on the position of LA cells. In ventrally located LA cells (n = 4), BA SPOs coincided with low-amplitude IPSPs (1.64 ± 0.06 mV; Fig. 3A), much lower than seen in BA neurons (6.3 ± 0.1 mV; t-test, P = 0.001). In more dorsally located LA cells (n = 5; Fig. 3B), few or no IPSPs were detected. Moreover, the IPSPs seen in ventrally located LA cells developed after the BA IPSPs (delay ranging between 18 and 25 ms). We also obtained paired BA-cortex recordings (n = 8; Fig. 3C) and paired BA-Ce (n = 6; Fig. 3D) recordings but observed no correlates of BA SPOs in these cells.
Fig. 3.
Origin and distribution of SPOs. A–D: paired patch recordings. In all cases, a projection cell was recorded in the BA (black) simultaneously with a second neuron (red) in LA (A and B), the adjacent cortex (C), or CE (D). On the left are schemes showing the position of recorded cells. In the middle panels, the maximal downward slope of each SPO was detected in the BA cell (black), and these times were used to align the activity of the 2nd cell (red). On the right are averages of the traces shown in the middle panel. All tests were conducted at a membrane potential of −60 mV obtained by depolarizing current injection. Rest (in mV) was −70 for the LA cell and −72 for the BA cell of A, −74 for the LA and −79 for the BA cell of B, −71 for the cortical and −68 for the BA cell of C, −74 for the CE cell and −73 for the BA cell of D.
To further test whether cortical inputs are involved in generating BA SPOs, in two experiments, the slices were prepared with knife cuts isolating the amygdala from the surrounding cortex on all sides. In all principal BA cells recorded in these experiments (n = 6), we observed SPOs that were indistinguishable from those seen in intact slices. Indeed, they occurred at an average frequency of 0.53 ± 0.1 Hz and were 9.3 ± 0.4 mV in amplitude (t-test, P ≥ 0.7).
Dependence of SPOs on cholinergic activity
The higher incidence of SPOs in choline rats compared with control and sucrose rats suggested that the mechanisms responsible for the generation of SPOs are regulated by cholinergic activity. To test this, we obtained patch recordings of BA cells and administered various drugs affecting cholinergic neurotransmission (Fig. 4). Addition of the nicotinic receptor antagonist mecamylamine (10 μM) to the ACSF abolished SPOs in four of six tested cells (Fig. 4A), reduced their incidence in a fifth one (Fig. 4B), and had no effect in the last. In contrast, addition of the muscarinic receptor antagonist atropine sulfate (1.5 μM, n = 7) always led to the disappearance of the SPOs (Fig. 4, B and C).
Fig. 4.
Sensitivity of SPOs to cholinergic antagonists. Addition of mecamylamine (10 μM; A) or atropine (1.5 μM, C) to the ACSF abolishes SPOs in 2 different projection cells. Portion of the data in dashed red lines is shown with a faster time base below. B: a 2nd example in which the application of mecamylamine did not completely abolish SPOs. Subsequently adding atropine, however, eliminated these events. All tests were conducted at a membrane potential of −60 mV obtained by depolarizing current injection. Rest (in mV) was −74 in A, −69 in B, and −76 in C.
Overall, the sensitivity of SPOs to cholinergic antagonists, coupled to the contrasting incidence of SPOs in choline versus control and sucrose rats, suggest that ambient levels of acetylcholine (ACh) regulate SPO-generating mechanisms. If this is true, one would expect that control and sucrose slices devoid of SPOs could be made to generate SPOs by raising ACh levels with a cholinesterase inhibitor. To test this, we recorded 23 BA neurons before and during the application of neostigmine in a wide range of concentrations (0.05–30 μM). However, we were unable to induce SPOs in all tested cells. In our view, these negative findings lend themselves to several interpretations. First, it is possible that the higher incidence of SPOs in slices prepared in choline does not reflect a cholinergic dependence but the impact of another unidentified factor. Another possibility is that SPOs are dependent on ACh but in a narrow range of concentrations, which we were unable to achieve. Finally, it is possible that the increase in ACh levels produced by neostigmine differs from that produced by preparation of the slices in choline. With neostigmine present, one would expect a global increase of ACh concentration, whereas in choline slices with no neostigmine present, the ACh levels would likely not be uniform and differentially affect particular cell types. We favor the latter interpretation.
Variations in intracellular correlates of SPOs as a function of cell identity
As mentioned above, SPOs typically appeared as long-lasting IPSPs of large amplitude that recurred at a low frequency in presumed projection cells (Fig. 1). However, the sensitivity of SPOs to CNQX and picrotoxin (Fig. 2), coupled to their persistence after isolating the BA from cortex with knife cuts, implied that SPOs arose from the interplay between glutamatergic and GABAergic BA neurons. To shed light on the nature of this interplay, we next analyzed the behavior of presumed interneurons in relation to SPOs.
Given the heterogeneous firing patterns of identified interneurons described in previous studies (see references in methods), BA neurons were classified as local-circuit cells when they generated very brief spikes (≤0.6 ms at half-amplitude; 0.42 ± 0.09 ms, n = 4; Fig. 5A, red). Compared with regular spiking projection cells (P cells), these presumed interneurons had a significantly higher input resistance and a more depolarized resting potential [t-test, interneurons (n = 4) vs. projection cells (n = 43): Rin, 335.5 ± 40.6 vs. 133.5 ± 12.4 MΩ, P = 0.0003; resting potential, −62.6 ± 3.8 vs. −71.5 ± 1 mV, P = 0.037; spike duration at half-amplitude, 0.42 ± 0.09 vs, 1.24 ± 0.14 ms, P = 0.007].
Fig. 5.
Opposite correlates of SPOs in interneurons and principal BA cells. A: voltage responses to current pulses in a presumed interneuron (red) and a projection cell (black). Middle panel shows superimposed action potentials generated by the 2 cells showing shorter spike duration in interneuron. B: cell-attached recording of same interneuron as in A showed spontaneous bursts of action potentials recurring at the frequency of SPOs. C: activity of simultaneously recorded interneuron and projection cell (same cells as in A) shows that periods of firing in the interneuron coincides with inhibitory postsynptic potentials (IPSPs) in the projection cell. D: simultaneously recorded SPO correlates in interneuron (red) and projection cell (black). We used the IPSP onset, as seen in the projection cell, to align the activity of the 2 neurons. Note faster time base in D than in C. Rest was −61 mV in the presumed interneuron (red) and −72 mV in the projection cell (black).
These presumed interneurons were recorded simultaneously with a projection cell to examine the temporal relationship between the intracellular correlates of SPOs in the two cell types. In two cases, the interneurons were recorded in cell-attached mode before establishing the whole cell recording configuration. These juxtacellular recordings showed that presumed interneurons generated spikes clusters that recurred at the frequency of SPOs (Fig. 5B). After establishing the whole cell configuration (Fig. 5, C and D), the SPO-related IPSPs of projection cells were seen to coincide with excitatory, and typically, suprathreshold events in the interneurons (Fig. 5, C and D). On average, interneurons fired 7.83 ± 0.11 spikes per SPO.
Although the general rule was for interneurons and projection cells to, respectively, undergo depo- and hyperpolarization during SPOs, we did encounter a few cells (n = 9) that also showed EPSPs and spikes during SPOs, even though they could not be classified as interneurons. To determine the identity of these cells, we performed a series of experiments (n = 17) where projection cells could be unambiguously identified as such by performing injections of a retrograde tracer in the mPFC (Fig. S1A)1 or ventral striatum (Fig. S1B) 3–5 days before the in vitro experiments.
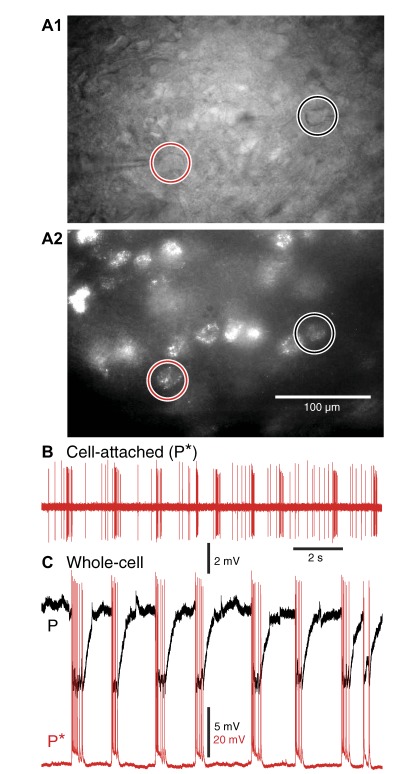
In keeping with previous tracing studies (see references in Introduction), these injections led to widespread retrograde labeling in the BLA (Fig. S1, C and D). Of the 32 retrogradely labeled BA projection cells we recorded in these experiments (Fig. 6A), most (27 or 84%) displayed inhibitory SPOs (Fig. 6C, black) and a minority (5 or 16%; Fig. 6C, red) showed the opposite profile of activity. Hereafter, these unusual projection cells will be referred to as P* cells. Importantly, in two of these five P* cells, prior recording in cell-attached mode confirmed that SPO-related firing was not caused by dialysis of the cells by the pipette solution (Fig. 6B). Although the repetitive firing properties, input resistance, resting potential, and spike durations in these two subsets of projection cells were undistinguishable (t-test, P ≥ 0.18), spike amplitudes were significantly higher in P* cells than in typical projection cells (t-test, P = 0.02, 93.2 ± 4.0 vs. 85.0 ± 2.6 mV).
Fig. 6.
A subset of projection cells (P*) undergo depolarization and spiking during the spontaneous IPSPs seen in most projection cells (P). The photomicrographs in A1 and A2 show the same BA region in brightfield mode (A1) or with FITC filter (A2). Red and black circles, respectively, mark the retrogradely labeled P* and P cells that were simultaneously recorded. B: cell-attached recording of P* cell shown in A and C. C: activity of simultaneously recorded P* and P neurons (same cells as in A) shows that periods of firing in P* cell coincides with IPSPs in P cell. Rest was −76 mV for the P cell and −71 mV for the P* cell.
Although the above establishes that P* cells are projection cells, the method we used leaves open the possibility that they use GABA as a transmitter. To test this, we combined retrograde tracing with fluorescently labeled green latex microbeads and GAD67 immunohistochemistry. As in the previous experiments, the tracer was injected in the mPFC (n = 2) or ventral striatum (n = 2). In keeping with previous reports in cats and guinea pigs (Apergis-Schoute et al. 2007; Paré and Smith 1994), we could not find a single double-labeled cell in the BA nuclei (Fig. S2), suggesting that projection cells and GABAergic neurons constitute two nonoverlapping populations of neurons in the BA. Therefore it is most likely that P* cells are glutamatergic projection cells.
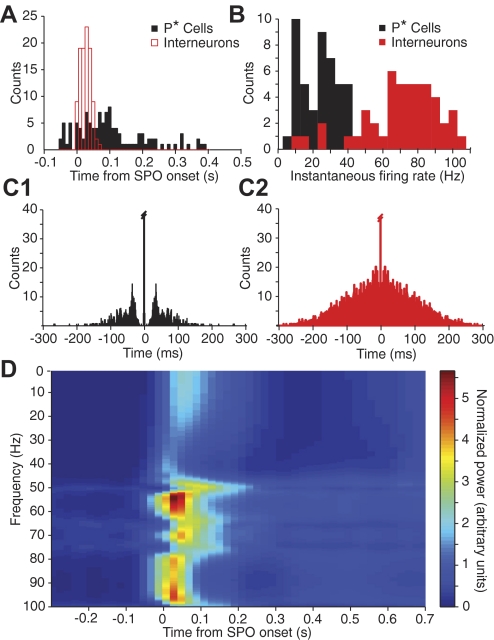
Last, we examined the temporal coordination between the activity of interneurons and the two types of projection cells during the SPOs (Fig. 7). First, during paired recordings of P cells with either identified P* cells (n = 5) or presumed interneurons (n = 4), we detected the onset of SPO-related IPSPs in P cells and examined relative spike timing in the other two cell types. As shown in the perievent histograms of firing of Fig. 7A, this analysis showed that presumed interneurons (Fig. 7A, red) began to fire at the onset of IPSPs and typically ceased firing within 90 ms. In contrast, spiking in P* cells (Fig. 7A, black) was more distributed in time (from –50 to 400 ms with respect to IPSP onset). Analysis of instantaneous firing rates in the two cell types (Fig. 7B) showed that presumed interneurons reached higher firing rates (mode at around 80 Hz, average of 70.3 ± 3.2 Hz) compared with P* cells (always below 40 Hz; 23.3 ± 1.6 Hz; t-test, P < 0.0001). Here, note that, although A and B of Fig. 7 look similar, they provide different information. Figure 7A shows when P* cells and interneurons fire, whereas Fig. 7B does not show when they fire but at what frequency. Autocorrelograms of SPO-related firing in the two cell types showed a trend for rhythmic firing in the 25- to 35-Hz range for P* cells (Fig. 7C1) but irregular activity in the presumed interneurons (Fig. 7C2).
Fig. 7.
Activity of P* cells (black) and interneurons (red) during SPOs. A: perievent histogram of P* cell and interneuron firing computed around the onset of SPOs, as seen in simultaneously recorded P cells. Each count represents the time between SPO onset and the 1st action potential encountered in the P* cell or interneuron. All available cells and spontaneous IPSPs combined. Note that P* cells sometimes fired before IPSP onset. B: instantaneous firing rates of P* cells and interneurons during SPOs. C: autocorrelogram of firing in same 2 cell types. D: time-dependent power fluctuations of intracellular potential in relation to SPOs, as recorded in P cells. See text and Fig. S2 for method used to obtain the spectrogram.
Because surgical isolation of the BLA does not abolish SPOs, the analyses of Fig. 7, A–C suggest the following scenario for the generation of SPOs. For reasons still undetermined, a few P* cells begin firing, strongly exciting interneurons. In turn, the latter inhibit the majority of projection cells. If indeed SPOs are generated in and restricted to the BA, membrane fluctuations associated with SPOs in P cells should reflect inputs from interneurons and possibly P* cells. To test this idea, we computed power spectrograms of membrane potential fluctuations (80-ms windows, 20-ms steps, 1–100 Hz, 0.2-Hz bins) around the onset of SPOs (see Fig. S3 for method). Consistent with our hypothesis, we observed that the power in two ranges of frequencies was significantly increased during the early phase of SPOs relative to baseline (paired t-test, P < 0.0001; Fig. 7D): in the 1- to 25-Hz range (63 ± 5% increase in power) and in the 50- to 100-Hz range (223 ± 6% increase in power). Although this could be coincidental, it is interesting to note that these two range of frequencies, respectively, match the instantaneous firing rates of P* cells (<40 Hz) and interneurons (∼80 Hz) during the early phase of SPOs (Fig. 7B).
DISCUSSION
This study analyzed the mechanisms underlying the genesis of a slow periodic inhibitory oscillation previously observed in the BA in vitro (Chung and Moore 2009a,b; Rainnie 1999). Consistent with these earlier reports, we found that this oscillation consisted of large IPSPs that occurred almost synchronously in the vast majority of projection neurons. Using paired recordings, we observed that, irrespective of the distance between projection cells, these IPSPs occurred almost synchronously and were generally not preceded by EPSPs. In addition, we obtained strong evidence that these IPSPs are generated within the BA. Indeed, the inhibitory SPOs had little or no correlate in neighboring amygdala nuclei or cortical fields, and they persisted after interruption of all extrinsic inputs to the amygdala with knife cuts. Expectedly, presumed interneurons fired repetitively during the initial phase of the IPSPs, at ∼50–100 Hz. However, the IPSPs were not only sensitive to picrotoxin but also CNQX, suggesting that interactions between glutamatergic and GABAergic BA neurons are involved. In keeping with this, a small subset of anatomically identified projection cells (15%) began firing shortly before IPSP onset and presumably drove interneuronal firing. The significance of these findings is discussed in the following text.
Identification of cell types
The validity of our interpretations depends on the reliability of the criteria we used to identify different BLA cell types. Here, we distinguished principal cells from interneurons using action potential duration. Indeed, most types of BLA interneurons generate much briefer action potentials than principal cells (reviewed in Pape and Paré 2010 and Sah et al. 2003). The fast-spiking cells we recorded also had a significantly higher input resistance and more depolarized membrane potential than presumed principal cells. However, it was reported that some cholecystokinin-expressing (CCK+) interneurons generate long-duration spikes (Jasnow et al. 2009), raising the possibility that we misclassified some interneurons as principal cells. Although we cannot exclude this possibility, given that CCK+ interneurons account for a minute proportion of BLA interneurons, such instances of misclassifications, if they occurred, had to be very infrequent. Nevertheless, recognizing this limitation, we complemented our electrophysiological criteria with tracing techniques. Using this approach, projection cells could be unambiguously identified as such when they could be retrogradely labeled from known projections of the BLA. Moreover, because it was reported in various cortical regions that some GABAergic neurons contribute long-range projections (Apergis-Schoute et al. 2007; Jinno et al. 2007), we also verified whether this was the case in the amygdala. As we had reported before in cats (Paré and Smith 1994) and guinea pigs (Apergis-Schoute et al. 2007), no instances of GABAergic projection cell could be found in the rat BLA. Thus we are confident that P and P* cells are indeed glutamatergic projection neurons.
Inhibition as a major regulator of activity within the BLA
Although the BLA is endowed with an extremely divergent system of excitatory connections between projection cells (Paré et al. 1995b; Smith and Paré 1994), most principal cells of the BLA show very low firing rates (i.e., see Bordi et al. 1993; Gaudreau and Paré 1996; Paré and Gaudreau 1996). Several factors contribute to this paradoxical situation. First, projection cells are endowed with a calcium-dependent K+ conductance that can be activated when glutamatergic synapses cause Ca2+ entry via NMDA receptors, thereby shunting EPSPs (Chen and Lang 2003; Danober and Pape 1998; Faber et al. 2005; Lang and Paré 1997b). Second, the spontaneous activity of projection cells in vivo is dominated by large amplitude IPSPs (Lang and Paré 1997a) mediated by GABAA and GABAB receptors after GABA release by local-circuit cells (Danober and Pape 1998; Martina et al. 2001; Rainnie et al. 1991; Washburn and Moises 1992b). Third, interneurons receive a much lower number of inhibitory synapses (Pan et al. 2009; Smith et al. 1998) and their IPSPs lack a GABAB component (Martina et al. 2001). Instead, the IPSPs they display are apparently pure GABAA IPSPs that reverse at more depolarized potentials than in projection cells (near to the spike threshold), because of a contrasting regulation of intracellular chloride in the two cell types (Martina et al. 2001).
This study further establishes the determining influence of inhibition on the activity of projection cells. That most principal BA neurons showed synchronized IPSPs despite the compromised connectivity of the slice points to the remarkable efficiency of the BA inhibitory network. This makes perfect sense given that these inhibitory mechanisms effectively gate the induction of synaptic plasticity in the BLA (Bissière et al. 2003; Faber et al. 2005; Tully et al. 2007).
Contrasting correlates of SPOs in different types of BA projection cells
The vulnerability of inhibitory SPOs to picrotoxin and CNQX implied that their genesis depends on interactions between glutamatergic and GABAergic BA neurons. Support for this notion came from recordings of anatomically identified projection cells, a low proportion of which showed EPSPs and firing in relation to SPOs, rather than the typical periodic IPSPs. These observations suggest that the BA contains at least two subtypes of projection cells. Other than generating spikes of differing amplitudes (P* > P), these two classes of projection cells do not differ in their electroresponsive properties. What mainly distinguishes them is the magnitude of inhibitory influences they are subjected to. These results add to earlier observations indicating that the BA contains at least two distinct types of projection cells that vary in their connectivity with neurons within and outside the amygdala. Two examples are provided below.
Although tract-tracing studies indicate that principal BA cells project to Ce (Krettek and Price 1978; Paré et al. 1995b; Pitkanen et al. 1997), electrical stimulation of the mPFC, which backfires a large proportion of projection cells (Likhtik et al. 2005), does not elicit firing in Ce neurons (Quirk et al. 2003). This suggests that the BA contains at least two subsets of principal cells, some projecting to mPFC but not Ce and others projecting to Ce but not mPFC. The P* cells we recorded may correspond to the former, given that no EPSPs were seen in Ce in relation to SPOs.
Similarly, a recent study identified two subsets of BA cells in a fear conditioning paradigm (Herry et al. 2008). Some cells (termed fear neurons) acquired responses to auditory conditioned stimuli (CS) as a result of fear conditioning and lost them after extinction training. The second class (termed extinction neurons) remained unresponsive to the CS after conditioning but acquired CS responses after extinction training. Interestingly, these two cell types showed contrasting connections with the mPFC and hippocampus with the fear cells receiving inputs from the hippocampus but not mPFC and the extinction cells showing the opposite (Herry et al. 2008).
Multiple regulatory mechanisms of inhibitory SPOs
We observed that the incidence of SPOs was much higher in slices prepared in an ACSF with choline chloride substituted for NaCl. This suggested that ambient levels of ACh have a determining impact on the network mechanisms generating SPOs. Consistent with this, we found that cholinergic receptor antagonists, especially against the muscarinic family, could abolish SPOs. Similarly, previous work showed that drugs acting at a variety of G protein–coupled receptors could reduce, abolish, or enhance SPOs. For instance, Rainnie (1999) reported that serotonin inhibited SPOs. In addition, Chung and Moore (2009a,b) found that cholecystokinin and corticotropin-releasing factor enhanced SPOs, whereas neuropeptide Y or somatostatin attenuated them. For some of these compounds, such as cholecystokinin (Chung and Moore 2009a) and serotonin (Rainnie 1999), evidence for a direct action on BA interneurons was obtained. An important challenge for future studies will be to determine what interneuron subtype(s) are involved in generating the SPOs.
Possible mechanisms for the initiation of SPOs
Three main observations support the view that P* cells play a determining role in the initiation of SPOs. First, the SPOs seen in BA do not depend on extrinsic inputs to BA because they survive after isolation of BA with knife cuts. Second, SPOs vanished after CNQX application, implying that glutamatergic inputs to interneurons are essential. Third, some P* cells fired just before SPOs, whereas interneuronal firing always coincided with the onset of SPOs.
Together, this suggests that SPOs result from the firing of P* cells, leading to the recruitment of interneurons and the consequent inhibition of P cells. At present, the reason why P* cells begin firing in the first place is unclear. It is possible that P* cells are strongly interconnected such that random firing of a few of them by background inputs, provided it occurs in short time window, leads to a self-reinforcing positive feedback of excitation among P* cells. In this scenario, the fact that P* cells are apparently subjected to minimal inhibition would play a permissive role. Once initiated, rapid and essentially synchronized propagation of SPOs throughout BA would be supported by diverging connections between P* cells and electrical coupling between interneurons (Muller et al. 2005; Woodruff and Sah 2007).
Another possibility, compatible with the above, is that some or all P* cells, because of unusual chloride homeostatic mechanisms, are actually excited by GABAergic inputs. This idea is supported by previous studies showing that some GABAergic inputs are excitatory in cortical (Khirug et al. 2008; Szabadics et al. 2006) and BLA (Woodruff et al. 2006) circuits. Future studies should address this question now that P* cells can be readily identified on the basis of their distinct behavior in relation to SPOs.
GRANTS
This material is based on work supported by the National Institute of Mental Health Grants RO1 MH-073610 and MH-083710 to D. Paré.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci 25: 9680–9685, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apergis-Schoute J, Pinto A, Paré D. Muscarinic control of long-range GABAergic inhibition within the rhinal cortices. J Neurosci 27: 4061–4071, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EP, Paz R, Paré D. Gamma oscillations coordinate amygdalo-rhinal interactions during learning. J Neurosci 27: 9369–9379, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissière S, Humeau Y, Lüthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci 6: 587–592, 2003 [DOI] [PubMed] [Google Scholar]
- Bordi F, LeDoux J, Clugnet MC, Pavlides C. Single-unit activity in the lateral nucleus of the amygdala and overlying areas of the striatum in freely behaving rats: Rates, discharge patterns, and responses to acoustic stimuli. Behav Neurosci 107: 757–769, 1993 [DOI] [PubMed] [Google Scholar]
- Carlsen J. Immunocytochemical localization of glutamate decarboxylase in the rat basolateral amygdaloid nucleus, with special reference to GABAergic innervation of amygdalostriatal projection neurons. J Comp Neurol 273: 513–526, 1988 [DOI] [PubMed] [Google Scholar]
- Chen JC, Lang EJ. Inhibitory control of rat lateral amygdaloid projection cells. Neuroscience 121: 155–166, 2003 [DOI] [PubMed] [Google Scholar]
- Chung L, Moore SD. Cholecystokinin excites interneurons in rat basolateral amygdala. J Neurophysiol 102: 272–284, 2009a [DOI] [PubMed] [Google Scholar]
- Chung L, Moore SD. Neuropeptides modulate compound postsynaptic potentials in basolateral amygdala. Neuroscience 164: 1389–1397, 2009b [DOI] [PubMed] [Google Scholar]
- Danober L, Pape HC. Mechanisms and functional significance of a slow inhibitory potential in neurons of the lateral amygdala. Eur J Neurosci 10: 853–867, 1998 [DOI] [PubMed] [Google Scholar]
- Faber ES, Callister RJ, Sah P. Morphological and electrophysiological properties of principal neurons in the rat lateral amygdala in vitro. J Neurophysiol 85: 714–723, 2001 [DOI] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci 8: 635–641, 2005 [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Calcium-activated K+ (BK) channel inactivation contributes to spike broadening during repetitive firing in rat lateral amygdala neurons. J Physiol 552: 482–497, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci 22: 1618–1628, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner B, Brown TH. Morphology and physiology of neurons in the rat perirhinal-lateral amygdala area. J Comp Neurol 411: 613–642, 1999 [PubMed] [Google Scholar]
- Gaudreau H, Paré D. Projection cells of the lateral nucleus are virtually silent throughout the sleep-waking cycle. J Neurophysiol 75: 1301–1305, 1996 [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem 8: 148–155, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature 454: 600–606, 2008 [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Ressler KJ, Hammack SE, Chhatwal JP, Rainnie DG. Distinct subtypes of cholecystokinin (CCK)-containing interneurons of the basolateral amygdala identified using a CCK promoter-specific lentivirus. J Neurophysiol 101: 1494–1506, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khirug S, Yamada J, Afzalov R, Voipio J, Khiroug L, Kaila K. GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. J Neurosci 28: 4635–4639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Amygdaloid Projections to the Frontal Cortex and the Striatum in the Rat. J Comp Neurol 298: 40–49, 1990 [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex and adjacent olfactory structures to the entorhinal cortex and to the subiculum in the rat and cat. J Comp Neurol 172: 723–752, 1977a [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol 172: 687–722, 1977b [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol 178: 255–280, 1978 [DOI] [PubMed] [Google Scholar]
- Lang EJ, Paré D. Similar inhibitory processes dominate the responses of cat lateral amygdaloid projection neurons to their various afferents. J Neurophysiol 77: 341–352, 1997a [DOI] [PubMed] [Google Scholar]
- Lang EJ, Paré D. Synaptic and synaptically activated intrinsic conductances underlie inhibitory potentials in cat lateral amygdaloid projection neurons in vivo. J Neurophysiol 77: 353–363, 1997b [DOI] [PubMed] [Google Scholar]
- Lang EJ, Paré D. Synaptic responsiveness of interneurons of the cat lateral amygdaloid nucleus. Neuroscience 83: 877–889, 1998 [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Paré D. Prefrontal control of the amygdala. J Neurosci 25: 7429–7437, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Royer S, Paré D. Cell-type-specific GABA responses and chloride homeostasis in the cortex and amygdala. J Neurophysiol 86: 2887–2895, 2001 [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the mediodorsal thalamus and prefrontal cortex: A fluorescence retrograde transport study in the rat. J Comp Neurol 262: 46–58, 1987 [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cell types and intrinsic connections of the amygdala. In: The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction, edited by Aggleton JP. New York: Wiley-Liss, 1992, p. 67–96 [Google Scholar]
- McDonald AJ, Mascagni F. Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala. Neuroscience 105: 681–693, 2001 [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Res 943: 237–244, 2002 [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Parvalbumin-containing interneurons in the basolateral amygdala express high levels of the alpha1 subunit of the GABA A receptor. J Comp Neurol 473: 137–146, 2004 [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory—a century of consolidation. Science 287: 248–251, 2000 [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Synaptic connections of distinct interneuronal subpopulations in the rat basolateral amygdalar nucleus. J Comp Neurol 456: 217–236, 2003 [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Coupled networks of parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J Neurosci 25: 7366–7376, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BX, Dong YL, Ito W, Yanagawa Y, Shigemoto R, Morozov A. Selective gating of glutamatergic inputs to excitatory neurons of amygdala by presynaptic GABAb receptor. Neuron 61: 917–929, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Driesang RB. Ionic mechanisms of intrinsic oscillations in neurons of the basolateral amygdaloid complex. J Neurophysiol 79: 217–226, 1998 [DOI] [PubMed] [Google Scholar]
- Pape HC, Paré D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90: 419–463, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D. Role of the basolateral amygdala in memory consolidation. Prog Neurobiol 70: 409–420, 2003 [DOI] [PubMed] [Google Scholar]
- Paré D, Gaudreau H. Projection cells and interneurons of the lateral and basolateral amygdala: Distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. J Neurosci 16: 3334–3350, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Smith Y. GABAergic projection from the intercalated cell masses of the amygdala to the basal forebrain in cats. J Comp Neurol 344: 33–49, 1994 [DOI] [PubMed] [Google Scholar]
- Paré D, Pape HC, Dong JM. Bursting and oscillating neurons of the cat basolateral amygdaloid complex in vivo: Electrophysiological properties and morphological features. J Neurophysiol 74: 1179–1191, 1995a [DOI] [PubMed] [Google Scholar]
- Paré D, Smith Y, Paré JF. Intra-amygdaloid projections of the basolateral and basomedial nuclei in the cat: Phaseolus vulgaris-leucoagglutinin anterograde tracing at the light and electron microscopic level. Neuroscience 69: 567–583, 1995b [DOI] [PubMed] [Google Scholar]
- Paz R, Pelletier JG, Bauer EP, Paré D. Emotional enhancement of memory via amygdala-driven facilitation of rhinal interactions. Nat Neurosci 9: 1321–1328, 2006 [DOI] [PubMed] [Google Scholar]
- Pitkänen A. Connectivity of the rat amygdaloid complex. In: The Amygdala: A Functional Analysis, edited by Aggleton JP. Oxford: Oxford University Press, 2000, p. 31–115 [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. Ann NY Acad Sci 911: 369–391, 2000 [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci 20: 517–523, 1997 [DOI] [PubMed] [Google Scholar]
- Popescu AT, Popa D, Paré D. Coherent gamma oscillations couple the amygdala and striatum during learning. Nat Neurosci 12: 801–807, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23: 8800–8807, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol 82: 69–85, 1999 [DOI] [PubMed] [Google Scholar]
- Rainnie DG. Synchronous inhibitory synaptic potentials in simultaneously recorded neurons of the basolateral amygdala. Soc Neurosci Abstr 26: 651, 1999 [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Inhibitory transmission in the basolateral amygdala. J Neurophysiol 66: 999–1009, 1991 [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Intracellular recordings from morphologically identified neurons of the basolateral amygdala. J Neurophysiol 69: 1350–1362, 1993 [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Mania I, Mascagni F, McDonald AJ. Physiological and morphological characterization of parvalbumin-containing interneurons of the rat basolateral amygdala. J Comp Neurol 498: 142–161, 2006 [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ESL, De Armentia ML, Power J. The amygdaloid complex: Anatomy and physiology. Physiol Rev 83: 803–834, 2003 [DOI] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science 301: 846–850, 2003 [DOI] [PubMed] [Google Scholar]
- Smith Y, Paré D. Intra-amygdaloid projections of the lateral nucleus in the cat: PHA-L anterograde labeling combined with post-embedding GABA and glutamate immunocytochemistry. J Comp Neurol 342: 232–248, 1994 [DOI] [PubMed] [Google Scholar]
- Smith Y, Paré JF, Paré D. Cat intraamygdaloid inhibitory network: ultrastructural organization of parvalbumin-immunoreactive elements. J Comp Neurol 391: 164–179, 1998 [DOI] [PubMed] [Google Scholar]
- Sosulina L, Meis S, Seifert G, Steinhauser C, Pape HC. Classification of projection neurons and interneurons in the rat lateral amygdala based upon cluster analysis. Mol Cell Neurosci 33: 57–67, 2006 [DOI] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311: 233–235, 2006 [DOI] [PubMed] [Google Scholar]
- Tully K, Li Y, Tsvetkov E, Bolshakov VY. Norepinephrine enables the induction of associative long-term potentiation at thalamo-amygdala synapses. Proc Natl Acad Sci USA 104: 14146–14150, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci 12: 4066–4079, 1992a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Inhibitory responses of rat basolateral amygdaloid neurons recorded in vitro. Neuroscience 50: 811–830, 1992b [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Monyer H, Sah P. GABAergic excitation in the basolateral amygdala. J Neurosci 26: 11881–11887, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Networks of parvalbumin-positive interneurons in the basolateral amygdala. J Neurosci 27: 553–563, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.