Abstract
Background:
The palatal masticatory mucosa is widely used as a donor material in periodontal plastic surgery. The thickness of graft tissue is an important factor for the graft survival. The purpose of this study was to determine the thickness of palatal mucosa by a bone sounding technique. The association of age and gender with the thickness of palatal mucosa was also examined.
Materials and Methods:
Twenty four healthy subjects had participated in the study. The younger age group of 16-30 years consisted of 12 subjects of 7 females and 5 males, and the older age group of 31-54 years consisted of 12 subjects, of 5 females and 7 males. A bone sounding method using a periodontal probe was done to assess the thickness of palatal mucosa at 15 measurement sites defined according to the gingival margin and palatal line. Mann-Whitney test was used to determine the difference in mucosal thickness between both the groups.
Results:
The younger age group had thinner mucosa ranged from 2 to 3.1 mm in thickness than the older age group which ranged from 3.2 to 3.7 mm. In the same age group, females had thinner mucosa than males in the same age group. The mean thickness of palatal masticatory mucosa ranged from 2.5 to 3.7 mm.
Conclusion:
The younger subjects had thinner mucosa than older subjects. The canine and premolar areas appeared to be the most appropriate donor site for grafting procedures.
Keywords: Bone sounding, masticatory mucosa, periodontal probe
INTRODUCTION
Detailed information on thickness of different parts of the masticatory mucosa may be of considerable interest for several reasons. Palatal masticatory mucosa is widely used as a donor tissue in periodontal plastic surgery for root coverage procedures, for increasing the width of attached gingiva, and for alveolar ridge augmentation.[1–5] The volume of tissues that can be obtained from this donor site is important for the selection of treatment modalities and can affect the surgical outcome, particularly in ridge augmentation procedures to correct moderate and severe ridge resorption.[6] Several materials have been used for soft tissue augmentation. The main donor sites for connective tissue graft are the palate, maxillary tuberosity area, and edentulous sites. The success of this technique depends on the thickness of the graft tissue obtained (Kydd et al.). The graft obtained can shrink, if it is too thin, and there can be problems with revascularization and healing, if it is too thick (Morman et al.). Different methods have previously investigated the thickness of palatal masticatory mucosa, in edentulous individuals.[7–13]
There are relatively few reports on the thickness of masticatory mucosa in dentate individuals.[11,14] The thickness and volume of the tissue to be grafted from donor site are important factors in determining the appropriate treatment method and for predicting the prognosis. Thus, the aim of the present study was to determine the thickness of palatal masticatory mucosa associated with age and gender by using a bone-sounding technique.
MATERIALS AND METHODS
Twenty four healthy subjects from out patient department of SDM College of Dental Sciences, Dharwad, participated in this study. The younger age group consisted of 12 subjects (seven females and five males of 16-30 years). Older age group consisted of 12 subjects (five females and seven males of 31-54 years). Subjects with history of surgery in palate or tuberosity region, subjects with previous history or presence of any stomatological disease in the palate or tuberosity, pregnant women or lactating mothers, smokers, and subjects with tooth malpositions were excluded from the study. Subjects with healthy periodontal tissues, with complete dentition in the upper jaw with or without third molar, were included in the study. An informed consent from all the subjects was obtained after explaining the experimental procedure.
Upper arch alginate impressions were made to prepare the study model for each subject. Clear acrylic stents were prepared on each cast, for each subject. Measurement points were made as follows [Figure 1]: The 15 measurements points among lines b, c, d, Ca, P1, P2, M1, and M2 were chosen as the measurement points. Line a - gingival margin, line b – 3 mm from the gingival margin, line c and d - ¼ and ½ distances between line b and line P, respectively. Lines b, c, and d run parallel to line a. Lines Ca, P1, and P2 - mid-palatal aspect of the canine, first premolar and second premolar, respectively. Lines M1 and M2 – mesio-palatal cusps of the first and second molars, respectively). Fifteen measurement points were made on the cast, keeping mid palatine raphae and gingival margin as reference points [Figures 1 and 2]. Fissure diamond bur was used to create holes at the marked measurement points on the stent at 90° to the surface of the stent [Figure 3] the prepared stent provided a consistent location for the assessment of mucosal thickness.
Figure 1.
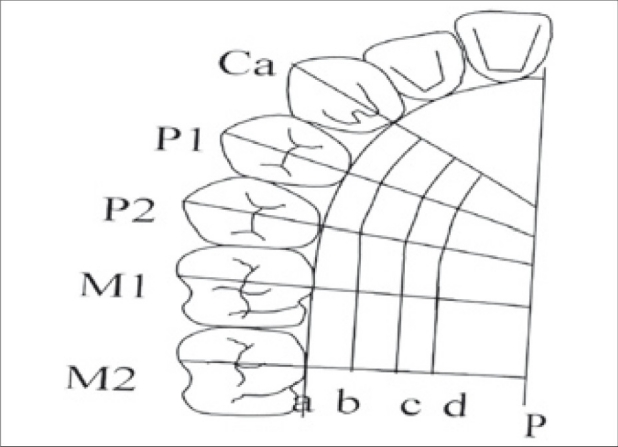
Schematic representation of measurement points
Figure 2.
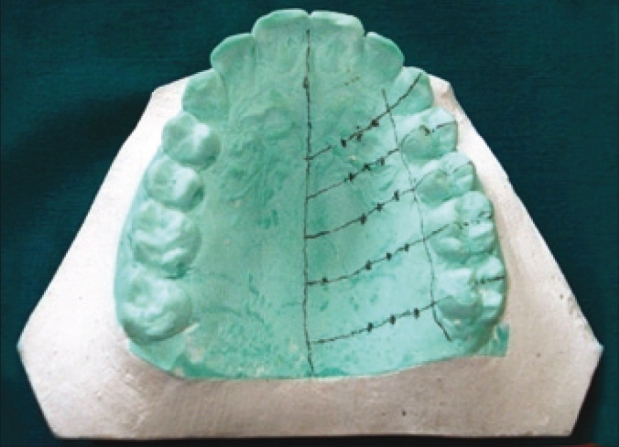
Measurement points marked on the cast
Figure 3.
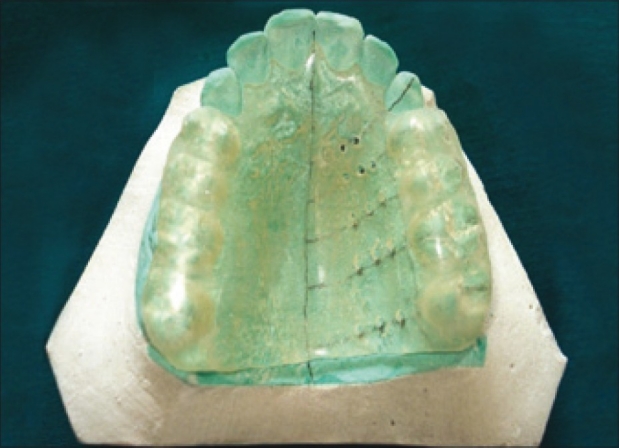
Acrylic Stent preparation with bur holes
The greater palatine and incisive nerves were blocked with 2% lidocaine, 1:100,000 epinephrine injection. The measurement points were marked with a Gentian Violet pencil on the palate, through the holes made on the stent [Figure 4]. Measurements were performed 20 minutes after the injection. The thickness of the palatal masticatory mucosa was measured by “bone sounding with periodontal probe, with a rubber stopper” [Figure 4]. The probe with the rubber stopper, securely in place, was then lined up to a 0.5 mm sterile stainless steel ruler. The value was rounded up to the nearest 0.5 mm. In cases where the measurements were too close to the 0.5 mm marking, magnification loupes were used. When the measurement point was on the rugae area, the base of the rugae, but not the hill, was chosen as the measurement point.
Figure 4.
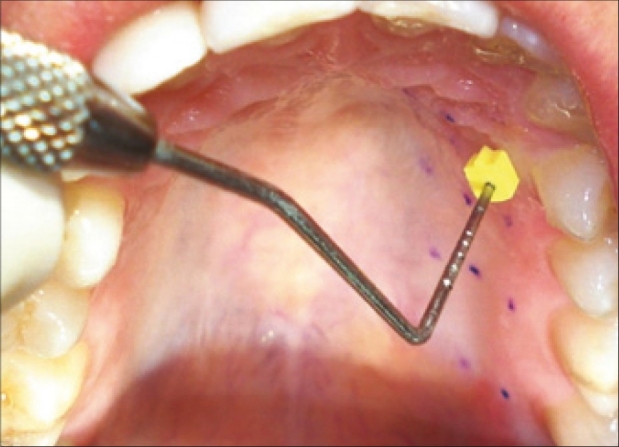
Periodontal probe with the rubber stopper
Statistical analysis was performed by Mann Whitney U test and paired t test.
RESULTS
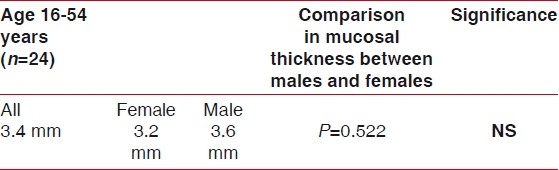
The younger age group had thinner mucosa ranged (2 to 3.1 mm) than the older age group [Table 1]. In the same age group, females had thinner mucosa than male, but the difference was not statistically significant [Table 2]. The mean thickness of palatal masticatory mucosa ranged between 2.5 to 3.7 mm.
Table 1.
Mean thickness of palatal masticatory mucosa in mm by age

Table 2.
Mean thickness of palatal masticatory mucosa in mm by gender
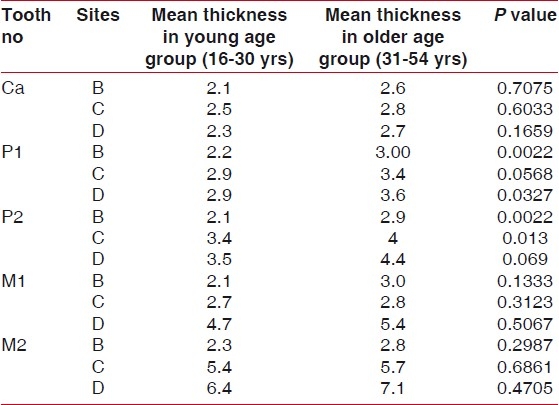
Analysis of the palatal masticatory mucosa at each measurement point indicated that palatal mucosa was thinnest at the canine region and thickest in the second molar region. The older age group had significantly thicker mucosa than younger age group, in relation to each measurement points, on both the premolars [Table 3].
Table 3.
Thickness of palatal masticatory mucosa at 15 measurement points in mm by age group
DISCUSSION
The bone sounding technique, a direct clinical measurement using periodontal probe and minimal local anesthesia along with a prepared acrylic stent to ensure consistent locations for the measurements, was employed in this study to assess the thickness of palatal mucosa. It has been previously suggested that this technique is relatively reliable for determining bone levels.[12,14–16] Twenty minutes of gap is given, after giving local anesthesia, to prevent false positive results due to blabbing of tissues.
The present study investigated the thickness of palatal masticatory mucosa in the subjects of age ranging from 16 to 54 years. The results demonstrated that mean thickness of palatal mucosa ranged between 2.5 to 3.7 mm among all participants. The study done by Nawarat Wara-aswapati et al., showed the mean thickness of 2 to 3.5 mm in Asian population.[6] Mucosal thickness was thinner in younger subjects as compared to older subjects. It is possible that the thickness of the ortho-keratinized epithelial layer of the hard palate mucosa increases with age, resulting in the thicker palatal mucosa in the older subjects. In addition, the hard palate possesses a sub mucosal layer, which contains various amounts of adipose tissue and small mucous glands. There are other factors that influence the mucosal thickness such as racial, genetic factors, and body weight. Free gingival graft which is commonly performed can lead to unaesthetic results due to keloid formation and color mismatch. The sub epithelial connective tissue graft results in a better aesthetic outcome, but requires thicker donor palatal tissue than the free gingival graft procedure. Although the palatal mucosa of the younger age group was thinner than the older age group, the mucosal thickness of the younger group ranged from 2 to 4.5 mm which is adequate for harvesting the graft in the younger age group.
Overall, the thickness of the palatal mucosa increased from canine to second molar areas and in sites farther away from gingival margin, with the exception of the first molar area on line c where significantly thinner mucosa was observed. This may be due to the palatal root of the first molar which will provide limited donor tissue for grafting [Table 3]. The maximum mucosal thickness was obtained at the line d of second molar because the probe must have-penetrated soft palate in this region.
The palatal neurovascular bundle, which is located approximately 7 to 17 mm from gingival margin of the third molar, may have an effect on the measurement, if probe penetrates into the neurovascular structures.[17] In this study, there were no clinical indications that the neurovascular bundle had been penetrated such as bleeding after probing, hematoma formation, or paresthesia.
Mucosal thickness was observed to be more in male as compared to female; however, it was not statistically significant. This was in accordance to the study done by Studer et al. The mucosal thickness as reported by Studer et al., in Caucasians, using the same measurement method as in this study, there was no significant difference in tissue thickness between men and women.[14] It was in contrast to study done by Nawarat Wara-aswapati. Terakura-compared an A mode ultrasonic device to bone probing and reported probing to be a reliable method showing a high correlation coefficient (0.95).[12] Among the studies, the difference in the mean thickness might be due to age, ethnicity, varying measurement methods, and the placement of measurement points.
CONCLUSION
The younger subjects had thinner mucosa than older subjects. The canine and premolar areas appear to be the most appropriate donor site for grafting procedures in both young and older individuals. The sub-epithelial connective tissue graft procedures can be considered as treatment modality in younger subjects also, since adequate amount of donor tissue can be obtained.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Bjorn H. Free transplantation of gingiva propria. Sven Tandlak Tidskr. 1963;22:6848. [Google Scholar]
- 2.Miller PD., Jr Root coverage with free gingival graft. Factors associated with incomplete coverage. J Periodontol. 1987;58:67481. doi: 10.1902/jop.1987.58.10.674. [DOI] [PubMed] [Google Scholar]
- 3.McGuire MK. Coverage of the denuded root surface using the free soft tissue graft. J Am Dent Assoc. 1990;121:2779. doi: 10.14219/jada.archive.1990.0244. [DOI] [PubMed] [Google Scholar]
- 4.Langer B, Langer L. Subepithelial connective tissue graft technique for root coverage. J Periodontol. 1985;56:71520. doi: 10.1902/jop.1985.56.12.715. [DOI] [PubMed] [Google Scholar]
- 5.Siebert JS. Reconstruction of deformed partially edentulous ridges, using full thickness onlay grafts.Part I technique and wound healing. Compend Contin Educ Dent. 1983;4:43753. [PubMed] [Google Scholar]
- 6.Wara-aswapati N, Pitiphat W, Chandrapho N, Rattanayatikul C, Karimbux N. Thickness of palatal masticatory mucosa associated with age. J Periodontol. 2001;72:140712. doi: 10.1902/jop.2001.72.10.1407. [DOI] [PubMed] [Google Scholar]
- 7.Lytle RB. The management of abused oral tissues in complete denture construction. J Prosthet Dent. 1957;7:27–42. [Google Scholar]
- 8.Ostulnd SG. The effect of complete dentures on the gum tissues.A histological and histopathological investigation. Acta Odontol Scand. 1958;16:140. [Google Scholar]
- 9.Turek D. A histologic comparison of the edentulous denture and nondenture bearing tissues. J Prosthet Dent. 1965;15:41934. doi: 10.1016/s0022-3913(65)80010-5. [DOI] [PubMed] [Google Scholar]
- 10.Daly CH, Wheeler JB., 3rd The use of ultrasonic thickness measurement in the clinical evaluation of the oral soft tissues. Int Dent J. 1971;21:41829. [PubMed] [Google Scholar]
- 11.Kydd WL, Daly CH, Wheeler JB., 3rd The thickness measurement of masticatory mucosa on vivo. Int Dent J. 1971;21:43041. [PubMed] [Google Scholar]
- 12.Terakura T. Non invasive measurement of the thickness of oral tissues. Nippon Hotet su shika Gakkai Zasshi. 1986;30:140211. [PubMed] [Google Scholar]
- 13.Fakukita H, Tsutomo Y, Fakomoto A, Sawadak FT, Sunanda I. Development and application of an ultrasonic imaging system for dental diagnosis. J Clin Ultrasound. 1985;13:597–600. doi: 10.1002/1097-0096(199010)13:8<597::aid-jcu1870130818>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.Studer SP, Allen EP, Rees TC, Koubo A. The thickness of masticatory mucosa in the human hard palate and tuberosity as potential donor sites for ridge augmentation procedures. J Periodontol. 1997;68:14551. doi: 10.1902/jop.1997.68.2.145. [DOI] [PubMed] [Google Scholar]
- 15.Ursell MJ. Relationships between alveolar bone levels measured at surgery, estimated by transgingival probing and clinical attachment level measurements. J Clin Periodontol. 1989;16:81–6. doi: 10.1111/j.1600-051x.1989.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 16.Mealey B, Neubauer M, Butzin C, Waldrop T. Use of furcal bone accuracy of furcation diagnosis. J Periodontol. 1994;65:64957. doi: 10.1902/jop.1994.65.7.649. [DOI] [PubMed] [Google Scholar]
- 17.Reiser GM, Bruno JF, Mahan PE, Larkin LH. The sunepithelial connective tissue graft palatal donor site: Anatomic considerations for surgeons. Int J Periodontics Restorative Dent. 1996;16:131–7. [PubMed] [Google Scholar]


