Abstract
Guided bone regeneration (GBR) in implant therapy is especially useful for implant placement with dehiscence defects or fenestration defects. In alveolar ridges with marked facial/buccal depressions or in knifeedge alveolar crests, the position and direction of fixture placement is restricted. Improvement of alveolar ridge morphology becomes possible with GBR. This article describes a case in which the fenestration defect around an implant was treated by the application of platelet rich fibrin, a second generation platelet concentrate along with bone graft, and guided tissue regeneration membrane.
Keywords: Guided bone regeneration, growth factors, platelet rich fibrin
INTRODUCTION
Implant therapy based on the principle of osseointegration has seen a remarkable expansion of its application in dentistry, in recent years. In the last decade, dental implants have become a reliable procedure for the treatment of partially or completely edentulous jaws. The lack of bone adjacent to an implant can be considered a true “bony defect” and several techniques have been proposed to promote defect fill with newly formed bone. One of the most popular procedures is guided bone regeneration (GBR), which involves placing a membrane over the defect to create a secluded space into which osteogenic cells can migrate and remain undisturbed over the exposed part of the implant.
A challenge in the reconstruction of periodontal structures is the targeted delivery of growth promoting molecules to the tooth root surface. Polypeptide growth factors are molecules identified in the periodontal tissues that have been implicated in the growth and differentiation of cells from the periodontal tissues.[1]
Platelet rich fibrin, which is a second generation platelet concentrate, offers the surgeon an access to growth factors with a simple and available technology. These growth factors which are autologous, nontoxic and non immunogenic, enhance and accelerate the normal bone regeneration pathways.[2]
This article presents a case report in which a fenestration defect around an implant was treated by GBR with platelet rich fibrin (PRF)-enhanced bone graft and the PRF smeared barrier membrane for guided bone regeneration.
CASE REPORT
A 30-year-old male patient reported with the chief complaint of his discolored cantilever bridge in the upper front tooth region. Intra oral examination revealed missing 21 and a cantilever bridge, with 11 as an abutment [Figures 1 and 2].
Figure 1.
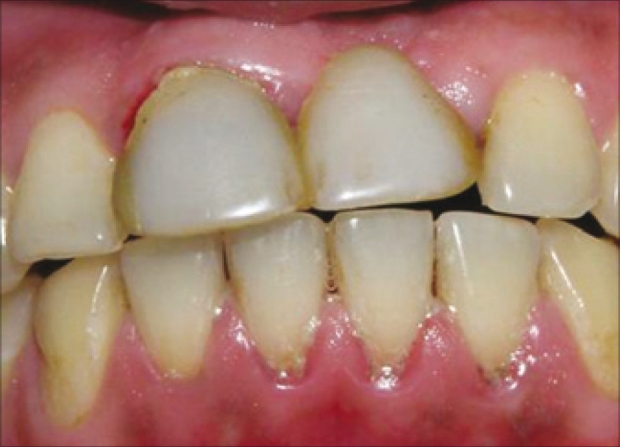
Pre-operative view
Figure 2.
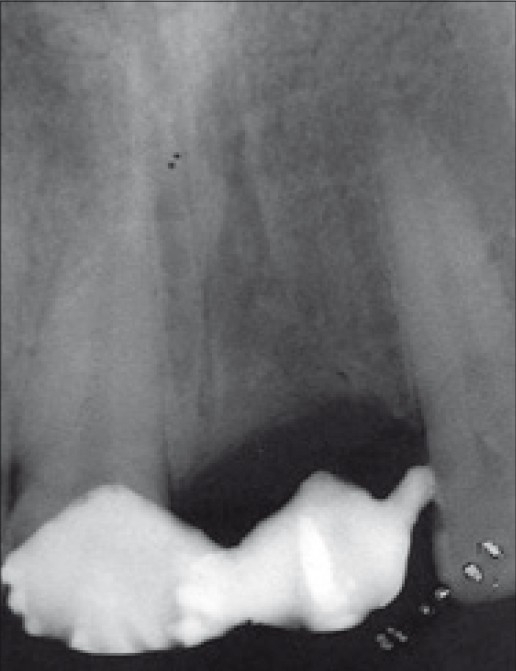
Pre-operative X-ray
History revealed that the tooth was extracted two years back due to trauma. X-ray showed adequate bone support. The treatment planned was to give delayed loading implant replacement and metal ceramic crown for the patient in the region 21, and a separate metal ceramic crown for 11.
Preparatory phase
Preparation of the patient included scaling and root planing of the entire dentition and oral hygiene instructions. Eleven was prepared for use as abutment.
Surgical technique
Under local anesthesia, a full – thick mucoperi-osteal flap was raised. A two – piece implant (zimmer) of diameter 4.25 mm and length 13 mm was placed in the region, 21 and the flap was sutured. After suture removal, the cantilever bridge was modified and given as temporary restoration [Figures 3–5].
Figure 3.
11 prepared
Figure 5.
Post-operative radiograph
Figure 4.
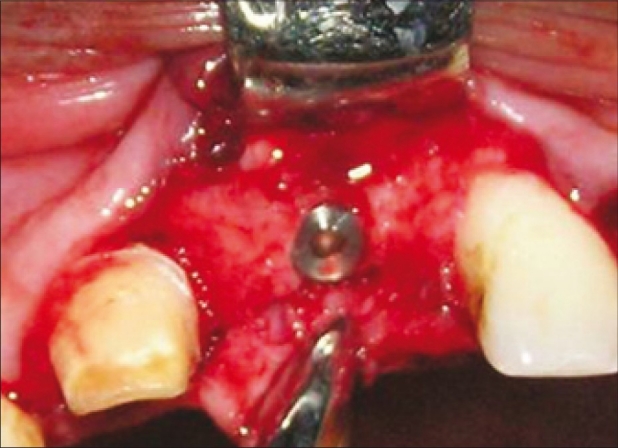
Implant with cover screw
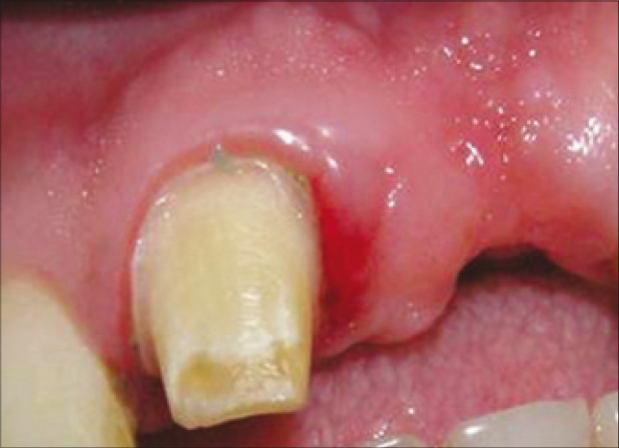
When the patient was reviewed after one month, there was a concavity in the buccal aspect of the region, 21 [Figure 6]. The region was examined with a bone meter, which revealed a buccolingual width of 5 mm. A fenestration defect was suspected. Treatment was planned to treat the fenestration defect with a combination of bone graft, PRF and guided tissue regeneration (GTR) membrane.
Figure 6.
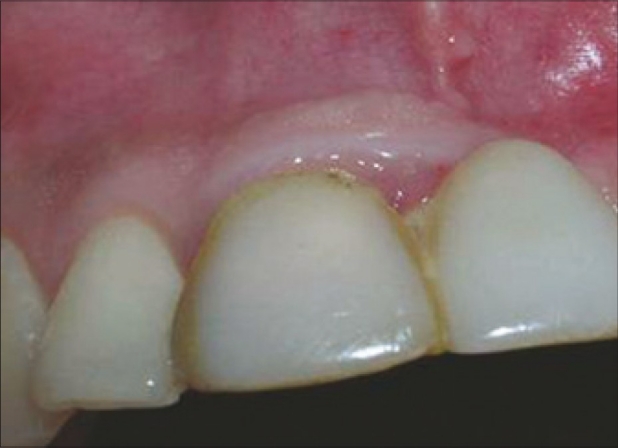
One month after implant placement
A full – thick mucoperiosteal flap was raised with two vertical releasing incisions, beyond the mucogingival junction, which revealed a class I fenestration defect [Figure 7].[3]
Figure 7.
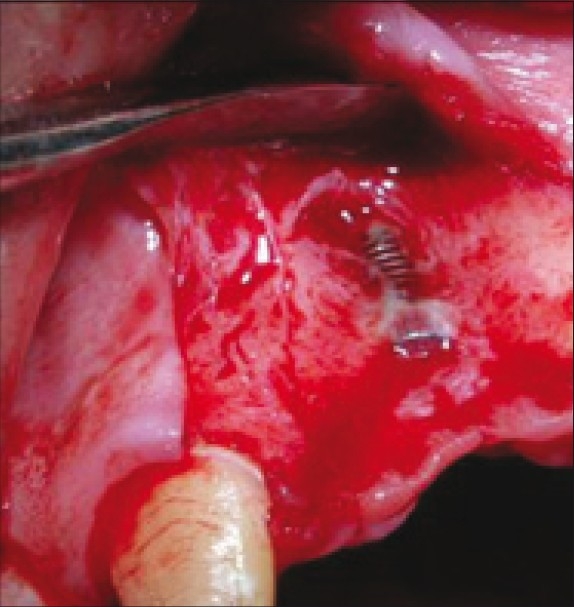
Fenestration defect around an implant
The fenestration defect was planned to be treated by placement of bone graft and PRF mixture and resorbable GTR and PRF membrane over the defect.
Preparation of the PRF
The patient's blood samples were taken in the operating room during the surgery. Immediately after the blood draw, the dried monovettes (without anticoagulant) were centrifuged at 2700 rpm for 12 minutes in a tabletop centrifuge [REMYR laboratories].
The resultant product consists of the following three layers [Figure 8]:
Figure 8.
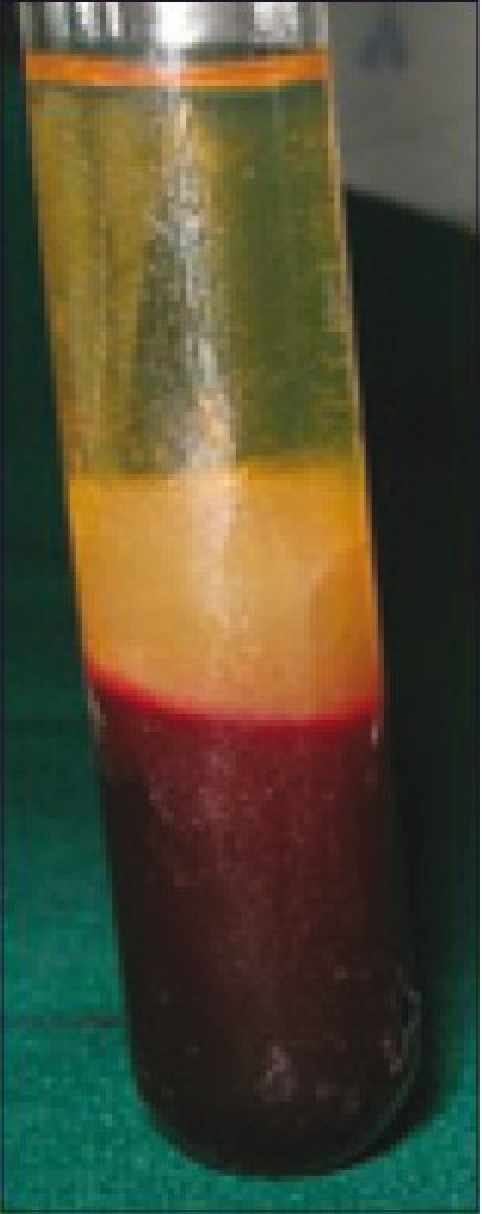
PRF in test tube
The topmost layer consisting of acellular platelet poor plasma
PRF clot in the middle
Red Blood Cells (RBCs) at the bottom.
Because of the absence of an anticoagulant, blood begins to coagulate as soon as it comes in contact with the glass surface. Therefore, for successful preparation of PRF, speedy blood collection and immediate centrifugation, before the clotting cascade is initiated is absolutely essential.[2]
The PRF clots were recovered and used in two ways:[4]
Some were placed in sterile cups and cut in few millimeter fragments. Then they were mixed with bioglass particles. The mixture obtained constituted an easy- to-use homogeneous graft material [Figures 9–11]
Others were packed tightly in two sterile compresses inorder to obtain resistant fibrin membranes which could be placed on the grafting material along with the resorbable membrane before wound closure [Figure 12].
Figure 9.
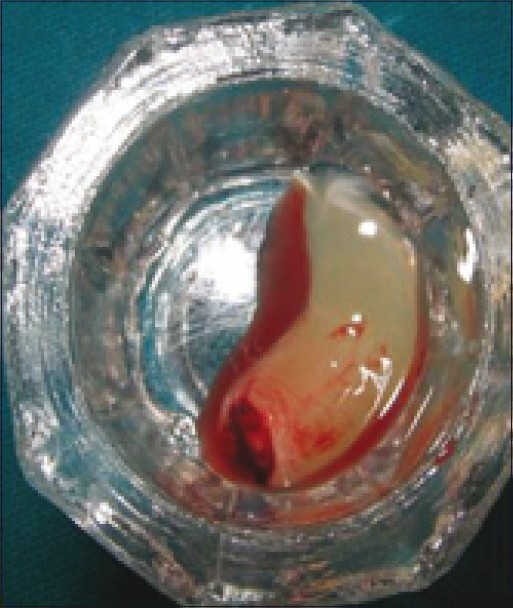
PRF gel
Figure 11.
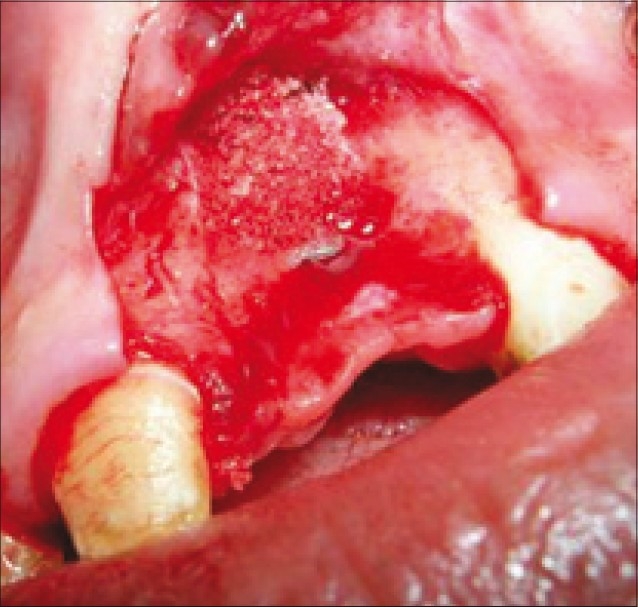
Bone graft and PRF mixture placed over defect
Figure 12.
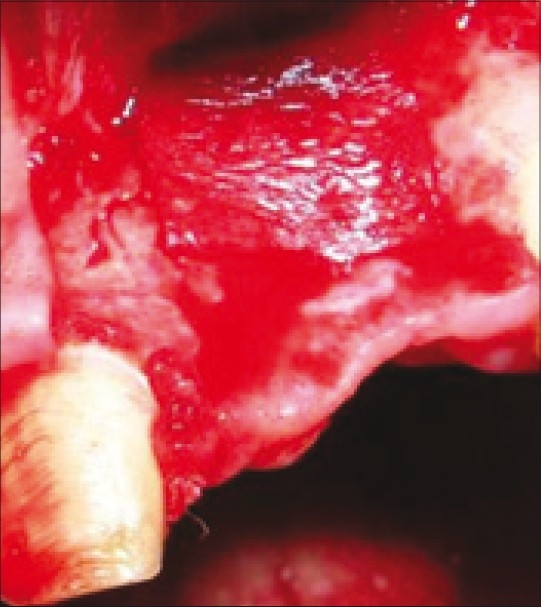
PRF and GTR membrane placed over defect
Figure 10.
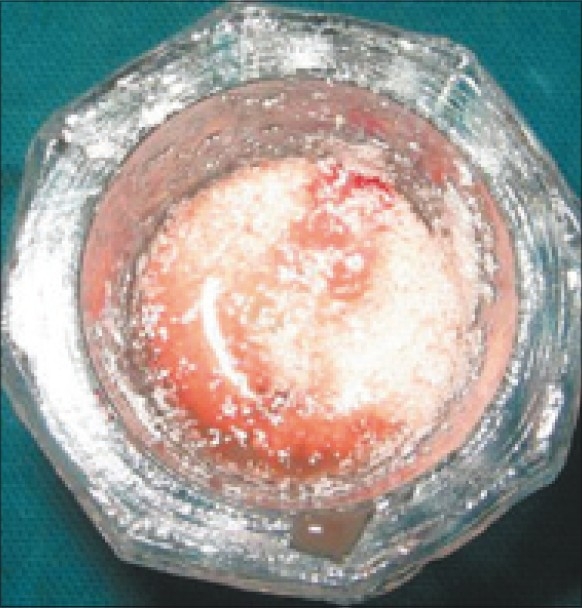
Bone graft mixed with PRF
Care was taken that the membrane extended 3-4 mm apically and mesiodistally. Later, the flap was coronally positioned and sutured [Figure 13].
Figure 13.
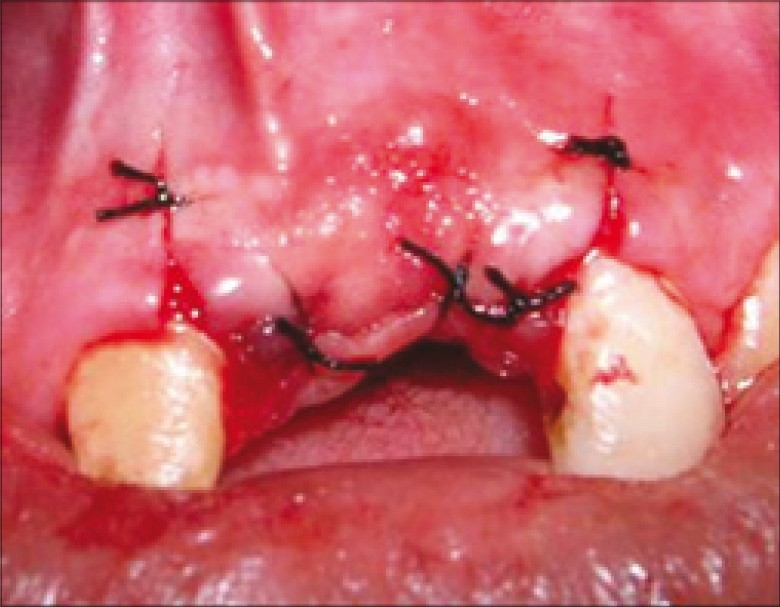
Flap sutured
The patient was given antibiotics (amoxycillin 500 mg tid for five days) and analgesics (ibuprofen 400 mg and paracetamol 500 mg twice daily for three days) and post-operative instructions were given. Antibiotics were prescribed to control any post-operative infections. Chlorhexidine (0.2%) mouth rinse was prescribed for four weeks after surgery.
Healing
The sutures were removed 10 days after the procedure. The surgical site was examined for uneventful healing. There was no post – operative complications and healing was satisfactory. The patient did not have any post-operative morbidity.
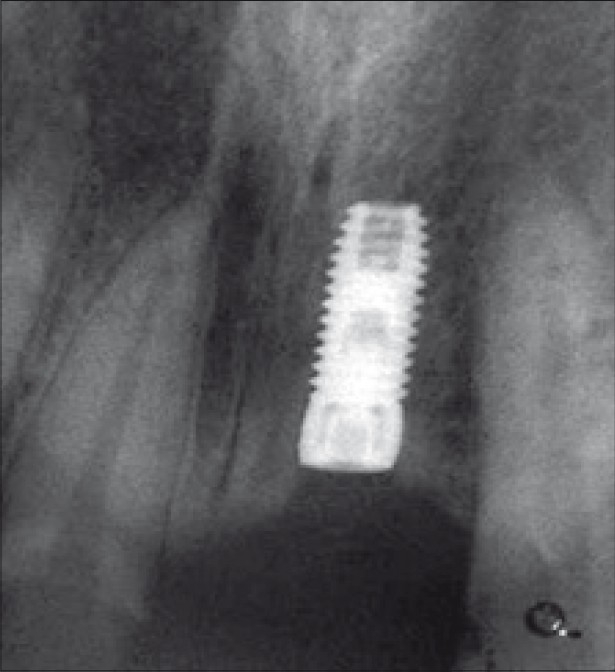
Six months after the GBR treatment, intra-oral examination with the bone meter revealed adequate buccolingual width of the ridge of 7 mm. In order not to disturb the minimal amount of bone that would have formed over the fenestration defect, reentry was not performed. The implant was uncovered and a healing abutment was connected to allow emergence of the implant through the soft tissues, thus facilitating access to the implant from the oral cavity and final restoration was placed [Figure 14].
Figure 14.
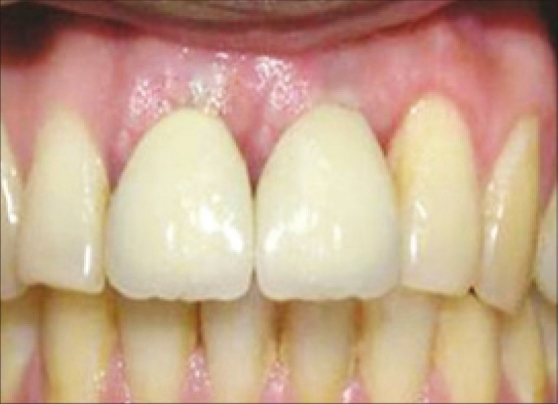
Final restoration
DISCUSSION
GBR is based on the principle of guided tissue regeneration, and was first performed in an experimental dog study.[5] This technique was clinically tested in the treatment of ridge deformities. Improvements in this technique have led to its wide-scale clinical applications to augment deficient alveolar ridges, treat implant fenestration or dehiscences, and permit immediate implant placement in large alveolar sockets.[6]
A minimum buccolingual width of 6 mm is recommended for placement of implants without undesirable complications. The most common complication associated with the placement of implants in narrow alveolar ridges or in an ideal position for esthetics is a dehiscence or fenestration defect.
A fenestration is a vestibular or linguopalatal defect as an expression of a bone thickness deficiency that creates partial exposure of an implant that is completely surrounded by bone.[3] In class I fenestrations, the implant surface penetrates the wall of bone by an insignificant amount and is located within the envelope of bone. In class II fenestrations, there is a convexity and a significant portion of the implant is exposed outside the envelope of bone for reasons of restorability.
In this case-report, there was class I fenestration defect around the implant and to group II of the classification given by Daniel Buser, 1994,[7] in which the prosthetically guided placement of an implant results in exposure of the buccal implant surface.
At present, it can be stated that biodegradable membranes have the potential to support bone formation if they are supported by bone graft material to resist collapse and if they are long-lasting enough to maintain their barrier function for extended periods in small to moderate bone defects.[8]
The degradation and resorption kinetics of a membrane for use in GBR should be set such that it remains intact for at least 6-9 months in large volume defects and then should be completely metabolized after 12-15 months.
In a recent systematic review, a reasonable comparison between bioresorbable and non-resorbable membranes could not be drawn due to lack of well designed studies.[9] In this case report, we used GTR membrane (Healiguide), which is made of collagen and PRF to treat the defect. This is a significant benefit to the patient and represents an important step in the development of GBR procedures. For successful outcomes with GBR, the factors as outlined by Mellonig et al. were followed.[10]
Recent clinical and histologic findings suggest that the use of platelet concentrates have technical benefits and may enhance bone regeneration when used in conjunction with bone grafts.[11] The amplification of platelet derived growth factor (PDGF) and transforming growth factor (TGF) beta is seen as an available and practical tool for enhancing the rate of bone formation and the final quality of bone formed.[12]
PRF has many advantages over platelet rich plasma (PRP). It eliminates the redundant process of adding anticoagulant as well as the need to neutralize it.[13] It has been shown from the literature that it increases the rate of clinical graft consolidation, and PRF- enhanced grafts produce more mature and dense bone than do grafts without PRF.[13,14] PRF is in the form of a platelet gel and can be used in conjunction with bone grafts, which offers several advantages including promoting wound healing, bone growth and maturation, graft stabilization, wound sealing and hemostasis and improving the handling properties of graft materials.[15] In an experimental study which used osteoblast cell cultures to investigate the influence of PRP and PRF on proliferation and differentiation of osteoblasts, it was found that PRF had a superior influence over PRP. Also, bone augmentation grafts may act as space-maintaining devices to allow coronal migration of periodontal progenitor cells.[16]
The technique of applying biomaterials to support bioresorbable membranes avoid the risks associated with harvesting autogenic bone.[17] Bioglass is preferred for its high bioactivity, because of which the reaction layers appear to form within minutes of its implantation, and the osteogenic cells freed by the surgery rapidly colonize the particles. Use of bioactive glass results in more rapid filling of the defects, which may result from more rapid accumulation of bone morphogenic proteins and other growth factors on the surface of bioactive particles, due to its high bioactive index. However, the bioactive glass in this case has no role beyond a scaffold and its absence of use would have produced the same result.
The development of biomaterials, ideally coupled with the incorporation of bone growth factors and bioactive peptides, represents an important line of research in this direction. Recent systematic reviews regarding the survival rate of implants into sites with regenerated/augmented bone using barrier membranes varied between 79% and 100% with the majority of studies indicating more than 90% after at least one year of function.[9]
Thus, in this case report, a fenestration defect was effectively treated by the application of growth factors both to the bone graft and GTR membrane.
CONCLUSION
At present, large bone defects are regularly augmented with autogenous block grafts and membranes. The use of synthetic materials would result in lower surgical risks and lower morbidity in the augmentation procedure and would represent an important step forward in simplifying bone regeneration techniques.
Although a meta-analysis of studies in the regeneration of intrabony defects with bone grafts has been reported, no such analysis has been made till date in evaluating the use of platelet concentrates alone or with bone grafts in the treatment of bony defects. We hope that this case-report would become a part of meta-analysis in the future to help plan an evidence-based treatment.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Lekovic V, Camargo PM, Weinlaender M, Vasilic N, Kenney EB. Comparison of platelet rich plasma, bovine porous bone material and guided tissue regeneration versus platelet rich plasma and bovine porous bone material in the treatment of intrabony defects. A re-entry study. J Periodontol. 2002;73:198–205. doi: 10.1902/jop.2002.73.2.198. [DOI] [PubMed] [Google Scholar]
- 2.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): A second generation platelet concentrate: Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Dahlin C, Lekholm U, Becker W. Treatment of fenestration and dehiscence bone defects around oral implants using the GTR technique: A propective multicenter study. Int J Oral Maxillofac Implants. 1995;10:312. [PubMed] [Google Scholar]
- 4.Choukron J, Diss A, Simonpieri A. Platelet –rich f brin: A second generation platelet concentrate. Part V: Histologic evaluation of PRF effects of bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:299–303. doi: 10.1016/j.tripleo.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Siebert J, Nyman S. Localized ridge augmentation in dogs: A pilot study using hydroxyapatite and membranes. J Periodontol. 1990;61:157–65. doi: 10.1902/jop.1990.61.3.157. [DOI] [PubMed] [Google Scholar]
- 6.Simion M, Carano A, Piatelli A. GBR using resorbable and nonresorbable membrane – A compared histologic study in humans. Int J Oral Implants. 1996;7:735–42. [PubMed] [Google Scholar]
- 7.Buser D, Dahlin C, Schenk RK. Guided bone regeneration in implant dentistry. London: Quintessence Publishing Co, Inc; 1994. [Google Scholar]
- 8.Mehta Guided tissue regeneration. J Indian Dent Assoc. 1999;70:86–9. [Google Scholar]
- 9.Chiapasco M, Zaniboni M, Boisco M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin Oral Implant Res. 2006;17:136–59. doi: 10.1111/j.1600-0501.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 10.Mellonig JT, Nevins M. Guided bone regeneration of bone defects associated with implants: An evidence-based outcome assessment. Int J Periodontics Restorative Dent. 1995;15:168–85. [PubMed] [Google Scholar]
- 11.Kassolis JD, Rosen PS, Reynolds MA. Alveolar ridge and sinus augmentation utilizing platelet rich plasma in combination with freeze dried bone allograft; case series. J Periodontol. 2000;71:1654–61. doi: 10.1902/jop.2000.71.10.1654. [DOI] [PubMed] [Google Scholar]
- 12.Lynch SE, Genco RJ, Marx RE. Quinte; Tissue engineering: Applications in maxillofacial surgery and periodontics. London: Quintessence Publishing Co, Inc; 1999. pp. 71–82. [Google Scholar]
- 13.Par Wiltfang J, Terheyden H, Gassling V, Acyl A. Platelet rich plasma (PRP) vs. platelet rich plasma (PRP) vs platelet rich fibrin (PRF): Comparison of growth factor content and osteoblast proliferation and differentiation in the cell culture. In: Report of the 2nd international symposium on growth Factors (SyFac. 2005 [Google Scholar]
- 14.Nkenke E, Schultze-Mosgau S, Radespiel-Tröger M, Kloss F, Neukam FW. Morbidity of harvesting with chin grafts – A prospective study. Clin Oral Implant Res. 2001;12:495–500. doi: 10.1034/j.1600-0501.2001.120510.x. [DOI] [PubMed] [Google Scholar]
- 15.Sunitha Raja V, Munirathnam Naidu E. Platelet-rich fibrin: Evolution of a second generation platelet concentrate – A review. Indian J Dent Res. 2008;19:42–7. doi: 10.4103/0970-9290.38931. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez AR, Sheridan PJ, Kupp LI. Is platelet rich plasma the perfect enhancement factor. A current review? Int J Oral Maxillofac Implants. 2003;18:93–103. [PubMed] [Google Scholar]
- 17.Yuan H, de Bruijn JD, Zhang X, van Blitterswijk CA, de Groot K. Bone induction by porous glass ceramic made from Bioglass (45S5) J Biomed Mater Res. 2001;58:270–6. doi: 10.1002/1097-4636(2001)58:3<270::aid-jbm1016>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]


