To the Editor
Pseudoxanthoma elasticum (PXE) is an autosomal recessive disorder characterized by ectopic mineralization of soft connective tissues (Li et al, 2009a; Uitto et al, 2010). The clinical manifestations derive primarily from the involvement of three organ systems, the skin, the eyes, and the cardiovascular system. There is considerable phenotypic, both intra- and inter-familial, heterogeneity with respect to the age of onset and the severity of the disease. Furthermore, in some families the involvement of one of the organ systems predominates, so that in some families the primary manifestations are in the skin with little eye or cardiovascular involvement, while in others, the ocular problems are the major cause of morbidity. The eye manifestations characteristically consist of angioid streaks that are due to mineralization of an elastin-rich Bruch’s membrane behind the pigmented retina (Booij et al, 2010). Mineralization of this membrane causes ruptures of blood vessels with subsequent neovascularization, associated with bleeding to the eye, and leading to progressive loss of visual acuity and, occasionally, blindness.
Classic PXE is caused by mutations in the ABCC6 gene, which encodes a putative transmembrane transporter protein, ABCC6, expressed primarily in the baso-lateral surface of hepatocytes (Pfendner et al, 2007). ABCC6 has been shown in in vitro experiments utilizing inside-out insect cell vesicles to function as an efflux pump which transports anionic small molecular weight conjugates (Ilias et al, 2002). However, the physiologic ligands in vivo and the precise role of ABCC6 in the pathomechanistic pathways leading to peripheral connective tissue mineralization are currently unknown.
Well over 300 distinct mutations have been identified in the ABCC6 gene, consisting of premature termination codon (PTC)-causing mutations as a result of nonsense mutations or out-of-frame small insertions or deletions (Pfendner et al, 2007). In addition, large genomic deletions resulting in loss of several exons or the entire gene, and occasionally including flanking genes as well, have been identified (Chassaing et al, 2007). Finally, a number of missense mutations, particularly those affecting the nucleotide binding folds (NBF1 and NBF2), two protein domains critical for the function of ABCC6 as a transmembrane transporter protein, have been identified. Careful examination of the mutation database in the context of phenotypic variability in PXE has not revealed any clear cut genotype-phenotype correlations (Pfendner et al, 2007). However, specific mutations in ABCC6 have been recently suggested to be associated with development of angioid streaks. For example, the ABCC6 gene in patients with angioid streaks was scanned for putative mutations by single-strand conformation polymorphism technique, and a specific base substitution of c.G3803A was identified in exon 27, resulting in a change in codon from CGG to CAG (p.R1268Q) (Mizutani et al, 2006). Highly significant differences were observed in both genotype and in allelic frequencies of p.R1268Q between patients with angioid streaks and control subjects (p < 0.001, p < 0.002, respectively; Chi-square test). The authors concluded that the missense mutation p.R1268Q is associated with the disease state of angioid streaks. Subsequent examination of a cohort of 54 Japanese patients with angioid streaks, identified six mutations (p.R419Q, p.E422K, c.2542delG, Del_exon23, c.3774-3775insC, and p.E1427K) as causal mutations for this eye disease (Sato et al, 2009).
The Molecular Diagnostics Laboratory at Jefferson Institute of Molecular Medicine in Philadelphia has over the past decade examined patients with pseudoxanthoma elasticum for mutations in ABCC6. Based on the previous suggestions that the p.R1268Q mutation may be associated with angioid streaks, we have reviewed our PXE mutation database, consisting of a total of 118 individuals with PXE, for the presence of this missense mutation. We identified a total of 11 individuals who were either heterozygous or homozygous for p.R1268Q (Fig. 1a and Table 1). All these individuals had skin findings consistent with PXE, and in most cases this diagnosis was confirmed by skin biopsy, revealing characteristic features of PXE, i.e., ectopic mineralization of pleiomorphic elastic structures in the mid or upper dermis. All individuals who had the p.R1268Q mutation also showed angioid streaks, associated with other ocular findings, causing considerable morbidity (blindness, loss of visual acquity) (Fig. 1b and Table 1). Examination of the clinical findings of the remaining 107 patients revealed that, at the time of DNA testing, 64 (60%) of them had documented ocular findings. This frequency of ocular involvement differs from that noted in patients with p.R1268Q (100%) (p = 0.0069); Fisher’s exact test).
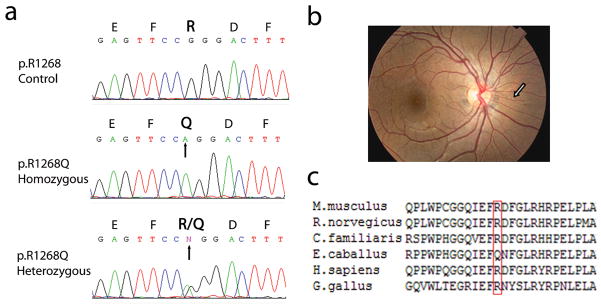
Figure 1.
Demonstration of the mutation p.R1268Q and conservation of the R1268 in the ABCC6 gene. (a) Genomic DNA was isolated from peripheral blood leukocytes and used as template for PCR amplification of exon 27 of the gene. Direct sequencing of the PCR product revealed either homozygous or heterozygous replacement of arginine (R) by glutamine (Q) reflecting G-to-A nucleotide substitution (arrows) at position 3803 in patients with angioid streaks. (b) Presentation of angioid streaks in patient AS11 (from Li et al, 2010, with permission); (c) The arginine residue in position 1268 of the human protein is conserved in several (Mus musculus, Rattus norvegicus, Canis familiaris, and Gallus gallus) but not all (Equus caballus) vertebrate proteins. The alignment was performed with ClustalW2 Program (http://www.ebi.ac.uk/Tools/Clustalw2).
Table 1.
Mutation analysis and clinical features of patients with p.R1268Q mutation in the ABCC6 gene1)
| Patient | Age (sex) | Mutation 1 | Mutation 2 | R1268Q status | Eye findings | Skin findings consistent with PXE | Biopsy proven |
|---|---|---|---|---|---|---|---|
| AS1 | 67(M) | 179delG | G1354R | het | AS, moderate to severe visual problems | + | ND |
| AS2 | 45(M) | R1141X | NP | het | AS, central vision loss due to trauma, macular degeneration | NP | − |
| AS3 | 24(F) | del23-29 | 1868-5T→G | hom | AS, drusen, visual problems | + | + |
| AS4 | 76(F) | 2542delG | R419Q | het | AS, retinal hemorrhage, cataract, macular degeneration with neovascularization | + | + |
| AS5 | 51(M) | N370D | 3107Del3 | het | AS | + | + |
| AS6 | 22(F) | G1100E | R1339C | het | AS | + | + |
| AS7 | 62(M) | del23-29 | ? | hom | AS, macular degeneration, blind | NP | ND |
| AS8 | ?(F) | A950T | A950T | hom | AS | + | ND |
| AS9 | 50(F) | 4063del18 | 998+2delTG | het | AS | + | ND |
| AS10 | 48(F) | G1302R | K1490X | het | AS, drusen, macular degeneration | + | + |
| AS11 | 18(F) | R1221H | R1221H | hom | AS | + | + |
AS, angioid streaks; NP, not present; ND, not done; het, heterozygous; hom, homozygous.
One of the diagnostic features of PXE is that the onset of clinical manifestations is delayed, and the diagnosis is often not made until teens or the third or fourth decade of life. The ocular manifestations, particularly angioid streaks, are often accompanied with PXE, but they are usually noted later than the cutaneous findings. As indicated above, a number of specific mutations in the ABCC6 gene, including p.R1268Q, have been suggested to be associated with angioid streaks. This specific mutation was initially identified in families with PXE and was suggested to be pathogenic (Ringpfeil et al, 2000). Subsequently, this mutation was found at the homozygous state in apparently healthy volunteers, leading the authors to conclude that the p.R1268Q mutation in the ABCC6 gene is an inconsequential polymorphism which does not cause PXE (Germain et al, 2000). The frequency of the p.R1268Q allele in control population of Caucasian ancestry was reported to be 0.19 (n = 62) (Germain et al., 2000). At the same time, the genotypic frequency of homozygous A/A (corresponding to Q) in Japanese population with angioid streaks was reported to be 16% (14/44), while in control subjects the corresponding frequency was 2.1% (3/154) (Mizutani et al, 2006). In our cohort, p.R1268Q substitution was associated with two other mutations in the ABCC6 gene in 10 patients, while in one patient heterozygous for p.R1141X mutation accompanied p.R1268Q in trans, and no additional mutations in this gene could be identified by complete sequencing of the PCR products corresponding to all exons and flanking intronic sequences. This approach does not exclude the possibility of a mutation in the regulatory elements, such as 5′ UTR or the promoter region of ABCC6. It should be noted that in our cohort of a total of 118 patients with PXE, mutations in the ABCC6 gene were identified in 104 of them (88.1%). Finally, it was noted that arginine in the position 1268 is conserved in a number of vertebrate orthologs of the ABCC6 gene (Fig. 1c).
Recent studies have indicated that another gene, GGCX, encoding γ-glutamyl carboxylase, can harbor mutations in PXE, either in form of compound heterozygous missense mutations, or a missense mutation in one allele of GGCX and a PTC-causing mutation in one allele of ABCC6 (Vanakker et al, 2007; Li et al, 2009b,c). Furthermore, recent studies utilizing the Abcc6−/− mice which recapitulate the genetic, histopathologic and ultrastructural features of PXE, have suggested the importance of the GGCX gene as a modulator of the PXE phenotype (Li and Uitto, 2010). Finally, it has been suggested that single nucleotide polymorphisms in the VEGFA gene show significant association with the development of severe retinopathy in patients with PXE (Zarbock et al, 2009). These findings suggest that the PXE phenotypes, including the ocular manifestations, due to mutations in the ABCC6 gene can be modulated by genetic factors. Collectively, these observations and our findings of association of angioid streaks with the p.R1268Q mutation, suggest that this sequence variant may contribute to the development and/or severity of angioid streaks in patients with PXE due to mutations in the ABCC6 gene. Early identification of angioid streaks may help to prevent development of ocular complications by allowing institution of effective therapy to prevent neovascularization, the cause of loss of visual acuity and blindness in patients with PXE (Uitto et al., 2010).
Acknowledgments
GianPaolo Guercio and Carol Kelly assisted in preparation of this publication. This study was supported by USPHS DHHS, NIH/NIAMS Grant R01 AR28450 (JU). QL is recipient of the Dermatology Foundation Research Career Development Award.
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Booij JC, Baas DC, Beisekeeva J, Gorgels TG, Bergen AA. The dynamic nature of Bruch’s membrane. Prog Retin Eye Res. 2010;29:1–18. doi: 10.1016/j.preteyeres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Chassaing N, Martin L, Bourthoumieu S, Calvas P, Hovnanian A. Contribution of ABCC6 genomic rearrangements to the diagnosis of pseudoxanthoma elasticum in French patients. Hum Mutat. 2007;28:1046. doi: 10.1002/humu.9509. [DOI] [PubMed] [Google Scholar]
- Germain DP, Perdu J, Remones V, Jeunemaitre X. Homozygosity for the R1268Q mutation in MRP6, the pseudoxanthoma elasticum gene, is not disease-causing. Biochem Biophys Res Comm. 2000;274:297–301. doi: 10.1006/bbrc.2000.3101. [DOI] [PubMed] [Google Scholar]
- Iliás A, Urban Z, Seidl TL, et al. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6) J Biol Chem. 2002;227:16860–16867. doi: 10.1074/jbc.M110918200. [DOI] [PubMed] [Google Scholar]
- Li Q, Jiang Q, Pfendner E, Váradi A, Uitto J. Pseudoxanthoma elasticum: Clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Derm. 2009a;18:1–11. doi: 10.1111/j.1600-0625.2008.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Schurgers LJ, Smith ACM, Tsokos M, Uitto J, Cowen EW. Co-existent pseudoxanthoma elasticum and vitamin K-dependent coagulation factor deficiency. Am J Pathol. 2009b;174:534–539. doi: 10.2353/ajpath.2009.080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Grange DK, Armstrong NL, et al. Mutations in the GGCX and ABCC6 genes in a family with pseudoxanthoma elasticum-like phenotypes. J Invest Dermatol. 2009c;129:553–563. doi: 10.1038/jid.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Török L, Kocsis L, Uitto J. Mutation analysis (ABCC6) in a family with pseudoxanthoma elasticum: presymptomatic testing with prognostic implications. Br J Dermatol (in press), 2010. PMID20491760 Mizutani Y, Nakayama T, Asai S, et al. ABCC6 mutation in patients with angioid streaks. Int J Biomed Sci. 2006;2:9–14. doi: 10.1111/j.1365-2133.2010.09856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfendner EG, Vanakker OM, Terry SF, et al. Mutation detection in the ABCC6 gene and genotype-phenotype analysis in a large international case series affected by pseudoxanthoma elasticum. J Med Genet. 2007;44:621–628. doi: 10.1136/jmg.2007.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringpfeil F, Lebwohl MG, Christiano AM, Uitto J. Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci USA. 2000;97:6001–6006. doi: 10.1073/pnas.100041297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Nakayama T, Mizutani Y, Yuzawa M. Novel mutations of ABCC6 gene in Japanese patients with angioid streaks. Biochem Biophys Res Comm. 2009;380:548–553. doi: 10.1016/j.bbrc.2009.01.117. [DOI] [PubMed] [Google Scholar]
- Uitto J, Li Q, Jiang Q. Pseudoxanthoma elasticum: molecular genetics and putative pathomechanisms. J Invest Dermatol. 2010;130:661–670. doi: 10.1038/jid.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanakker OM, Martin L, Gheduzzi D, et al. Pseudoxanthoma elasticum-like phenotype with cutis laxa and multiple coagulation factor deficiency represents a separate genetic entity. J Invest Dermatol. 2007;127:507–510. doi: 10.1038/sj.jid.5700610. [DOI] [PubMed] [Google Scholar]
- Zarbock R, Hendig D, Szliska C, Kleesiek K, Götting C. Vascular endothelial growth factor gene polymorphisms as prognostic markers for ocular manifestations in pseudoxanthoma elasticum. Hum Mol Genet. 2009;18:3344–3351. doi: 10.1093/hmg/ddp259. [DOI] [PubMed] [Google Scholar]