Abstract
Pseudoxanthoma elasticum (PXE), a multi-system disorder characterized by ectopic mineralization of soft connective tissues, is caused by mutations in the ABCC6 gene. The pathomechanistic details of the mineralization process are largely unknown, but oxidative stress has been suggested to play a role. In this study, we tested Abcc6−/− mice, which serve as a model of PXE, for markers of the oxidative stress in the liver and serum. The total antioxidant capacity as well as markers of protein oxidation and lipid peroxidation suggested the presence of chronic oxidative stress. Feeding these mice for five months with a diet supplemented with antioxidants (vitamins C and E, selenium and N-acetylcysteine) countered the oxidative stress but did not modify the ectopic mineralization process. These results suggest that the Abcc6−/− mice suffer from chronic oxidative stress but this does not contribute to connective tissue mineralization, the hallmark of PXE.
Introduction
Pseudoxanthoma elasticum (PXE) is an autosomal recessive, multi-system disorder affecting the skin, eyes and the cardiovascular system, with considerable morbidity and mortality (Neldner and Struk, 2002; Ringpfeil et al., 2006). The characteristic pathologic lesion displays mineralization of soft connective tissues in affected organs. This disease is caused by mutations in the ABCC6 gene which encodes multidrug resistance-associated protein 6 (MRP6), an ATP-binding cassette protein, which serves as a putative transmembrane transporter (see Pfendner et al., 2007). The ABCC6 gene is expressed primarily in the baso-lateral surface of hepatocytes, to a lesser degree in the proximal tubules of kidneys, and at very low level, if at all, in the resident cells in tissues affected in PXE (Scheffer et al., 2002; Matsuzaki et al., 2005). The mechanisms leading from ABCC6 mutations to the ectopic mineralization of soft connective tissues remain unresolved and the molecules serving as potential ligands for MRP6 in vivo are currently unknown.
Two general hypotheses have been advanced to explain the potential pathomechanisms of PXE. The “metabolic hypothesis” postulates that in the absence of ABCC6 activity in the liver, alterations in the levels of circulatory factors that are physiologically required to prevent premature mineralization occur (Uitto et al., 2001; Jiang and Uitto, 2006). This hypothesis has been supported by observations that serum from patients with PXE or from transgenic Abcc6−/− mice, which recapitulate the features of this disease, lack the capacity to prevent calcium/phosphate precipitation in vitro (Jiang et al., 2007). Alternatively, the “PXE cell hypothesis” postulates that altered cellular activities in the resident cells, as a result of ABCC6 deficiency, lead to local mineralization (Quaglino et al., 2000). In addition, PXE has been suggested to be a disorder of oxidative stress, as fibroblast cultures derived from the skin of patients with PXE demonstrate changes in oxidative stress markers in vitro (Pasquali-Ronchetti et al., 2006).
We have recently developed a transgenic mouse, Abcc6−/−, by targeted disruption of the corresponding gene (Klement et al., 2005). These mice demonstrate progressive mineralization of soft tissues in organs affected in PXE, including the skin, the eyes, and the arterial blood vessels; thus, these mice serve as a model system to study PXE. One of the intriguing features of these mice is extensive mineralization of the connective tissue capsule surrounding the vibrissae in the muzzle skin, which can be demonstrated as early as the 5th week of age. It has been suggested, therefore, that mineralization of the connective tissue capsule serves as an early biomarker of the overall mineralization process (Klement et al., 2005). In this study, we have utilized this mouse model (Abcc6−/−) to explore potential signs of oxidative stress in PXE, and we have monitored the effects of feeding these mice with a diet supplemented with antioxidants.
Results
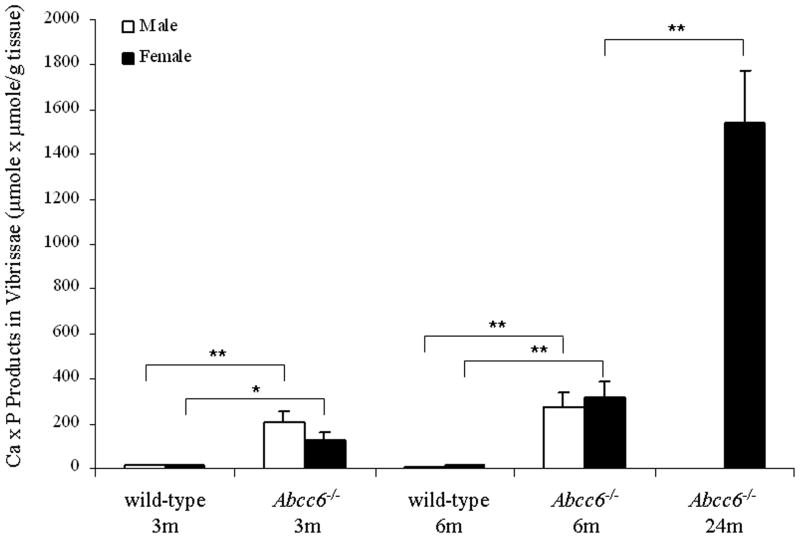
As indicated, the Abcc6−/− mice demonstrate progressive mineralization of the connective tissue capsule surrounding the bulb of vibrissae. This early morphologic observation (Klement et al., 2005) was further confirmed here by computerized morphometric analysis of mice up to 24 months of age (Table 1), as well as by determination of the calcium and phosphate contents of the muzzle skin containing the vibrissae (Fig. 1). There was progressive mineralization, as determined by the chemical assays of calcium x phosphate product, which in knockout (KO) mice at the age of 3, 6 and 24 months exceeded that measured in the muzzle skin of wild-type (WT) mice by about 13, 24 and 130-fold, respectively (Fig. 1). The progressive mineralization of vibrissae also reflected the presence of Ca/P deposits in other organs, such as the eyes, kidneys, and the heart (not shown).
Table 1.
Quantitation of Connective Tissue Mineralization in Vibrissae of Abcc6−/− Micea
| Mouse | Age (mo) | Gender (no) | Mineralized vibrissae (per mouse) | Total vibrissae (per mouse examined) | Percent mineralizedb | Area of mineralization per total area of vibrissaeb | Foldc | P valued | P valuee |
|---|---|---|---|---|---|---|---|---|---|
| wild-type | 24 | M/F | 0 | 0 | 0 | 0.00 | |||
| Abcc6−/− | 3 | M (7) | 7.2 | 10.8 | 68.3 ± 7.2 | 0.93 ± 0.18 | 1.00 | ||
| Abcc6−/− | 3 | F (6) | 4.5 | 8.5 | 53.1 ± 9.2 | 0.94 ± 0.28 | 1.01 | 0.50 | |
| Abcc6−/− | 6 | M (5) | 5.6 | 8.6 | 64.6 ± 10.3 | 1.82 ± 0.56 | 1.96 | 0.08 | |
| Abcc6−/− | 6 | F (4) | 5.8 | 8.3 | 67.8 ± 11.9 | 2.16 ± 0.95 | 2.32 | 0.31 | 0.09 |
| Abcc6−/− | 24 | M (3) | 20.5 | 25.0 | 81.8 ± 4.3 | 7.39 ± 1.87 | 7.95 | 0.0003 | |
| Abcc6−/− | 24 | F (4) | 17.1 | 21.5 | 80.9 ± 7.5 | 5.03 ± 1.43 | 5.41 | 0.18 | 0.0043 |
Skin sections containing vibrissae were stained with H&E stain and examined by computerized morphometric analyses.
Values presented as mean ± SE.
Calculated using the 3 month old Abcc6−/− male mice as 1.00.
Compared between male and female in each group.
Compared with gender-matched 3 month old Abcc6−/− mice.
Figure 1.
Demonstration of progressive mineralization, measured by chemical assay of calcium and phosphate, of connective tissue in the muzzle skin containing vibrissae in Abcc6−/− mice, as compared to their corresponding wild-type counterparts at 3 months and 6 months of age. At 24 months of age, only female Abcc6−/− mice were available. (Values are mean ± SE, n = 3–7; *p<0.05, **p<0.01).
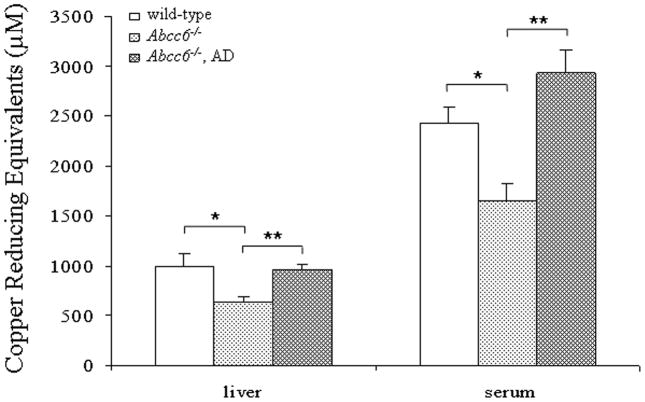
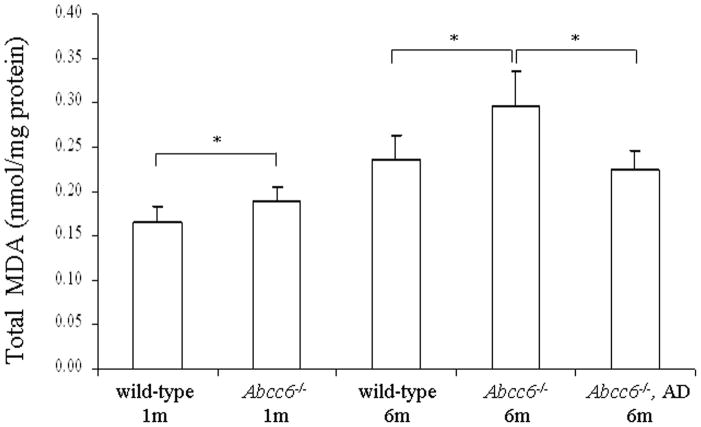
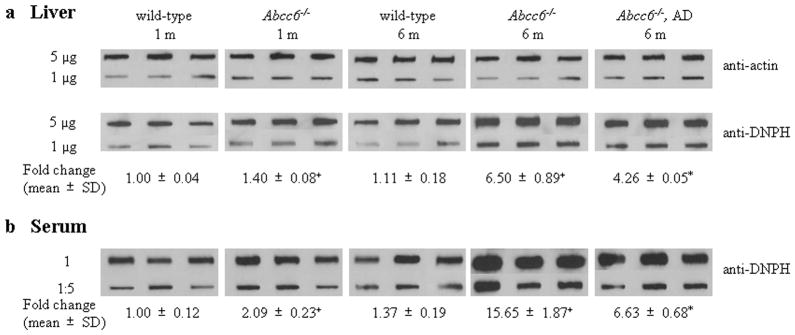
To explore the potential of oxidative stress in PXE, Abcc6−/− (KO) mice in comparison to WT mice were first examined for markers of the oxidative stress by measuring the total antioxidant power, based on copper reducing equivalence in liver extracts and in serum of mice at 6 months of age. This measurement is an overall marker of the oxidative stress in animals (Prior and Cao, 1999). Both liver and serum samples demonstrated reduced antioxidant capacity (Fig. 2). Next, the levels of hepatic malondialdehyde (MDA) as a marker of lipid peroxidation was determined in mice at 1 month and 6 months of age. Both WT and KO mice at 6 months of age demonstrated increased levels of MDA in comparison to their counterparts at 1 month of age, reflecting general cumulative increase in oxidative damage with age (Richwine et al., 2005). However, both 1-month and 6-month old KO mice demonstrated higher levels of MDA than the corresponding WT mice (Fig. 3). The same animals were also used for measurement of protein oxidation both in the liver and serum by blot analysis utilizing anti-dinitrophenyl hydrazine (DNPH) antibodies. These measurements revealed a significant increase, both in the liver and serum, particularly in 6-month old KO mice, the highest values in the serum reaching 11.4-fold increase as compared to WT mice of the same age (Fig. 4). Thus, the KO mice display evidence for oxidative stress but whether this is a cause or consequence of the pathological mineralization is not resolved by these measurements.
Figure 2.
Assay of total antioxidant capacity in the liver extracts and in serum of 6-month old wild-type or Abcc6−/− mice fed with standard diet, or Abcc6−/− mice fed diet supplemented with antioxidants (AD). (Values are mean ± SE, n = 4; *p<0.05, **p<0.01).
Figure 3.
Assay of total MDA in liver extracts, reflecting lipid peroxidation, in wild-type as well as Abcc6−/− mice fed with standard diet, or Abcc6−/− mice on antioxidant diet (AD). (Mean ± SE, n = 4; *p<0.05).
Figure 4.
Analysis of the degree of protein oxidation in the liver (a) and in the serum (b) of wild-type and Abcc6−/− mice fed with standard diet, as well as Abcc6−/− mice on antioxidant diet (AD). Extracts of liver protein (5 or 1 μg), as well as 0.5 μl serum, either undiluted (1) or diluted 1:5, were blotted onto nitrocellulose filters and incubated with an anti-DNPH antibody. The loading of liver protein was verified by incubation with an anti-actin antibody (top panel). The fold change of values determined by densitometry of the chemiluminescent signal is indicated below the Figures, referring the wild-type 1-month old mice as 1.00. (*p<0.01, compared with age-matched Abcc6−/− mice on normal diet; +p<0.01, compared with age-matched wild-type mice).
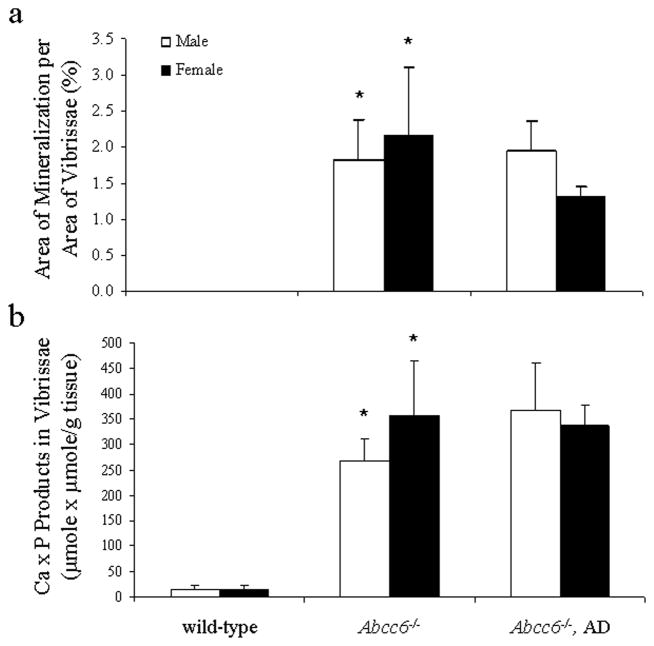
To explore the potential causal relationship between the oxidative stress and the connective tissue mineralization, the mice were fed a diet supplemented with antioxidants, viz. vitamin C, vitamin E, selenium, and N-acetylcysteine (NAC), in quantities indicated in Materials and Methods. The experimental diet started at 4 weeks of age, before the mineralization of the connective tissue capsule in vibrissae is histologically evident, and was continued for an additional five months. The KO mice on antioxidant diet were subjected to measurements of markers of oxidative stress as above and the values were compared with KO mice of the same age but fed with standard diet. The total antioxidant power in KO mice fed the experimental diet returned to normal, and the hepatic MDA levels as well as protein oxidation both in the liver and serum were significantly reduced (Figs. 2–5). These observations indicate that the antioxidant diet was effective, as reflected by these markers. At the same time, however, the extent of tissue mineralization in the KO mice, as determined either by computerized morphometric analysis of vibrissae (Fig. 5a) or by chemical assay of calcium and phosphate levels in muzzle skin containing the vibrissae (Fig. 5b), was not altered by the experimental diet. Furthermore, the mineralization affecting the kidneys and the eyes was not altered by the antioxidant diet (not shown). These observations suggest that the oxidative stress in Abcc6−/−mice is not causative of mineralization, but may rather reflect the general disease state of these mice.
Figure 5.
Effects of antioxidant diet (AD) on the degree of mineralization of the connective tissue, as determined by computerized morphometric analysis of vibrissae (a) or by chemical assay of calcium and phosphate in muzzle skin (b). (Mean ± SE, n = 4–5; *p<0.01, compared with wild-type mice). Note that there is no mineralization in vibrissae of the wild-type mice, as examined by light microscopy (a); see Table 1.
Discussion
Oxidative stress, an imbalance between the production of reactive oxygen species and the system’s ability to counteract their deleterious effects, can result in tissue damage, the primary targets being proteins, lipids and DNA. Oxidative stress is encountered in a number of degenerative disease processes, as exemplified by atherosclerosis (Lankin et al., 2005; Madamanchi et al., 2005). Furthermore, chronologic aging is associated with oxidative stress, possibly explaining some of the age-associated degenerative changes (Ames et al., 1993). In this study, we explored the presence of oxidative stress in PXE utilizing a transgenic animal model that recapitulates the genetic, histopathologic and ultrastructural features of this progressive mineralization disorder (Klement et al., 2005).
The results of our study, utilizing three independent markers of oxidative stress, provided evidence of chronic oxidative stress, as reflected by reduced total antioxidant capacity and the presence of enhanced protein oxidation and lipid peroxidation markers. These findings in intact animals support previous observations made on cultured fibroblasts from patients with PXE and suggesting mild, chronic oxidative stress in this disease (Pasquali-Ronchetti et al., 2006). The key observation in our study was, however, that antioxidant diet, which was clearly effective in counteracting the oxidative stress, did not modify the process of aberrant mineralization of connective tissues in PXE mice. Since ectopic mineralization is the pathologic hallmark of this disease responsible for the clinical manifestations, it is unlikely that oxidative stress plays a major causal role in the pathomechanism of this disease. It is more likely that oxidative stress is reflection of generalized condition of these animals as a consequence of the disease, as encountered in a number of clinical situations and in chronologic aging (Ames et al., 1993; Livrea et al., 1998; Lankin et al., 2005; Madamanchi et al., 2005)
Our findings have implications for potential treatment of PXE. Specifically, previous demonstrations of oxidative stress in cultured PXE fibroblasts (Pasquali-Ronchetti et al., 2006), a finding corroborated by our in vivo study, prompted the authors to suggest that ingestion of antioxidants may be beneficial in counteracting the disease process in PXE. Our findings do not support such suggestion, although carefully controlled clinical trials in patients with PXE would potentially provide an unequivocal answer.
Materials and Methods
Mice and diet
Abcc6−/− mice
The PXE mouse model Abcc6−/− (KO) mouse was developed by targeted inactivation of the Abcc6 gene (Klement et al, 2005). KO and WT mice were maintained in the Animal Facility of the Thomas Jefferson University in a temperature- and humidity-controlled environment under 12-h light/dark cycles and had free access to water. Mice were sacrificed at different ages, sections of the muzzle skin containing vibrissae, as well as the eyes, kidneys and heart were stained using the H&E and Alizarin Red, and tissue mineralization in vibrissae was quantified by computerized morphometric analysis of H&E-stained sections and by chemical assays for calcium and phosphate, as described previously (Jiang et al., 2007; La Russo et al., 2007; Li et al., 2007).
Antioxidant diet
The Abcc6−/− mice at four weeks of age were divided into two groups. The first group was fed a standard Lab Diet 5010 (PMI Nutrition, Brentwood, MO). The second group was fed the same diet but supplemented with antioxidants containing vitamin E (500 IU/kg), vitamin C (500 mg/kg), selenium (2 mg/kg) and NAC (3g/kg) (Miquel et al., 1995; Richwine et al., 2005). Mice were continued on the experimental diet for five additional months.
Oxidative stress markers
Total antioxidant power assay
Total Antioxidant Power Colorimetric Assay kit was used to assay total antioxidant capacity in the mouse liver and sera (Oxford Biomedical Research, Inc., Oxford, MI). The method measures colorimetrically the amount of Cu+ derived from Cu++ by the action of all antioxidant moieties in the sample.
Lipid peroxidation
Lipid peroxidation in the liver tissue was evaluated by measuring total MDA with the BIOXYTECH MDA-586 assay kit (Oxis Research, Portland, OR). Briefly, liver tissues were homogenized in the presence of 5 mM butylated hydroxytoluene. Extracts were centrifuged at 10,000 g for 10 min at 4°C, and 0.1 N HCl and 500 mM butylated hydroxytoluene (pH 1-2) was added to the supernatant and incubated for 80 min at 60°C to hydrolyze Schiff base adducts of MDA. The samples were centrifuged at 3,000 g for 10 min at 4°C, and the supernatant was used to determine concentrations of total MDA. Protein concentration in the supernatant was measured with a bicinchoninic acid reagent kit (Pierce, Rockford, IL).
Protein oxidation
Amounts of oxidized proteins containing carbonyl groups were measured using an OxyBlot™ Protein Oxidation Detection Kit (Chemicon International, Temecula, CA). Briefly, 5 and 1 μg of liver protein extracted with RIPA buffer (Sigma, St. Louis, MO) or 0.5 μl serum sample, either undiluted or diluted 1:5, were derivatized with 1x DNPH for 15–30 min, followed by neutralization with a solution containing glycerol and β-mercaptoethanol. The derivatized protein samples were blotted to nitrocellulose membrane using Bio-Dot SF Microfiltration Apparatus (Bio-Rad, Hercules, CA) and blocked. The blot was incubated overnight with a rabbit anti-DNPH antibody (1:150) at 4°C, followed by incubation with goat anti-rabbit secondary HRP-conjugated IgG antibody (1:300) for 1 hr at room temperature. Equal loading of liver proteins was verified by incubation with an anti-actin antibody (1:7,500) (Calbiochem, San Diego, CA) for 1 hr at 4°C overnight, followed by incubation with horse anti-mouse HRP-conjugated antibody (1:2,000) (Cell Signaling Technology, Inc., Danvers, MA) for 1 hr at room temperature. Bands were developed using ECL plus Western Blotting Detection System (Amersham Biosciences, Piscataway, NJ) with nonsaturating exposures. The films were scanned and the signals quantified with a Kodak Image Station 440 CF using Kodak 1D 3 imaging analysis software (Eastman Kodak, Rochester, NY).
Statistical analysis
Statistical differences between the means in various groups of mice were analyzed by Student’s one-tailed t-test.
Acknowledgments
The authors thank Carol Kelly for assistance and acknowledge helpful suggestions by Sharon Terry, PXE International. Dr. Jan Hoek (Jefferson Medical College) provided expert comments on our study. Jennifer LaRusso and Noriko Udagawa assisted in mouse genotyping. These studies were supported by NIH/NIAMS grants R01 AR28450 and R01 AR52627. Dr. Jiang is recipient of a Research Career Development Award from the Dermatology Foundation.
Abbreviations
- PXE
pseudoxanthoma elasticum
- KO
knock-out
- WT
wild-type
- MDA
malondialdehyde
- DNPH
dinitrophenyl hydrazine
- NAC
N-acetylcysteine
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
The medical ethical committee of Thomas Jefferson University approved all described studies. The animal protocols were approved by the Thomas Jefferson University’s Institutional Animal Care and Use Committee.
References
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Uitto J. Pseudoxanthoma elasticum: a metabolic disease? J Invest Dermatol. 2006;126:1440–1. doi: 10.1038/sj.jid.5700267. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Li Q, Uitto J. Aberrant mineralization of connective tissue in a mouse model of pseudoxanthoma elasticum: systemic and local regulatory factors. J Invest Dermatol. 2007;127:1392–402. doi: 10.1038/sj.jid.5700729. [DOI] [PubMed] [Google Scholar]
- Klement JF, Matsuzaki Y, Jiang Q-J, Terlizzi J, Choi HY, Fujimoto N, et al. Targeted ablation of the Abcc6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol. 2005;25:8299–310. doi: 10.1128/MCB.25.18.8299-8310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankin VZ, Lisina MO, Arzamastseva NE, Konovalova GG, Nedosugova LV, Kaminnyi AI, et al. Oxidative stress in atherosclerosis and diabetes. Bull Exp Biol Med. 2005;140:41–3. doi: 10.1007/s10517-005-0406-z. [DOI] [PubMed] [Google Scholar]
- LaRusso J, Jiang Q, Li Q, Uitto J. Ectopic mineralization of connective tissue in Abcc6−/−mice: effects of dietary modifications and a phosphate binder – a preliminary study. Exp Derm. 2007 doi: 10.1111/j.1600-0625.2007.00645.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Jiang Q, LaRusso J, Klement JK, Sartorelli AC, Belinsky M, et al. Targeted ablation of Abcc1 or Abcc3 in Abcc6−/− mice does not modify the ectopic mineralization process. Exp Derm. 2007 doi: 10.1111/j.1600-0625.2007.00621.x. (in press) [DOI] [PubMed] [Google Scholar]
- Livrea MA, Tesoriere L, Maggio A, D’Arpa D, Pintaudi AM, Pedone E. Oxidative modification of low-density lipoprotein and atherogenetic risk in β-thalassemia. Blood. 1998;92:3936–42. [PubMed] [Google Scholar]
- Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- Matsuzaki Y, Nakano A, Jiang QJ, Pulkkinen L, Uitto J. Tissue-specific expression of the ABCC gene. J Invest Dermatol. 2005;125:900–05. doi: 10.1111/j.0022-202X.2005.23897.x. [DOI] [PubMed] [Google Scholar]
- Miquel J, Ferrandiz ML, De Juan E, Sevilla I, Martinez M. N-acetylcysteine protects against age-related decline of oxidative phosphorylation in liver mitochondria. Eur J Pharmacol. 1995;292:333–5. doi: 10.1016/0926-6917(95)90041-1. [DOI] [PubMed] [Google Scholar]
- Neldner KH, Struk B. Pseudoxanthoma elasticum. In: Royce PM, Steinmann B, editors. Connective tissue and its heritable disorders. Molecular, genetic and medical aspects. Wiley-Liss, Inc; New York: 2002. pp. 561–583. [Google Scholar]
- Pasquali-Ronchetti I, Garcia-Fernandez MI, Boraldi F, Quaglino D, Gheduzzi D, De Vincenzi Paolinelli C. Oxidative stress in fibroblasts from patients with pseudoxanthoma elasticum: possible role in the pathogenesis of clinical manifestations. J Pathol. 2006;208:54–61. doi: 10.1002/path.1867. [DOI] [PubMed] [Google Scholar]
- Pfendner E, Terry SF, Vourthis S, McAndrew P, McClain MR, Fratta S, et al. Mutation detection in the ABCC6 gene and analysis in a large international case series affected by pseudoxanthoma elasticum. J Med Genet. 2007 doi: 10.1136/jmg.2007.051094. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior RL, Cao G. In vivo total antioxidant capacity: comparison of different analytical methods. Free Radic Biol Med. 1999;27:1173–81. doi: 10.1016/s0891-5849(99)00203-8. [DOI] [PubMed] [Google Scholar]
- Quaglino D, Jr, Boraldi F, Barbieri D, Croce A, Tiozzo R, Pasquali-Ronchetti I. Abnormal phenotype of in vitro dermal fibroblasts from patients with pseudoxanthoma (PXE) Biochim Biophys Acta. 2000;1501:51–62. doi: 10.1016/s0925-4439(00)00007-7. [DOI] [PubMed] [Google Scholar]
- Richwine AF, Godbout JP, Berg BM, Escobar CJ, Millard DK, et al. Improved psychomotor performance in aged mice fed diet high in antioxidants is associated with reduced ex vivo brain interleukin-6 production. Brain Behav Immun. 2005;19:512–20. doi: 10.1016/j.bbi.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ringpfeil F, McGuigan K, Fuchsel L, Kozic H, Larralde M, Lebwohl M, et al. Pseudoxanthoma elasticum is a recessive disease characterized by compound heterozygosity. J Invest Dermatol. 2006;126:782–6. doi: 10.1038/sj.jid.5700115. [DOI] [PubMed] [Google Scholar]
- Scheffer GL, Hu X, Pijnenborg AC, Wijnholds J, Bergen AA, Scheper RJ. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest. 2002;82:515–8. doi: 10.1038/labinvest.3780444. [DOI] [PubMed] [Google Scholar]
- Uitto J, Pulkkinen L, Ringpfeil F. Molecular genetics of pseudoxanthoma elasticum: a metabolic disorder at the environment-genome interface? Trends Mol Med. 2001;7:13–7. doi: 10.1016/s1471-4914(00)01869-4. [DOI] [PubMed] [Google Scholar]