Abstract
Relations between changes in children's cognitive performance and changes in sleep problems were examined over a 3-year period, and family socioeconomic status, child race/ethnicity, and gender were assessed as moderators of these associations. Participants were 250 second- and third-grade (8–9 years old at Time 1) boys and girls. At each assessment, children's cognitive performance (Verbal Comprehension, Decision Speed) was measured using the Woodcock-Johnson III Tests of Cognitive Abilities, and sleep problems (Sleepiness, Sleep/Wake Problems) were collected via self-report. Individual growth models revealed that children who reported increases in Sleepiness exhibited little growth in Verbal Comprehension over time compared with their peers who reported decreases in Sleepiness, resulting in a nearly 11-point cognitive deficit by the end of the study. These associations were not found for Sleep/Wake Problems or Decision Speed. Child race/ethnicity and gender moderated these associations, with Sleepiness serving as a vulnerability factor for poor cognitive outcomes, especially among African American children and girls. Differences in cognitive performance for children with high and low Sleepiness trajectories ranged from 16 to 19 points for African American children and from 11 to 19 points for girls. Results build substantially on existing literature examining associations between sleep and cognitive functioning in children and are the first to demonstrate that children's sleep trajectories over 3 waves were associated with changes in their cognitive performance over time.
Keywords: cognitive performance, sleep problems, group differences, individual growth modeling
Partial sleep deprivation and problems initiating and maintaining sleep impact the lives of around 25% of normally developing children (Meltzer & Mindell, 2006). Furthermore, around 10% of elementary school children experience daytime sleepiness (Owens, Spirito, McGuinn, & Nobile, 2000). Approximately 21% of 7- to 10-year-olds and 19% of 11- and 12-year olds are tired during the day; 8% of the former age group and 7% of the latter fall asleep during the day (Stein, Mendelsohn, Obermeyer, Amromin, & Benca, 2001). Sleep problems have a profound effect on multiple aspects of children's cognition (Sadeh, 2007), including impaired attention, learning, and memory (Owens, 2009). Sleep has also been related to cognitive functioning and academic performance in children who have neither clinically significant sleep disorders nor cognitive impairments (e.g., Buckhalt, El-Sheikh, Keller, & Kelly, 2009).
The vast majority of sleep studies with children have been cross-sectional. Because sleep processes are dynamic, with rapid changes exhibited across childhood and adolescence, understanding how these processes change over time is a critical step in investigating associations between sleep and other outcomes. Furthermore, because the acquisition of knowledge and skills is a central developmental task in childhood and later knowledge acquisition is built upon what has previously been learned, any periodic failure to learn a basic skill may jeopardize subsequent achievement. Thus, understanding how changes in sleep relate to changes in children's cognitive functioning is critical for supporting optimal development.
Cross-Sectional Studies of Sleep and Cognition
Schutte (2009) conducted a meta-analysis of 35 studies that correlated sleep and cognitive/academic outcomes at one point in time among children without sleep disorders and found that more sleep problems were associated with poorer outcomes, although the overall effect size was somewhat small (Cohen's d = .20). In another meta-analysis of 17 studies, Dewald, Meijer, Oort, Kerkhof, and Bögels (2010) found that sleep quality, duration, and sleepiness all related independently and in expected directions to school performance. Sleepiness had the largest effect size (r = −.133) followed by sleep quality (r = .096) and sleep duration (r = .069); larger associations were found for studies that included younger children. In the few studies with samples large enough to test path models using structural equation modeling, similar significant single-order correlations have been found (El-Sheikh, Buckhalt, Cummings, Keller, & Acebo, 2007).
Experimental manipulation of sleep has also provided strong evidence that partial or total sleep deprivation degrades cognitive performance in adults, but such studies with children are scarce. Nevertheless, a few studies have shown that restricting sleep has a deleterious effect on children's cognitive processing (Fallone, Acebo, Seifer, & Carskadon, 2005; Randazzo, Muehlbach, Schweitzer, & Walsh, 1998). Conversely, extending sleep (1-hr-earlier bedtime) has been found to improve performance on multiple cognitive tasks when compared with mild sleep restriction (1-hr-later bedtime; Sadeh, Gruber, & Raviv, 2003).
Longitudinal Studies of Sleep and Cognition
Not much is known about longitudinal relations between sleep problems and children's cognitive functioning. To our knowledge, only two pertinent longitudinal studies have been conducted. Touchette and colleagues (2007) demonstrated relations between early mother report of nighttime sleep duration and later cognitive functioning; shorter sleep duration at age 2 predicted lower receptive vocabulary at age 6. In another study that followed children over a 2-year period, Buckhalt et al. (2009) discovered that poor sleep at age 8 was related to lower cognitive performance at age 10, controlling for autoregressive effects. Neither of these studies, however, examined how changes in sleep problems, marked by an initial level and a rate of change over time, were related to change trajectories in cognitive performance, which is the focus of the present study.
Socioeconomic Status, Ethnicity, and Sleep
In comparison to European Americans (EAs) and children from higher income backgrounds, multiple parameters of poor sleep have been observed in either low-income or African American (AA) children (e.g., Crosby, LeBourgeois, & Harsh, 2005). However, only a few studies have examined economic disadvantage or minority status as a moderator of relations between sleep and cognitive problems. In one study with an independent sample, socioeconomic status (SES; controlling for ethnicity) and ethnicity (controlling for SES) moderated the relation between sleep and cognitive performance; more optimal sleep was a protective factor against cognitive performance difficulties especially for either AA children or those from lower socioeconomic backgrounds (Buckhalt, El-Sheikh, & Keller, 2007). In a 2-year follow-up, the same moderation effect was found (Buckhalt et al., 2009). To our knowledge, this is the only study in which SES moderation effects over time have been investigated and suggests that the effect of SES on relations between sleep and cognitive performance may be enduring.
A conceptual framework for these relations has been explicated by Buckhalt (2011) and Buckhalt and colleagues (2009), who proposed that the effects of SES and race/ethnicity may be understood in terms of a health disparities hypothesis (Carter-Pokras & Baquet, 2002). The premise that sleep problems may cause disproportionate harm to economically disadvantaged or ethnic minority children is based on an aggregation of risk perspective and the fact that these individuals are likely exposed to more lifelong and concurrent stressors than their counterparts of higher economic stance or majority status (e.g., Evans & English, 2002).
Gender and Sleep
Mean comparisons on a number of sleep dimensions have suggested differences between boys and girls. A meta-analysis of 30 studies of children ages 9 to 18 in 23 countries showed that girls slept more than boys (Olds, Blunden, Petkov, & Forchino, 2010). However, this literature has yielded many inconsistent findings, with some studies reporting poorer sleep quality for girls (e.g., Meijer, Reitz, Dekovic, Van Den Wittenboer, & Stoel, 2010), while others report opposite findings (Buckhalt et al., 2007; El-Sheikh, Buckhalt, Mize, & Acebo, 2006). Furthermore, higher levels of and more frequent daytime sleepiness have been reported for girls (Gaina et al., 2007) and for boys (El-Sheikh, Kelly, Buckhalt, & Hinnant, 2010).
An important question, then, is whether differences in boys' and girls' sleep influence the relationship between sleep and other outcomes. A few longitudinal studies have examined gender as a moderator of the link between sleep and children's psychological adjustment; however, findings are inconsistent, with some reporting null effects (El-Sheikh et al., 2010) and others reporting nuanced interaction effects that vary across internalizing and externalizing symptoms (Meijer et al., 2010). Recent findings with the present sample indicate that changes in sleep problems over 3 years had a more negative effect on girls' depressive symptoms (El-Sheikh, Bub, Kelly, & Buckhalt, 2011). Since these differences have been found for adjustment, we examined gender as a moderator of the sleep–cognitive outcomes link.
The Current Study
The present study is the first to examine whether (a) changes in children's sleep problems predict changes in their cognitive performance over a 3- year period and (b) these relations differ by sociodemographic factors and gender. No study has considered simultaneously the associations between longitudinal trajectories in sleep problems and cognitive performance. Here, we utilized three waves of data gathered over a 3-year period to investigate developmental trajectories, marked by an intercept and a rate of change, of both sleep problems and cognitive performance over time. We treated these change indices (i.e., intercept and rate of change) of sleep problems as predictors of cognitive performance trajectories over the same time frame. Thus, we were able to gain a better understanding of the interaction between these two dynamic processes. Additionally, we explored within-individual differences in sleep problems over time (i.e., growth) rather than focusing on between-individual differences. Finally, we used an economically and ethnically diverse sample in an effort to disentangle the effects of family SES from those of race/ethnicity.
We expected that elevated sleep problems at intercept (initial level) and increasing sleep problems (slope) would predict diminished cognitive performance at intercept and slower increases in cognitive performance over time. Sleep problems are conceptualized along a continuum and are indicated by increased self-reported problems using two scales of a well-established instrument (Sleep Habits Survey; Wolfson & Carskadon, 1998), Sleepiness and Sleep/Wake Problems. These scales measure different but related aspects of sleep. Sleepiness is concerned with the consequences of sleep problems for daytime functioning, whereas Sleep/Wake Problems relate to behaviors associated with the process of going to bed and sleep, with sleeping itself, and with waking up. Dewald and colleagues (2010) conducted metaanalyses of relations between school performance and measures of sleep duration, quality, and sleepiness and concluded that these sleep parameters should be treated as separate constructs because they index different facets of sleep and have different associations with child functioning.
Because Sleepiness and Sleep/Wake Problems have been associated with negative child outcomes, we expected a similar pattern of effects for each index. Furthermore, because earlier work with independent samples found differences in associations between sleep and child outcomes by family SES and child ethnicity, we sought to extend the assessment of such effects using a new sample of children and three study waves, the first such assessment in the literature. On the basis of existing evidence (Buckhalt et al., 2007, 2009), we expected that relations between sleep problems and cognitive functioning would be more pronounced for AA children or those from lower economic backgrounds. Given the novelty of investigating gender as a moderator in this context, this examination was considered exploratory.
Method
Participants
The study consisted of three waves. At Time 1 (T1), participants were 128 girls and 123 boys in the second or third grade, along with their parents. Children were recruited from public school districts in a semirural community in the Southeastern United States, and exclusion criteria included having a diagnosis of attention-deficit/hyperactivity disorder, mental retardation, learning disability, or chronic physical illness. The majority of children (74%) lived with both biological parents; 26% lived in reconstituted families. Parents were married or cohabiting for an extended period. Families came from a wide range of economic backgrounds. The majority of families reported income levels between $20,000 and $35,000 at all three waves, although there was considerable variability among families within and across waves. Specifically, 4%–8% of families reported income levels < $10,000; 8%–12% between $10,000 and $12,000; 18%–24% between $20,000 and $35,000; 20%–22% between $35,000 and $50,000; 21%–22% between $50,000 and $75,000; and 15%–21% > $75,000. Mothers and fathers reported having at least some college education, on average, at T1 (45% of mothers and 41% of fathers), Time 2 (T2; 44% of mothers and 41% of fathers), and Time 3 (T3; 35% of mothers and 27% of fathers). The ethnic composition of the sample was similar to that of the community: 66% EA and 34% AA. Both EA and AA families were over-sampled across a wide socioeconomic range. Using pubertal status criteria developed by Petersen, Crockett, Richards, and Boxer (1988), 94% of children at T1 were classified by parents as prepubertal.
Attrition and missing data
Of the 251 children and their families originally enrolled in the study, 214 (106 boys) returned for T2 (85% retention rate; M = 12.84 months between T1 and T2, SD = 2.06 months). About one year later (T3), 194 children (92 boys) and their families returned for a third wave of data collection (91% retention rate from T2; M = 11.34 months between T2 and T3, SD = 1.62 months). In the case of divorce or separation, a custodial parent was invited to participate along with the child. Reasons for attrition included participants' hectic schedule, lack of interest, not responding to phone messages, and geographic relocation. Contingency table analyses and mean equivalence tests were performed to investigate differences between participants with complete data (n = 194) and nonparticipants (n = 57) on key demographic characteristics, including child age, race/ethnicity, gender, and pubertal status, as well as level of maternal education and family SES. Participating children and families did not differ significantly from the 57 families who were recruited but lost to follow-up on any of these factors.
Procedure
This study is part of a larger longitudinal investigation in which relations between family functioning and children's adjustment are examined and only pertinent procedures are mentioned. At each study wave, children and their parents visited our research laboratory. A research assistant administered cognitive tests to each child. Mothers and fathers completed questionnaires in separate rooms while a research assistant administered questionnaires to children. All measures were completed during each study wave, and families were compensated $200 at T1, $250 at T2, and $300 at T3 for their participation. This study was approved by the university's institutional review board.
Measures
Cognitive performance
Direct assessments of children's cognitive performance were collected using the Woodcock-Johnson Tests of Cognitive Abilities III (WJIII; Woodcock, McGrew, & Mather, 2001). The WJIII is a well-normed measure of cognitive abilities with demonstrated reliability and validity. For the current study, measures of two broad factors—Crystallized Intelligence (Gc) and Processing Speed (Gs)—were selected. Verbal Comprehension is the single best index of Gc, as it has a .90 factor loading for the 9- to 13-year-old age group in the WJIII standardization sample (Woodcock et al., 2001). It is comprised of four subtests: Picture Vocabulary, Synonyms, Antonyms, and Verbal Analogies, with split-half reliability coefficients of .88 to .90 across ages 8 to 12. Decision Speed is the best single index of the Gs factor, with a .72 factor loading for 9- to 13-year-olds and Rasch reliability coefficients of .84 to .86 for 8- to 12-year-olds (Woodcock et al., 2001). The Decision Speed test measures attentional focus and visual processing speed. Because we analyzed these measures longitudinally, we used vertically equated item response theory (IRT)-scaled scores (i.e., W scores), which essentially reflect an individuals' deviation from a criterion score and are thus equatable over time (Rasch, 1960).
These tests were chosen for their representation of two important, yet very different, aspects of cognitive functioning. Crystallized Intelligence is the strongest of all the factorial components of general intelligence and is among the most stable of ability factors. Over time, children tend to maintain their rank order in a group, although they may demonstrate considerable mean-level changes in their Gc. Processing Speed, on the other hand, is more likely to manifest day-to-day variations such as would be expected in conditions of mental fatigue or sleepiness. Although much research has associated sleep deficits in children with cognitive functioning in speeded tasks a short time afterward (Sadeh et al., 2003), only one longitudinal study has found that factors thought to be more enduring (e.g., verbal ability) may be related to sleep parameters over a longer period of time (Buckhalt et al., 2009).
Time
Time was represented by a single variable coded to reflect data collection at each assessment: T1, T2, and T3. We centered time at the first assessment so that the intercept in growth models would represent children's cognitive performance or sleep problems at T1.
Children's sleep
Children completed via interview the Sleep Habits Survey (SHS; Wolfson & Carskadon, 1998). The SHS is widely used and has demonstrated reliability and validity for school-age children (Acebo & Carskadon, 2002; Buckhalt et al., 2007, 2009). Two scales were used in analyses: Sleepiness (α = .70–.74 across waves) and Sleep/Wake Problems (α = .73–.83 across waves). The Sleepiness scale assesses whether children fell asleep or struggled to stay awake while performing daily activities within the past 2 weeks (e.g., attending a performance, in class, watching TV). The Sleep/Wake Problems scale assesses oversleeping, unscheduled sleep, irregular sleep times, and staying up late at night. Higher scores on both scales indicate more sleep problems.
Child characteristics
Information on child gender and race/ethnicity was based on mothers' reports. Child gender and ethnicity were represented by dichotomous variables (1 = boy, 0 = girl; 1 = AA, 0 = EA).
Socioeconomic status
We created separate composite variables at T1, T2, and T3 from family income and mother and father education. Maternal reports of total family income were gathered and classified into one of the following categories: (a) < 10,000; (b) $10,000 to $20,000; (c) $20,000 to $35,000; (d) $35,000 to $50,000; (e) $50,000 to $75,000; or (f) >$75,000. Mother's and father's years of education were also gathered. Following the recommendations of Braveman and colleagues (2005), the indicators were standardized and then summed to create separate composite variables representing overall SES at T1, T2, and T3.
Child age
Child age in months at each assessment was included to account for any age-related differences in cognitive functioning or sleep.
Puberty status
Ratings on the Puberty Development Scale (Petersen et al., 1988) are 1 (prepubertal), 2 (early pubertal), 3 (midpubertal), 4 (late pubertal), and 5 (postpubertal).
Results
Analysis Plan
Means, standard deviations, and intercorrelations among primary study variables were examined in preliminary analyses. Next, we fit unconditional growth models to examine whether children's cognitive performance or sleep problems changed over time. We represented change over time for each of the performance and sleep variables by linear growth and examined estimates of the population average initial level and population average rate of change. Next, for Sleepiness and Sleep/Wake Problems, we fit separate Level 1 growth models for each individual in the data set using ordinary least squares regression to generate an estimate of initial level and rate of change for every individual in the analytic sample (Willett, 1997). A total of four growth parameters were generated and retained for use in subsequent analyses (Singer & Willett, 2003).
Using these estimates as predictors in our subsequent analyses, we fit a taxonomy of growth models in which changes in children's cognitive performance were predicted by estimates of children's initial level and rate of change in sleep problems, controlling for family SES, child race/ethnicity, gender, age in months, and pubertal status. We predicted children's initial level of Verbal Comprehension (or Decision Speed) at T1 from their initial level of Sleepiness (or Sleep/Wake Problems) at T1; their rate of change in Verbal Comprehension (or Decision Speed) between T1 and T3 was predicted by both their initial level and rate of change in Sleepiness (or Sleep/Wake Problems). Finally, we added to the model interactions between estimates of children's initial level and rate of change in sleep problems and family SES, child race/ethnicity, and child gender. To illustrate the magnitude of these moderated associations, we plotted the fitted cognitive performance growth trajectories for prototypical children with low (1 SD below the mean) and high (1 SD above the mean) initial levels and rates of change in sleep problems.
All analyses were conducted in Stata Version 10. Model fit was assessed by comparing the within, between, and total R2 statistics across model specifications. Larger R2 statistics suggest more variation is explained and thus indicate a better fit. A multiple imputation (MI) procedure was used to impute data on key predictors and demographic control variables (Widaman, 2006). Estimates for each missing data point were derived from a population regression line and include a random error term for each individual. By generating numerous estimates, the MI procedure creates a set of plausible values for each missing data point based on other available information in the data set (Rubin, 1987). This set of values represents the uncertainty about which value is right to impute and allows the researcher to make statistically valid inferences. Parameter estimates presented below reflect the average associations across multiply imputed data sets and are based on a sample size of 250.
Preliminary Analyses
Sample descriptives for the outcome, key predictor, and control variables are presented in Table 1. Correlations among outcome and predictor variables are presented in Table 2. Verbal Comprehension and Decision Speed scores increased steadily between T1 and T3. Correlations suggest that differences among children in cognitive performance were relatively constant over time. Children's Sleepiness decreased somewhat across the study period, while their Sleep/Wake Problems increased between T1 and T2 and decreased between T2 and T3 (see Table 1). Correlations suggest modest between-individual stability in sleep problems across time.
Table 1. Descriptive Statistics for Outcome, Predictor, and Control Variables by Assessment (n = 250).
| Variable | Time 1 mean (SD) or % | Time 2 mean (SD) or % | Time 3 mean (SD) or % |
|---|---|---|---|
| Outcome variables | |||
| Verbal Comprehension | 486.26 (12.59) | 494.78 (11.88) | 499.62(11.42) |
| Decision Speed | 488.01 (15.87) | 502.08 (16.41) | 510.55 (16.86) |
| Predictor variables | |||
| Sleepiness | 15.67 (5.00) | 14.21 (4.73) | 13.66 (4.20) |
| Sleep/Wake Problems | 18.78 (6.02) | 19.79 (7.58) | 17.87 (6.01) |
| Moderators | |||
| Gender (boys) | 49.00% | 48.85% | 48.85% |
| Ethnicity (African American) | 35.46% | 35.94% | 35.94% |
| Total family income | 3.87 (1.46) | 3.89 (1.50) | 4.10(1.46) |
| Mother's education | 4.80 (0.94) | 4.83 (0.97) | 4.79 (1.06) |
| Father's education | 4.66(1.01) | 4.67 (1.04) | 4.64(1.00) |
| Socioeconomic status composite | -0.002 (2.45) | 0.012 (2.45) | 0.105 (2.33) |
| Control variables | |||
| Child age (in months) | 98.71 (8.64) | 111.70(9.46) | 123.30(11.99) |
| Puberty status | 1.39 (0.33) | 1.56(0.44) | 1.72(0.56) |
Table 2. Intercorrelations Among Outcome and Predictor Variables Across Times 1, 2, and 3 (n = 250).
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Verbal Comprehension T1 | — | |||||||||
| 2. Verbal Comprehension T2 | .77*** | — | ||||||||
| 3. Verbal Comprehension T3 | .72*** | .80*** | — | |||||||
| 4. Decision Speed T1 | .32*** | .27*** | .24** | — | ||||||
| 5. Decision Speed T2 | .25*** | .23** | .26*** | .63*** | — | |||||
| 6. Decision Speed T3 | .12 | .16* | .26*** | .60*** | .73*** | — | ||||
| 7. Sleepiness intercept | –.18** | –.19** | –.20** | –.09 | –.16* | –.06 | — | |||
| 8. Sleepiness slope | –.05 | –.07 | –.12 | .03 | .07 | .00 | –.63*** | — | ||
| 9. Sleep/Wake Problems intercept | .03 | .04 | .04 | –.09 | –.15* | –.12 | .39*** | –.21*** | — | |
| 10. Sleep/Wake Problems slope | .05 | –.01 | –.01 | .10 | .10 | .11 | –.06 | .29*** | –.41*** | — |
Note. T1 = data collected at Time 1; T2 = data collected at Time 2; T3 = data collected at Time 3.
p < .05.
p < .01.
p < .001.
Individual growth models indicated that children's cognitive performance and sleep problems changed significantly over time. The average Verbal Comprehension score at the start of the study was 486.53 (p < .001) and increased by approximately 7 points per assessment (π1i = 6.91, p < .001), while the average Decision Speed score at the start of the study was 488.72 (p < .001) and increased by more than 11 points per assessment (π1i= 11.08, p < .001). This indicates that Verbal Comprehension may be somewhat more stable than Decision Speed. Nevertheless, there was considerable variability in both variables, with approximately 77% of the variability in Verbal Comprehension and 65% of the variability in Decision Speed over time attributable to differences between children rather than to factors unique to the individual. These preliminary findings suggest that variables differentiating children from one another (e.g., race/ethnicity, gender) might be more powerful predictors of differences in performance than variables differing within individuals over time (e.g., economic background).
Findings for changes in children's sleep problems over time were somewhat mixed. Children's Sleepiness at T1 was 15.54 (p < .001) and decreased significantly over time (π1i= −1.01, p < .001), suggesting that the consequences of sleep deficiencies for daytime functioning may diminish over time. In contrast, although children's initial level of Sleep/Wake Problems was significantly different from zero (π0i= 19.17, p < .001), this variable did not change over time (π1i = −0.363, p > .10), suggesting there is considerable stability within individuals. Approximately 70% of the variability in Sleepiness and 71% of the variability in Sleep/Wake Problems are due to factors unique to the individual rather than to differences between children.
Do Changes in Sleep Problems Predict Changes in Cognitive Performance?
Parameter estimates from the fitted growth curve models predicting changes in Verbal Comprehension (Models 1–4) or Decision Speed (Models 5–8) from changes in Sleepiness or Sleep/Wake Problems are presented in Tables 3 and 4. Although we found no evidence that children's initial level of Sleepiness or Sleep/Wake Problems at T1 was related to their T1 Verbal Comprehension (Model 1, Tables 3 and 4) or Decision Speed (Model 5, Tables 3 and 4), we did identify an association between children's initial level of Sleepiness and their rate of change in Verbal Comprehension as well as between their rate of change in Sleepiness and their rate of change in Verbal Comprehension. Children with higher Sleepiness at T1 showed slower increases in Verbal Comprehension compared to children with lower Sleepiness at T1. Verbal Comprehension increased by approximately 20 points per assessment when children's T1 Sleepiness was average, but for children who reported high Sleepiness (1 SD) at T1, their Verbal Comprehension scores increased by only 15.5 points per assessment.
Table 3. Models Predicting Changes in Cognitive Functioning From Changes in Child-Reported Sleepiness (n = 250).
| Parameter | Verbal comprehension | Decision speed | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | Model 8 | |
| Intercept (γ00) | 451.59*** | 451.64*** | 450.10*** | 452.51*** | 451.65*** | 451.16*** | 449.96*** | 451.90*** |
| Sleepiness_I (γ01) | −.16 | −.16 | −.04 | −.27 | −.23 | −.27 | −.08 | −.301 |
| SES composite | 0.68* | 0.43 | 0.70** | 0.68* | 0.398 | 1.79 | 0.41 | 0.406 |
| AA | −5.79*** | -5.86*** | -0.99 | −5.76*** | −2.32 | −2.05 | 2.88 | −2.25 |
| Boy | 2.18 | 2.20 | 2.13 | −1.68 | −8.46*** | −8.44*** | −8.54*** | −11.30 |
| Age | .40*** | .40*** | .40*** | .41*** | .46*** | .48*** | .46*** | .48*** |
| Puberty | −1.09 | −1.09 | −1.27 | −1.28 | −0.55 | −0.78 | −0.82 | −0.77 |
| Sleepiness_I × SES | .02 | −.09 | ||||||
| Sleepiness_I × AA | −.30 | −.32 | ||||||
| Sleepiness_I × Boy | .24 | .18 | ||||||
| Rate of change (γ10) | 19.95*** | 19.70*** | 18.04*** | 22.07*** | 24.94*** | 24.84*** | 20.38*** | 28.34*** |
| Sleepiness_I (γ11) | −.22* | −.21* | −.12 | −.39** | −.12 | −.13 | .13 | −.41* |
| Sleepiness_S (γ12) | −.36** | −.32* | −.13 | −.62** | −.19 | −.23 | .26 | −.53 |
| SES composite | 0.04 | −0.10 | 0.02 | 0.03 | 0.41 | 0.50 | 0.37 | 0.39 |
| AA | 0.10 | 0.25 | 3.33 | −0.006 | 2.26* | 2.12 | 10.76* | 2.14 |
| Boy | −0.41 | −0.41 | −0.29 | −5.91* | −1.98 | −2.02 | −1.77 | −11.00** |
| Age | −.13*** | −.13*** | −.12*** | −.13*** | −.14** | −.15** | −.14** | −.14** |
| Puberty | 0.84 | 0.81 | 0.92 | 1.01 | 0.17 | 0.26 | 0.30 | 0.36 |
| Sleepiness_I× SES | .02 | −.01 | ||||||
| Sleepiness_S × SES | .09 | −.06 | ||||||
| Sleepiness_I × AA | −.24 | −.60* | ||||||
| Sleepiness_S × AA | −.51* | −1.00* | ||||||
| Sleepiness_I × Boy | .39* | .63* | ||||||
| Sleepiness_S × Boy | .51* | .65 | ||||||
| Variance components | ||||||||
| Within subjects | 5.77 | 5.76 | 5.76 | 5.75 | 9.40 | 9.41 | 9.36 | 9.37 |
| Between subjects | 8.54 | 8.54 | 8.43 | 8.53 | 11.66 | 11.51 | 11.66 | 11.63 |
| Goodness-of-fit statistics | ||||||||
| R2 within | .5895 | .5919 | .5921 | .5948 | .5800 | .5784 | .5856 | .5850 |
| R2 between | .3508 | .3536 | .3580 | .3586 | .3248 | .3352 | .3302 | .3320 |
| R2 total | .4036 | .4067 | .4097 | .4111 | .3745 | .3812 | .3777 | .3806 |
Note. SES = socioeconomic status; AA = African American; _I = population average intercept at Time 1 for Sleepiness; _S = population average slope of Sleepiness between Time 1 and Time 3.
p < .05.
p < .01.
p < .001.
Table 4. Models Predicting Changes in Cognitive Functioning From Changes in Child-Reported Sleep/Wake Problems (n = 250).
| Parameter | Verbal comprehension | Decision speed | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | Model 8 | |
| Intercept (γ00) | 446.51*** | 446.83*** | 445.69*** | 444.90*** | 452.04*** | 452.14*** | 449.68*** | 457.36*** |
| Sleep/Wake Problems_I (γ01) | .16 | .15 | .19 | .23 | −.21 | −.24 | −.08 | −.48* |
| SES composite | 0.64* | 1.12 | 0.65* | 0.64* | 0.42 | 2.91* | 0.43 | 0.43 |
| AA | −6.56*** | −6.58*** | −4.93 | −6.59*** | −2.46 | −2.48 | 3.56 | −2.49 |
| Boy | 2.23 | 2.22 | 2.24 | 4.95 | −8.24*** | −8.40*** | −8.21*** | −17.94** |
| Age | .39*** | .39*** | .39*** | .39*** | .46*** | .47*** | .46*** | .46*** |
| Puberty | −0.85 | −0.86 | −0.77 | −0.67 | −0.43 | −0.73 | −0.36 | −0.72 |
| Sleep/Wake Problems_I × SES | −.03 | −.13 | ||||||
| Sleep/Wake Problems_I × AA | −.08 | −.31 | ||||||
| Sleep/Wake Problems_I × Boy | −.14 | .50 | ||||||
| Rate of change (γ10) | 18.02*** | 17.96*** | 16.88*** | 19.68*** | 23.32*** | 23.33*** | 22.66*** | 20.98*** |
| Sleep/Wake Problems_I (γ11) | −.06 | −.05 | .00 | −.15 | .02 | −.001 | .06 | .12 |
| Sleep/Wake Problems_S (γ12) | −.07 | −.07 | −.04 | −.13 | .14 | .11 | .17 | −.06 |
| SES composite | 0.06 | −0.21 | 0.07 | 0.08 | 0.41 | 0.66 | 0.41 | 0.37 |
| AA | −0.31 | −0.25 | 2.39 | −0.30 | 1.85 | 1.99 | 3.39 | 1.73 |
| Boy | −0.24 | −0.26 | −0.22 | −3.65 | −1.88 | −1.99 | −1.88 | 1.64 |
| Age | −.13*** | −.13*** | −.13*** | −.13*** | −.15** | −.15 | −.15** | −.14** |
| Puberty | 0.84 | 0.83 | 0.84 | 0.77 | 0.15 | 0.34 | 0.13 | 0.23 |
| Sleep/Wake Problems_I× SES | .02 | −.01 | ||||||
| Sleep/Wake Problems_S × SES | .02 | .001 | ||||||
| Sleep/Wake Problems_I × AA | −.14 | −.08 | ||||||
| Sleep/Wake Problems_S × AA | −.08 | −.07 | ||||||
| Sleep/Wake Problems_I × Boy | .18 | −.17 | ||||||
| Sleep/Wake Problems_S × Boy | .10 | .46 | ||||||
| Variance components | ||||||||
| Within subjects | 5.76 | 5.77 | 5.76 | 5.75 | 9.38 | 9.41 | 9.40 | 9.35 |
| Between subjects | 8.85 | 8.86 | 8.87 | 8.91 | 11.71 | 11.56 | 11.70 | 11.74 |
| Goodness-of-fit statistics | ||||||||
| R2 within | .5927 | .5933 | .5948 | .5958 | .5806 | .5804 | .5807 | .5861 |
| R2 between | .3195 | .3210 | .3195 | .3178 | .3249 | .3417 | .3291 | .3286 |
| R2 total | .3797 | .3805 | .3797 | .3794 | .3765 | .3904 | .3790 | .3799 |
Note. SES = socioeconomic status; AA = African American; _I = population average intercept at Time 1 for Sleep/Wake Problems; _S = population average slope of Sleep/Wake Problems between Time 1 and Time 3.
p < .05.
p < .01.
p < .001.
Similarly, increases, or less rapid decreases, in Sleepiness were associated with less rapid increases in cognitive performance between T1 and T3, though the effect was quite small. Children whose rate of change in Sleepiness was high and increasing had a 19-point increase in Verbal Comprehension per assessment (compared to a 20-point gain for a child with average rates of change in Sleepiness over time). Decreases in Sleepiness over time (− 1 SD) resulted in a 21.5-point increase in Verbal Comprehension per assessment. Although our predictor and control variables explained some of the variation in our outcomes (approximately 8% in Verbal Comprehension and 10% in Decision Speed), the majority of variation remains to be explained. Together, these findings suggest that high levels of Sleepiness at T1 and increasing Sleepiness over time represent a risk for growth in Verbal Comprehension but not for growth in Decision Speed over time. Sleep/Wake Problems had no effect on children's cognitive performance.
Are There Sociodemographic and Child Gender Differences in the Effects of Changes in Sleep Problems on Changes in Cognitive Performance?
To determine whether high initial levels of sleep problems or increases in sleep problems over time are more detrimental to some children than others, we tested a series of interactions between the growth parameters for changes in children's sleep, and family and child demographics. We found no moderation effects for our family income/education composite for either Sleepiness (Models 2 and 5, Table 3) or Sleep/Wake Problems (Models 2 and 5, Table 4). We did, however, find that child race/ethnicity and gender significantly moderated the effects of Sleepiness (but not Sleep/Wake Problems) on Verbal Comprehension (Models 3 and 4, Table 3) and Decision Speed (Models 7 and 8, Table 3). Specifically, child race/ethnicity moderated the effect of rate of change in Sleepiness on the rate of change in Verbal Comprehension as well as the effect of Sleepiness at T1 and the rate of change between T1 and T3 on the rate of change in Decision Speed. Increases (1 SD) in Sleepiness over time appeared to be a risk factor for AA children's Verbal Comprehension, while decreases (− 1 SD) in Sleepiness appeared to be a protective factor (Model 3, Table 4). AA children whose Sleepiness increased over time (labeled High Sleepiness in Figure 1A) showed very little growth in Verbal Comprehension between T1 and T3. In contrast, although the Verbal Comprehension scores of AA children with high and low rates of change in Sleepiness were relatively similar at the start of the study (484.6 vs. 487.9, respectively), those children whose Sleepiness decreased over time (labeled Low Sleepiness in Figure 1A) demonstrated considerable growth in Verbal Comprehension across the study period (approximately 17 points). High rates of change in Sleepiness also had a detrimental effect on EA children's Verbal Comprehension such that their scores increased more slowly than the scores of EA children with low rates of change in Sleepiness, but the effect was not as strong as that for AAs.
Figure 1.
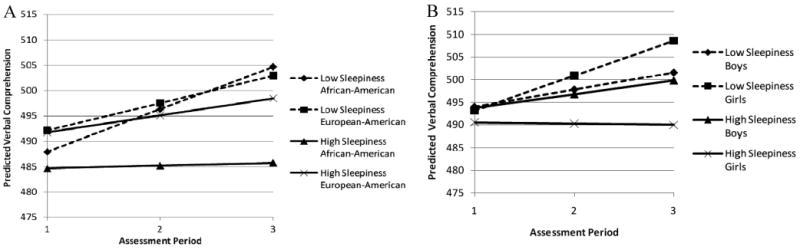
Fitted Verbal Comprehension growth trajectories associated with low (1 SD below the mean) initial levels and rates of change in Sleepiness and high (1 SD above the mean) initial levels and rates of change in Sleepiness. Panel A illustrates moderation by child race/ethnicity, and Panel B illustrates moderation by child gender. Trajectories were estimated from a model controlling for child age in months and pubertal status.
The pattern of findings for Decision Speed was somewhat different. Child race/ethnicity moderated the effects of both initial level and rate of change in Sleepiness on rate of change in Decision Speed such that high Sleepiness (1 SD) was a risk factor for AAs while low Sleepiness (− 1 SD) was a protective factor (see Figure 2A). AA children with high initial levels of Sleepiness and increases in Sleepiness over time had slower increases in Decision Speed. In contrast, AA children with low Sleepiness at T1 and decreases in Sleepiness over time demonstrated significantly greater increases in Decision Speed. The resulting difference in Decision Speed scores was just over 15 points by the end of the study. Note that the Decision Speed scores of these prototypical groups were separated by fewer than 4 points at the start of the study. There was little effect of Sleepiness on changes in EAs' Decision Speed scores over time. Thus, Sleepiness appears to be a greater risk factor for AA than EA children. Nevertheless, both Verbal Comprehension and Decision Speed scores increased more slowly for children with lower than higher levels of Sleepiness regardless of their race/ethnicity.
Figure 2.
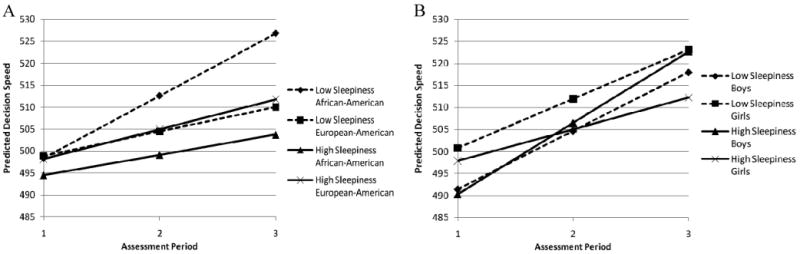
Fitted Decision Speed growth trajectories associated with low (1 SD below the mean) initial levels and rates of change in Sleepiness and high (1 SD above the mean) initial levels and rates of change in Sleepiness. Panel A illustrates moderation by child race/ethnicity, and Panel B illustrates moderation by child gender. Trajectories were estimated from a model controlling for child age in months and pubertal status.
Child gender also moderated the associations between sleep problems and children's cognitive performance. On average, boys' and girls' Verbal Comprehension scores at T1 were similar regardless of their Sleepiness (see Figure 1B). When we examined the effects of Sleepiness on growth in Verbal Comprehension, however, the detrimental effects that Sleepiness has on children's cognitive performance was clear. Girls with high initial levels of Sleepiness and increases in Sleepiness (1 SD; labeled High Sleepiness in Figure 2B) over time demonstrated no growth in Verbal Comprehension between T1 and T3. In contrast, boys with high Sleepiness demonstrated positive growth in Verbal Comprehension over time, though this growth was lower than that of either boys or girls with low initial levels and decreases in Sleepiness (−1 SD; labeled Low Sleepiness in Figure 1B). Girls with low Sleepiness at T1 and decreases in Sleepiness over time showed the greatest growth in Verbal Comprehension, suggesting that low Sleepiness is a protective factor for girls while high Sleepiness is a significant risk factor. By the end of the study period, more than 18 points separated girls with low versus high levels of Sleepiness on Verbal Comprehension. Boys, however, seemed to perform similarly on Verbal Comprehension regardless of their Sleepiness (fewer than 2 points separated boys with high versus low Sleepiness).
Gender moderation was only found for the effect of children's initial level of Sleepiness on rate of change in Decision Speed (see Figure 2B). Again, girls were more affected by Sleepiness than boys. Specifically, girls with high initial levels of Sleepiness at T1 had the lowest rates of increase in Decision Speed over time, resulting in a nearly 11-point deficit in Decision Speed scores between girls with low and high initial levels of Sleepiness by the end of the study. Boys' initial Decision Speed scores at T1 were nearly identical, regardless of whether their initial Sleepiness scores were high (490.3) or low (491.4); at T3, Decision Speed scores of boys with high and low initial levels of Sleepiness were not significantly different.
Discussion
Strong indications from many studies with cross-sectional designs and a few experimental studies have been that poorer cognitive performance is seen when children's sleep is compromised. Whether those relations are maintained over time has been unknown. Likewise unknown has been the shape of change trajectories in sleep problems and their relations to change trajectories in cognitive performance. Our findings contribute to existing literature in important ways and are the first to demonstrate that initial levels of Sleepiness as well as changes in Sleepiness are related to changes in children's Verbal Comprehension over time; for children whose Sleepiness increased (or decreased less rapidly) over 3 years, their Verbal Comprehension increased less rapidly. This is the first study of its kind to demonstrate longitudinal associations between changes in sleep problems and changes in cognitive performance and highlights the importance of contemporaneous longitudinal assessments of these two dynamic systems.
Verbal Comprehension is a test that measures an important cognitive ability, and it has high reliability coefficients in extended test intervals and strong links with academic achievement (Woodcock et al., 2001). Links with Sleepiness over time may be due to downward pressures in verbal acquisition accumulating over time. If poorer sleep is related to lower cognitive performance at any one point in time and if sleep problems are chronic rather than episodic, it stands to reason that problems may magnify over time, as academic skills incompletely mastered at an early stage may hinder later skill attainment. That Decision Speed does not show links with Sleepiness may be related to slightly lower Rasch reliabilities and substantially lower extended time test–retest reliabilities in standardization studies (Woodcock et al., 2001), a difference that is even more pronounced in our sample (rs = .67–.70 for Verbal Comprehension and .50–.57 for Decision Speed). Processing speed develops rapidly during the age period we studied, and a greater range of individual differences may obscure generalizations based on age (Kail, 1991). Differential associations between sleep and various cognitive parameters highlight the importance of examining multiple facets of cognition for a better elucidation of associations with sleep problems. Both sleep and cognitive development are dynamic and intertwined, and both systems may develop at different rates in individual children.
Importantly, findings were evident for children's Sleepiness but not their Sleep/Wake Problems. Because there are individual differences in the effects of sleep disruptions on children's daytime sleepiness and associated functioning, some children may experience more deleterious effects of poor sleep, while others may be more resilient even though their sleep/wake behaviors are similar. Consistent with differential findings reported for Sleepiness and Sleep/Wake Problems in relation to cognitive functioning (e.g., Anderson, Storfer-Isser, Taylor, Rosen, & Redline, 2009) and current recommendations in the literature (Dewald et al., 2010), our findings support the importance of separate assessments of various sleep parameters.
Moderation effects for race/ethnicity and gender demonstrate that trajectories of sleep and cognitive functioning are not the same for all children. These findings are the first in the literature to demonstrate that gender and race/ethnicity are significant moderators of the link between sleep and cognitive performance over three waves and are important for understanding vulnerability and protection associated with individual and group differences. Differences in cognitive ability and academic achievement by children of different racial/ethnic groups have been among the most studied and most controversial phenomena in American education. Our results indicate that the effects of Sleepiness on children's Verbal Comprehension were more pronounced for AA compared with EA children. Whereas increases in Sleepiness over time served as a vulnerability factor for AAs, decreases in Sleepiness were associated with considerable growth in cognitive performance. Even though we reduced confounding race/ethnicity and SES by recruiting children across a wide range of educational and economic backgrounds and both racial/ethnic groups, there was still a significant, albeit low correlation of the two at each time period of testing. Recall however, that analyses of ethnicity controlled for economic background, and thus, we were able to disentangle these effects to some degree.
Although lower SES AA children may be jeopardized by SES-related health disparities, AA children of all SES levels may have elevated risk for sleep problems and thus poorer cognitive functioning. Good sleep depends upon a sufficient oxygen supply; thus, although speculative, the higher rates of asthma as well as high blood pressure among AA children may help explain some of the observed associations (Muntner, He, Cutler, Wildman, & Whelton, 2004). Furthermore, subcultural norms and customs likely play a role in ethnic differences observed. For example, AA children are more likely to take naps (Crosby et al., 2005) and to share bedrooms than EAs, even when the ratio of number of inhabitants to number of bedrooms is considered (Buckhalt et al., 2007). Future explication of variables associated with ethnicity that may clarify their moderating role in the context of sleep and child outcomes is imperative.
Gender differences in sleep problems are inconsistent (Gaina et al., 2007; Laberge et al., 2001), and only a few studies have examined gender as a moderator of the sleep–child functioning link. Ours are the first results to demonstrate that child gender is a moderator of relations between changes in sleep problems and changes in children's cognitive performance. High Sleepiness acted as a risk factor for lower Verbal Comprehension among girls but not boys. These findings are consistent with recent evidence from the present sample indicating that initial levels and increases in Sleepiness over 3 years have a more negative effect on girls' depressive symptoms over time (El-Sheikh et al., 2011). Sleep disruptions are linked to compromised functioning of the prefrontal cortex (PFC), which is associated with cognitive, behavioral, and emotional problems (Dahl, 1996). The PFC develops rapidly during late childhood and early adolescence, and its maturation is associated with pubertal development (Blakemore, 2008). Earlier pubertal development and perhaps earlier brain development among girls may lead to greater vulnerability in the context of sleep problems for girls but not boys in early adolescence. However, given that puberty status was controlled in analyses, this explanation is very tentative. Follow-up assessments of this sample as they manifest pubertal maturation should provide more clarity.
The results and conclusions drawn from this work must be viewed in the context of the study's limitations. Sleep was measured via self-report rather than with more objective techniques of actigraphy or polysomnography. Self-report measures have been determined to have sufficient reliability and validity for many purposes, but the correspondence with less subjective methods has been inconsistent and often low. Nevertheless, persuasive arguments have been made that all sleep measurement methods offer their unique and defensible source of data about children's sleep (Sadeh, 2008). Similarly, assessments of sleepiness through self-reports is an established methodology in the sleep literature. That our findings supporting the moderating role of ethnicity in the sleep problems–cognitive functioning link are synchronous with those based on actigraphic assessments of sleep either cross-sectionally (Buckhalt et al., 2007) or longitudinally through two waves of data (Buckhalt et al., 2009) bolsters conclusions. Another study limitation is that we did not examine other economic adversity-, ethnicity-, or gender-related variables such as perceived discrimination, stress exposure, family functioning, and gender roles that may underlie the observed associations. Although investigating relations over three study waves is a major advance in this literature, four or more assessment periods would allow for examining nonlinear relations.
To conclude, these data extend the knowledge base about enduring relations between sleep and cognitive performance in middle childhood. Our study is one of very few longitudinal studies of sleep in children and the first to study the relation between changes in sleep and cognitive functioning over three study waves. Further replication and extensions, including more longitudinal studies, are needed, but sufficient evidence exists to justify considerable concern about children's sleep and to warrant prevention and intervention programs. Interventions for children with sleep disorders have been successful in improving sleep and associated cognitive deficits (Chervin et al., 2006; Mindell & Owens, 2009). Attention now needs to extend to high-risk children and to interventions designed to mitigate the deleterious consequences of insufficient sleep over the transition to puberty.
Acknowledgments
This research was supported by National Institutes of Health Grant R01-HD046795. We acknowledge contributions made by staff of our research laboratory for data collection. We also thank the school personnel,children, and parents who participated.
Contributor Information
Kristen L. Bub, Human Development and Family Studies, Auburn University
Joseph A. Buckhalt, College of Education, Auburn University
Mona El-Sheikh, Human Development and Family Studies, Auburn University.
References
- Acebo C, Carskadon MA. Influence of irregular sleep patterns on waking behavior. In: Carskadon MA, editor. Adolescent sleep patterns: Biological, social, and psychological influences. New York, NY: Cambridge University Press; 2002. pp. 220–235. [Google Scholar]
- Anderson B, Storfer-Isser A, Taylor HG, Rosen CL, Redline S. Associations of executive function with sleepiness and sleep duration in adolescents. Pediatrics. 2009;123:e701–e707. doi: 10.1542/peds.2008-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S. Socioeconomic status in health research: One size does not fit all. JAMA. 2005;294:2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- Buckhalt JA. Insufficient sleep and the socioeconomic status achievement gap. Child Development Perspectives. 2011;5:59–65. doi: 10.1111/j.1750-8606.2010.00151.x. [DOI] [Google Scholar]
- Buckhalt JA, El-Sheikh M, Keller P. Children's sleep and cognitive functioning: Race and socioeconomic status as moderators of effects. Child Development. 2007;78:213–231. doi: 10.1111/j.1467-8624.2007.00993.x. [DOI] [PubMed] [Google Scholar]
- Buckhalt JA, El-Sheikh M, Keller PS, Kelly RJ. Concurrent and longitudinal relationships between children's sleep and cognitive functioning: The moderating role of parent education. Child Development. 2009;80:875–892. doi: 10.1111/j.1467-8624.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- Carter-Pokras O, Baquet C. What is a “health disparity”? Public Health Reports. 2002;117:426–434. doi: 10.1093/phr/117.5.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervin RD, Ruzicka DL, Giordani BJ, Weatherly RA, Dillon JE, Hodges EK, Guire KE. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117:e769–e778. doi: 10.1542/peds.2005-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby B, LeBourgeois MK, Harsh J. Racial differences in reported napping and nocturnal sleep in 2-to-8-year-old children. Pediatrics. 2005;115:225–232. doi: 10.1542/peds.2004-0815D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE. The regulation of sleep and arousal: Development and psychopathology. Development and Psychopathology. 1996;8:3–27. doi: 10.1017/S0954579400006945. [DOI] [Google Scholar]
- Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bögels SM. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep Medicine Reviews. 2010;14:179–189. doi: 10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Bub KL, Kelly R, Buckhalt JA. Sociodemographic differences in children's sleep and adjustment: A residualized change analysis. 2011 doi: 10.1037/a0030223. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA, Cummings EM, Keller PS, Acebo C. Child emotional insecurity and academic achievement: The role of sleep disruptions. Journal of Family Psychology. 2007;21:29–38. doi: 10.1037/0893-3200.21.1.29. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA, Mize J, Acebo C. Marital conflict and disruption of children's sleep. Child Development. 2006;77:31–43. doi: 10.1111/j.1467-8624.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Kelly RJ, Buckhalt JA, Hinnant JB. Children's sleep and adjustment over time: The role of the socioeconomic context. Child Development. 2010;81:870–883. doi: 10.1111/j.1467-8624.2010.01439.x. [DOI] [PubMed] [Google Scholar]
- Evans GW, English K. The environment of poverty: Multiple stress exposure, psychophysiological stress, and socioemotional adjustment. Child Development. 2002;73:1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Fallone G, Acebo C, Seifer R, Carskadon MA. Experimental restriction of sleep opportunity in children: Effects on teacher ratings. Sleep. 2005;28:1561–1567. doi: 10.1093/sleep/28.12.1561. [DOI] [PubMed] [Google Scholar]
- Gaina A, Sekine M, Hamanishi S, Chen X, Wang H, Yamagami T, Kagamimori S. Daytime sleepiness and associated factors in lapanese school children. Journal of Pediatrics. 2007;151:518–522. doi: 10.1016/j.jpeds.2007.04.036. [DOI] [PubMed] [Google Scholar]
- Kail R. Developmental change in speed of processing during childhood and adolescence. Psychological Bulletin. 1991;109:490–501. doi: 10.1037/0033-2909.109.3.490. [DOI] [PubMed] [Google Scholar]
- Laberge L, Petit D, Simard C, Vitaro F, Tremblay RE, Montplasir J. Development of sleep patterns in early adolescence. Journal of Sleep Research. 2001;10:59–67. doi: 10.1046/j.1365-2869.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- Meijer AM, Reitz E, Dekovic M, Van Den Wittenboer GLH, Stoel RD. Longitudinal relations between sleep quality, time in bed and adolescent problem behaviour. Journal of Child Psychology and Psychiatry. 2010;51:1278–1286. doi: 10.1111/j.1469-7610.2010.02261.x. [DOI] [PubMed] [Google Scholar]
- Meltzer LJ, Mindell JA. Sleep and sleep disorders in children and adolescents. Psychiatric Clinics of North America. 2006;29:1059–1076. doi: 10.1016/j.psc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Mindell JA, Owens JA. A clinical guide to pediatric sleep: Diagnosis and management of sleep problems. New York, NY: Lippincott, Williams, & Wilkins; 2009. [Google Scholar]
- Muntner P, He J, Cutler JA, Wildman RP, Whelton PK. Trends in blood pressure among children and adolescents. JAMA. 2004;291:2107–2113. doi: 10.1001/jama.291.17.2107. [DOI] [PubMed] [Google Scholar]
- Olds T, Blunden S, Petkov J, Forchino F. The relationships between age, geography, and time in bed in adolescents: A meta-analysis of data from 23 countries. Sleep Medicine Reviews. 2010;14:371–378. doi: 10.1016/j.smrv.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Owens JA. Neurocognitive and behavioral impact of sleep disordered breathing in children. Pediatric Pulmonology. 2009;44:417–422. doi: 10.1002/ppul.20981. [DOI] [PubMed] [Google Scholar]
- Owens JA, Spirito A, McGuinn M, Nobile C. Sleep habits and sleep disturbance in elementary school-aged children. Journal of Developmental and Behavioral Pediatrics. 2000;21:27–36. doi: 10.1097/00004703-200002000-00005. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Randazzo AC, Muehlbach MJ, Schweitzer PK, Walsh JK. Cognitive function following acute sleep restriction in children ages 10-14. Sleep: Journal of Sleep Research & Sleep Medicine. 1998;21:861–868. [PubMed] [Google Scholar]
- Rasch G. Probabilistic models for some intelligence and attainment tests. Chicago, IL: University of Chicago Press; 1960. [Google Scholar]
- Rubin DB. Multiple imputation for nonresponse in surveys. New York, NY: Wiley; 1987. [Google Scholar]
- Sadeh A. Consequences of sleep loss or sleep disruption in children. Sleep Medicine Clinics. 2007;2:513–520. doi: 10.1016/j.jsmc.2007.05.012. [DOI] [Google Scholar]
- Sadeh A. Commentary: Comparing actigraphy and parental report as measures of children's sleep. Journal of Pediatric Psychology. 2008;33:406–407. doi: 10.1093/jpepsy/jsn018. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: What a difference an hour makes. Child Development. 2003;74:444–455. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]
- Schutte R. Sleep and cognition in school-age children. Paper presented at the Pediatric Sleep Medicine Meeting; Denver, CO. 2009. Oct, [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Stein MA, Mendelsohn J, Obermeyer WH, Amromin J, Benca R. Sleep and behavior problems in school-aged children. Pediatrics. 2001;107:1–9. doi: 10.1542/peds.107.4.e60. [DOI] [PubMed] [Google Scholar]
- Touchette E, Petit D, Séguin JR, Boivin M, Tremblay RE, Montplaisir JY. Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep: Journal of Sleep and Sleep Disorders Research. 2007;30:1213–1219. doi: 10.1093/sleep/30.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widaman K. Best practices in quantitative methods for developmentalists: III. Missing data: What to do with or without them. Monographs of the Society for Research in Child Development. 2006;71(3):42–64. doi: 10.1111/j.1540-5834.2006.07103001.x. [DOI] [PubMed] [Google Scholar]
- Willett JB. Measurement of change. In: Keeves JP, editor. Educational research, methodology and measurement: An international handbook. 2nd. Oxford, England: Pergamon Press; 1997. pp. 327–334. [Google Scholar]
- Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Development. 1998;69:875–887. [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather M. Woodcock-Johnson III Tests of Cognitive Abilities. Rolling Meadows, IL: Riverside Publishing; 2001. [Google Scholar]


