Abstract
Variation in height and body proportions is relatively well understood at the inter-population level, but less is known about intra-population variation. This study explores intra-population variation in body proportions among 172 (88 female; 84 male) adult rural Amazonians. We test the hypotheses that: 1) stunting is associated with changes in proportions and fatness; 2) the sexes express different proportions in response to similar environmental stress and 3) female growth is negatively affected by the costs of reproduction. We examined height, sitting height and total leg length in subsamples based on sex and nutritional status (stunted/non-stunted) in relation to biocultural factors including access to food and healthcare and female reproductive history parameters. Differences in proportions were examined using the Quick-Test (Tsutakawa and Hewett, 1977); correlation analyses were employed to detect associations between anthropometric data and body fatness, and female reproductive history parameters. We found significantly higher rates of stunting among females (X2=5.31; p=0.02; RR=1.4). Stunted individuals exhibited relatively shorter legs than non-stunted individuals (p=0.02), although this was not found in within-sex analyses. A significant negative correlation was found between leg length index and fatness (p<0.01). Lastly, females exhibited relatively shorter legs than males (p=0.0003) and, among females, height and leg length were significantly positively correlated with age-at-first-birth (p<0.02) suggesting that adolescent pregnancy may negatively affect growth in this population. Our findings provide insights for the study of intra-population variation in body proportions and highlight the importance of biocultural data in interpreting the pattern of variation observed in living and past populations.
Keywords: Body Proportions, Stunting, Reproductive costs, Growth, Ribeirinhos
Human populations exhibit phenotypic variation at least some of which is likely the result of long-term selection and adaptation to the environment. Among other aspects of such variation, differences in height have been documented among populations inhabiting different regions of the world and subject to different environmental conditions. The study of height change over time and space has revealed that variation in body size is generally associated with a population’s overall environmental quality and life conditions (Boas, 1912; Lasker, 1952; Meredith, 1976; Garn, 1987; Formicola and Giannecchini, 1999; Friedman et al., 2000; Loesch et al., 2000; Steckel, 2004; Gerhards, 2005; Maat, 2005; Gustafsson et al., 2007; Giannecchini and Moggi-Cecchi, 2008). Specifically, height has been shown to vary among human populations in relation to a variety of environmental factors, including climatic conditions, diet, socio-economic status, and disease (Rona and Chinn, 1991; Waterlow and Schürch, 1993; Bogin, 1999; Wadsworth et al., 2001; Bogin et al., 2002; Mascie-Taylor and Lasker, 2005). Improvements in sanitation, medical care and access to resources in industrialized countries over the past century have provided evidence for phenotypic plasticity in height (Eveleth and Tanner, 1990; Bogin, 1999). In particular, individuals benefitting from better nutrition and overall living conditions achieve taller adult stature. This increase in height is commonly accompanied by allometric changes in body proportions generally attributable to a relative elongation of the lower limb (Tanner et al., 1982; Bogin et al., 2002), especially the distal segment (Holliday, 1997; Jantz and Jantz, 1999; Sylvester et al, 2008; Auerbach and Sylvester, 2011).
Human skeletal growth is a heterochronic process, with different body segments growing at different rates during different phases of development (Bass et al., 1999; Humphrey, 1999; Bradney et al., 2000; Nyati et al., 2006). The lower limb bones, especially the distal segments, tend to reach adult size earlier than the trunk (Bass et al., 1999; Bradney et al., 2000). This difference in the timing and tempo of skeletal growth implies that growth disruptions during different stages of the life course can affect body proportions (Karlberg, 1989). Given that childhood is characterized by fast long bone growth promoted by somatotropin (Nilsson et al., 1994), perturbations during this growth period typically have the greatest impact on long-term development and are expressed in shorter lower limb length (Karlberg, 1989; Allen, 1994). Conversely, improved conditions can promote better lower limb growth (Bogin et al., 2002).
Nonetheless, while it is well accepted that differences in overall life conditions contribute to variation in height and proportions, the impact of specific factors on growth is not clearly understood. Indeed, most studies examining height and body proportions have addressed biological variation by comparing different contemporaneous populations exposed to different environmental conditions (Stinson, 1990; Holliday, 1997; Katzmarzyk and Leonard, 1998; Weinstein, 2005) or by examining how changes in life conditions over time in the same population affect changes in growth (Zakrzewski, 2003; Temple et al., 2008). While these studies have the merit of documenting skeletal growth variation between populations across space and time, less is known of the degree and causes of variation within human populations (i.e., intra-population variation). In this study, we define intra-population variation as the biological variation observable within a single population, which is relatively homogeneous in terms of environmental, biological and cultural factors. In this sense, a suitable population for this kind of analysis is a population whose individual members: 1) are exposed to the same environmental conditions – with no major variation in climatic, geophysical and ecological conditions; 2) share a similar biological history and exhibit biological continuity – affected by minimal external gene flow – for an extended period of time; and 3) show relative cultural stability, with no sudden changes in social organization, subsistence strategy and cultural beliefs. According to this definition, the subject of intra-population studies may not be restricted to a cross-sectional examination of biological variation at a specific point in time, but may also examine variation in the same population over time, provided that there are no major changes in the population’s eco-bio-cultural milieu. This is particularly true when studying variation within past human populations, where limited sample size and dating issues may not allow researchers to restrict the analysis to a specific point in time.
Arguably, intra-population variation arises because each individual’s height and body proportions are the outcome of a unique life history. In a vacuum, an individual’s skeletal growth would follow a genetically predetermined trajectory and would result in adult height and proportions typical of the individual’s population of origin. However, in reality, environmental conditions interact with an individual’s genotype in determining actual growth trajectories and end results (Sultan and Stearns, 2005). Depending on their duration and timing, environmental growth disruptions (e.g. malnutrition and disease) may play a major role in shaping the adult phenotype (Martorell, 1989; Martorell et al., 1994). It has been suggested that chronic growth insults may have more severe long-term effects on skeletal growth than single stress episodes (Tanner, 1992: Golden, 1994). Additionally, evidence indicates that suboptimal growth conditions during childhood – the fastest growth phase in human development – typically have the greatest impact on long-term development and expression of adult height and proportions (Karlberg, 1989; Allen, 1994). However, human growth is a relatively plastic process and a variety of factors can intervene and affect the final achieved stature and proportions. First, it has been observed that, under improved conditions, individuals previously exposed to stress may fully recover (catch-up growth) from the effects of environmental perturbations (Martorell et al, 1994; Cameron et al, 2005). Catch-up growth may occur by an increase in growth rates and/or an elongation of the growth period by delaying maturation (Gafni and Baron, 2000). Second, it has been argued that the sexes may respond differently to environmental stress, with females being more buffered than males against growth disruptions (Stini 1969). Stinson (1985), in a review of the literature, found that while there is empirical evidence to support the hypothesis of female buffering during the prenatal period, data collected during the period of postnatal growth in a range of human populations are mixed with some showing evidence of female buffering and others not. According to Stinson (1985), one reason for these mixed results may be the confounding effects of the preferential treatment of sons observed in many human societies. Third, considering that under stressful conditions growth may be delayed and continue beyond an individual’s teen years (Roche and Davila, 1972; Riley et al., 1989), it is possible that growth may be affected by the costs of reproduction, especially in females (Scholl et al., 1993; Scholl et al., 1994; Casanueva et al., 2006; Rah et al., 2008). While a number of studies have shown that the conflict between maternal growth and reproduction results in smaller infant size at birth, it is unclear how maternal growth may be affected (Scholl et al., 1993). As a consequence, the complexity of human growth, the unpredictability of its disruptions, and the plastic response of different genotypes to stress hinder interpreting variation in skeletal growth.
A way to improve our understanding of the causes and mechanisms of variation in height and body proportions is to complement auxological data with biocultural information. Biocultural approaches in human biology recognize that culture is an intrinsic aspect of human nature and plays an integral role in shaping the environment in which growth and development take place (Dufour, 2006). Under this approach, biological variation, including variation in stature, is interpreted as a response to the complex biosocial environment. Consequently, recording and measuring both biological and cultural variables is essential for improving our understanding of the causes of biological variation.
By adopting a biocultural approach, this study aims to contribute to the literature on human growth variation by examining height and body proportions at the intra-population level. Our sample includes 172 rural Amazonian adults (18–77 years) and is particularly well suited for addressing our research questions in that the population (1) is relatively genetically homogeneous, meaning that there are no distinct ethnic groups and due to restrictions on land-use due to the proximity of the communities to the Caxiuanã National Forest, created in 1961, no recent influx of genetically distinct populations into the area (Lisboa, 2002); (2) does not exhibit a large degree of variation in economic status (i.e., wage labor opportunities were limited and all practiced subsistence farming) and (3) shared a common biocultural environment, meaning individual members shared a common set of beliefs and ideals as well as a common environment where they were exposed to similar environmental stress (nutritional, infectious disease) during most of the period of growth and development. Long-term, longitudinal fieldwork conducted by BAP in the region provide us with detailed biocultural data including demographic patterns, and reproductive histories, as well as ethnographic data on cultural beliefs and practices regarding gender roles, diet and work patterns, health and reproduction critical for interpreting data on human growth patterns (Piperata and Dufour, 2007; Piperata, 2007; Piperata et al., 2011a).
The goal of this study is to draw on this rich data set to address models of human growth and test the following research hypotheses:
Severe growth retardation is associated with changes in body proportions and body fatness. Assuming that the lower limb is the segment most affected by environmental perturbations, it is expected that growth retardation will be due primarily to relatively shorter legs than trunks (Bogin et al., 2002). This is expected to occur similarly in the sexes. Additionally, since it has been observed that early growth retardation may also affect adult body fatness because of the inter-relatedness of physiological mechanisms involved in energetic efficiency and low fat oxidation, (Frisancho, 2007; Hoffman et al., 2000; Sawaya et al., 2004), a negative association between leg length and body fatness is expected.
Males and females exhibit different body proportions in response to similar environmental stress. Based on the hypothesis of greater male susceptibility to environmental insults, all other factors being equal, males are expected to show a greater negative response to stress than females. Specifically, since it has been shown that the lower limb is more affected by negative environmental conditions than the trunk (Bogin et al 2002), males are expected to have relatively shorter lower limbs than females.
Female skeletal growth in suboptimal conditions is negatively affected by the costs of reproduction. It is expected that female growth will be negatively impacted by the costs of reproduction and that significant associations between reproductive history parameters and growth will be found.
MATERIALS AND METHODS
People and field site
This study explored intra-population variation in height and proportions in a population of Brazilian Ribeirinhos living in upper-land (terra firme) communities located in and around the Caxiuanã National Forest in the State of Pará (Fig. 1) (Piperata, 2007). The local people self-identified as Ribeirinhos (also referred to as Caboclos in the literature). They are a mixed ethnicity (Amerindian/Portuguese and to a lesser extent African) group formed during the colonization of the Amazon region by the Portuguese in the 16th century (Harris, 1998).
Figure 1.
Map of the field site.
The communities are rural, had no electricity or running water, and only a few households had pit toilets. The majority of households used the forest and river for waste disposal. Water for cooking and drinking was collected from the river or, in a few cases, from hand-dug wells, and trash was burned, buried, or dumped in the river. While people were aware of public health messages regarding the transmission of disease via water, efforts to treat drinking water were uncommon and, when practiced, inconsistent. For example, BAP observed the use of chlorine drops to treat drinking water in a small number of households. However, the amount of chlorine used was insufficient for the volume of water, household members drank treated and untreated water on the same days and, the results of a household survey revealed that no household reported purchasing or using chlorine, or any other type of water treatment, on a regular basis. While we are unaware of a local study of water quality, the fact that gastrointestinal infections were the second most commonly reported cause of morbidity implies that water-borne illnesses are an issue in these communities.
Homes sat on stilts, were made of wood and covered with one of three materials, palm fronds, ceramic tile, or an industrialized, fire retardant material referred to as Brasilite. Most households consisted of a nuclear family but some included extended kin. Average household size was 7.6 (range: 3–15). The region, in general, is considered part of a black-water river system (Costa et al., 2002). Such ecosystems are characterized by acidic water and soils and are known for their relatively low productivity relative to other Amazonian ecosystems (Moran, 1993). All the people practiced slash and burn agriculture with bitter manioc (Manihot esculenta) as their staple crop. Manioc was consumed primarily in the form of farinha, a toasted meal, and was the most important source of calories and carbohydrates in the diet (Murrieta et al., 2008; Piperata et al., 2011b). Fish, and, to a lesser extent, hunted game, were the most important sources of protein, and açaí (Euterpe oleracea), a local palm fruit consumed primarily in the form of a juice, was an important seasonal source of calories (Piperata et al., 2011b). While the people cultivated, fished, hunted, and collected the majority of the food they consumed, they were also actively involved in and dependent upon the regional market economy. Men and women shared the work associated with the cultivation and processing of manioc, which included clearing the forest, burning, planting, weeding, harvesting and finally, processing the roots into farinha. Fishing, hunting and the collection of açaí were primarily male activities, although women cleaned and prepared the fish and game and extracted the açaí juice. Women were also responsible for all housework and childcare.
Access to medical care was limited. The closest clinic and hospital were located in the town of Portel, which was an eight-hour boat ride from the communities. Due to the costs of fuel and what were described by the local people as poor services due to limited medical staff and medications, trips to town for care were limited to what were perceived as emergency situations (i.e., snake bites and broken bones). As part of a household survey conducted in 2002, people were asked to list the most common causes of illness. Their responses indicated that respiratory infections (i.e., flu, sinus congestion), gastrointestinal problems (i.e., diarrhea, vomiting, parasitic infections), skin related issues (i.e., rashes, itching) and general aches and pains related to heavy labor and/or arthritis were the most common causes of morbidity. Distance to town also limited the uptake of pre- and postnatal health services. The majority of women relied on a local midwife to assess their condition during pregnancy and assist with birth. Hospital births were rare (<10%) and were limited to instances where the fetus was identified as being breach by the midwife or, in a few cases, where the mother was young (14–15 years) and her parents, out of fear, paid to have her transported to town to deliver her first child. Follow-up neonatal care was also limited and typically ceased after the infant was fully vaccinated. Overall, Ribeirinhos are subject to chronic stress – as evidenced by high rates of growth retardation – which has been associated with nutritional stress during growth and development and poor access to healthcare (Silva and Crews, 2006; Murrieta et al., 1998; Giugliano et al., 1981, 1984).
Anthropometric and biocultural data
This study included a total of 172 (88 female; 84 male) adults between 18–77 years of age. Anthropometric data were collected between March and August of 2002 while the biocultural data were collected over a two-year period (2002–2004) when BAP lived and worked in the communities. All data collection methods were reviewed and approved by the Human Research Committee at the University of Colorado-Boulder (HRC no. 1001.2) and by similar committees in Brazil.
In order to assess intra-population variation in height and body proportions, we examined height, sitting height and total leg length in the different population subsamples: male vs. female; stunted vs. non-stunted; stunted male vs. non-stunted male; stunted female vs. non-stunted female (Table 1). Anthropometric measurements were taken at either the local schoolhouse or in individual homes. The schoolhouses had level, cement floors. When anthropometric data were collected in an individual’s home, a level was used to find the most appropriate area for taking the measurements. All measurements were taken following standardized procedures (Lohman et al., 1988). Heights and sitting heights were recorded to the nearest 0.1 cm using a Seca portable stadiometer. Total leg length was calculated as the difference between height and sitting height (Frisancho, 2008). All women who were in the final trimester of a pregnancy were measured multiple times pre- and post-partum. Anthropometric data recorded two months after birth were used in the analyses reported here to avoid methodological issues associated with height variation due to vertebral compression and postural changes during pregnancy (Scholl et al., 1993). Individual z-scores for height-for-age (HAZ) were calculated using the National Health and Nutrition Examination Surveys (NHANES III) reference values provided in Frisancho (2008). HAZ were used to assign individuals to different growth-outcome groups, i.e., stunted and non-stunted. Stunting was defined as a low HAZ (z-score < −2) (WHO, 1995).
TABLE 1.
Descriptive statistics for anthropometric data and age by subsample.
| Height | Sitting Height | Total Leg Length | Age | ||
|---|---|---|---|---|---|
| Males (Total) | N | 84 | 84 | 84 | 84 |
| Min | 136.9 | 72.5 | 64.4 | 18 | |
| Max | 179.4 | 91.0 | 94.7 | 77 | |
| Mean | 160.4 | 83.2 | 77.2 | 36.0 | |
| St Dev | 7.1 | 4.0 | 4.8 | 14.2 | |
| Females (Total) | N | 88 | 88 | 88 | 88 |
| Min | 122.0 | 70.9 | 43.8 | 18 | |
| Max | 160.2 | 87.2 | 78.8 | 66 | |
| Mean | 146.5 | 78.0 | 68.5 | 32.9 | |
| St Dev | 5.5 | 3.0 | 4.6 | 12.8 | |
| Male Stunted | N | 32 | 32 | 32 | 32 |
| Min | 136.9 | 72.5 | 64.4 | 18 | |
| Max | 159.8 | 90.8 | 82.9 | 77 | |
| Mean | 153.6 | 80.1 | 73.4 | 40.1 | |
| St Dev | 5.3 | 4.1 | 3.8 | 15.6 | |
| Male Non Stunted | N | 52 | 52 | 52 | 52 |
| Min | 159.0 | 80.2 | 73.7 | 18 | |
| Max | 179.4 | 91.0 | 94.7 | 66 | |
| Mean | 164.6 | 85.1 | 79.5 | 33.4 | |
| St Dev | 4.1 | 2.5 | 3.7 | 12.7 | |
| Female Stunted | N | 50 | 50 | 50 | 50 |
| Min | 122.0 | 70.9 | 43.8 | 18 | |
| Max | 149.3 | 82.0 | 74.5 | 66 | |
| Mean | 143.1 | 76.9 | 66.3 | 34.4 | |
| St Dev | 4.6 | 2.9 | 4.5 | 12.4 | |
| Female Non Stunted | N | 38 | 38 | 38 | 38 |
| Min | 147.8 | 74.8 | 66.6 | 18 | |
| Max | 160.2 | 87.2 | 78.8 | 65 | |
| Mean | 150.9 | 79.5 | 71.4 | 30.8 | |
| St Dev | 3.0 | 2.4 | 2.7 | 13.1 | |
To explore the association between longitudinal growth outcomes and body fatness, percent body fat was calculated from skinfold thickness measurements (triceps, subscapular) by using the formulae developed by Durnin and Womersley (1974). Skinfolds were measured in triplicate to the nearest 0.5 mm using Lange skinfold calipers.
Adult ages were based on the individual’s recall and, when available, were crosschecked with legal documents such as government-issued identification cards. Structured interviews were used to gather detailed biocultural data on the individual participants including household size and composition and women’s reproductive histories. Reproductive histories were conducted with each of the 88 adult women and included information on age at menarche, age at birth of first child, parity and date of birth of each child. The birth dates of the individual children were used to calculate inter-birth intervals and their average. Semi-structured and unstructured interviews with adult women and men, along with ethnographic data collected via participant observation, were used to gather information on daily life and local beliefs regarding gender (work and access to resources). Detailed dietary data collected in 2002 on a sample of 23 lactating women (Piperata and Dufour, 2007) and dietary data collected in 2009 on a sample of 52 children (unpublished) are used to complement the observational data on gender and access to resources mentioned above.
Data analysis
To test the hypotheses that stunting would be associated with changes in body proportions and, that males and females would respond differently to environmental stress, we employed the “Quick-Test” devised by Tsutakawa and Hewett (1977). First, we plotted log-transformed sitting height over log-transformed height data and fitted a line with a slope of 1.0 through the grand mean of the combined samples being compared. This fit line represents a perfectly isometric relationship between the variables under examination for the pooled samples. Consequently, we employed the “Quick-Test,” which allowed us to test the null hypothesis that the joint distribution of data points above and below the fit line is the same for distinct samples (Tsutakawa and Hewett, 1977). This test has been used to identify differences in body proportions in a range of taxa (Swartz, 1997; Maie et al., 2007; Temple et al., 2008).
The association between linear growth retardation and body fatness was explored by correlation analyses between body fatness and the leg length index (calculated as [(total leg length/height)*100]). All correlation analyses were controlled for age to account for age-related changes in body fatness.
Raw and log-transformed data, rather than z-scores, were used in all analyses. While z-scores have the advantage of standardizing raw data by sex and age, we preferred using raw data in order to avoid masking part of the biological variation within the population by standardizing it by means of an extraneous reference sample. A potential problem with using raw data lies in possible age-related changes in height and sitting height, primarily due to vertebral compression. To evaluate the potential effects of age on the data, we compared age distributions across subsamples (male vs. female; stunted vs. non-stunted; stunted male vs. non-stunted male; stunted female vs. non-stunted female) using the Mann-Whitney or Kruskal-Wallis tests, depending on the number of subsamples being compared. These non-parametric tests are preferable to the parametric ANOVA because they are applicable even when the distribution of the data departs from normality (Zar, 1999).
Lastly, we explored the association between linear growth (height, sitting height, total leg length and leg length index) and female reproductive history parameters (age at menarche, age at first birth, parity, and inter-birth interval between first and second birth) using correlation analysis. Given the existence of a secular trend in age at first birth (see below), the obvious association between older age and parity, and the well-known phenomenon of age-related reduction in trunk height, we controlled for age in all correlation analyses. Statistical analyses were performed using PASW Statistics 18.0 and Microsoft Excel 2007. Statistical significance is defined as p 0.05.
RESULTS
Changes in body proportions associated with stunting
The result of the Mann-Whitney test on age distributions of males and females in our sample was non-significant (p=0.14). Therefore, we first tested the hypothesis that stunting was associated with changes in body proportions in the entire sample, regardless of sex. We plotted log-height over log-sitting height for the sex-combined sample, fitted the joint distribution by a line of slope equal to 1.0 through the grand mean of the samples (Fig. 2), and employed the Quick-Test to test for differences in proportions between stunted and non-stunted individuals. The results of the test were significant (p = 0.02). As expected, stunted individuals tended to fall above the line, indicating that they had relatively shorter legs. Considering the hypothesis that males and females may be differentially affected by environmental stress, we explored the relationship between stunting and proportions in the sex-specific subsamples.
Figure 2.
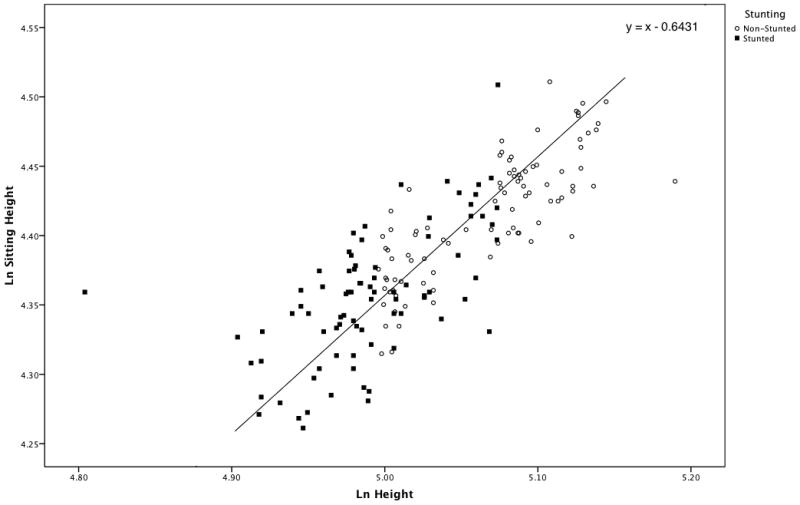
Scatter plot of Sitting Height over Height for stunted and non-stunted individuals, sexes combined. The line has a slope of 1.0 and passes through the grand mean (5.029, 4.385) of the samples. Stunted individuals tend to cluster above the line, indicating that they are characterized by relatively shorter legs, while non-stunted individuals, who are more numerous below the line, exhibit relatively longer legs.
Males
Age distributions between stunted and non-stunted individuals in the population were tested using the Mann-Whitney test and revealed a significant difference in the male subsample (p = 0.04). This difference was due to the higher occurrence of stunting among older individuals. When the oldest individual in the population (77 years old) was removed from the comparison, the age distributions of the two subsamples were not significantly different (p = 0.07). Therefore, we plotted log-sitting height over log-height for stunted and non-stunted males (Fig. 3). The results indicated no difference in the relationship between height and sitting between stunted and non-stunted males (p = 0.51). In other words, stunting (or the process of stunting) appears to be isometric and not accompanied by detectable changes in sitting height/height proportions.
Figure 3.
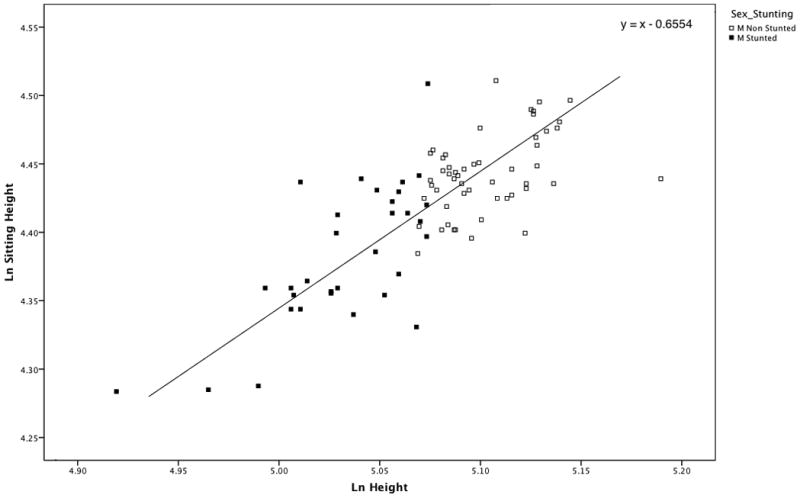
Scatter plot of Sitting Height over Height for stunted and non-stunted males (M). The line has a slope of 1.0 and passes through the grand mean (5.068, 4.413) of the samples. The two samples are equally distributed above and below the line, indicating that there are no differences in body proportions between stunted and non-stunted individuals.
Females
The difference in the age distribution of stunted and non-stunted females in the population was non-significant (p = 0.09). Figure 4 is the scatter plot of log-sitting height over log-height of stunted and non-stunted females in the population. The result of the “Quick-Test” was non-significant (p = 0.12) meaning there was no significant difference in the relationship between height and sitting height in stunted and non-stunted females.
Figure 4.
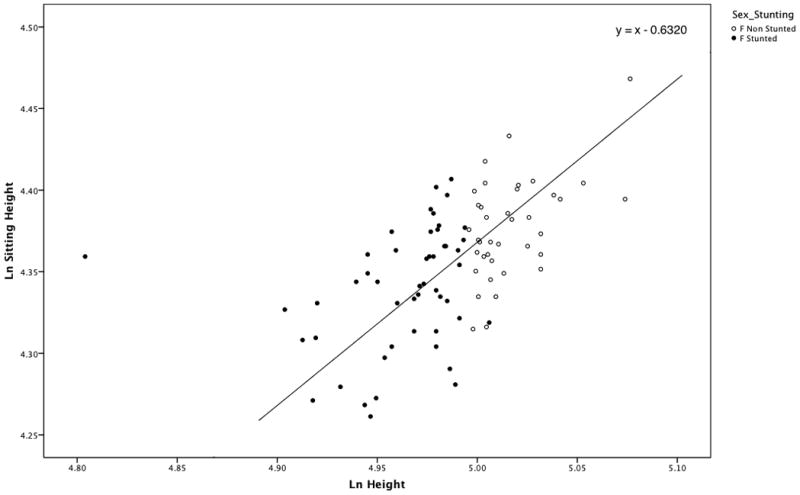
Scatter plot of Sitting Height over Height for stunted and non-stunted females (F). The line has a slope of 1.0 and passes through the grand mean (4.670, 4.678) of the samples. The two samples are equally distributed above and below the line, indicating that there are no differences in body proportions between stunted and non-stunted individuals.
Association between growth retardation and body fatness
We tested the hypothesis that growth faltering would be associated with increased body fatness, with short individuals exhibiting greater percent body fat. The results revealed a significant negative correlation between the leg length index ((leg length/stature)*100) and percent body fat among males (r = −0.33; p = 0.02) and females (r = −0.44; p = 0.00002) (Table 2).
TABLE 2.
Correlation analysis between body fatness and leg length index, controlled for age
| Sample | Control | Variable | Leg Length Index | |
|---|---|---|---|---|
| Males | Age | Body Fatness | Correlation | −0.329 |
| Significance (2-tailed) | 0.0021 | |||
| Degrees of freedom | 81 | |||
| Females | Age | Body Fatness | Correlation | −0.437 |
| Significance (2-tailed) | 0.000022 | |||
| Degrees of freedom | 85 |
Significant at 0.01 level.
Significant at 0.0001 level.
Sex differences in response to environmental stress
The overall prevalence of stunting in our sample was 48% and the mean height-for-age z-score for the combined sex sample was −2.0. The mean height-for-age z-scores in the male and female subsamples were −1.9 and −2.2, respectively. A significantly higher rate (X2 = 5.31; p = 0.02; RR = 1.4) of stunting was detected in females (50/88 = 57%) than in males (32/84 = 38%).
We employed the “Quick-test” to test the hypothesis that males and females would exhibit differences in body proportions in response to similar environmental stress. Since there was no significant difference in age distributions of males and females (p = 0.14), we plotted log-sitting height over log-height for males and females and fitted the joint distribution by a line of slope equal to 1.0 through the grand mean of the male and female samples (Fig. 5). The “Quick-Test” result was significant (p = 0.0003), indicating that the relationship between height and sitting height in the sample was significantly different between the sexes. Specifically, males tended to have relatively shorter trunks (sitting height) than females and thus, tended to cluster below the line.
Figure 5.
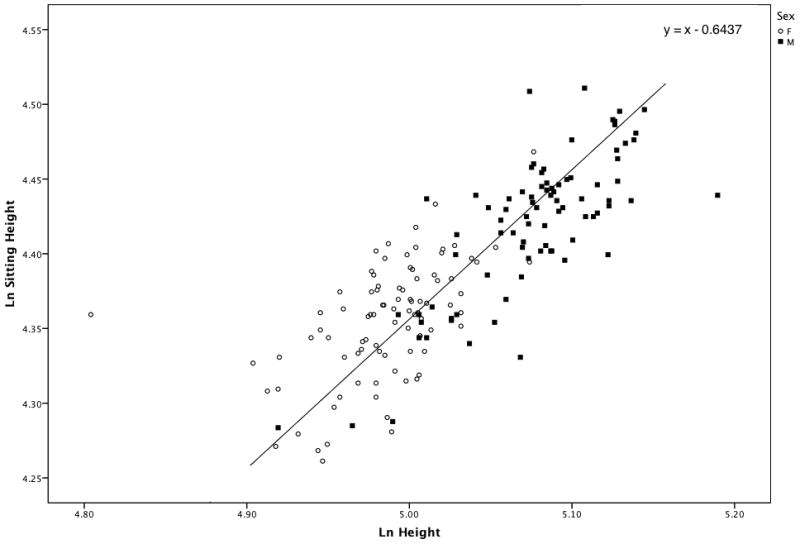
Scatter plot of sitting height over height for males (M-squares) and females (F-circles). The line has a slope of 1.0 and passes through the grand mean (5.031, 4.388) of the samples. Females tend to cluster above the line, and males below the line, indicating that they have relatively shorter legs than males.
Relationship between skeletal growth and reproductive characteristics in females
Average age at menarche was 12.8 ± 1.1 years and average age at the birth of the first child was 17.6 ± 2.3 years. On average, women breastfed for 14.2 ± 4.4 months and the average inter-birth interval was 28.4 ± 12.0 months. Average total parity for women over the age of 45 was 10.3 ± 4.8 children (Table 3). Age at first birth showed a significant decline over time (Fig. 6) with the trend of younger women giving birth earlier than older women (Kruskall-Wallis test, p = 0.02). Age at menarche did not differ among age groups (p = 0.66). However, parity expectedly increased with age (p < 0.001). Due to the relationship between reproductive life history variables and age, we controlled for age in all correlation analyses (Table 4). Age at first birth was the only reproductive history parameter that was significantly correlated with the anthropometric variables. Specifically, positive correlations were found between age at first birth and height (r = 0.29; p = 0.01), age at first birth and total leg length (r = 0.41; p = 0.001), and age at first birth and the leg length index (r = 0.39; p = 0.001). There was no relationship between age at first birth and sitting height (r = −0.04; p = 0.72). That is, those with earlier ages at first birth were shorter in stature and had proportionally shorter legs.
TABLE 3.
Reproductive characteristics of the women (n=88)
| Min | Max | Mean | St Dev | |
|---|---|---|---|---|
| Menarche | 11 | 16 | 12.8 | 1.1 |
| Age at First Birth | 13 | 24 | 17.8 | 2.3 |
| Breastfeeding duration | 4.3 | 25.6 | 13.4 | 4.3 |
| Inter-birth Interval | 10.75 | 73.0 | 27.5 | 10.7 |
| Parity | 1.0 | 15.0 | 6.2 | 4.0 |
| Parity (women > 45 yrs) (n= 15) | 2.0 | 15.0 | 10.3 | 3.8 |
Figure 6.
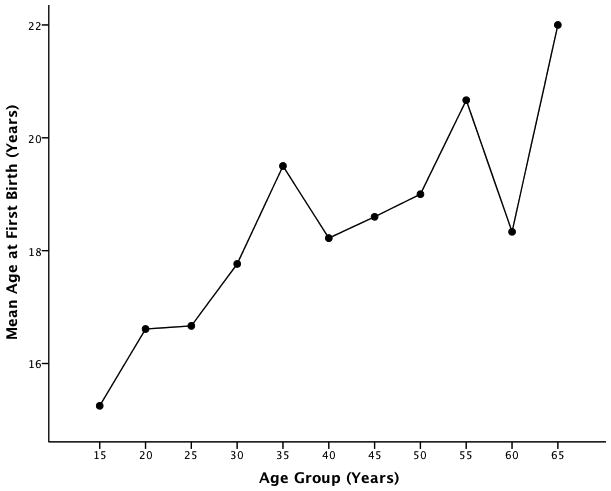
Secular trend in mean age at first birth among Ribeirinhos.
TABLE 4.
Correlation analysis between anthropometric data and reproductive history parameters, controlled for age and age at first birth.
| Control | Variable | Height | Sitting Height | Total Leg Length | Leg Length Index | |
|---|---|---|---|---|---|---|
| Age | Menarche | Correlation | 0.195 | 0.123 | 0.158 | 0.077 |
| Significance (2-tailed) | 0.098 | 0.298 | 0.182 | 0.517 | ||
| Degrees of freedom | 71 | 71 | 71 | 71 | ||
| Age at First Birth | Correlation | 0.289 | −0.044 | 0.412 | 0.388 | |
| Significance (2-tailed) | 0.0181 | 0.723 | 0.0012 | 0.0012 | ||
| Degrees of freedom | 65 | 65 | 65 | 65 | ||
| Parity | Correlation | −0.133 | −0.021 | −0.145 | −0.123 | |
| Significance (2-tailed) | 0.220 | 0.850 | 0.180 | 0.255 | ||
| Degrees of freedom | 85 | 85 | 85 | 85 | ||
| Age at first child | Inter-birth Interval | Correlation | −0.135 | −0.134 | −0.072 | 0.020 |
| Significance (2-tailed) | 0.389 | 0.392 | 0.645 | 0.897 | ||
| Degrees of freedom | 41 | 41 | 41 | 41 | ||
Significant at 0.05 level.
Significant at 0.001 level.
DISCUSSION
Changes in body proportions associated with stunting
We tested the hypothesis that stunting would be associated with allometric changes in body proportions, with shorter individuals exhibiting relatively shorter legs. We detected a significant difference between stunted and non-stunted individuals in the sex-combined sample, with stunted individuals exhibiting significantly shorter legs than non-stunted individuals. This result matches expectations and is consistent with what has been reported by other authors (Bogin et al., 2002). However, this result should be interpreted with caution in light of the absence of significant differences in the sex-specific samples (see below). In particular, given the significantly higher rate (X2 = 5.31; p = 0.02; RR = 1.4) of stunting in females (50/88 = 57%) than in males (32/84 = 38%), it is possible that the differences observed may be attributable to sex-related differences in body proportions rather than body shape changes associated with growth retardation. The observed disparity in stunting rates between the sexes may be due, at least in part, to lack of catch-up growth, especially during adolescence, and/or increased nutritional stress associated with reproductive costs during growth experienced by females in this population, which are discussed in further detail below.
The unexpected lack of significant differences in body proportions between stunted and non-stunted individuals in the sex-specific samples may indicate that height reduction in the population is rather isometric and that the stress leading to stunting affected trunk and lower limb length equally. Additionally, confounding factors such as age-related changes in trunk length and catch-up growth may also explain our findings. For example, even though the age distributions of stunted and non-stunted individuals did not significantly differ, stunting was more prevalent among older individuals. Numerous authors have reported age-related height reduction attributable to the compression of the vertebral column over time (Galloway, 1988; Cline et al., 1989; Chandler and Bock, 1991; Giles, 1991). This reduction varies in relation to a variety of factors, such as age at onset of height reduction due to age-related vertebral compression, sex, and population differences in the extent and timing of height loss. According to Trotter and Gleser (1951), beginning at age 30, average loss in stature is approximately 0.06 cm per year. While negligible in the short term, this small annual reduction in stature would add up over decades leading to a noticeable decline in total height in older age. Therefore, the fact that we found no difference in proportions between stunted and non-stunted individuals may be related to different age-related changes in the anthropometric variables tested. Additionally, catch-up growth during adolescence (see below) may partly reverse the effects of stunting, therefore eliminating differences in body proportions between the stunted and non-stunted sub-samples.
Association between growth retardation and body fatness
Research has shown that severe growth faltering is a major sign of poor health and is associated with a number of adverse health outcomes, including compromised immune competence, poor psychological performance, diminished productivity, reduced reproductive potential, and increased mortality risk (Martorell, 1989; Paajanen et al., 2010). In addition, researchers have observed a relationship between growth faltering and a predisposition toward obesity (Hoffman et al., 2003; Leonard et al., 2008). Frisancho (2007), using data from the National Health and Nutrition Survey (NHANES III), explored the association between body fatness and leg length index, which is a good indicator of individual developmental histories due to its sensitivity to early growth disruptions. In that study, an association between low leg length index values and increased body fatness was detected, supporting the hypothesis that growth faltering is associated with increased risk of obesity (Frisancho, 2007). We explored whether a similar association between growth retardation and increased body fatness also existed among Brazilian Ribeirinhos. In agreement with expectations, we found a significant negative correlation between leg length index and percent body fat. The correlation was stronger in females than males, suggesting that males and females experienced different (or differently timed) environmental stresses, which led them to exhibit different growth outcomes and body fatness.
Sex differences in response to environmental stress
Based on the hypothesis of greater male susceptibility to environmental stress (i.e., greater female buffering) (Stinson, 1985), we expected the male and female Ribeirinhos in this study to respond differently to the same environmental stress (i.e., intra-population variation). As evidence suggests that the lower limb is more susceptible to environmental stress than the trunk (Bogin et al., 2002), all other factors being equal, we expected males to exhibit relatively shorter legs than females. In contrast to our expectations, the results indicate that while male and female Ribeirinhos in the sample expressed different body proportions, males exhibited relatively shorter trunks (i.e., longer legs) than females. While contrary to our expectations, the differences in trunk/lower limb proportions detected in this study are consistent with a general pattern observed in analyses of worldwide variation in relative limb lengths, which have shown that males tend to have relatively longer lower limbs than females (Leonard and Katzmarzyk, 2010). Drawing on long-term ethnographic research, which included data obtained via interviews and participant observation, and detailed dietary data, there are several possible factors that could be invoked to explain this finding, including differential access to resources between the sexes and sex-specific energetic stress associated with reproduction during adolescence.
Access to resources over the period of growth and development
As discussed earlier, according to Stinson (1985) a major confounding factor that may explain the weak support for female buffering during the postnatal period is the preferential treatment of sons, especially in terms of access to food and medical care, observed in many societies. Brazilian society, like those throughout Latin America, is patriarchal and exhibits male dominance (Neuhouser, 1989). Several studies of child health conducted in Latin America have found evidence of greater dietary adequacy (Frongillo and Bégin, 1993; Dewey, 1980), better access to healthcare (Delgado et al., 1982; Dewey, 1980, 1983; Larme, 1997) and better nutritional status (Frongillo and Bégin, 1993; Dewey, 1980, 1983) among boys. However, studies conducted in Brazil indicate that the pattern of parental-child investment is complex and there is little evidence of male bias in access to resources (Emerson and Souza, 2007; Silva and Crews, 2006; Thomas, 1994).
Ribeirinhos identify with the larger Brazilian society and can also be described as male dominant; however, data collected during long-term research in these communities provide little evidence for an increased desire for or preferential treatment of sons, especially during the period of most rapid growth.
As part of the reproductive history interview, women were asked about their ideal family size and composition. The most common response to the question “How many children do you want?” was “O que Deus quiser” or “What God wants or desires.” This response indicates that women felt they had little control over their total parity and this was due, in large part, to their limited access to birth control. Another common response to this question was “um casal” which literary translates as “a married couple” but in this context means “one girl and one boy.” Only in a few instances, where a woman was pregnant and already had a large number of children of one sex, was a preference for the opposite sex expressed.
The lack of preference for one sex over the other may be related to the fact that adults recognized the contributions of both boys and girls in the household. Girls provided significant support to their mothers from an early age, caring for younger siblings, hauling water and doing laundry. The contribution of boys started around 10–12 years when they began fishing and then later (~15 years) when they started to hunt and assist their fathers with heavy labor such as clearing the forest to plant a new manioc garden, cut wood and haul heavy loads.
Ribeirinhos do not observe a preferential residence pattern. Thus, upon marriage, it was equally likely for daughters and sons to choose to build their homes close to that of their parents. In cases where daughters lived near their parents it was common to observe mothers and daughters time their domestic and agricultural activities to coincide so that they could enjoy the company and assistance of one another as they worked. Sons, when living close to their natal home, often continued hunting and fishing with their fathers and fathers and sons assisted one another clear their respective manioc gardens. Fathers and sons also coordinated their economic activities and commonly traveled together to town to trade.
Long-term field research, including 22-months living in the communities provided BAP many opportunities to observe the ways boys and girls were treated. In terms of healthcare, there is little evidence to support preferential access for males. As mentioned earlier, access to healthcare was limited for all community members due to the distance between the communities and town and people only tended to seek medical care in emergency situations. Common illnesses, such as respiratory and gastrointestinal infections, were rarely treated with western medicine. Both types of illnesses were typically allowed to run their course although, in the case of gastrointestinal infections, mothers often prepared medicinal teas made from local plants to treat symptoms.
Direct observations of hundreds of meals provide no evidence of the preferential feeding of boys over girls. Dietary data collected in 2009 on 52 children aged 4–16 years support these observations as statistical analyses revealed no significant sex-based difference in energy (t = 1.2; p = 0.23) or protein (t = 1.1; p = 0.29) adequacy (un-published). In addition, Piperata (2007) found no difference in average height-for-age z-scores or rates of stunting between male and female children and, in a longitudinal study, Piperata et al. (2011a) found no difference in the rates of catch-up growth between males and females during the childhood or juvenile stages. Taken together these data provide little support for the preferential treatment of males in these communities, at least during the period of most rapid growth.
While there is no evidence for the preferential treatment of male children, there are two local practices or norms that could potentially induce sex-related differences in access to resources, specifically food, during adolescence that may explain the higher rates of stunting and proportionally shorter legs found among adult females. First, direct observations and interviews with mothers revealed a particular concern with the dietary intakes of their older, adolescent, sons. As mothers saw it, adolescent and adult males required more food than other members of the household due to the energy they expended in subsistence work including manioc cultivation, fishing and hunting. Adolescent boys, along with their fathers, were often served first at mealtimes and food was often set-aside for them if they were absent from the household when a meal was served. Even though this practice did not appear to compromise the dietary intakes of other children in the household, it is possible that adolescent males benefitted from improved nutrition and that this, in turn, allowed for catch-up growth during adolescence.
Second, adolescent males exhibited much greater mobility than their female counterparts and only males, including adolescents, participated in wage labor jobs. Houses in most communities were strung along the river’s edge and were anywhere between a five and 30 minute canoe ride from one another. It was far more common to see males traveling to other households than females and local custom dictated that visitors be offered food. While a highly sugared cup of coffee was the most common offering, a visitor that arrived during a meal was almost always invited to eat. Those employed in wage labor (e.g., small-scale timber operations, scientific field station) were fed by their employers and these meals could be substantial such as large servings of rice and beans, along with farinha and tinned, fatty meats. Thus, both of these activities may have provided males the opportunity to secure resources outside of their own households, options unavailable to adolescent females.
In conclusion, even though male and female Ribeirinhos appear to be exposed to the same environmental conditions during infancy and childhood, potentially better access to resources for males during adolescence may explain some of the differences in body proportions observed in this study. Specifically, improved nutrition in adolescence may support catch-up growth in males that exhibited relatively longer legs than their female counterparts.
Female skeletal growth and the costs of reproduction
An additional factor that may have contributed to the higher rates of stunting and sex differences in relative trunk/limb proportions observed among adults in this population is the energetic stress of reproduction on females during the period of growth and development.
Several studies (Scholl et al., 1993; Scholl et al., 1994; Casanueva et al., 2006; Rah et al., 2008) have reported that early pregnancy can conflict with fetal growth. In these instances, the mother’s body may divert nutrients for its own use, including growth, leading to adverse birth outcomes (Frisancho et al., 1983; Scholl et al., 1994; Wallace et al., 2001; Duvan et al., 2010). Fewer studies have addressed the impact that early reproduction may have on maternal growth, especially under sub-optimal environmental conditions. Recent studies conducted among Mexican and Bangladeshi adolescents suggest that, in poorer settings, pregnancy during adolescence may lead to maternal growth cessation (Casanueva et al., 2006; Rah et al., 2008). However, it is important to note that both studies were conducted over a relatively brief period during the postpartum (1–6 months). Thus, it remains unclear if maternal growth resumed at some later point. Here, we adopted a different approach and explored the association between the final outcome of linear growth and reproductive history parameters such as age at menarche, age at first birth and parity.
It has been argued that age at menarche, reflecting sexual maturation, may show an association with estrogen-induced epiphyseal closure and consequently with growth cessation (Porcu et al, 1994). We found no significant correlation between age at menarche and anthropometric variables. However, this lack of relationship should be considered with caution as older women may not have been able to accurately recall their age at menarche and instead may have reported values similar to those of the younger women (daughters and granddaughters) around them who experienced the event more recently.
The positive correlation between age at first birth and total leg length suggests that early reproduction had a negative impact on the mother’s own skeletal growth. Even though correlation does not imply causation, it seems reasonable to attribute the observed relatively shorter lower limb in females to nutritional costs associated with pregnancy and lactation. The data collected indicate a declining age at firth birth among females in this population (see Fig. 6) and thus the increased occurrence of pregnancy prior to the completion of skeletal growth. Data collected between 2002 and 2009 indicate that adolescents in these communities become sexually active early in their teens (14–15 years) and, with limited access to contraception, teen pregnancy was common.
A detailed, longitudinal study of maternal dietary intake, energy expenditure and mobilization of body stores during lactation conducted in these same communities found that while women’s energy and protein intakes came close to meeting their normal daily energy needs, they were insufficient for meeting the additional energy demands of reproduction, especially lactation, forcing women to rely on their own energy reserves (Piperata and Dufour, 2007). Therefore, it is conceivable that women reproducing at a time when their own growth was not complete experienced greater nutritional stress compared to their adolescent male counterparts.
Additional evidence to support this idea comes from comparisons of the rates of catch-up growth among male and female adolescents in this population. While Piperata et al. (2011a) found no difference in the rates of catch-up growth among children and juveniles, marked differences were found among adolescents. Ninety percent of adolescent males compared with only 8% of adolescent females exhibited signs of catch-up growth. Reproductive histories revealed that most of the adolescent girls had been pregnant, at least once, during the study period leading Piperata and colleagues to suggest that the sex-related difference in catch-up growth during adolescence was likely attributable to the costs of pregnancy and lactation on the growing female body.
Lastly, we explored whether multiple pregnancies may be associated with more severe female growth retardation than a single pregnancy. To this end, we analyzed the correlation between anthropometric variables and parity (controlling for age), as well as between anthropometric variables and inter-birth interval between first and second birth, controlling for age at first birth. In both cases, no significant correlations were found. Our results suggest that the nutritional costs (and possibly the increased estrogen levels) associated with pregnancy and lactation, occurring concomitantly with skeletal growth, may inhibit any further gains in height.
It is interesting to note that, in our sample, growth retardation associated with early reproduction was limited to leg length, while sitting height was not affected. It has been argued that, based on growth heterochrony, different timing and duration of growth insults may result in altered adult body proportions (Bass et al., 1999; Bradney et al., 2000). Specifically, negative growth outcomes are expected to be greater for those body segments that are growing faster and/or are further from growth completion at the time of the insult. There is evidence indicating that the appendicular skeleton grows faster than the trunk during childhood, while the trunk growth velocity increases at the onset of puberty (Bass et al., 1999; Bradney et al., 2000). On this basis, one would expect stress experienced later in growth – such as adolescent pregnancy and lactation – to have a greater impact on the axial rather than the appendicular skeleton. However, our findings do not match expectations.
This discrepancy may be explained in terms of developmental plasticity and growth canalization. First, while the models reported above may be valid for individuals from affluent societies, they may not be applicable to individuals chronically exposed to environmental stress, such as those included in our study. It is possible that the human body responds to a more stressful environment by plastically adjusting growth rates and duration, as has been observed in other species (Bateson et al., 2004). This hypothesis finds support in the delayed maturation patterns observed in several populations from developing countries (Bogin, 1999). Therefore, the negative impact of reproduction on leg length may be due to the fact that, unlike their peers living in more affluent settings, adolescent mothers in the developing world are farther from having reached adult leg length. Second, regardless of the tempo of growth of different body segments, it is possible that sitting height may be more canalized, hence less variable, than leg length. Specifically, sitting height may be more constrained in size in relation to the size of the vital organs located in the chest and abdomen. Given the increased spatial demand in the torso associated with pregnancy, it seems reasonable to assume that leg length may be preferentially sacrificed.
To further examine this interpretation, we explored whether, in our sample, trunk length was more canalized than leg length by using an F-test. The results indicate that the trunk is significantly more canalized than the leg among females (F = 1.955; p = 0.00008), but not among males (F = 1.988; p = 0.09). This evidence suggests that, at least in our sample, a greater canalization of trunk length may be associated with the specific demands of female reproduction. Our study highlights the potential magnitude of reproduction’s impact on the final outcome of growth, but does not provide insights on the specific causes and mechanisms of reproduction-related growth retardation. Future research focused on early pregnancy and maternal growth may enlighten the relationship between somatic growth and specific environmental conditions.
These results highlight the difficulties intrinsic in testing the hypothesis of greater male environmental susceptibility. In fact, while sex-related differences in environmental susceptibility may exist, they are not easily tested because the environmental conditions experienced by males and females after birth may differ at different stages of development, even in populations that do not openly privilege children of either sex. Based on our observations, this seems to be the case among the Ribeirinhos included in this study. Consequently, the hypothesis of greater male susceptibility can find no support or refutation in our analysis.
Insights for studies of human growth in the past
The study of human growth in the past is a central component of bioarchaeological investigation (Hoppa and Fitzgerald, 1999; Wright and Yoder, 2003). In fact, bioarchaeologists commonly rely on skeletal growth to gain important insights on life conditions and health in past populations (Saunders and Hoppa, 1993; Larsen, 1997; Pietrusewsky et al., 1997; Saunders, 2008; Maat, 2005). Exemplary cases of height variation over time and in relation to major population events (subsistence shifts, population contact, climate change) have been reported and demonstrated the value of using skeletal growth in reconstructing life in the past (Cohen and Armelagos, 1984; Larsen, 1994, 1995; Gerhards, 2005; Formicola and Holt, 2007; Gustafsson et al, 2007). Nonetheless, the relationship between early life conditions and growth in the past is often equivocal (Larsen, 1997; Temple, 2008; Klaus and Tam, 2009). Indeed, interpretation of growth in the past can be complicated by the difficulties intrinsic in reconstructing a population’s environment.
The present study underscores the importance of considering biocultural factors when studying human growth, especially when intra-group variation is examined. In this study, information on reproductive history and cultural practices in food distribution was crucial in interpreting and understanding the pattern of biological variation observed. While such information is likely unavailable when studying growth in past populations, it is nonetheless important to take into consideration the possible role of a number of biocultural variables on the final outcome of growth. Social status differences, belief systems, cultural practices privileging offspring of a certain sex, and reproductive customs – among a large number of other factors – should be investigated as much as possible, because they can clearly have a notable impact on body size and proportions.
In conclusion, we argue that studies on the living can provide important insights for our understanding of life in the past. As bioarchaeology becomes increasingly multidisciplinary (Larsen, 2006), it is important that perspectives and approaches typical of human biology are adopted and applied to the study of growth in the past.
CONCLUSIONS
This study examined intra-population variation in body proportions among rural Amazonian adults in relation to a number of biocultural variables. Our results indicated that:
Stunting in this population is not accompanied by significant changes in body proportions. Significant correlations are found between the leg length index and increased body fatness, even though they may be associated with different underlying causes, including differential opportunities for catch-up growth for males and females and to the costs of female reproduction.
Males and females in this population exhibited significantly different body proportions. Specifically, females expressed overall more compromised growth outcomes. This result contrasts with the hypothesis of greater male susceptibility, which postulates that males would exhibit greater responses to environmental stress. The availability of rich biocultural data on this population allowed us to explain this unexpected finding as due to greater stress experienced by females in association with reproduction. Therefore, our analysis does not provide support or refutation for the hypothesis of greater male susceptibility. Rather, it highlights the difficulties intrinsic in testing this hypothesis when biological and cultural factors interplay with allegedly comparable stress experienced by individuals of different sexes.
Among all reproductive history parameters, age-at-first-birth was significantly correlated with terminal height and leg length. This result suggests that early reproduction may induce the cessation of growth in female adolescents, at least in underprivileged settings. While early pregnancy has generally been associated with poor birth outcomes, to our knowledge this is the first study that provides evidence for negative maternal growth outcomes in relation to early reproduction. Future research focused on early pregnancy and maternal growth may enlighten the relationship between somatic growth and specific environmental conditions.
The use of biocultural data is extremely useful in understanding and interpreting biological variation at the intra-population level and – whenever possible – should be incorporated in the study of both living and past populations.
Acknowledgments
Grant Sponsor: Wenner-Gren Foundation for Anthropological Research Inc. (Grant # 6861), National Science Foundation (Grant # BCS 0201936)
We are grateful to the people in the seven communities, who took the time to take part in this research. We also thank our Brazilian colleagues Dr. Rui Murrieta, Dr. Walter Neves, Dr. Peter Mann de Toledo and Dr. Ima Guimarães for the institutional support that made this work possible. Furthermore, we thank Paul Sciulli for his statistical advice and Clark Spencer Larsen for his helpful comments. We are also grateful to the Editor-in-Chief and three anonymous reviewers for their insightful comments and suggestions for revision, which notably improved the quality of the manuscript.
LITERATURE CITED
- Allen LH. Nutritional influences on linear growth: a general review. Eur J Clin Nutr. 1994;48:S75–S89. [PubMed] [Google Scholar]
- Auerbach BM, Sylvester AD. Allometry and apparent paradoxes in human limb proportions: implications for scaling factors. Am J Phys Anthropol. 2011;144:382–391. doi: 10.1002/ajpa.21418. [DOI] [PubMed] [Google Scholar]
- Bass S, Delmas PD, Pearce G, Hendrich E, Tabensky A, Seeman E. The differing tempo of growth in bone size, mass, and density in girls is region-specific. J Clin Invest. 1999;104:795–804. doi: 10.1172/JCI7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Mirazón Lahr M, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Boas F. Changes in the bodily form of descendants of immigrants. Am Anthropol. 1912;14:530–562. [Google Scholar]
- Bogin B, Smith P, Orden A, Varela Silva M, Loucky J. Rapid change in height and body proportions of Maya American children. Am J Hum Biol. 2002;14:753–761. doi: 10.1002/ajhb.10092. [DOI] [PubMed] [Google Scholar]
- Bogin B. Patterns of human growth. 2. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Bradney M, Karlsson MK, Duan Y, Stuckey S, Bass S, Seeman E. Heterogeneity in the growth of the axial and appendicular skeleton in boys: implications for the pathogenesis of bone fragility in men. J Bone Miner Res. 2000;15:1871–1878. doi: 10.1359/jbmr.2000.15.10.1871. [DOI] [PubMed] [Google Scholar]
- Cameron N, Preece MA, Cole TJ. Catch up growth or regression to the mean? Recovery from stunting revisited. Am J Hum Biol. 2005;17:412–417. doi: 10.1002/ajhb.20408. [DOI] [PubMed] [Google Scholar]
- Casanueva E, Roselló-Soberón ME, De-Regil LM, del Carmen Arguelles M, Céspedes MI. Adolescents with adequate birth weight newborns diminish energy expenditure and cease growth. J Nutr. 2006;136:2498–2501. doi: 10.1093/jn/136.10.2498. [DOI] [PubMed] [Google Scholar]
- Chandler PJ, Bock RD. Age changes in adult stature: trend estimation from mixed longitudinal data. Ann Hum Biol. 1991;18:433–440. doi: 10.1080/03014469100001732. [DOI] [PubMed] [Google Scholar]
- Cline MG, Meredith KE, Boyer JT, Burrows B. Decline of height with age in adults in a general population sample: estimating maximum height and distinguishing birth cohort effects from actual loss of stature with aging. Hum Biol. 1989;61:415–425. [PubMed] [Google Scholar]
- Cohen MN, Armelagos GJ. Paleopathology at the origins of agriculture: Editors’ summation. In: Cohen MN, Armelagos GJ, editors. Paleopathology at the origins of agriculture. Orlando: Academic Press; 1984. pp. 585–601. [Google Scholar]
- Costa ML, Kern DC, von Behling H, Borges MS. Geologia. In: Lisboa PLB, editor. Caxiuanã, Populacções Tradicionais, Meio Físico & Diversidade Biológica. Belém: Museu Paraense Emílio Goeldi; 2002. pp. 179–206. [Google Scholar]
- Delgado HL, Valverde V, Belizan JM, Klein RE. Diarrheal disease, nutritional status and health care: analysis of their inter-relationships. Ecol Food Nutr. 1982;12:229–234. doi: 10.1080/03670244.1983.9990720. [DOI] [PubMed] [Google Scholar]
- Dewey KG. The impact of agricultural development on child nutrition in Tabasco, Mexico. Med Anthropol. 1980;4:21–54. [Google Scholar]
- Dewey Nutrition survey in Tabasco, Mexico: nutritional status of preschool children. Am J Clin Nutr. 1983;37:1010–1019. doi: 10.1093/ajcn/37.6.1010. [DOI] [PubMed] [Google Scholar]
- Dufour DL. Biocultural approaches in human biology. Am J Hum Biol. 2006;18:1–9. doi: 10.1002/ajhb.20463. [DOI] [PubMed] [Google Scholar]
- Durnin JVGA, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- Duvan CI, Turhan NÖ, Onaran Y, Gümüs, Yuvaci H, Gözdemir E. Adolescent pregnancies: maternal and fetal outcomes. New J Med. 2010;27:113–116. [Google Scholar]
- Emerson PM, Souza AP. Child labor, school attendance and intrahousehold gender bias in Brazil. World Bank Econ Rev. 2007;21:301–316. [Google Scholar]
- Eveleth PB, Tanner JM. Worldwide variation in human growth. 2. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- Formicola V, Giannecchini M. Evolutionary trends of stature in Upper Paleolithic and Mesolithic Europe. J Hum Evol. 1999;36:319–333. doi: 10.1006/jhev.1998.0270. [DOI] [PubMed] [Google Scholar]
- Formicola V, Holt BM. Resource availability and stature decrease in Upper Palaeolithic Europe. J Anthropol Sci. 2007;85:147–155. [Google Scholar]
- Friedman DS, Kettel Kahn L, Serdula MK, Srinivasan SR, Berenson GS. Secular trends in height among children during 2 decades. Arch Pediatr Adolesc Med. 2000;154:155–161. doi: 10.1001/archpedi.154.2.155. [DOI] [PubMed] [Google Scholar]
- Frisancho AR, Matos J, Flegel P. Maternal nutritional status and adolescent pregnancy outcome. Am J Clin Nutr. 1983;38:739–746. doi: 10.1093/ajcn/38.5.739. [DOI] [PubMed] [Google Scholar]
- Frisancho AR. Relative leg length as a marker to track developmental history of individuals and populations: growth delay and increased body fat. Am J Hum Biol. 2007;19:703–710. doi: 10.1002/ajhb.20676. [DOI] [PubMed] [Google Scholar]
- Frisancho AR. Anthropometric standards: an interactive nutritional reference of body size and body composition for children and adults. Ann Arbor: The University of Michigan Press; 2008. [Google Scholar]
- Frongillo EA, Bégn F. Gender bias in food intake favors male preschool Guatemalan children. J Nutr. 1993;123:89–96. doi: 10.1093/jn/123.2.189. [DOI] [PubMed] [Google Scholar]
- Gafni RI, Baron J. Catch-up growth: possible mechanisms. Pediatr Nephrol. 2000;14:616–619. doi: 10.1007/s004670000338. [DOI] [PubMed] [Google Scholar]
- Galloway A. Estimating actual height in the older individual. J Forensic Sci. 1988;33:126–136. [PubMed] [Google Scholar]
- Garn SM. The secular trend in size and maturational timing and its implications for nutritional assessment. J Nutr. 1987;117:817–823. doi: 10.1093/jn/117.5.817. [DOI] [PubMed] [Google Scholar]
- Gerhards G. Secular variation in the body stature of the inhabitants of Latvia (7th millennium BC – 20th c. AD) Acta Med Lituanica. 2005;12:33–39. [Google Scholar]
- Giannecchini M, Moggi-Cecchi J. Stature in archeological samples from Central Italy: methodological issues and diachronic changes. Am J Phys Anthropol. 2008;135:284–292. doi: 10.1002/ajpa.20742. [DOI] [PubMed] [Google Scholar]
- Giles E. Corrections for age in estimating older adults’ stature from long bones. J Forensic Sci. 1991;36:898–901. [PubMed] [Google Scholar]
- Giugliano R, Giugliano L, Shrimpton R. Estudos nutricionais das populações rurais da Amazônia 1-Várzea do Rio Solimões. Acta Amazônica. 1981;11:773–788. [Google Scholar]
- Giugliano R, Shrimpton R, Marinho HA, Giugliano LG. Estudos nutricionais das populações rurais da Amazônia. II. Rio Negro. Acta Amazônica. 1984;14:427–449. [Google Scholar]
- Golden JSR. Is complete catch-up possible for stunted malnourished children? Eur J Clin Nutr. 1994;48:S58–S70. [PubMed] [Google Scholar]
- Goodman AH, Thomas RB, Swedlund AC, Armelagos GJ. Biocultural perspectives on stress in prehistoric, historical, and contemporary population research. Yrbk Phys Anthropol. 1988;31:169–202. [Google Scholar]
- Goodman AH. On the interpretation of health from skeletal remains. Curr Anthropol. 1993;34:281–288. [Google Scholar]
- Gustafsson A, Weredelin L, Tullberg BS, Lindenfors P. Stature and sexual dimorphism in Sweden, from the 10th to the end of the 20th century. Am J Hum Biol. 2007;19:861–870. doi: 10.1002/ajhb.20657. [DOI] [PubMed] [Google Scholar]
- Harris M. What it means to be Caboclo: some critical notes on the construction of Amazonian Caboclo society as an anthropological object. Critique Anthropol. 1998;18:83–95. [Google Scholar]
- Hoffman DJ, Sawaya AL, Verreschi I, Tucker KL, Roberts SB. Why are nutritionally stunted children at increased risk of obesity? Studies of metabolic rate and fat oxidation in shantytown children from Sao Paulo, Brazil. Am J Clin Nutr. 2003;72:702–707. doi: 10.1093/ajcn/72.3.702. [DOI] [PubMed] [Google Scholar]
- Holliday TW. Body proportions in Late Pleistocene Europe and modern human origins. J Hum Evol. 1997;32:423–448. doi: 10.1006/jhev.1996.0111. [DOI] [PubMed] [Google Scholar]
- Hoppa RD, Fitzgerald CM. From head to toe: integrating studies from bones and teeth in biological anthropology. In: Hoppa RD, Fitzgerald CM, editors. Human growth in the past. Studies from bones and teeth. Cambridge Stud Biol Evol Anthropol. Vol. 25. Cambridge: Cambridge University Press; 1999. pp. 1–31. [Google Scholar]
- Humphrey LT. Growth patterns in the modern human skeleton. Am J Phys Anthropol. 1998;105:57–72. doi: 10.1002/(SICI)1096-8644(199801)105:1<57::AID-AJPA6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Jantz LM, Jantz RL. Secular change in long bone length and proportion in the United States, 1800–1970. Am J Phys Anthropol. 1999;110:57–67. doi: 10.1002/(SICI)1096-8644(199909)110:1<57::AID-AJPA5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Karlberg J. A biologically-oriented mathematical model (ICP) for human growth. Acta Paediatr Scand, Suppl. 1989;350:70–94. doi: 10.1111/j.1651-2227.1989.tb11199.x. [DOI] [PubMed] [Google Scholar]
- Katzmarzyk PT, Leonard WR. Climatic influences on human body size and proportions: ecological adaptations and secular trends. Am J Phys Anthropol. 1998;106:483–503. doi: 10.1002/(SICI)1096-8644(199808)106:4<483::AID-AJPA4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Klaus HD, Tam ME. Contact in the Andes: bioarchaeology of systemic stress in colonial Mórrope, Peru. Am J Phys Anthropol. 2009;138:356–368. doi: 10.1002/ajpa.20944. [DOI] [PubMed] [Google Scholar]
- Larme AC. Healthcare allocation and selective neglect in rural Peru. Soc Sci Med. 1997;44:1711–1723. doi: 10.1016/s0277-9536(96)00373-5. [DOI] [PubMed] [Google Scholar]
- Larsen CS. In the wake of Columbus: postcontact native population biology of the Americas. Yrbk Phys Anthropol. 1994;37:109–154. [Google Scholar]
- Larsen CS. Biological changes in human populations with agriculture. Ann Rev Anthropol. 1995;24:185–213. [Google Scholar]
- Larsen CS. Bioarchaeology: interpreting behavior from the human skeleton. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Larsen CS. The changing face of bioarchaeology: an interdisciplinary science. In: Buikstra JE, Beck LA, editors. Bioarchaeology: the contextual analysis of human remains. Amsterdam; Boston: Academic Press; 2006. pp. 359–374. [Google Scholar]
- Lasker GW. Environmental growth factors and selective migration. Hum Biol. 1952;24:262–289. [PubMed] [Google Scholar]
- Leonard WR, Katzmarzyk PT. Body size and shape: climatic and nutritional influences on human body morphology. In: Muehlenbein MP, editor. Human evolutionary biology. Cambridge: Cambridge University Press; 2010. pp. 157–169. [Google Scholar]
- Leonard WR, Sorensen M, Mosher MJ, Spitsyn V, Comuzzie AG. Reduced fat oxidation and obesity risks among the Buryat of southern Siberia. Am J Hum Biol. 2009;21:664–670. doi: 10.1002/ajhb.20903. [DOI] [PubMed] [Google Scholar]
- Lisboa PLB. Caxiuanã: Populações tradicionais, meio físico and diversidade biológica. Belém: Museu Paraense Emílio Goeldi; 2002. [Google Scholar]
- Loesch DZ, Stokes K, Huggins RM. Secular trend in body height and weight of Australian children and adolescents. Am J Phys Anthropol. 2000;111:545–556. doi: 10.1002/(SICI)1096-8644(200004)111:4<545::AID-AJPA9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- Maat GJR. Two millennia of male stature development and population health and wealth in the Low Countries. Int J Osteoarchaeol. 2005;15:276–290. [Google Scholar]
- Maie T, Schoenfuss HL, Blob RW. Ontogenetic scaling of body proportions in waterfall-climbing gobiid fishes from Hawai’i and Dominica: implications for locomotor function. Copeia. 2007;3:755–764. [Google Scholar]
- Martorell R, Khan LK, Schroeder DG. Reversibility of stunting: epidemiologic findings in children from developing countries. Eur J Clin Nutr. 1994;48:S45–S57. [PubMed] [Google Scholar]
- Martorell R. Body size, adaptation and function. Hum Org. 1989;48:15–20. [Google Scholar]
- Mascie-Taylor CGN, Lasker GW. Biosocial correlates of stature in a British national cohort. J Biosoc Sci. 2005;37:245–251. doi: 10.1017/s0021932004006558. [DOI] [PubMed] [Google Scholar]
- Meredith HV. Findings from Asia, Australia, Europe, and North America on secular change in mean height of children, youths, and young adults. Am J Phys Anthropol. 1976;44:315–326. doi: 10.1002/ajpa.1330440214. [DOI] [PubMed] [Google Scholar]
- Moran E. Human adaptive strategies in Amazonian blackwater ecosystems. Am Anthropol. 1993;93:361–382. [Google Scholar]
- Murrieta RSS, Bakri MS, Adams C, Oliveira PSS, Strumpf R. Consumo alimentar e ecologia de populações ribeirinhas em dois ecossistemas amazônicos: um estudo comparativo. Rev Nutr Campinas. 2008;21:123S–133S. [Google Scholar]
- Nyati LH, Norris SA, Cameron N, Pettifor JM. Effect of ethnicity and sex on the growth of the axial and appendicular skeleton of children living in a developing country. Am J Phys Anthropol. 2006;130:135–141. doi: 10.1002/ajpa.20318. [DOI] [PubMed] [Google Scholar]
- Pietrusewsky M, Douglas MT, Ikehara-Quebral RM. Assessment of health and disease in the prehistoric inhabitants of the Mariana Islands. Am J Phys Anthropol. 1997;104:315–342. doi: 10.1002/(SICI)1096-8644(199711)104:3<315::AID-AJPA4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Piperata BA, Spence JE, Da Gloria P, Hubbe M. The nutrition transition in Amazonia: Rapid economic change and its impact on growth and development in Ribeirinhos. Am J Phys Anthropol. 2011a doi: 10.1002/ajpa.21459. [DOI] [PubMed] [Google Scholar]
- Piperata BA, Ivanova SA, Da-Gloria P, Veiga G, Polsky A, Spence JE, Murrieta RSS. Nutrition in transition: Dietary patterns of Amazonian women during a period of economic change. Am J Hum Biol. 2011b;23:458–469. doi: 10.1002/ajhb.21147. [DOI] [PubMed] [Google Scholar]
- Piperata BA. Nutritional status of Ribeirinhos in Brazil and the nutrition transition. Am J Phys Anthropol. 2007;133:868–878. doi: 10.1002/ajpa.20579. [DOI] [PubMed] [Google Scholar]
- Piperata BA, Dufour DL. Diet, energy expenditure, and body composition of lactating Ribeirinha women in the Brazilian Amazon. Am J Hum Biol. 2007;19:722–734. doi: 10.1002/ajhb.20628. [DOI] [PubMed] [Google Scholar]
- Porcu E, Venturoli S, Fabbri R, Paradisi R, Longhi M, Sganga E, Flamigni C. Skeletal maturation and hormonal levels after the menarche. Arch Gynecol Obstet. 1994;255:43–46. doi: 10.1007/BF02390674. [DOI] [PubMed] [Google Scholar]
- Rah JE, Christian P, Shamim AA, Arju UT, Labrique AB, Rashid M. Pregnancy and lactation hinder growth and nutritional status of adolescent girls in rural Bangladesh. J Nutr. 2008;138:1505–1511. doi: 10.1093/jn/138.8.1505. [DOI] [PubMed] [Google Scholar]
- Riley AP, Huffman SL, Chowdury AKM. Age at menarche and postmenarcheal growth in rural Bangladeshi females. Ann Hum Biol. 1989;16:347–359. doi: 10.1080/03014468900000472. [DOI] [PubMed] [Google Scholar]
- Roche AF, Davila GH. Late adolescent growth in stature. Paediatrics. 1972;50:874–880. [PubMed] [Google Scholar]
- Rona RJ, Chinn S. Father’s unemployment and height of primary school children in Britain. Ann Hum Biol. 1991;18:441–448. doi: 10.1080/03014469100001742. [DOI] [PubMed] [Google Scholar]
- Saunders SR, Hoppa RD. Growth deficit in survivors and non-survivors: biological mortality bias in subadult skeletal samples. Yrbk Phys Anthropol. 1993;36:127–151. [Google Scholar]
- Saunders SR. Juvenile skeletons and growth-related studies. In: Katzenberg AM, Saunders SR, editors. Biological anthropology of the human skeleton. 2. New York: Wiley-Liss, Inc; 2008. pp. 117–147. [Google Scholar]
- Sawaya AL, Martina PA, Grillo LP, Florencio TT. Long-term effects of early malnutrition on body weight regulation. Nutr Rev. 2004;62:S127–S133. doi: 10.1111/j.1753-4887.2004.tb00082.x. [DOI] [PubMed] [Google Scholar]
- Scholl TO, Hediger ML, Cronk CE, Schall JI. Maternal growth during pregnancy and lactation. Horm Res. 1993;39(Suppl 3):59–67. doi: 10.1159/000182785. [DOI] [PubMed] [Google Scholar]
- Scholl TO, Hediger ML, Schall JI, Khoo CS, Fischer RL. Maternal growth during pregnancy and the competition for nutrients. Am J Clin Nutr. 1994;60:183–188. doi: 10.1093/ajcn/60.2.183. [DOI] [PubMed] [Google Scholar]
- Silva HP, Crews DE. Ecology of children’s growth: an example from transitional populations of the Brazilian Amazon. Int J Anthropol. 2006;21:97–109. [Google Scholar]
- Smith RJ. Use and misuse of the reduced major axis for line-fitting. Am J Phys Anthropol. 2009;140:476–486. doi: 10.1002/ajpa.21090. [DOI] [PubMed] [Google Scholar]
- Steckel RH. New Light on the “Dark Ages.” The remarkably tall stature of Northern European men during the medieval era. Soc Sci Hist. 2004;28:211–29. [Google Scholar]
- Stini AW. Nutritional stress and growth: sex difference in adaptive response. Am J Phys Anthropol. 1969;31:417–426. doi: 10.1002/ajpa.1330310316. [DOI] [PubMed] [Google Scholar]
- Stinson S. Sex differences in environmental sensitivity during growth and development. Yrbk Phys Anthropol. 1985;28:123–147. [Google Scholar]
- Stinson S. Variation in body size and shape among South American Indians. Am J Hum Biol. 1990;2:37–51. doi: 10.1002/ajhb.1310020105. [DOI] [PubMed] [Google Scholar]
- Sultan SE, Stearns SC. Environmentally contingent variation: phenotypic plasticity and norms of reaction. In: Hallgrìmsson B, Hall BK, editors. Variation. Amsterdam; Boston: Elsevier; 2005. pp. 303–332. [Google Scholar]
- Sylvester AD, Kramer PA, Jungers WL. Humans are not (quite) isometric. Am J Phys Anthropol. 2008;137:371–383. doi: 10.1002/ajpa.20880. [DOI] [PubMed] [Google Scholar]
- Swartz SM. Allometric patterning in the limb skeleton of bats: implications for the mechanics and energetics of powered flight. J Morphol. 1997;234:277–294. doi: 10.1002/(SICI)1097-4687(199712)234:3<277::AID-JMOR6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Growth as a measure of the nutritional and hygienic status of a population. Horm Res. 1992;38(Suppl 1):106–115. doi: 10.1159/000182580. [DOI] [PubMed] [Google Scholar]
- Tanner JM, Hayashi T, Preece MA, Cameron N. Increase in length of leg relative to trunk in Japanese children and adults from 1957 to 1977: comparison with British and with Japanese Americans. Ann Hum Biol. 1982;9:411–423. doi: 10.1080/03014468200005951. [DOI] [PubMed] [Google Scholar]
- Temple D. What can variation in stature reveal about environmental differences between prehistoric Jomon foragers? Understanding the impact of systemic stress on developmental stability. Am J Hum Biol. 2008;20:431–439. doi: 10.1002/ajhb.20756. [DOI] [PubMed] [Google Scholar]
- Temple DH, Auerbach BM, Nakatsukasa M, Sciulli PW, Larsen CS. Variation in limb proportions between Jomon foragers and Yayoi agriculturalists from prehistoric Japan. Am J Phys Anthropol. 2008;137:164–174. doi: 10.1002/ajpa.20853. [DOI] [PubMed] [Google Scholar]
- Thomas D. Like father, like son: like mother, like daughter: parental resources and child height. J Hum Resour. 1994;29:950–988. [Google Scholar]
- Trotter M, Gleser GC. Estimation of stature from long limb bones of American Whites and Negroes. Am J Phys Anthropol. 1952;10:463–514. doi: 10.1002/ajpa.1330100407. [DOI] [PubMed] [Google Scholar]
- Trotter M, Gleser GC. The effect of ageing on stature. Am J Phys Anthropol. 1951;9:311–324. doi: 10.1002/ajpa.1330090307. [DOI] [PubMed] [Google Scholar]
- Tsutakawa RK, Hewett JE. Quick test for comparing two populations with bivariate data. Biometrics. 1977;33:215–219. [PubMed] [Google Scholar]
- Vilallpando S, Flores-Huerta S, Hernandez-Beltran M, Ramirez-Grande ME. Nutritional status of a nation-wide sample of rural Mexican populations. Rev Int Clin. 1992;44:21–30. [PubMed] [Google Scholar]
- Wadsworth ME, Hardy RJ, Paul AA, Marshall SF, Cole TJ. Leg and trunk length at 43 years in relation to childhood health, diet and family circumstances; evidence from the 1946 national birth cohort. Int J Epidemiol. 2002;31:383–90. [PubMed] [Google Scholar]
- Wallace J, Bourke D, Da Silva P, Aitken R. Nutrient partitioning during adolescent pregnancy. Reproduction. 2001;122:347–375. doi: 10.1530/rep.0.1220347. [DOI] [PubMed] [Google Scholar]
- Waterlow JC, Schürch B, editors. Causes and mechanisms of linear growth retardation. London: United Nation University Press; 1993. [Google Scholar]
- Weinstein KJ. Body proportions in ancient Andeans from high and low altitudes. Am J Phys Anthropol. 2005;128:569–585. doi: 10.1002/ajpa.20137. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Physical status: the use and interpretation of anthropometry. Geneva: World Health Organization; 1995. p. 452. [Google Scholar]
- Wright LE, Yoder CJ. Recent progress in bioarchaeology: approaches to the osteological paradox. J Archaeol Res. 2003;11:43–70. [Google Scholar]
- Zakrzewski SR. Variation in ancient Egyptian stature and body proportions. Am J Phys Anthropol. 121:219–229. doi: 10.1002/ajpa.10223. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]


