Abstract
Purpose of review
Expanding rates of acute kidney injury (AKI) coupled with increasing awareness of its short and long-term sequelae have focused efforts to identify patients at risk for developing this disease and its complications. This review details recent attempts to identify novel risk factors for AKI, describe further refinements in the diagnostic and prognostic approach to this disease using biological markers of injury, and highlights features of AKI that independently predict poor long-term outcomes.
Recent Findings
The presence of proteinuria predicts the development of AKI independently of glomerular filtration rate (eGFR). Initial results from a large prospective study of AKI biomarkers in cardiac surgery indicate lower agreement with serum creatinine as an AKI standard than observed in early studies. AKI severity and duration are important predictors of chronic kidney disease (CKD) and long-term mortality. A minority of patients surviving AKI with decreased kidney function is seen by a nephrologist.
Summary
While the pathophysiologic link unclear, proteinuria is an easily measurable risk factor for AKI worth considering before anticipated procedures or medication exposures with nephrologic risk. Investigation extending beyond agreement with serum creatinine is needed to fully understand the diagnostic and prognostic value of AKI biomarkers. Severity and duration are components of AKI that can help risk-stratify survivors in need of monitoring or nephrology referral.
Keywords: Acute Kidney Injury, Epidemiology, Biomarkers, Proteinuria, Chronic Kidney Disease
Introduction
Acute Kidney Injury (AKI) is an increasingly common disease that strongly associates with poor short- and long-term morbidity and mortality.[1-8] Disappointingly, only marginal improvements in the survival have been observed with little evidence supporting the use of tested pharmacotherapies in established disease.[9-11] With recent well-executed trials also indicating a therapeutic ceiling in conventional renal replacement therapies may have been achieved,[12-14] emphasis on developing strategies to prevent AKI and its long-term consequences has grown. In this review, we discuss recent additions to the AKI literature focusing on novel risk factors for developing AKI, ongoing efforts to refine the diagnostic and prognostic approach to this disease with emerging biomarkers of injury, and attempts to better identify survivors at risk for poor longitudinal outcomes.
New risk factors for AKI
As the incidence of AKI increases,[1, 2] attempts to identify those at risk for its development and complications continue. Previous work has consistently uncovered several risk factors across different clinical settings including the presence of advanced age, diabetes, male gender, African American race, and factors related to the underlying procedure or illness. Among the most potent appears to be the presence of underlying kidney dysfunction as defined by clearance.[15] For example, Pannu recently confirmed earlier findings by Hsu[15] describing a robust step-wise increase in the risk for severe AKI with an advancing CKD stage (adjusted Odds Ratio (OR) 18.3 (95% CI, 16.5-20.3)) relative to those with preserved eGFR in a population-based setting.[16**]
Recent efforts to add to these data have quantified the risk of AKI conferred by the presence of underlying proteinuria as recently reviewed by Hsu.[17-19**] Huang add to these data by demonstrating that preoperative proteinuria assessed by simple dipstick measurement was independently associated with the risk of developing AKI following cardiac surgery even after adjusting for underlying CKD stage and diabetes.[20] Collectively, these findings argue against the notion that associations between higher serum creatinine values and the risk for AKI may be confounded by the effects of ascertainment bias when using a fixed changes in serum creatinine to define AKI.[21] As proteinuria itself is not a component of the definition, these studies reinforce the link between underlying structural damage and the risk for adverse renal events and uncover an easily detectable risk factor not routinely measured before procedures or exposures carrying intrinsic risk for renal injury. They also highlight the need to determine the pathophysiologic link between AKI and proteinuria and the extent to which the latter is truly modifiable.
Refining the Diagnostic Approach to AKI
While modern consensus criteria have helped standardize the approach to the diagnosis and staging of AKI,[22, 23] the use of incrementally smaller changes in serum creatinine carry inherent specificity limitations and place a premium on the accurate determination of baseline kidney function. The lack of a uniform approach to the latter has recently been shown to compound the risk for AKI misclassification, hindering effective comparisons of this disease between settings.[24-26*] Adding to the uncertainty is the identification of additional confounders including fluid balance. In a post-hoc analysis of the NHLBI-sponsored Fluid and Catheter Treatment Trial (FACTT),[27**] Liu et al. examined the occurrence of AKI in 1000 critically ill patients with the Acute Respiratory Distress Syndrome randomized to a fluid conservative versus fluid liberal management strategy. After adjusting serum creatinine measurements for fluid balance, notable increases in the incidence of AKI were noted in each study arm [(conservative, 57% vs. 51%, p=0.04)(liberal 66% vs. 58%, p=0.007)]. Comparable mortality rates between those patients in whom the AKI diagnosis was “masked” versus those with known AKI before fluid correction were also observed (31% vs. 38%, p=0.18). These findings suggest that in addition to being an important prognostic factor,[28, 29*] variations in fluid balance can cause the diagnosis of AKI to be missed or delayed in high-risk patients when using serum creatinine-based definitions alone. As further iterations of these definitions are refined, these limitations continue to underscore the need to effectively segregate evolving aspects of injury from changes in function.
Towards that end, the emerging story of biological markers of AKI continues to unfold with initial reports from large prospective cohorts (Table 1) assembled to address earlier study limitations of size, generalizability, and a paucity of hard end points. The most notable of these include the initial publications from the NHLBI-sponsored Translational Research Investigating Biomarker Endpoints in AKI (TRIBE-AKI) consortium,[30, 31**] a multicenter prospective study of 1219 adults and 311 children undergoing cardiac surgery in the United States and Canada. The primary goal of these studies was to determine if perioperative levels of urine and plasma neutrophil gelatinase-associated lipocalin (u,p NGAL) and Interleukin-18 (u,p IL-18) could predict a doubling of baseline serum creatinine, the need for acute dialysis, or other important endpoints. Surprisingly, in contrast to earlier smaller studies, diagnostic testing revealed only moderate performance when added to a clinical model for diagnosing a composite outcome of doubling of baseline serum creatinine levels or dialysis in the adult study with AUCs of 0.67 (p=0.12) and 0.70 (p=0.01) for uNGAL and pNGAL, respectively, and 0.74 (p=0.03) for uIL-18. Individual performance of each biomarker within the pediatric study was substantially less robust than earlier reports with AUCs for uNGAL and pNGAL of 0.71 (95%CI: 0.63-0.79) and 0.56 (0.46-0.66), respectively and 0.64 (95% CI: 0.58-0.70) for uIL-18. However, after adjustment for demographic and intraoperative risk factors, adult patients within the highest quintile of post-operative pNGAL and uIL-18 levels were still found to have between a 5 to 7 fold incremental risk for developing severe AKI when compared to those in the lowest quintile, with uNGAL not showing a statistically significant association. In pediatric patients, the highest quintile of uNGAL and uIL-18 levels conferred an approximate 4-7 fold incremental risk relative to the lowest quintile with pNGAL not demonstrating a significant association. Net Reclassification index (NRI), which quantifies the global ability of biomarkers to correctly reclassify individuals with and without severe AKI to their respective higher or lower risk categories beyond a clinical model, indicated a 22-25% improvement in overall reclassification for uIL-18 in both studies, a 14-17% improvement for urine and plasma NGAL in the pediatric study, and an 18% improvement for pNGAL in the adult study.
Table 1.
Summary of the Recent Prospective AKI Biomarker Studies
| Authors and year | Clinical Settings | Sample Size | Biomarkers | Endpoint | Summary of Findings |
|---|---|---|---|---|---|
| Parikh et al, 2011[30, 31**] | Undergoing cardiac surgery | 1219 adults 311 pediatric | pNGAL, uNGAL, uIL-18 | AKI prediction In-hospital and ICU LOS, RRT, mortality | Adult: AUCs for severe AKI diagnosis uNGAL 0.67, pNGAL 0.70, uIL-18 0.74. After multivariate adjustment, highest quintiles of postoperative levels of pNGAL and uIL-18 had between a 5- to 7-fold incremental risk of severe AKI, uNGALnot significant. |
| Pediatric: AUCs for severe AKI diagnosis uNGAL 0.71, pNGAL 0.56, uIL-18 0.72. After multivariate adjustment, the highest quintiles of uNGAL and uIL-18 levels 4- 7-fold incremental risk for AKI relative to the lowest quintiles, pNGAL not sign. | |||||
| 22 to 25% improvement in net reclassification index for uIL-18 in both studies, a 14 to 17% improvement for plasma and urine NGAL in the pediatric study, and an 18% improvement for pNGAL in the adult study. | |||||
| All biomarkers showed a significant ability to predict harder clinical endpoints including survival and duration of care (ventilation, ICU, and hospital days). | |||||
| Haase et al, 2011[32**] | Cardiac Surgery + ICU (pooled cohorts) | 2322 adults (10 studies) | NGAL | RRT, Mortality, LOS | NGAL-/Cr- (55.8%), NGAL+/Cr- (19.2%), NGAL-/Cr+ (4.6%), NGAL+/Cr+ (20.4%). Graded stepwise increase in risk for RRT, mortality, and length of stay. NGAL provides prognostic information beyond creatinine alone. |
| Krawczeski et al, 2011[33] | Cardiac surgery | 220 pediatric | uNGAL, uKIM-1, uL-FABP,uIL-18 | Timing of biomarker elevation and AKI prediction (50% increase in SCr) | Earliest significant elevation after CPB (NGAL 2 hrs., IL-18 and L-FABP 6 hrs., and KIM-1 12 hrs.). AUC for AKI at 2 hrs. clinical model 0.74, + uNGAL 0.85. At 6 hrs., clinical model 0.72, + uNGAL 0.91, + uIL-18 0.84, + L-FABP 0.77. At 12 hrs. clinical model 0.72,+ KIM-1 0.79. Net reclassification and integrated discrimination improved for each biomarker at varying time points. |
| Endre et al, 2011[34] | ICU | 528 adults | uGGT, uAP, uNGAL uCystatin-C,uKIM-1 uIL-18 | Diagnosis of AKI on entry, early diagnosis of AKI, RRT, Mortality | AUC for AKI on entry or prediction (all < 0.7). AUC for prediction of RRT (7 days) for NGAL, Cystatin C, IL-18 b/w 0.7-0.8. AUCS for prediction of death (7 days) all < 0.7. Prediction of AKI seemed to improve in the subset of those with abnormal eGFR < 60 ml/min/1.73m2 and varied with time from presumed insult. |
| Krawczeski et al, 2011[35] | Cardiac surgery | 374 neonates and children | pNGAL, uNGAL | Early AKI Diagnosis, severity of AKI, Hospital LOS | Ability of early post-operative uNGAL and pNGAL to predict AKI in 48 hours (0.3 mg/dl increase creatinine in neonates, 50% increase in non-neonates). AUC from 2-48 hours following CPB ranged from 0.88-0.97 though most robust at 2-hour time point. In non-neonates, strong correlation observed between 2-hour NGAL levels and length of stay, and severity/duration of AKI. |
| De Geus et al, 2011[36] | ICU | 632 adults | pNGAL, uNGAL | Early Diagnosis of AKI | AUC for early diagnosis of AKI using RIFLE R (pNGAL 0.77 ± 0.05, uNGAL 0.80 ± 0.04). Performance improved with increasing AKI severity. Biomarker levels improved discrimination, calibration, and net reclassification of a clinical model for severe AKI (RIFLE F). |
| Doi et al, 2011[37] | Medical-surgical mixed ICU | 339 adults | uL-FABP, NGAL, IL-18, N-acetyl β-DG, Albumin | Early Diagnosis of AKI (50% increase in SCr), Mortality | AUC for early diagnosis of AKI (uL-FABP 0.75, uNGAL 0.70, uIL-18 0.69, uNAG 0.62, urinary albumin 0.69). AUC for prediction of 14 day mortality uL-FABP 0.90, uNGAL 0.83, uIL-18 0.83, uL-FABP + uNGAL = 0.93). |
| Shlipak et al, 2011[38] | Undergoing cardiac surgery (TRIBE-AKI) | 1147 adults | Serum Cystatin-C | Presurgical Risk Stratification for AKI | Presurgical cystatin c performed better than creatinine/eGFR for estimating risk of AKI. Adjusted ORs for intermediate and worst kidney function by cystatin C were 1.9 (95% CI, 1.4-2.7) and 4.8 (95% CI, 2.9-7.7) compared with 1.2 (95% CI, 0.9-1.7) and 1.8 (95% CI, 1.2-2.6) for creatinine. After adjustment for clinical predictors, AUC for AKI was 0.70 without kidney markers, 0.69 with creatinine, and 0.72 with cystatin C. Cystatin C improved AKI risk classification compared with creatinine, based on a net reclassification index of 0.21 (P < 0.001). |
| Srisawat et al 2011[39] | Hospitalized with pneumonia | 181 adults | pNGAL | Prediction of recovery from RIFE- Failure during hospitalization) | pNGAL alone predicted failure to recovery with AUC 0.74. Clinical model + NGAL did not improve AUC, but did improve net reclassification index by 17%. |
| Perry et al, 2010[40] | Undergoing cardiac surgery | 879 adults | pNGAL | Early Diagnosis of AKI (50% increase in SCr) | pNGAL values did not reliably predict subsequent AKI (AUC immediately post-CPB 0.64, POD#1 0.67) though did associate independently after multivariate adjustment (for levels 353.5 ng/ml)(odds ratio, 2.3; 95% CI: 1.5–6.5) |
| Siew et al, 2010[41] | Mixed ICU population | 451 adults | uIL-18 | Early Diagnosis of AKI, RRT, mortality | uIL-18 was not reliable predictor of AKI but did predict composite outcome of death and RRT within 28 days |
AKI; Acute kidney injury, ICU;Intensive care unite, LOS;length of stay, RRT;renal replacement therapy, pNGAL; plasma neutrophil gelatinase associated lipocalin, uNGAL; urine neutrophil gelatinase associated lipocalin, uKIM-1; urine kidney injury molecule -1, uL-FABP; urine liver fatty acid binding protein, N-acetyl β-DG; N-acetyl β-Dglucosaminidase, CRE;creatinine, AUC;under curve area, eGFR; estimated glomerular filtration rate
Given its known limitations, extending examination beyond whether these markers agree with creatinine will be necessary to determine their additional clinical usefulness. In the TRIBE-AKI studies, all biomarkers demonstrated a significant ability to predict harder clinical endpoints including survival and duration of care beyond conventional clinical parameters. More recently, Haase et al.[32**] examined pooled data from several prospective NGAL studies to determine the prognosis of patients grouped according to agreement between biomarker and creatinine levels regarding AKI status. Not surprisingly, the patients at lowest and highest risk for dialysis or mortality were those in whom both NGAL and creatinine data were both either depressed or elevated, respectively (Figure 1). However, patients in whom NGAL levels were alone (NGAL+/Creatinine-) were observed to be at higher risk for dialysis initiation than their NGAL negative counterparts (NGAL-/Creatinine-). These same patients (NGAL+/Creatinine-) were also at higher risk for mortality than among those in whom creatinine levels indicated injury but NGAL levels were not elevated (NGAL-/Creatinine +). While limited by the use of pooled data, these findings support the hypothesis that novel biomarkers are providing valuable prognostic information beyond changes in creatinine alone. Numerous efforts to determine the clinical usefulness of these new markers for helping to detect AKI, provide prognostic information, determine recovery, and predict long-term outcomes are ongoing.
Figure 1. Incidence of Renal-related Events Grouped by Biomarker and Creatinine Status.
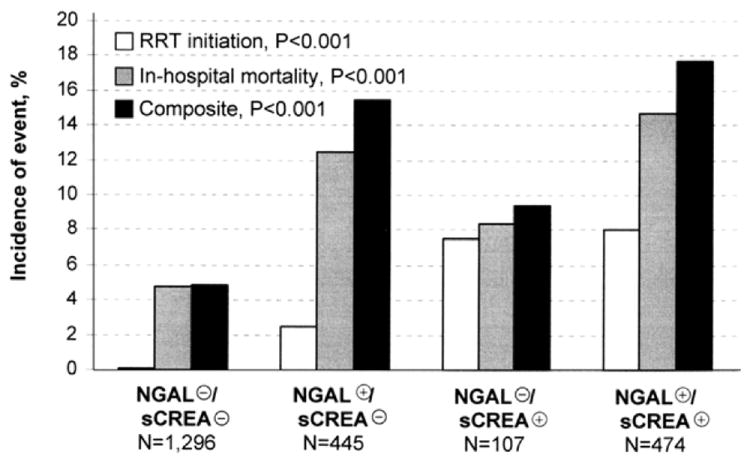
(previously published).[32] Haase, et al. using pooled data from 10 prospective observational studies of AKI, examined how different NGAL and creatinine profiles associated with the risk for in-hospital mortality and the need for renal replacement therapy in 2,322 critically ill patients. A stepwise increase in the risk of subsequent renal replacement therapy was observed -NGAL(−)/sCREA(−): 0.0015% versus NGAL(+)/sCREA(−): 2.5% (odds ratio: 16.4, 95% confidence interval: 3.6 to 76.9, p < 0.001), NGAL(−)/sCREA(+): 7.5%, and NGAL(+)/sCREA(+): 8.0%, respectively. Similar trends were findings were observed with hospital mortality (4.8%, 12.4%, 8.4%, 14.7%, respectively) and their combination (4-group comparisons: all p<0.001).
Long-Term Outcomes and Care Processes in Survivors of AKI
Observations indicating steady increases in AKI incidence[1, 2] coupled with potential improvements in short-term survival[9, 42] have implied a growing population of AKI survivors. While the reasons for the growth in AKI rates are not clear, contributions from parallel increases in the rates of sepsis, CKD, and the advancing age of the population are likely contributors.[43-45] The result has been increased focus on the long-term sequelae of this disease and its potential public health implications. Several large observational studies have already established the association between AKI and the subsequent risk for long-term mortality and decline in renal function.[46-48]
Building upon this emerging body of literature, recent efforts have focused on isolating individual factors that confer higher risk for developing CKD and its complications. A pair of recent studies performed within the Department of Veterans Affairs Healthcare System explored the association between indices of AKI severity and the risk for subsequent development of CKD and mortality. Chawla et al.[49*] developed risk stratification tools that predicted the development of stage IV CKD in 5351 patients with a primary discharge diagnosis of AKI and cross-validated them in 11,589 patients admitted for myocardial infarction or pneumonia. All models performed well (AUCs ≥ 0.81) with the model most easily implemented including age, baseline kidney function, time at risk, AKI severity, and serum albumin levels. Within this model, incremental increases in RIFLE stage (adjusted OR 4.43) and the need for dialysis (adjusted OR 53.18) were the most potent independent predictors of stage IV CKD during 5-year follow-up period.
Another recent study retrospectively examined if the magnitude of post-operative changes in serum creatinine independently predicted the development and progression of CKD or mortality in 29,388 patients undergoing cardiac surgery.[50**] Approximately 70% of patients were without evidence of impaired kidney function at baseline (eGFR < 60 ml/min/1.73 m2) with an overall modest distribution of AKI severity (>90% experiencing less than a doubling of serum creatinine). Even within this milder AKI phenotype, a monotonic relation was observed between incrementally worse injury (<25%, 25-49%, 50-99%, >100% increases in serum creatinine) and the risk for incident CKD (adjusted hazard ratios (aHRs), 2.1, 4.0, 5.5, and 6.6, respectively, p<0.01), progression of CKD stage in those with pre-existing kidney dysfunction (aHRs 2.5, 3.8, 4.4, 8.0, p<0.01), and mortality (aHRs 1.4, 1.9. 2.8, and 5.0, p<0.01) at 3 months. The risks observed attenuated modestly over time but persisted up to at least 5 years following surgery.
Another potential harbinger of injury severity not captured in current definitions of AKI is the duration of injury. Two recent studies in surgical patients examined the persistence of injury as an added dimension in predicting poor outcomes among AKI survivors. Coca[51**] examined how the duration of injury (≤2 days, 3-6 days, ≥7 days) added incremental value to AKI Network stages in predicting long-term mortality (median follow-up 3.7 years) in 35,302 diabetic Veterans undergoing non-cardiac surgery. While confirming earlier associations between AKIN stage and long-term mortality risk, the investigators results were driven by the subgroup of patients with the longest duration of injury. (Figure 2) A dose-dependent increase in the risk for mortality with longer injury duration was observed and often provided more prognostic information than AKIN stage alone. For example, extended duration with mild injury (AKIN Stage 1) conferred a nearly 2-fold higher risk for mortality than in those with severe (AKIN Stage 3) injury but of short duration (≤2 days). A subsequent study using identical duration cutoffs was performed in a cohort of 4,987 cardiac surgery patients in the northeastern United States.[52**] Similar incremental risk for long-term mortality was observed with increasing duration of injury. Whether duration may be a surrogate indication of the nature or extent of parenchymal injury or add to conventional real-time risk stratification tools (e.g. urine sediment or sodium retention) is unclear. However, these data suggest it as an important component of injury worth incorporating into future AKI classification scheme iterations.
Figure 2. Longitudinal Mortality Rates by Magnitude and Duration of AKI.
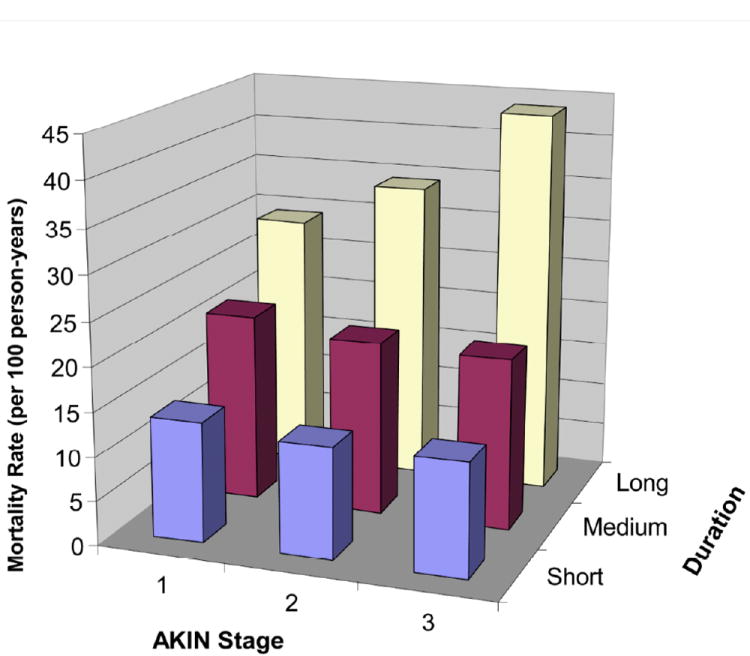
(previously published).[51] Coca, et al. examined the relationship between duration and magnitude of AKI in 35,302 diabetic patients following cardiac surgery and long-term survival. No increase in mortality rate was observed with increasing AKI severity of short or medium duration. However, the mortality rate increased by duration of AKI within each AKIN stage. The mortality rate for those with AKIN Stage 1 and long duration of AKI is more than 2-fold higher than for those with AKIN Stage 3 and short duration of AKI.[51]
The majority of studies examining the longitudinal effects of AKI have compared patients with an isolated episode of AKI during hospitalization to those without a concomitant event. However, few have considered the risk conferred by multiple AKI events. Thakar [53**] recently followed a high-risk cohort of 3,679 diabetic patients, 62% with baseline proteinuria, within an integrated health care system for the development of stage IV CKD over a mean of 5 years. Despite overall preserved baseline kidney function (mean eGFR 81 +/- 26 ml/min/1.73 m2), fourteen percent of the population experienced an AKI event with nearly one-third of this group experiencing multiple events. Patients experiencing an AKI event were twice more likely to reach stage IV CKD than those who did not (24.6% versus 12.9%, p<0.01). Multivariate Cox regression analysis identified the presence of any AKI to be associated with an aHR of 3.5 (95% CI: 2.7-4.6) with each subsequent episode of AKI further doubling that risk (HR 2.02: 95% CI: 1.78-2.30). In addition to confirming earlier associations between prior AKI and future CKD, these findings suggest that AKI often begets further AKI and uncover a potential limitation of current study designs that restrict ascertainment of AKI or non-AKI status to a single time point.
As the interaction between AKI and CKD becomes clearer, improved understanding of how to optimally care for this growing population will be needed. One potential quality indicator for high-risk patients following AKI is the rate of nephrology referral. We examined the likelihood of nephrology referral among 3,929 survivors of AKI whose last eGFR was < 60 ml/min/1.73 m2 30 days following peak injury in a United States Department of Veterans Affairs database.[54**] Time to nephrology referral was determined over the subsequent 12-month period treating improvement in kidney function, dialysis initiation, and death as competing risks. Overall mortality during the surveillance period was 22%. The cumulative incidence of nephrology referral before dying, initiating dialysis, or experiencing an improvement in kidney function was 8.5% (95% CI: 7.6-9.4) within the entire cohort. (Figure 3) The absolute referral rate among the subgroup of survivors at 12 months who neither improved kidney function nor initiated dialysis was only 19%. These data suggest only a minority of at-risk survivors following AKI is seen by a nephrologist. Of note, a substantial proportion of patients in this study (44%) experienced improvements in eGFR to > 60 ml/min/1.73 m2 by the end of the surveillance period. Taken together, these data highlight the need for detailed prospective studies that can identify survivors at highest risk following AKI, examines the potential benefit of nephrology referral, and the optimal patient care models to meet this need.
Figure 3. Cumulative Incidences of Nephrology Referral, Dialysis Initiation, Improvement in Kidney Function, and Death Analyzed as Competing Risks.
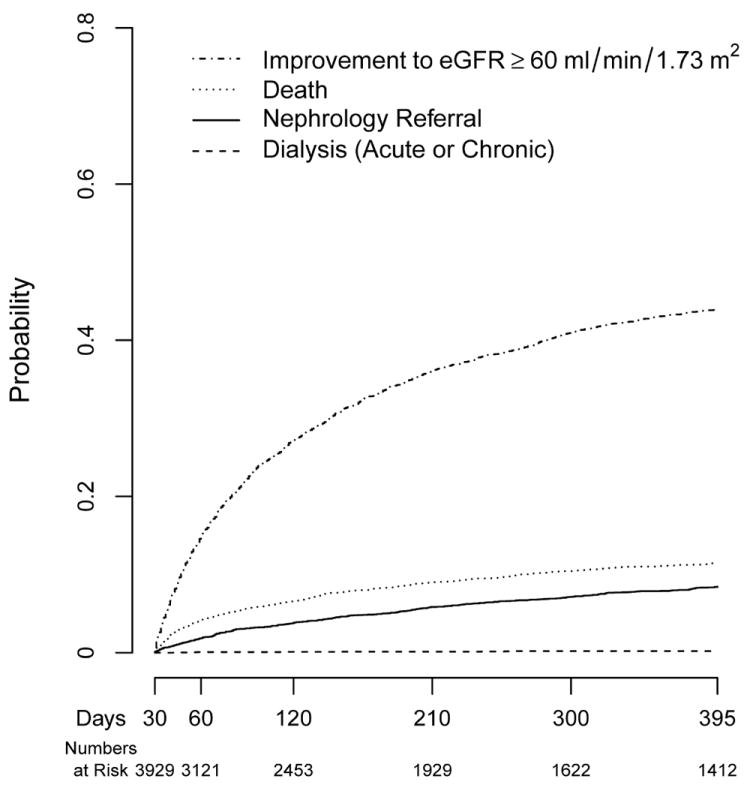
(previously published).[54] Siew, et al. examined outpatient nephrology referrals among 3929 survivors of AKI whose last eGFR up to 30 days following peak injury was < 60 ml/min/1.73 m2 in a multi-center Veterans Affairs Cohort. Cumulative incidences of the prespecified outcomes as competing risks during the 12-month surveillance period (30-395 days following peak injury) are shown. Beginning at 30 days following peak injury, the cumulative incidences of first improving kidney function to an eGFR >60 ml/min/1.73 m2, dying, being referred to nephrology or receiving dialysis were 44.0% (95% CI: 42.4-45.5), 11.5% (95% CI: 10.5-12.5), 8.5% (95% CI: 7.6-9.4), and 0.2% (95% CI: 0.1-0.4), respectively.
Conclusion
In summary, steady increases in the observed rate of AKI coupled with increasing awareness of its association with poor short- and long-term outcomes has driven efforts to better determine those at highest risk. Recent work has helped to reinforce the link between CKD as a risk factor for AKI by uncovering proteinuria as a novel risk factor not routinely assessed, further examined the ability of novel markers of injury to provide important diagnostic and prognostic information beyond creatinine alone in larger, more adequately phenotyped cohorts, and identified potent predictors of the long-term complications of AKI and potential opportunities to improve care of this population.
Key Points.
Baseline proteinuria is an easily measurable risk factor for the development of AKI.
Early results from larger multicenter studies investigating the diagnostic role of novel AKI biomarkers have been less robust agreement with creatinine-defined AKI than in earlier studies.
Further research that extends beyond how much AKI biomarkers markers agree with serum creatinine is desperately needed to determine their clinical utility.
There is a growing population of AKI survivors at risk for the development of CKD and its complications.
AKI severity and duration are important determinants of poor long-term outcome and can help stratify those that may benefit from closer monitoring or nephrology referral.
Acknowledgments
EDS is supported by a grant 1K23 DK088964-01A1 from the National Institutes for Diabetes and Digestive and Kidney Diseases. SMD is supported by International Society of Nephrology/Turkish Society of Nephrology fellowship award. We would like to thank Andrew J. Vincz for his technical assistance.
Footnotes
Conflict of Interest: EDS has a consulting arrangement with Alere, Inc.
Contributor Information
Edward D. SIEW, Division of Nephrology and Hypertension, Vanderbilt University Medical Center, Nashville, TN, edward.siew@vanderbilt.edu
Serpil M. DEGER, Division of Nephrology and Hypertension, Vanderbilt University Medical Center, Nashville, TN, serpil.deger@vanderbilt.edu
References
- 1.Hsu CY, McCulloch CE, Fan D, et al. Community-based incidence of acute renal failure. Kidney Int. 2007;72:208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 3.Mehta RL, Pascual MT, Soroko S, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66:1613–1621. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 4.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. Jama. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 5.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 6.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009 doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. Jama. 2009;302:1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 9.Waikar SS, Curhan GC, Wald R, et al. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17:1143–1150. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- 10.Hirschberg R, Kopple J, Lipsett P, et al. Multicenter clinical trial of recombinant human insulin-like growth factor I in patients with acute renal failure. Kidney Int. 1999;55:2423–2432. doi: 10.1046/j.1523-1755.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 11.Lewis J, Salem MM, Chertow GM, et al. Atrial natriuretic factor in oliguric acute renal failure. Anaritide Acute Renal Failure Study Group. Am J Kidney Dis. 2000;36:767–774. doi: 10.1053/ajkd.2000.17659. [DOI] [PubMed] [Google Scholar]
- 12.Palevsky PM, Zhang JH, O’Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellomo R, Cass A, Cole L, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 14.Pannu N, Klarenbach S, Wiebe N, et al. Renal replacement therapy in patients with acute renal failure: a systematic review. JAMA : the journal of the American Medical Association. 2008;299:793–805. doi: 10.1001/jama.299.7.793. [DOI] [PubMed] [Google Scholar]
- 15.Hsu CY, Ordonez JD, Chertow GM, et al. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–107. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Pannu N, James M, Hemmelgarn BR, et al. Modification of outcomes after acute kidney injury by the presence of CKD. Am J Kidney Dis. 2011;58:206–213. doi: 10.1053/j.ajkd.2011.01.028. This is a large population-based studies showing a graded increase in the risk for AKI with worsening baseline eGFR. [DOI] [PubMed] [Google Scholar]
- 17*.Grams ME, Astor BC, Bash LD, et al. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol. 2010;21:1757–1764. doi: 10.1681/ASN.2010010128. Two population-based cohort studies demonstrating baseline proteinuria as an independent risk factor for AKI and its complications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.James MT, Hemmelgarn BR, Wiebe N, et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet. 2010;376:2096–2103. doi: 10.1016/S0140-6736(10)61271-8. Two population-based cohort studies demonstrating baseline proteinuria as an independent risk factor for AKI and its complications. [DOI] [PubMed] [Google Scholar]
- 19**.Hsu RK, Hsu CY. Proteinuria and reduced glomerular filtration rate as risk factors for acute kidney injury. Curr Opin Nephrol Hypertens. 2011;20:211–217. doi: 10.1097/MNH.0b013e3283454f8d. A prospective cohort study demonstrating baseline proteinuria as an independent risk factor for AKI following cardiac surgery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang TM, Wu VC, Young GH, et al. Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. J Am Soc Nephrol. 2011;22:156–163. doi: 10.1681/ASN.2010050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh P, Rifkin DE, Blantz RC. Chronic kidney disease: an inherent risk factor for acute kidney injury? Clinical journal of the American Society of Nephrology : CJASN. 2010;5:1690–1695. doi: 10.2215/CJN.00830110. [DOI] [PubMed] [Google Scholar]
- 22.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008;73:538–546. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 23.Cruz DN, Bagshaw SM, Ronco C, Ricci Z. Acute kidney injury: classification and staging. Contrib Nephrol. 2010;164:24–32. doi: 10.1159/000313717. [DOI] [PubMed] [Google Scholar]
- 24.Siew ED, Matheny ME, Ikizler TA, et al. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int. 2010;77:536–542. doi: 10.1038/ki.2009.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Lafrance JP, Miller DR. Defining acute kidney injury in database studies: the effects of varying the baseline kidney function assessment period and considering CKD status. Am J Kidney Dis. 2010;56:651–660. doi: 10.1053/j.ajkd.2010.05.011. Cohort studies demonstrating how the use of different estimates of baseline function can affect the accuracy of AKI classification) [DOI] [PubMed] [Google Scholar]
- 26*.Bagshaw SM, Uchino S, Cruz D, et al. A comparison of observed versus estimated baseline creatinine for determination of RIFLE class in patients with acute kidney injury. Nephrol Dial Transplant. 2009;24:2739–2744. doi: 10.1093/ndt/gfp159. Cohort studies demonstrating how the use of different estimates of baseline function can affect the accuracy of AKI classification) [DOI] [PubMed] [Google Scholar]
- 27**.Liu KD, Thompson BT, Ancukiewicz M, et al. Acute kidney injury in patients with acute lung injury: Impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med. 2011;39:2665–2671. doi: 10.1097/CCM.0b013e318228234b. Secondary analysis of the Fluid and Catheter Treatment Trial (FACTT) demonstrating how the diagnosis of AKI can be missed or delayed by failure to correct serum creatinine measurements for fluid balance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–427. doi: 10.1038/ki.2009.159. AKI cohort studies describing the negative prognostic influence of fluid overload. [DOI] [PubMed] [Google Scholar]
- 29*.Grams ME, Estrella MM, Coresh J, et al. Fluid balance, diuretic use, and mortality in acute kidney injury. Clinical journal of the American Society of Nephrology : CJASN. 2011;6:966–973. doi: 10.2215/CJN.08781010. AKI cohort studies describing the negative prognostic influence of fluid overload. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Parikh CR, Devarajan P, Zappitelli M, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. Primary results from the landmark TRIBE-AKI consortium examining the predictive ability of neutrophil gelatinase-associated lipocalin and Interleukin-18 to provide diagnostic and prognostic information in high-risk patients undergoing cardiac surgery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Parikh CR, Devarajan P, Zappitelli M, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011;22:1737–1747. doi: 10.1681/ASN.2010111163. Primary results from the landmark TRIBE-AKI consortium examining the predictive ability of neutrophil gelatinase-associated lipocalin and Interleukin-18 to provide diagnostic and prognostic information in high-risk patients undergoing cardiac surgery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–1761. doi: 10.1016/j.jacc.2010.11.051. Pooled data analysis indicating that NGAL can provide additional prognostic information in predicting the need for RRT or mortality beyond creatinine alone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krawczeski CD, Goldstein SL, Woo JG, et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol. 2011;58:2301–2309. doi: 10.1016/j.jacc.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endre ZH, Pickering JW, Walker RJ, et al. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int. 2011;79:1119–1130. doi: 10.1038/ki.2010.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krawczeski CD, Woo JG, Wang Y, et al. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr. 2011;158:1009–1015. e1001. doi: 10.1016/j.jpeds.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 36.de Geus HR, Bakker J, Lesaffre EM, le Noble JL. Neutrophil gelatinase-associated lipocalin at ICU admission predicts for acute kidney injury in adult patients. Am J Respir Crit Care Med. 2011;183:907–914. doi: 10.1164/rccm.200908-1214OC. [DOI] [PubMed] [Google Scholar]
- 37.Doi K, Negishi K, Ishizu T, et al. Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit. Crit Care Med. 2011;39:2464–2469. doi: 10.1097/CCM.0b013e318225761a. [DOI] [PubMed] [Google Scholar]
- 38.Shlipak MG, Coca SG, Wang Z, et al. Presurgical serum cystatin C and risk of acute kidney injury after cardiac surgery. Am J Kidney Dis. 2011;58:366–373. doi: 10.1053/j.ajkd.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srisawat N, Murugan R, Lee M, et al. Plasma neutrophil gelatinase-associated lipocalin predicts recovery from acute kidney injury following community-acquired pneumonia. Kidney Int. 2011;80:545–552. doi: 10.1038/ki.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perry TE, Muehlschlegel JD, Liu KY, et al. Plasma neutrophil gelatinase-associated lipocalin and acute postoperative kidney injury in adult cardiac surgical patients. Anesth Analg. 2010;110:1541–1547. doi: 10.1213/ANE.0b013e3181da938e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siew ED, Ikizler TA, Gebretsadik T, et al. Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin J Am Soc Nephrol. 2010;5:1497–1505. doi: 10.2215/CJN.09061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thakar CV, Worley S, Arrigain S, et al. Improved survival in acute kidney injury after cardiac surgery. Am J Kidney Dis. 2007;50:703–711. doi: 10.1053/j.ajkd.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 43.Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000-2007) Chest. 2011;140:1223–1231. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 44.Coca SG. Acute kidney injury in elderly persons. Am J Kidney Dis. 2010;56:122–131. doi: 10.1053/j.ajkd.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson S, Eldadah B, Halter JB, et al. Acute kidney injury in older adults. J Am Soc Nephrol. 2011;22:28–38. doi: 10.1681/ASN.2010090934. [DOI] [PubMed] [Google Scholar]
- 46.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney international. 2011 doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Wu VC, Huang TM, Lai CF, et al. Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney international. 2011;80:1222–1230. doi: 10.1038/ki.2011.259. A meta-analysis and two observational cohort studies describing the association between AKI and the risk for long-term mortality and decline in kidney function. [DOI] [PubMed] [Google Scholar]
- 48*.James MT, Ghali WA, Tonelli M, et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney international. 2010;78:803–809. doi: 10.1038/ki.2010.258. A meta-analysis and two observational cohort studies describing the association between AKI and the risk for long-term mortality and decline in kidney function. [DOI] [PubMed] [Google Scholar]
- 49*.Chawla LS, Amdur RL, Amodeo S, et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79:1361–1369. doi: 10.1038/ki.2011.42. A meta-analysis and two observational cohort studies describing the association between AKI and the risk for long-term mortality and decline in kidney function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Archives of internal medicine. 2011;171:226–233. doi: 10.1001/archinternmed.2010.514. Two large Veterans Affairs Studies showing how the severity of AKI is a robust predictor of long-term mortality or decline in kidney function. [DOI] [PubMed] [Google Scholar]
- 51**.Coca SG, King JT, Jr, Rosenthal RA, et al. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney international. 2010;78:926–933. doi: 10.1038/ki.2010.259. Two large Veterans Affairs Studies showing how the severity of AKI is a robust predictor of long-term mortality or decline in kidney function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Brown JR, Kramer RS, Coca SG, Parikh CR. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg. 2010;90:1142–1148. doi: 10.1016/j.athoracsur.2010.04.039. Two large observational cohorts demonstrating how duration of AKI predicts the risk for long-term survival independently of AKI severity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53**.Thakar CV, Christianson A, Himmelfarb J, Leonard AC. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011;6:2567–2572. doi: 10.2215/CJN.01120211. Two large observational cohorts demonstrating how duration of AKI predicts the risk for long-term survival independently of AKI severity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54**.Siew E, Peterson JF, Eden SK, Hung AM, Speroff T, Ikizler TA, Matheny ME. Outpatient Nephrology Referral Rates after Acute Kidney Injury. J Am Soc Nephrol. 2011:23. doi: 10.1681/ASN.2011030315. A study demonstrating low nephrology referral rates among survivors of AKI with diminished kidney function in the year following hospital discharge. [DOI] [PMC free article] [PubMed] [Google Scholar]


