Abstract
Background:
Local delivery of antimicrobial has resulted in good clinical outcome along with scaling and root planing. The present study is carried out to evaluate and compare the efficacy of local delivery of 10% doxycycline hyclate in adjunct to scaling and root planing in the treatment of periodontitis.
Materials and Methods:
A randomized crossover split mouth design was performed, a total number of 130 sites from 4 patients, 63 sites from patients with aggressive periodontitis and 67 sites from chronic periodontitis patients were selected and divided into scaling and root planing (SRP) group, SRP and doxycycline group and doxycycline alone group. Clinical parameters viz. plaque index, modified gingival index, bleeding index, clinical attachment level (CAL), and sub gingival temperature were evaluated on day 0, 15th, 45th, and 90th day. CAL recording was performed only on day 0 and 90th day.
Results:
In 90 days study, all the three groups showed significant reduction in clinical parameters. But on comparison, SRP and doxycycline group showed better results than doxycycline alone group and SRP alone group.
Conclusion:
The results of this study demonstrated that doxycycline hyclate 10% gel (Atridox) is as effective as SRP in reducing the clinical signs of periodontitis.
Keywords: Aggressive periodontitis, antimicrobial agents, chronic periodontitis, doxycycline hyclate (Atridox), local drug delivery, subgingival temperature
INTRODUCTION
The greatest therapeutic challenge associated with the successful treatment of periodontitis involves the ability to alter/eliminate the bacteria that cause the infection. Increasing knowledge of anaerobic bacteria as predominant agent in the development of periodontal disease has led to new treatment modalities, aiming primarily at suppression/elimination of specific periodontal pathogens. A less invasive approach in the management of periodontitis is administering an antimicrobial agent into the pocket, which is biodegradable and provides appropriate antimicrobial level for sustained period.
Recently, a local anti-infective treatment for periodontitis has been introduced that delivers a formulation of 10% doxycycline hyclate to the periodontal pocket in a biodegradable liquid polymer that solidifies on contact with the gingival crevicular fluid. As it biodegrades, this solid implant delivers doxycycline at high levels to the site for 7-14 days.
Atridox (Atrix Laboratories, NJ) is a recently developed gel system, which incorporates doxycycline in a syringeable gel system. An animal study by Polson et al.[1] in beagle dogs initially suggested beneficial aspects of locally delivered doxycycline. Studies undertaken by Polson et al.[2] when compared to sanguinarine indicated that 10% doxycycline hyclate is an effective means of reducing the clinical signs of adult periodontitis, Stoller et al.[3] indicated that this controlled release delivery system displays an appropriate pharmacokinetic profile for the delivery of doxycycline into periodontal pockets, Garrett et al.,[4] when compared to scaling and root planing indicated that 10% doxycycline hyclate is equally effective as scaling and root planing in reducing the clinical signs of adult periodontitis, Walker et al.,[5] showed that doxycycline treatment significantly reduced the anaerobic population in plaque and did not change the number of resistant bacteria. Most of the studies in literature have excluded aggressive periodontitis cases to assess the efficacy of local doxycycline therapy.
Therefore, an attempt has been made in this study to evaluate the efficacy of 10% doxycycline hyclate subgingivally delivered gel system in the treatment of aggressive periodontitis and chronic periodontitis with and without scaling and root planing (SRP).
MATERIALS AND METHODS
Study overview
A crossover split mouth design was performed. Four patients (both males and females), two diagnosed as aggressive periodontitis and two as chronic periodontitis were selected from outpatient Department of Periodontics, College of Dental Sciences, Davangere, Karnataka, India. This study reports the results of the relative clinical efficacy of each of three periodontal treatments over a 90 day period.
Screening procedures
Eligibility was determined by conducting a periodontal evaluation at pre-baseline screening visits to assess current disease status, periodontal treatment history, and medical history. Subjects were included if they gave signed consent, have diagnosed as aggressive periodontitis and chronic periodontitis (American Academy of Periodontology (AAP) -1999), at least two quadrants where each of these quadrants contained periodontal pockets measuring ≥5 mm and ≤7 mm with bleeding gentle on probing on clinical examination, and radiographic evidence of bone loss. Sites with these criteria were termed as tests sites.
The following criteria excluded subject participation in the study suffering from known systemic disease, if they had contraindicated medications, pregnancy or active lactation, using chemotherapeutics during past six months, allergy to periodontal dressing, known hypersensitive to doxycycline or lignocaine and if they have received any surgical or non – surgical periodontal therapy for the past six months of the baseline visits. Ethical clearance was obtained from the ethical committee of College of Dental Sciences, Davangere, Karnataka, India. All participants gave informed consent.
Treatment procedure
A split mouth design was used in this study for the delivery of treatment. Each quadrant of the subject's mouth was assigned to one of three treatments: SRP alone, SRP and doxycycline and doxycycline alone only.
Baseline measurements included plaque index (Silness and Loe, 1964),[6] bleeding index (Ainamo and Bay, 1975),[7] modified gingival index (Lobene, Weatherford, and Rose, l986),[8] and clinical attachment level using University of North Carolina (UNC)-15 mm (Hu-Friedy, USA) periodontal probe and sublingual temperature using modified thermometer, Micro care.
Evaluations
At the baseline visit and at the follow up evaluation at 15th, 45th, and 90th days, measurements were made of plaque index, bleeding index, modified gingival index, and sub gingival temperature. Whereas clinical attachment level recording was done at baseline and at 90th day. All periodontal measurements were made using a periodontal probe graduated in 1 mm increment. Reading was rounded to the nearest mm [Figure 1].
Figure 1.
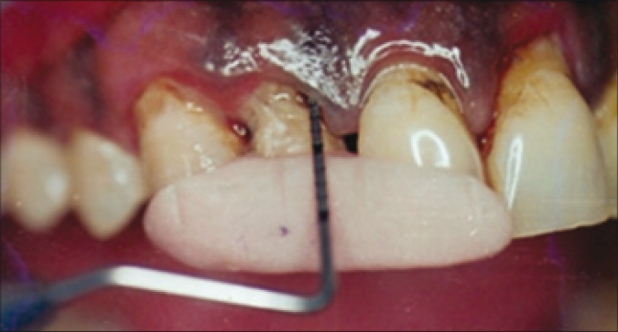
Probing with acrylic stent
After recording the clinical parameters at each site in selected patients, a through scaling and root planing was done using hand instruments and ultrasonic scalers in SRP alone group and SRP and doxycycline group sites.
Application of doxycycline hyclate 10% gel
Preparation for use
The two components of the formulations were provided in two separate syringes that were coupled together 15 minutes prior to use and mixed for 100 cycles. Once mixed, the coupled syringes were allowed to set at room temperature for 15 minutes and then mixed for another 10 cycles before use.
Product administration
By the use of 2/3 gauge cannula attached to the delivery syringe, the above prepared product was slowly expressed into the periodontal pocket starting from the base of the pocket until it reached the gingival margin [Figure 2]. Following the withdrawal of the cannula tip from the pocket a moist curette was used to pack any overflow of material down into the pocket. Then treated quadrants were covered with periodontal dressing to achieve retention of the product into the pocket.
Figure 2.
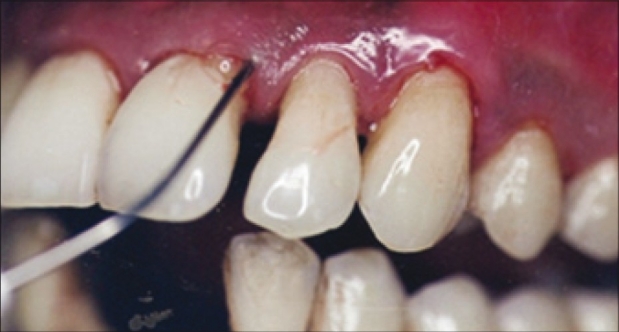
Application of doxycycline hyclate gel
Patient instructions
After introducing doxycycline gel, patients were instructed not to brush or floss on the treated area for seven days, avoid hard or sticky foods for seven days and not to touch the area with tongue, finger or tooth pick. Oral hygiene instructions were given to each patient to brush with roll on technique. The patients were also instructed to report immediately, if the material or pack is dislodged before the scheduled recall visit or if pain, swelling or any other problem occurs. The patients were recalled after seven days for the removal of periodontal dressing.
On the subsequent recall visits, the clinical parameters and any adverse effects were recorded.
Statistical method
Analysis of variance (ANOVA) F test was used to detect the differences between treatments, the paired t test was used to detect differences between two sample periods within the same treatments, and the unpaired t test was used to detect differences between treatments at the same sample periods. All statistical tests were applied using 5% level of significance.
RESULTS
All the clinical parameters, i.e., plaque index, modified gingival index, bleeding index, clinical attachment level, and subgingival (Delta) temperature showed significant reduction on all the days from the baseline.
The mean reduction in plaque index and modified gingival index showed higher reduction in doxycycline alone group and SRP alone group compared to SRP and doxycycline group. The mean reduction in bleeding index and clinical attachment level shows higher reduction in SRP and doxycycline group compared to doxycycline alone group and SRP alone group. The difference between the groups for plaque index, bleeding index, and clinical attachment was not significant except for modified gingival index between SRP and doxycycline group and doxycycline alone group showed statistically significant (P=0.01). The reduction in delta temperature showed higher reduction in SRP and doxycycline group and doxycycline alone group compared to SRP alone group. The reduction in delta temperature between groups was not significant except between doxycycline alone group and SRP alone group which showed statistically significant (P<0.01).
The results of this study are presented in Tables 1 and 2 and Figures 3–7.
Table 1.
Comparison of various clinical parameters between different groups at different time intervals
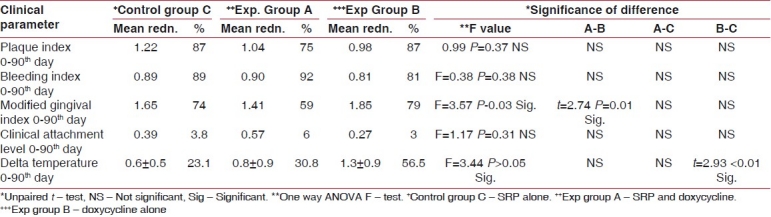
Table 2.
Comparison of various clinical parameters within each group at different time intervals
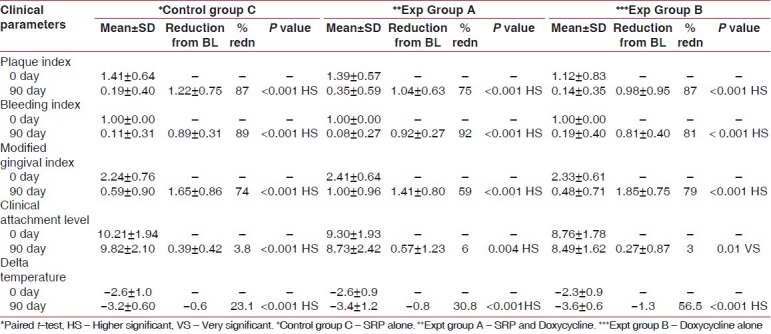
Figure 3.
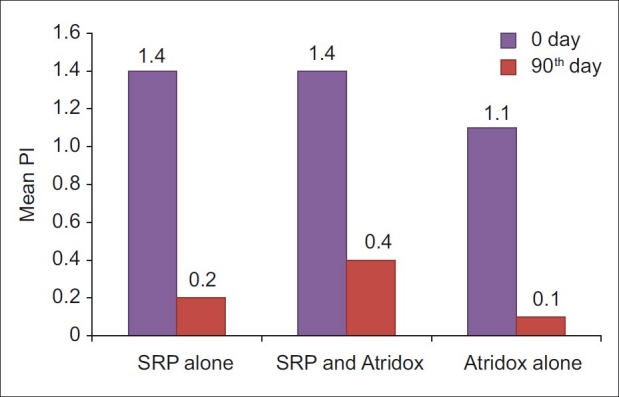
Plaque index score at 0 and 90th day in SRP alone, SRP and Atridox and Atridox alone groups
Figure 7.
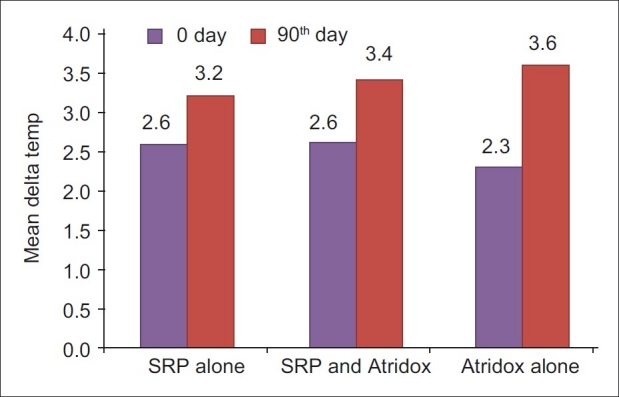
Delta temperature (-ve values) at 0 and 90th day in SRP alone, SRP and Atridox and Atridox alone groups.
Figure 4.

Bleeding Index score at 0 and 90th day in SRP alone, SRP and Atridox and Atridox alone groups
Figure 5.

Modified gingival index score at 0 and 90th day in SRP alone, SRP and Atridox and Atridox alone groups.
Figure 6.
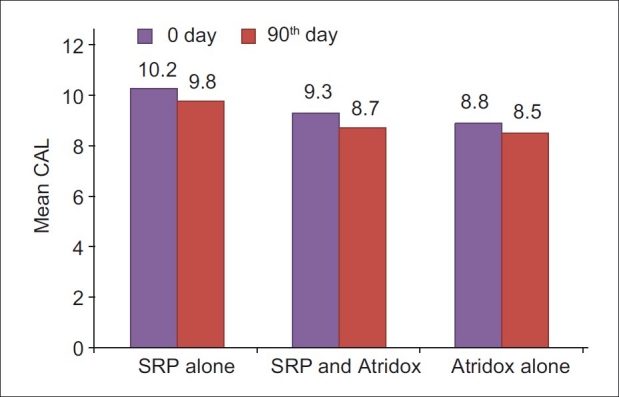
Clinical attachment level score at 0 and 90th day in SRP alone, SRP and Atridox and Atridox alone groups
DISCUSSION
The Atridox product which is chosen for the study delivers 10% doxycycline hyclate to the pocket environment over a 7-day period. Stoller et al.[3] have shown Gingival crevicular fluid (GCF) concentrations to peak with a concentration of 1473–1986 μg/ml at 2 hours and gradually decreased to 309 μg/ml at the end of 7 days which was above the minimum inhibiting concentration for doxycycline. However, minimal GCF drugs levels were obtained with oral doxycycline (2.53 μg/ml) when compared to systemic doxycycline.
In the present study, an attempt is made to evaluate the effectiveness of doxycycline hyclate 10% dental gel (Atridox) in the treatment of periodontal pockets with/without scaling and root planing in Periodontitis patients.
Plaque index
The mean reduction in plaque score for control group from baseline to 90th day was 87%, which was highly significant (P<0.001). This was consistent with the finding of Garrett et al.,[4] and Wennstrom et al.[9] This improvement observed from 0-90th day could be due to adequate maintenance of oral hygiene which was instructed to each patient. The mean reduction in plaque score for SRP and doxycycline group from baseline to 90th day was 75%, which was highly significant (P<0.001). This finding was similar to that of Wennstrom et al.[9] The mean reduction in plaque score for doxycycline alone group from baseline to 90th day was 87%, which was highly significant (P<0.001). This finding was similar to that of Polson et al.[2] and Garrett et al.[10]
Mean reduction in plaque scores between SRP and doxycycline group and doxycycline alone group, doxycycline alone group and SRP alone group, and SRP and doxycycline group and SRP alone group were not significant. This difference between doxycycline alone group and SRP alone group was similar to the observations of Garrett et al.,[4] and Garrett et al.[10] The difference between SRP and doxycycline group and SRP alone group was similar to the observation of Wennstrom et al.[9]
Bleeding index
The mean reduction in bleeding score for SRP alone group from baseline to 90th day was 89%, which was highly significant (P<0.001). This observation was in accordance with the finding of Polson et al.[1] Wennstrom et al.,[9] Garrett et al.[10] and Taner et al.[11] The mean reduction in bleeding score for experimental SRP and doxycycline group from baseline to 90th day was 92%, which was highly significant (P<0.001). This finding was consistent with the observation of Wennstrom et al.,[9] Ozcan et al.,[12] and Taner et al.[11] The mean reduction in bleeding score for experimental doxycycline alone group from baseline to 90th day was 81%, which was highly significant (P<0.001). This observation was in accordance with the finding of Polson et al.,[1] Garrett et al.,[10] Ozcan et al.,[12] and Taner et al.[11]
On comparison of reduction of bleeding scores between SRP and doxycycline group and doxycycline alone group, between doxycycline alone group and SRP alone group and between SRP and doxycycline group and SRP alone group the difference was not significant. The difference in reduction of bleeding scores between SRP and doxycycline group and SRP alone group was similar to the finding of Garrett et al.[10]
Modified gingival index
The mean reduction in modified gingival score from baseline to 90th day was 74% for SRP alone group, 59% for SRP and doxycycline group, and 79% for doxycycline alone group. The reduction m modified gingival scores was highly significant (P<0.001) within all the groups.
On comparison between the groups, the reduction of the modified gingival scores was not significant between SRP and doxycycline group and SRP alone group and between doxycycline alone group and SRP alone group except between SRP and doxycycline group and doxycycline alone group it was significant (P=0.01).
Clinical attachment level
The mean reduction in clinical attachment level (CAL) for control group from baseline to 90th day was 3.8%, which was highly significant (P<0.001). This finding was similar to the observation of Wennstrom et al.,[9] Garrett et al.[10] and Garrett et al.[4] The mean reduction in CAL for SRP and doxycycline group from baseline to 90th day was 6%, which was very significant (P=0.004). This was similar to the findings of Wennstrom et al.[9] The mean reduction in CAL for doxycycline alone group from baseline 90 day was 3%, which was very significant (P=0.01). This was similar to the findings of Garrett et al.,[4] Polson et al.,[1] Polson et al.,[13] Wennstrom et al.,[9] Ozcan et al.,[12] and Taner et al.[11]
The mean reduction in CAL between SRP and doxycycline group and doxycycline alone group was not significant. The mean reduction is CAL between SRP and doxycycline group and SRP alone group were not significant. This finding was similar to the observation of Wennstrom et al.[9] The mean reduction in CAL between doxycycline alone group and SRP alone group was not significant. This finding was similar to the observation of Garrett et al.,[10] and Garrett et al.[4]
Delta temperature
The mean reduction in delta temperature from baseline to 90th day was 23.11% for SRP alone group, 30.8% for SRP and doxycycline group, and 56.5% for doxycycline alone group. The reduction in delta temperature was highly significant (P<0.001) in all groups.
On comparison, the reduction of delta temperature was not significant between SRP and doxycycline group and doxycycline alone group and between SRP and doxycycline group and SRP alone group, except between doxycycline alone group and SRP alone group, which was significant (P<0.01). This significant difference in doxycycline alone group and SRP alone group was similar of Kung et al.[14]
No adverse reaction was observed by the clinician and the patients at the gel administered sites in this study. However, Wennstrom et al.,[9] in their study demonstrated mild to moderate gingival soreness and thermal sensitivity following treatment, Polson et al.[2] in their study demonstrated one patient with periodontal abscess at treated site in doxycycline group.
Several studies comparing SRP with doxycycline dental gel have revealed similar results, which indicates either SRP or doxycycline dental gel alone may be favorable (Garrett et al., Garrett et al.).[10,4] In one study combination therapy (SRP+doxycycline) comparing with SRP alone revealed similar results Wennstrom et al.[9]
The measurement of subgingival temperature is dealt rarely in periodontal literature with regard to local drug delivery which has been one of the measurements in the study.
The study is self funded with the financial restriction, limited number of patients and with good number of sites were treated based on site specific entity of periodontal disease. No attempt was made to compare aggressive v/s chronic periodontitis cases. Further, Randomized clinical trials (RCTs) with larger number of patients with microbial evaluation could provide better results. The use of doxycycline hyclate for treatment of periodontal patients is a good recommended considering the results of the study. However, non availability of the drug in Indian scene is a major drawback.
CONCLUSION
This study demonstrated that doxycycline hyclate 10% gel (Atridox) is as effective as SRP in reducing the clinical signs of periodontitis.
However, further studies are needed to determine which type of patients and lesions will benefit most from the incorporation of locally delivered controlled release doxycycline as an adjunct to non surgical periodontal therapy.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Polson AM, Southard GL, Dunn RL, Yewey GL, Godowski KC, Polson AP, et al. Periodontal pocket treatment in beagle dogs using subgingival doxycycline from a biodegradable system.Initial clinical responses. J Periodontol. 1996;67:1176–84. doi: 10.1902/jop.1996.67.11.1176. [DOI] [PubMed] [Google Scholar]
- 2.Polson AM, Garrett S, Stoller NH, Bandt CL, Hanes PJ, Killoy WJ, et al. Multicenter comparative evaulation of subgingivaly delivered sanguinarine and doxycycline in the treatment of periodontitis I study design, procedures and management. J Periodontol. 1997;68:110–8. doi: 10.1902/jop.1997.68.2.110. [DOI] [PubMed] [Google Scholar]
- 3.Stoller NH, Johnson LK, Trapnell S, Harrold CQ, Garrett S. The pharmacokinetic profile of a biodegradable controlled release delivery system containing doxycycline compared to systemically delivered doxycycline in gingival crevicular fluid, saliva and serum. J Periodontol. 1998;69:1085–91. doi: 10.1902/jop.1998.69.10.1085. [DOI] [PubMed] [Google Scholar]
- 4.Garrett S, Johnson L, Drisko CH, Adams DF, Bandt C, Beiswanger B, et al. Two 9 Months multicenter studies evaluating locally delivered doxycycline hyclate 8.5%, placebo control oral hygiene and scaling and root planing in the treatment of periodontitis. J Periodontol. 1999;70:490–503. doi: 10.1902/jop.1999.70.5.490. [DOI] [PubMed] [Google Scholar]
- 5.Walker KC, Godowski L, Borden L, Lennon J, Nangó S, Stone C, et al. The effects of sustained release doxycycline on the anaerobic flora and antibiotic resistant patterns in subgingival plaque and saliva. J Periodontol. 2000;71:768–74. doi: 10.1902/jop.2000.71.5.768. [DOI] [PubMed] [Google Scholar]
- 6.Sillness J, Loe H. Periodontal diseases in pregnancy (2) correlation between oral hygiene and periodontal condition. Acta Odontol Scan. 1964;24:747–59. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 7.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229–35. [PubMed] [Google Scholar]
- 8.Lobene RR, Weatherford T, Rose NM, Lamm RA, Menaker L. A modified gingival index for use in clinical trials. Clin Prev Dent. 1986;8:3–6. [PubMed] [Google Scholar]
- 9.Wennstrom JL, Newman HN, MacNeill SR, Killoy WJ, Griffiths GS, Gillam DG, et al. Utilization of locally delivered doxycycline in non surgical treatment of chrome periodontitis, a comparative multicenter trial of two treatment approaches. J Clin Periodontol. 2001;28:753–61. doi: 10.1034/j.1600-051x.2001.280806.x. [DOI] [PubMed] [Google Scholar]
- 10.Garrett S, Adams GF, Bogle G, Donly K, Drisko CH, Hallmon WW, et al. The effect of locally delivered controlled release doxycycline or scaling and root planing on periodontal maintenance patients over 9 months. J Periodontol. 2000;71:22–30. doi: 10.1902/jop.2000.71.1.22. [DOI] [PubMed] [Google Scholar]
- 11.Tanner IL, Ozcan G, Doganay T, Iscanolu M, Taplamacioglu B, Gultekin SE, et al. Comparison of antibacterial effects on subgingival microflora of two different resorbable base materials containing doxycycline. J Nihon Univ Sch Dent. 1994;36:183–90. doi: 10.2334/josnusd1959.36.183. [DOI] [PubMed] [Google Scholar]
- 12.Ozcan G, Taner IL, Doğanay T, Iscanoğlu M, Taplamacioğlu B, Gültekin SE, et al. Use of membranes containing 20% cholorhexidine and 40% of doxycycline for treatment of chronic periodontal pockets. J Nihon Univ Sch Dent. 1994;36:191–8. doi: 10.2334/josnusd1959.36.191. [DOI] [PubMed] [Google Scholar]
- 13.Polson AM, Garrett S, Stoller NH, Bandt CL, Hanes PJ, Killoy WJ, et al. Multicenter comparative evaluation of subgingivaly delivered sanguinarine and doxycycline in the treatment of periodontitis. II Clinical Results J Periodontol. 1997;68(2):199–26. doi: 10.1902/jop.1997.68.2.119. [DOI] [PubMed] [Google Scholar]
- 14.Kung RT, Goodson JM. Temperature cools following local tetracycline treatment. J Dent Res. 1990;69(Spec issue) (Abs 271):142. [Google Scholar]


