Abstract
Objective
Atherosclerosis is a chronic inflammatory disease initiated by monocyte recruitment and retention in the vessel wall. An important mediator of monocyte endothelial interaction is the chemokine IL-8. The oxidation products of phospholipids, including Ox-PAPC, accumulate in atherosclerotic lesions and strongly induce IL-8 in human aortic endothelial cells (HAECs). The goal of this study was to identify the proximal events leading to induction of IL-8 by Ox-PAPC in vascular endothelial cells.
Methods and Results
In a systems genetics analysis of HAECs isolated from 96 different human donors, we showed that HBEGF transcript levels are strongly correlated to IL-8 induction by Ox-PAPC. The silencing and overexpression of HBEGF in HAECs confirmed the role of HBEGF in regulating IL-8 expression. HBEGF has been shown to be stored in an inactive form and activation is dependent on processing by a dysintegrin and metalloproteinases (ADAM) to a form that can activate EGF receptor (EGFR). Ox-PAPC was shown to rapidly induce HBEGF processing and EGFR activation in HAECs. Using siRNA we identified three ADAMs that regulate IL-8 induction and directly demonstrated that Ox-PAPC increases ADAM activity in the cells by substrate cleavage assay. We provide evidence for one mechanism of Ox-PAPC activation of ADAM involving covalent binding of Ox-PAPC to cysteine on ADAM. Free thiol cysteine analogs showed inhibition of IL-8 induction by Ox-PAPC, and both a cysteine analog and a cell surface thiol blocker strongly inhibited ADAM activity induction by Ox-PAPC. Using microarray analyses, we determined that this ADAM pathway may regulate as much as 30% of genes induced by Ox-PAPC in HAECs.
Conclusion
This study is the first report demonstrating a role for the ADAM-HBEGF-EGFR axis in Ox-PAPC induction of IL-8 in HAECs. These studies highlight a role for specific ADAMs as initiators of Ox-PAPC action and provide evidence for a role of covalent interaction of Ox-PAPC in activation of ADAMs.
Keywords: Oxidized phospholipids, atherosclerosis, endothelium, metaloproteinase, HBEGF, EGFR
INTRODUCTION
Oxidative stress in the vascular system has been shown to be an important factor in the development of inflammation, including the inflammatory response in atherosclerosis1. Previous studies suggested that oxidation products of phospholipids are mediators that link oxidative stress to the development of chronic inflammation in the vascular system2. The oxidation of phospholipids occurs by specific enzymes or non-enzymatically by reactive oxygen species (ROS), which accumulate in inflammatory sites3, 4. Oxidized phospholipids are present in lipoproteins5, and in addition, several reports showed that membrane lipid components of cells exposed to apoptosis, oxidative stress, and necrosis, also contain oxidized phospholipids5, 6.
In this study we employed Ox-PAPC (oxidized 1-palmitoyl-2-arachidonyl-sn-glycerol-3-phosphocholine) as a representative oxidized phospholipid. The parent molecule PAPC is an endogenous phospholipid, which is a component of both low-density lipoprotein (LDL) and cell membrane7. Previously we reported that Ox-PAPC strongly activates human aortic endothelial cell (HAEC) and regulates the expression of more than 1,000 genes8. Using microarray analysis we determined that Ox-PAPC induced multiple pathways including redox regulation (e.g., HO-1), unfolded protein response (UPR) (e.g., ATF3), procoagulant processes (e.g., TF), sterol synthesis (e.g., LDLR), and inflammation (e.g., IL-8). Multiple, independent pathways appear to mediate the response to Ox-PAPC. For example, we previously reported that VEGF receptor 2 (VEGFR2) and NADPH oxidase 4 (NOX4) are mediators for Ox-PAPC induced expression of IL-8 but not HO-19, 10, and that the anti-inflammatory molecule high-density lipoprotein (HDL) selectively inhibits Ox-PAPC induced IL-8 but not HO-1 expression. We also obtained evidence that the UPR signaling regulated both basal and Ox-PAPC induced IL-8 expression in HAECs11. However, we did not identify the primary event(s) regulating these pathways. In this study, we focused on IL-8, a gene highly induced by Ox-PAPC, in order to identify the primary events by which Ox-PAPC induces inflammation. IL-8 has been demonstrated to play an important role in monocyte recruitment and retention in atherosclerosis12. Thus understanding the mechanism regulating IL-8 expression is important to understand the inflammatory function of Ox-PAPC.
In a previous study we employed a systems genetics approach to identify genes regulated by Ox-PAPC. These studies involved microarray analysis of HAEC from 96 human donors. In the current study, to gain insight into the regulation of IL-8, we used the array data to identify genes whose expression levels showed significant correlation to IL-8 induction by Ox-PAPC. From this approach, we determined that basal expression of heparin-binding EGF-like growth factor (HBEGF) was the most correlated gene with IL-8 induction by Ox-PAPC.
HBEGF is a ligand for EGFR on the cell surface and requires processing by subfamily of metalloproteinase for its activation. In this report, we show that Ox-PAPC activation of specific subtypes of ADAM (a dysintegrin and metalloproteinase) leads to processing of HBEGF to an active form. We also demonstrate that, in response to Ox-PAPC, HBEGF binds to EGFR and leads to an increase of IL-8 expression in the cells. We present evidence that Ox-PAPC covalently binds to cysteine residues on ADAMs, leading to its activation. Using knockdown of one of the ADAM family members responsible for Ox-PAPC induction of IL-8, we show that this ADAM pathway may regulate as much as 30% of genes induced by Ox-PAPC. Thus, these studies combine systems genetics studies with cell biology studies to identify a novel mechanism of IL-8 regulation by Ox-PAPC in the vascular endothelium.
METHODs
(Further details are described in supplementary method section)
Cell treatment
Unless otherwise indicated HAEC were changed to M199 medium containing 1% fetal bovine serum for 30min before the start of the experiments. Cells were then incubated with or without Ox-PAPC in medium containing 1% serum. We used media as contral treatment because the bioactive components of Ox-PAPC if fractional and unoxidized PAPC is insoluble in the reaction media. In some experiments cells were pretreated with chemical inhibitors for 1 hr and co-treated with Ox-PAPC for the indicated times.
Transfection of plasmids or siRNAs
Cells were treated with plasmid or siRNA complexes with Lipofectamine 2000 (Invitrogen) for 4 hours in Opti-MEM medium (Invitrogen) and the medium was replaced with growth media. Cells were used for experiments after 2 days of cell growth. The specific silencing of target genes were confirmed by qRT-PCR and additionally Western blotting.
ADAM substrate cleavage assay
The activity of endogenous ADAMs in HAECs or of commercial recombinant ADAM10 (Calbiochem, active form containing 215–673aa of precursor ADAM10) were determined using a fluorogenic ADAM substrate (BioMol, Dabcyl-Leu-Ala-Gln-Ala-Homophe-Arg-Ser-Lys(5-FAM)-NH2). The product formation was determined by fluoroscence mesurement using excitation at 485nm and emission at 520nm.
Microarray data analysis
The expression levels of 22,000 transcripts after 4 hr treatment of control media and Ox-PAPC (40ug/ml) in HAECs isolated from 96 donors were analyzed using Affymetrix HT-HU133A microarrays as previously described13. In a separate array analysis, to examine the effects of ADAM10 silencing in gene expression profiles, HAECs were transfected with ADAM10 siRNA as described above. After treatment of cells with control media and Ox-PAPC (40ug/ml) for 4 hrs, total RNAs were extracted from the cells to analyze the expression level of 22,000 transcripts using Affimetrix array (GeneChip Human Genome U133 Plus 2.0) in UCLA microarray core facility. After data normalization using RMA using Affy R package with cutoff of FDR<0.05, the list of genes up- or downregulated by 1.5 fold by Ox-PAPC were considered as Ox-PAPC responsive genes. The genes sensitive to ADAM10 silencing were determined by more than 20% reversal of Ox-PAPC effect.
Statistical analysis
Two-tailed student t-test was used to evaluate the difference between two groups; p < 0.05 was regarded as a statistically significant.
RESULTS
IL-8 induction by Ox-PAPC is strongly correlated with levels of heparin-binding EGF-like growth factor (HBEGF) transcript in 96 donor endothelial cells
To find the primary events in Ox-PAPC induction of IL-8, we employed data from our previously reported systems biology approach13. In the previous report we showed significant variation of gene expression following Ox-PAPC treatment of HAECs isolated from 96 human donors, suggesting the presence of genetic factors regulating the EC response to Ox-PAPC. For this study we analyzed genome-wide transcript profiles of a subset of HAECs isolated from 96 donors13. In the HAECs from different donors we found a large variation in induction of IL-8 expression by Ox-PAPC (Figure 1). We confirmed a strong positive correlation of cellular IL-8 mRNA level to the IL-8 protein level in the cell supernatant using qRT-PCR and ELISA assay (data not shown). From this array data analysis we identified mRNA transcripts most correlated with the level of IL-8 induced by Ox-PAPC. The transcript across the sample population that most significantly correlated with IL-8 induction by Ox-PAPC was basal levels of IL-8 indicating a likely cis-acting regulatory element at the IL-8 locus. The second most significant was basal transcript levels of HBEGF (correlation p value of 3.46e-09). This strongly suggested that the basal expression level of HBEGF is an important determinant in Ox-PAPC regulation of IL-8 expression in HAECs.
Figure 1. Ox-PAPC induced IL-8 expression shows a high level of variation in HAECs isolated from 96 human donors.
HAECs were treated as duplicate with media (C) or Ox-PAPC (50ug/ml) for 4 hrs, and the IL-8 transcript levels were determined by microarray analysis as described in the method section. The intensity of signalings before (○) and after (●) Ox-PAPC treatment are shown on the Y axis, and the cell lines are shown along the X axis. The lines have been rank-ordered on the base of basal levels. Arrows indicate representative cell lines showing strong and weak induction of IL-8.
Ox-PAPC induction of IL-8 is regulated by HBEGF and its receptor EGF receptor
We next performed studies to validate the importance of HBEGF and its receptor EGFR in Ox-PAPC induction of IL-8 in endothelial cells. The silencing of HBEGF significantly reduced IL-8 expression in response to Ox-PAPC (Figure 2A). HBEGF siRNA transfection induced about 80% reducion in HAECs as determined by QRT-PCR (Suppl. Figure I). We confirmed the inhibition of Ox-PAPC induction of IL-8 protein by HBEGF silencing using Western blotting (Suppl. Figure IB) and ELISA (Suppl. Figure IC). We also demonstrated that overexpression of HBEGF in HAEC increased the induction of IL-8 by Ox-PAPC (Figure 2B). To further determine the role of HBEGF in Ox-PAPC induced IL-8 regulation, we employed HEK293 cells. HEK293 cells express EGFR but do not express HBEGF as determined by qRT-PCR (data not shown). Ox-PAPC alone does not induce IL-8 in 293 cells. However, the introduction of HBEGF into HEK293 cells caused these cells to become responsive to Ox-PAPC and showed induction of IL-8 expression (Figure 2C). The introduction of HBEGF expression alone without Ox-PAPC treatment did not induce IL-8 expression, suggesting that the maturation of HBEGF in response to Ox-PAPC is essential for IL-8 expression in the HEK293 cells. This indicated a mediating role of HBEGF in IL-8 induction by Ox-PAPC.
Figure 2. IL-8 induction is strongly regulated by HBEGF.
(A) HBEGF silencing decreased IL-8 induction by Ox-PAPC in HAECs. (B–C) Overexpression of HBEGF in HAECs (B) or the introduction of HBEGF in HEK293 cells (C) increased IL-8 expression induced by Ox-PAPC. Endogenous HBEGF level was undetectable in untransfected HEK293 cells. After 2 days following transfection of siRNA or HBEGF plasmid, cells were treated with Ox-PAPC (50ug/ml) for 4hrs and the IL-8 mRNA levels were determined by qRT-PCR. Western blotting and qRT-PCR were repeated at least three times and representative results shown. The silencing of target proteins was confirmed by qRT-PCR as shown in Suppl. Figure I. (D) Active HBEGF is increased in response to Ox-PAPC. HAECs were treated with Ox-PAPC (50ug/ml) for 0, 10, and 30min in duplicate, and the proteins in cell lysates were separated using SDS-PAGE. The formation of active HBEGF was determined by Western blotting. (E) Recombinant HBEGF significantly increased IL-8 in HAECs. HAECs were treated with HBEGF for 4 hrs and the levels of IL-8 were determined by qRT-PCR. The values were represented as mean + SD, *p<0.05 and ** p<0.01, N=3 for reactions.
We next determined whether Ox-PAPC could increase maturation of HBEGF to the active form. Previous reports showed that formation of active HBEGF could be detected in cell lysate14. Ox-PAPC rapidly induced processing of precursor HBEGF (23kDa) into the active forms (8–10 kDa) in HAEC (Figure 2D). This processing of HBEGF happened within 10 min of Ox-PAPC treatment, suggesting this process appears to be a proximal step for IL-8 induction. There was also an increase in the total amount of HBEGF precursor in the cells in 10–30min of Ox-PAPC treatment, possibly through enhanced translation of HBEGF in the cells. Processing may begin earlier but may not be detectable by our Western blotting method because of limited sensitivity.
As shown in Figure 2E, the commercially available recombinant HBEGF also caused a significant increase of the expression of IL-8 in HAECs although the fold induction was lower than Ox-PAPC. In array data showing the expression of EGFR ligands in HAECs, HBEGF was shown to be the only detectable EGFR ligand (Suppl. Table I).
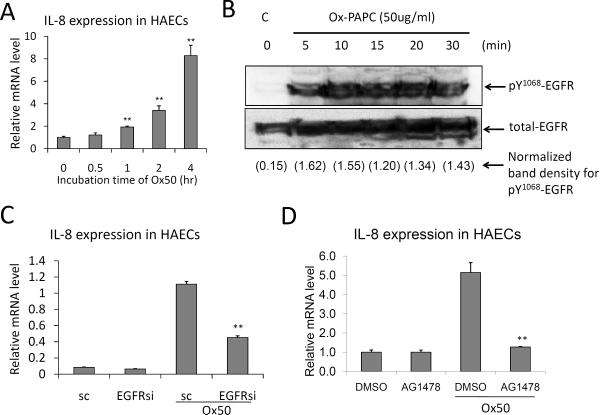
As shown in Figure 3A, Ox-PAPC caused a time-dependent increase of IL-8 expression in HAEC, being significantly increases only after 1 hour. HBEGF is a well-studied ligand of EGFR, but a role for EGFR in IL-8 induction by Ox-PAPC had not been previously reported. In this report, we demonstrated for the first time that Ox-PAPC rapidly activates EGFR in HAEC as determined by phosphorylation of Tyr1068 of the EGFR (Figure 3B). Either the silencing of EGFR or an EGFR inhibitor AG1478 significantly inhibited IL-8 induction by Ox-PAPC (Figure 3C and 3D). EGFR siRNA transfection showed 69% reduction of EGFR transcript levels in HAECs, which was confirmed by Western Blotting (Suppl. Figures IIA and IIB). We confirmed the EGFR silencing effects on IL-8 expression with the reduction of IL-8 protein levels in HAECs by Western blotting and in the cell supernatants shown by IL-8 ELISA. (Suppl. Figures IIC and IID). Taken together these data suggest that Ox-PAPC activates the maturation process of HBEGF in HAEC, leading to EGFR activation and IL-8 induction in the cells.
Figure 3. EGFR mediates the induction of IL-8 by Ox-PAPC in HAECs.
(A) Ox-PAPC increased IL-8 expression in HAECs in a time-dependent manner as determined by qRT-PCR. (B) Ox-PAPC activates EGFR. Cells were treated with Ox-PAPC (50ug/ml) for 0 to 30 min and the phosphorylation of Tyr1068 of EGFR was determined by Western blot using pY1068 -EGFR antibody. (C) EGFR silencing by siRNA transfection significantly reduced IL-8 induction by Ox-PAPC (50ug/ml, 4hrs). The mRNA content of IL-8 was determined by qRT-PCR. Multiple siRNAs for EGFR with different nucleotide sequences showed similar effects. The silencing of target proteins was confirmed by qRT-PCR and Western blotting as shown in Suppl. Figure II. (D) EGFR inhibitor reduced IL- expression by Ox-PAPC. Cells were pretreated with EGFR inhibitor AG1498 (10uM) for 1hr, and cotreated with Ox-PAPC (50ug/ml) for 4hrs. Vehicle (DMSO) was used as control treatment. The IL-8 mRNA levels were determined by qRT-PCR. Western blotting and qRT-PCR were repeated at least three times with reproducibility and representative results were shown. The values were represented as mean+SD, ** p<0.01, N=3 for reaction.
Specific metalloproteinases are mediators of IL-8 induction by Ox-PAPC in HAECs
It has previously been shown that EGFR signaling depends upon the processing of HBEGF by ADAM, a subfamily of metalloproteinase, in various cell types but not in endothelial cells15–18. First, we tested the effect of metalloproteinase inhibitors on IL-8 induction by Ox-PAPC. As shown Figure 4A, Ox-PAPC induction of IL-8 expression was inhibited by panmetalloproteinase inhibitor GM6001. Additional metalloproteinase inhibitors including epigallocatechin gallate (EGCG), phenanthroline, and EDTA showed similar inhibition effects (EGCG data shown in Suppl. Figure III). ADAM inhibitor TAPI-1 also significantly inhibited EGFR activation by Ox-PAPC in HAECs likely by inhibition of HBEGF processing in the cell surface (Figure 4B).
Figure 4. Specific ADAMs regulates IL-8 induction by Ox-PAPC in HAECs.
(A) Metalloproteinase mediates IL-8 expression in HAECs. HAECs were pretreated with the pan-metalloproteinase inhibitor GM6001 (10uM) for 1hr and co-treated with Ox-PAPC (50ug/ml, Ox50) for 4hrs, and the mRNA levels of IL-8 were determined by qRT-PCR. (B) Metalloproteinase mediates EGFR activation by Ox-PAPC in HAECs. Metalloproteinase inhibitor TAPI-1 reduced EGFR activation by Ox-PAPC in HAECs. Cells were pretreated with TAPI-1 (10uM) for 1 hr and co-treated with Ox-PAPC (50ug/ml) for 20min, and the relative EGFR activation was determined by Western blotting using pY1068 specific -EGFR antibody. (C, D) ADAM10, ADAM19, and ADAMTS4 mediate IL-8 expression by Ox-PAPC in HAECs. Gene silencing of ADAM10, ADAM19, and ADAMTS4 showed significant reductions in IL-8 expression by Ox-PAPC in HAECs. HAECs were treated and qRT-PCR performed as in (A). The silencing of target proteins was confirmed by qRT-PCR and Western blotting as shown in Suppl. Figures IV and V. Cell treatment and qRT-PCR were repeated at least three times and representative results shown. The values were mean values + SD, ** p<0.01, N=3 for each PCR reaction.
In order to identify the subtypes of ADAM that were responsible for Ox-PAPC induced IL-8 expression, we tested the effect of silencing of selected metalloproteinases expressed in HAECs. Approximately 20 subtypes of metalloproteinases are detectable in HAECs by microarray analysis (Suppl. Table II). Among them, we tested the effects of silencing of 10 metalloproteinases that were either highly expressed or had been shown to mediate atherosclerosis by other research groups (MMP1/-2/-9, ADAM10/-15/-17/-19, ADAMTS1/-4/-9)19, 20. From these silencing studies, as shown in Figures 4C and 4D, the ADAM10 knock-down modiestly reduced basal IL-8 levels and inhibited Ox-PAPC induction of IL-8 by about 60%. ADAM19 and ADAMTS4 silencings also decreased both basal and induced levels to the same extent. Silencing of the other subtypes did not cause changes of IL-8 expression (Suppl. Figure IV). The silencing efficiency and target specificity of siRNAs were confirmed using a representative group of ADAMs by qRT-PCR (Suppl. Figures VA–C) and Western blotting using ADAM specific antibodies (Suppl. Figures VD–F). We confirmed the inhibition of IL-8 expression by ADAM silencings using IL-8 ELISA, which showed that the ADAM silencings significantly reduced IL-8 secretion from HAECs (Suppl. Figures VG–H).
Ox-PAPC increases ADAM enzyme activity in HAECs
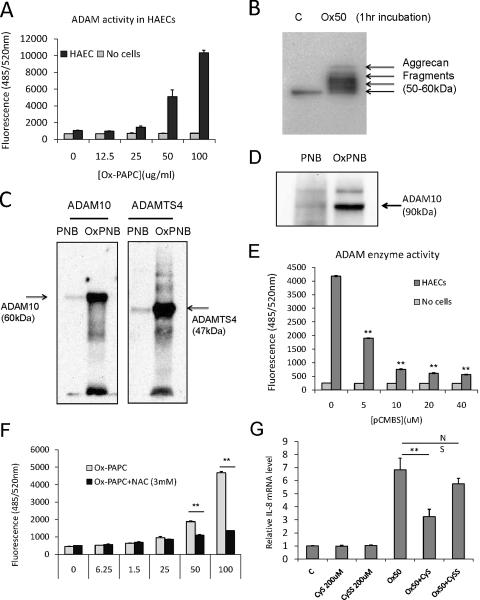
In order to verify upregulation of ADAM activity in response to Ox-PAPC we measured the processing of fluorogenic ADAM substrate21. For these studies cells were incubated with Ox-PAPC for one hour and the substrate was added for an additional hour. As shown in Figure 5A, Ox-PAPC dose-dependently increased processing of the ADAM substrate within 1hr of treatment. The pan-metalloproteinase inhibitors (EGCG or TAPI-1) dose-dependently inhibited the substrate processing validating the activity measurements (Suppl. Figure VIA–B). As an additional test of ADAM activation, HAECs were treated for 1 hour with Ox-PAPC and the processing of cellular aggrecan and release of products into the supernatant was detected (Figure VB). Aggrecan is a well-defined substrate for ADAMTS family22. These data demonstrated the upregulation of ADAM activity by Ox-PAPC in HAECs.
Figure 5. Ox-PAPC increases ADAM activities in HAECs and covalently interacts with ADAMs.
(A) Ox-PAPC dose-dependently increased ADAM activity in HAECs. HAEC were treated for 1 hour with different doses of Ox-PAPC and ADAM activity in the cells was determined using fluorogenic ADAM substrate as described in Methods section. (B) Ox-PAPC induced the digestion of endogenous ADAM-TS substrate aggrecan in HAECs. HAECs were treated with Ox-PAPC (50ug/ml) for 1hr, and the content of aggrecan fragments in the cell supernatants were determined by Western blotting using aggrecan-specific antibody. (C) Oxidized phospholipids covalently interact with recombinant ADAM10 and ADAMTS4 commercially available. Active form of ADAM10 and ADAMTS4 (1ug) were incubated with unoxidized and oxidized PAPE-N-biotin (50ug/ml) for 1 hr, and the reactions were run in SDS-PAGE in reducing condition. The presence of biotin-labeled protein was detected by probing with streptavidin-HRP. (D) Oxidized phospholipids also covalently interact with endogenous ADAM10 in HAECs. HAECs were incubated with unoxidized and oxidized PAPE-N-biotin (50ug/ml) for 1 hr, and the lysates in RIPA buffer were incubated with avidin-beads overnight for precipitation. After stringent washing with RIPA, the presence of ADAM10 biotin-labeled was detected by probing with ADAM10-specific antibody by Western blotting. (E) Cell surface free thiol is required for ADAM activation by Ox-PAPC in HAECs. HAECs were pretreated with differential doses of cell surface free thiol blocker p-chloromercuribenzene sulfonate (pCMBS) and Ox-PAPC (50ug/ml) for 1hr, and and ADAM activity as described in (A). The ranges of pCMBS used in this study did not show any obvious cell damage in HAECs. (F) The cysteine analog N-acetyl-L-cysteine (NAC) interacts with Ox-PAPC and inhibits ADAM activation in HAECs. HAECs were pretreated with different doses of NAC for 1hr in the presence of Ox-PAPC (50ug/ml), and ADAM activity as described in (A). (G) HAECs were pretreated with 200uM of cysteine or cystine for 1hr and cotreated with control media or Ox-PAPC (50ug/ml) for 4 hrs. The levels of IL-8 were determined by qRT-PCR. Western blotting and enzyme assay were repeated at least three times with reproducibility and representative results were shown. The values were represented as mean value + SD, ** p<0.01, N=3 for PCR or enzyme reactions.
Ox-PAPC interacts with cysteine residues of ADAMs
Studies from our group and others suggested that Ox-PAPC interaction with free thiols might be a mechanism by which Ox-PAPC activates ADAM enzyme23, 24. Previously our group reported that PEIPC, which is the most active lipid component in Ox-PAPC, can readily form Michael additions with reactive cysteine on proteins23. For some proteins covalent binding of Ox-PAPC was observed23. Other groups have shown that covalent interaction of metalloproteinase with electrophiles caused enhancement of enzyme activity25, 26. To examine a role for this thiol interaction in ADAM activation, we first determined the ability of Ox-PAPC to covalently interact with pure recombinant ADAM10 or ADAMTS4, which are commercially available. For this purpose, we used Ox-PAPE-N-biotin, which we have previously shown to act similarly to Ox-PAPC. Recombinant enzymes were incubated with unoxidized or oxidized PAPE-N-biotin to directly detect the covalent interaction between lipids with the enzymes. After incubation covalent adducts were detected with streptavidin-HRP. As shown in Figure 5C, Ox-PAPE-N-biotin, but not unoxidized PAPE-N-biotin, strongly interacted with recombinant ADAM10 and ADAMTS4. To determine if Ox-PAPC could covalently bind to endogenous ADAMs in cell culture, we treated cells with unoxidized or oxidized PAPE-N-biotin for 1hr. After precipitation of biotin-labeled proteins with neutravidin-beads, we detected the presence of ADAM10 by Western Blot. As shown in Figure 5D, only the oxidized form of the lipids covalently interacts with endogenous ADAM10 protein. We also detected significant covalent interactions of oxidized PAPE-N-biotin with ADAMTS4 (data not shown). We also tested the effects of p-chloromercuribenzenesulfonate (pCMBS), a cell-impermeable free thiol blocker, on ADAM activation by Ox-PAPC in HAECs. As shown in Figure 5E, pCMBS dose-dependently inhibited the Ox-PAPC induced ADAM activity of endothelial cells, suggesting the requirement of cell surface free thiol for the ADAM activation. A cysteine anlog N-acetyl-L-cysteine, which directly interacts with oxidized phospholipid, significantly inhibits ADAM enzyme activities induced by Ox-PAPC in HAECs (Figure 5F). In accordance with this hypothesis, the cysteine, which has free thiol, but not cystine a disulfide form of two cysteines, significantly reduced IL-8 induction by Ox-PAPC (Figure 5G).
We also tested a role of the p38 kinase signaling molecule in regulating ADAM activity in the cells as a previous report showed that another endogenous oxidative stress molecule, H2O2, activates ADAM via p38 in COS7 cells27. However, in HAECs the effect of p38 inhibitor was not observed. (Suppl. Figure VII), suggesting different mechanism explains ADAM activation in the two cell types.
Effects of ADAM 10 silencing on Ox-PAPC regulation of other genes
In addition to the induction of IL-8 expression by Ox-PAPC, we examined the effects of ADAM10 silencing on the Ox-PAPC regulated transcriptome in HAECs using microarray analysis. In this analysis, Ox-PAPC showed up-regulation of 301 genes and down-regulation of 256 genes by 1.5 fold with FDR <0.05. Among these genes approximately 30% of genes exhibited ADAM10-dependent regulation (Table 1). The lists and categories of these Ox-PAPC-regulated genes are shown in Suppl. Tables III–X. ADAM10-regulated genes are enriched in GO categories for response to cytokine stimulus, cell proliferation, and programmed cell death (Suppl. Tables of VI and X). From this array analysis we concluded that selective group of genes that are regulated by Ox-PAPC, are ADAM10-sensitive.
Table 1.
ADAM10 regulates the expression of multiple genes in response to Ox-PAPC*.
| Ox-PAPC regulated genes | ||||
|---|---|---|---|---|
| Up-regulated 301 (54%) | Down-regulated 256 (46%) | Total 557 | ||
| ADAM10-regulated | ADAM10-independent | ADAM10-regulated | ADAM10-independent | |
| 110 (20%) | 191 (34%) | 74 (13%) | 182 (33%) | |
| 251 (33%) | ||||
HAECs were transfected with ADAM10 siRNA for silencing. Cells were then incubated with control media or Ox-PAPC (40ug/ml) for 4 hrs in triplicate. The total RNAs were isolated for microarray chip analysis. The 22K transcripts were analyzed using Affimetrix microarray chips as described in the Methods section. The genes upregulated or downregulated by Ox-PAPC by 1.5 fold were determined. Among Ox-PAPC regulated genes, we identified genes that silencing of ADAM10 reversed the Ox-PAPC effects by more than 1.2 fold. Detailed procedures and lists of the genes and gene categories are provided in Supplementary Methods section and Suppl. Tables III–X.
DISCUSSION
We present evidence for a novel mechanism by which Ox-PAPC regulates the increased expression of the inflammatory gene IL-8 in vascular endothelial cells (Figure 6). In this signaling pathway, Ox-PAPC activates specific ADAMs that process HBEGF into active form. Active HBEGF then activates EGFR, leading to IL-8 induction. This pathway is activated in less than 10 minutes of Ox-PAPC treatment, significantly before IL-8 induction is observed, suggesting that it is upstream of many of Ox-PAPC effects, including IL-8 induction. The exact time course of formation of active HBEGF and the time course of EGFR activation are determined by methods which depend on sensitivity of each assay. Our study shows that both are very early events and that IL-8 induction by Ox-PAPC is heavily dependent on HBEGF. However, we cannot rule out the possibility that direct activation of EGFR may have some role in IL-8 induction. Previous reports showed that EGFR can be activated by covalent interaction with electrophiles like 4-hydroxynonenal for its activation28, 29.
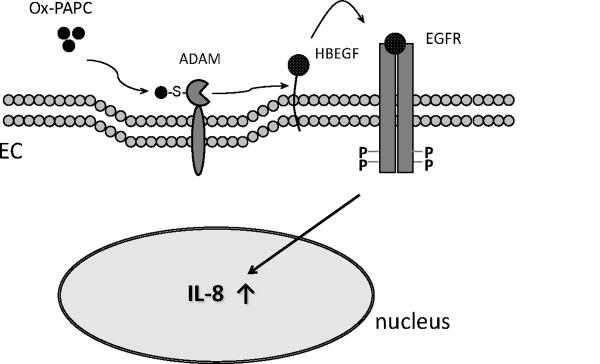
Figure 6. Hypothetical diagram depicting the proximal steps of Ox-PAPC signaling of IL-8 induction in vascular endothelial cells.
Ox-PAPC activates ADAMs on the vascular endothelial cell surface, and the activated ADAM processes HBEGF for EGFR activation, leading to IL-8 expression in the nucleus.
Although the role of ADAM in processing of ligands for EGFR is well defined30, there have previously been no reports of oxidized phospholipid activation of ADAMs. Though we have mainly focused on the effect of the ADAM-HBEGF-EGFR pathway on IL-8 induction, we also have presented evidence of a more broad importance of this pathway in induction of multiple genes by Ox-PAPC as shown in Table 1.
As mentioned above, a key player during EGFR transactivation process is HBEGF, which is a substrate of ADAM on the cell surface30. As shown in Suppl. Table I, the expression level of HBEGF is much higher than the other EGFR ligands in HAECs. Several groups have reported a correlation of HBEGF levels to the formation of atherosclerotic lesions31, 32. These observations suggest that the ADAM pathway described here is important in atherosclerosis.
Our data provide evidence for a mechanism of ADAM activation involving covalent interaction of Ox-PAPC with free thiols on ADAMs. We originally suspected that a previously reported mechanism for ADAM activation by H2O2, involving p38 activation, might also be involved. However, we saw no effect of P38 inhibition (Suppl. Figure VII). We demonstrated that pCMBS, a cell-impermeable free thiol blocker, strongly reduced ADAM activity in response to Ox-PAPC (Figure 5E). We previously reported that the most active lipid in Ox-PAPC for induction IL-8 is PEIPC. This lipid has an αβ unsaturated bond, adjacent to an epoxide bond, making it a strong electrophile for cysteine23. In previous microarray studies we observed that PEIPC regulates the expression of 80% of the genes regulated by Ox-PAPC33.
We also reported that Ox-PAPC can interact with proteins via covalent modification23. This study showed the direct interactions of oxidized phospholipids with recombinant or endogenous ADAM10 and ADAMTS4 (Figures 5C and 5D). Furthermore, incubation of Ox-PAPC with cysteine analog blocked the effect of Ox-PAPC on ADAM activity (Figure 5F). Previous reports showed the presence of `cysteine switch' in the inactivating domain of some ADAMs, which prevented activation of the enzyme30. When this cysteine is released from the interaction with Zn++ ion at the catalytic site, the enzyme becomes activated. The change of thiol modification of ADAM17 by oxidative stress or by thiol isomerase was shown to be a regulatory mechanism of enzyme activity24. The concept that Ox-PAPC can regulate metalloproteinase activity is supported by a report34.
Our studies demonstrated that at least three ADAM proteins are involved in IL-8 induction by Ox-PAPC: ADAM10, ADAM19, and ADAMTS4 (Figures 4C and D). In this report, we focused on ADAM10 because its effect was most specific for Ox-PAPC. Furthermore ADAM10 has been previously shown to be involved in processing of HBEGF30. However, it is clear that the other ADAMs also have a strong effect on IL-8 induction. ADAMTS4 has been shown to act to breakdown matrix molecules and we previously demonstrated that Ox-PAPC causes the breakdown of aggrecan, a protegoglycan35. We hypothesize that this breakdown releases HBEGF from the matrix, contributing to the activation of EGFR. ADAM19 is important in heart development36 and reported to be involved in the processing of neuregulin, an EGFR ligand37. Another group showed up-regulation of ADAMTS1 in the HUVEC by Ox-PAPC treatment38, suggesting it may also play a role in some Ox-PAPC regulatory events.
It is now becoming clear from studies from many labs including ours that a number of receptors participate in Ox-PAPC action. These effects depend on the cell type used and the oxidized phospholipids employed. It is also clear that even within one cell type more than one receptor can be involved in a particular effect. In this paper we report a role for MP in activation of the HBEGF/EGFR axis to induce IL-8 expression. We have previously shown that VEGFR2 also plays a role in IL-8 induction10. Silenicng of either VEGFR2 or EGFR does not cause complete inhibition of IL-8 induction by Ox-PAPC10. Thus these two receptors may have both separate and combined roles in regulating IL-8 induction and this interaction will be explored in future studies. These two receptors are the only ones we have shown to consistently control IL-8 induction by Ox-PAPC in HAEC.
The novel mechanism for the control of IL-8 expression reported in the current study was suggested by the results of a systems biology study from our group. This systems study examined at the role of natural variation in regulating the Ox-PAPC response in HAECs. Such an approach provides a filter for the many cell signaling pathways that might have controlled IL-8 induction and highlighted an unsuspected pathway of IL-8 induction. Furthermore, the fact that this pathway correlates with a natural variation suggests its importance in human endothelial cell inflammatory responses.
In summary, as depicted in Figure 6, we demonstrated a novel mechanism for regulation of IL-8 expression by Ox-PAPC in HAECs involving an ADAM-HBEGF-EGFR pathway. These studies highlight the potential importance of ADAM activation by Ox-PAPC as a regulator of the early events of atherosclerosis and suggest one mechanism by which ADAM may be activated involving direct binding to PEIPC or other cysteine binding molecules in Ox- PAPC. In contrast to cancer therapy, the therapeutic application of metalloproteinase inhibitors in cardiovascular diseases has received little attention mainly because of lack of information about the role of metalloproteinase in the development of atherosclerosis. In particular, the roles of ADAM are relatively new compared to the other types of metalloproteinases such as MMP2 and MMP939. Our studies and those of others suggest that ADAMs play an important role in the development of atherosclerosis.
Supplementary Material
Acknowledgements
We want to acknowledge to Nam Che for the preparation of the plasmid of HBEGF.
Sources of funding: This research was supported by NIH grants HL30568 (JAB and AJL), 1K99HL105577 (SD), and Ruth L. Kirschstein National Research Service Award T32HL69766 (JRS), and American Heart Association pre- and postdoctoral fellowships (CR, SDL).
Non-standard Abbreviations and Acronyms
- ADAM
a dysintegrin and metalloproteinase
- ADAMTS
ADAM-thrombospondin like domain
- EC
endothelial cell
- EGCG:
epigallocatechin gallate
- EGFR
epidermal growth factor receptor
- HAEC
human aortic endothelial cell
- HBEGF
heparin-binding EGF-like growth factor
- HO-1
heme oxygenase-1
- IL-8
interleukin-8
- MP
metalloproteinase
- Ox-PAPC
oxidized 1-palmitoyl-2-arachidonyl-sn-glycerol-3-phosphocholine
Footnotes
Disclosures: None.
REFERENCES
- 1.Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2311–2316. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 2.Levitan I, Volkov S, Subbaiah PV. Oxidized ldl: Diversity, patterns of recognition, and pathophysiology. Antioxid Redox Signal. 2010;13:39–75. doi: 10.1089/ars.2009.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stockl J. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2010;12:1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yla-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, Witztum JL, Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berliner JA, Leitinger N, Tsimikas S. The role of oxidized phospholipids in atherosclerosis. J Lipid Res. 2009;50(Suppl):S207–212. doi: 10.1194/jlr.R800074-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huber J, Vales A, Mitulovic G, Blumer M, Schmid R, Witztum JL, Binder BR, Leitinger N. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler Thromb Vasc Biol. 2002;22:101–107. doi: 10.1161/hq0102.101525. [DOI] [PubMed] [Google Scholar]
- 7.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 8.Gargalovic PS, Imura M, Zhang B, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Patel S, Nelson SF, Horvath S, Berliner JA, Kirchgessner TG, Lusis AJ. Identification of inflammatory gene modules based on variations of human endothelial cell responses to oxidized lipids. Proc Natl Acad Sci U S A. 2006;103:12741–12746. doi: 10.1073/pnas.0605457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S, gharavi NM, Honda H, Chang I, Kim B, Jen N, Li R, Zimman A, Berliner JA. A role for nadph oxidase 4 in the activation of vascular endothelial cells by oxidized phospholipids. Free Radic Biol Med. 2009;47:145–151. doi: 10.1016/j.freeradbiomed.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimman A, Mouillesseaux KP, Le T, Gharavi NM, Ryvkin A, Graeber TG, Chen TT, Watson AD, Berliner JA. Vascular endothelial growth factor receptor 2 plays a role in the activation of aortic endothelial cells by oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2007;27:332–338. doi: 10.1161/01.ATV.0000252842.57585.df. [DOI] [PubMed] [Google Scholar]
- 11.Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Baruch-Oren T, Berliner JA, Kirchgessner TG, Lusis AJ. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:2490–2496. doi: 10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- 12.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, Luster AD, Luscinskas FW, Rosenzweig A. Mcp-1 and il-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 13.Romanoski CE, Lee S, Kim MJ, Ingram-Drake L, Plaisier CL, Yordanova R, Tilford C, Guan B, He A, Gargalovic PS, Kirchgessner TG, Berliner JA, lusis AJ. Systems genetics analysis of gene-by-environment interactions in human cells. Am J Hum Genet. 2010;86:399–410. doi: 10.1016/j.ajhg.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. Egf receptor transactivation by g-protein-coupled receptors requires metalloproteinase cleavage of prohb-egf. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 15.Higashiyama S, Iwabuki H, Morimoto C, Hieda M, Inoue H, Matsushita N. Membrane-anchored growth factors, the epidermal growth factor family: Beyond receptor ligands. Cancer Sci. 2008;99:214–220. doi: 10.1111/j.1349-7006.2007.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berasain C, Perugorria MJ, Latasa MU, Castillo J, Goni S, Santamaria M, Prieto J, Avila MA. The epidermal growth factor receptor: A link between inflammation and liver cancer. Exp Biol Med (Maywood) 2009;234:713–725. doi: 10.3181/0901-MR-12. [DOI] [PubMed] [Google Scholar]
- 17.Schneider MR, Wolf E. The epidermal growth factor receptor ligands at a glance. J Cell Physiol. 2009;218:460–466. doi: 10.1002/jcp.21635. [DOI] [PubMed] [Google Scholar]
- 18.Fischer OM, Hart S, Gschwind A, Prenzel N, Ullrich A. Oxidative and osmotic stress signaling in tumor cells is mediated by adam proteases and heparin-binding epidermal growth factor. Mol Cell Biol. 2004;24:5172–5183. doi: 10.1128/MCB.24.12.5172-5183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 20.Edwards DR, Handsley MM, Pennington CJ. The adam metalloproteinases. Mol Aspects Med. 2008;29:258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moss ML, Rasmussen FH. Fluorescent substrates for the proteinases adam17, adam10, adam8, and adam12 useful for high-throughput inhibitor screening. Anal Biochem. 2007;366:144–148. doi: 10.1016/j.ab.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 22.Porter S, Clark IM, Kevorkian L, Edwards DR. The adamts metalloproteinases. The Biochemical journal. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gugiu BG, Mouillesseaux K, Duong V, Herzog T, Hekimian A, Koroniak L, Vondriska TM, Watson AD. Protein targets of oxidized phospholipids in endothelial cells. J Lipid Res. 2008;49:510–520. doi: 10.1194/jlr.M700264-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Willems SH, Tape CJ, Stanley PL, Taylor NA, Mills IG, Neal DE, McCafferty J, Murphy G. Thiol isomerases negatively regulate the cellular shedding activity of adam17. The Biochemical journal. 2010;428:439–450. doi: 10.1042/BJ20100179. [DOI] [PubMed] [Google Scholar]
- 25.Rajagopalan S, Meng XP, Ramasamy S, harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Oliver P, Lancaster JR, Jr, Schwarzenberger PO, Joshi MS, Cork J, Kolls JK. Reactive oxygen species mediate tumor necrosis factor alpha-converting, enzyme-dependent ectodomain shedding induced by phorbol myristate acetate. Faseb J. 2001;15:303–305. doi: 10.1096/fj.00-0371fje. [DOI] [PubMed] [Google Scholar]
- 27.Fischer OM, Hart S, Gschwind A, Prenzel N, Ullrich A. Oxidative and osmotic stress signaling in tumor cells is mediated by adam proteases and heparin-binding epidermal growth factor. Molecular and cellular biology. 2004;24:5172–5183. doi: 10.1128/MCB.24.12.5172-5183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W, Akhand AA, Kato M, Yokoyama I, Miyata T, Kurokawa K, Uchida K, Nakashima I. 4-hydroxynonenal triggers an epidermal growth factor receptor-linked signal pathway for growth inhibition. J Cell Sci. 1999;112(Pt 14):2409–2417. doi: 10.1242/jcs.112.14.2409. [DOI] [PubMed] [Google Scholar]
- 29.Suc I, Meilhac O, Lajoie-Mazenc I, Vandaele J, Jurgens G, Salvayre R, Negre-Salvayre A. Activation of egf receptor by oxidized ldl. Faseb J. 1998;12:665–671. doi: 10.1096/fasebj.12.9.665. [DOI] [PubMed] [Google Scholar]
- 30.Edwards DR, Handsley MM, Pennington CJ. The adam metalloproteinases. Mol Aspects Med. 2008;29:258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakata A, Miyagawa J, Yamashita S, Nishida M, Tamura R, Yamamori K, Nakamura T, Nozaki S, Kameda-Takemura K, Kawata S, Taniguchi N, Higashiyama S, Matsuzawa Y. Localization of heparin-binding epidermal growth factor-like growth factor in human coronary arteries. Possible roles of hb-egf in the formation of coronary atherosclerosis. Circulation. 1996;94:2778–2786. doi: 10.1161/01.cir.94.11.2778. [DOI] [PubMed] [Google Scholar]
- 32.Reape TJ, Wilson VJ, Kanczler JM, Ward JP, Burnand KG, Thomas CR. Detection and cellular localization of heparin-binding epidermal growth factor-like growth factor mrna and protein in human atherosclerotic tissue. J Mol Cell Cardiol. 1997;29:1639–1648. doi: 10.1006/jmcc.1997.0399. [DOI] [PubMed] [Google Scholar]
- 33.Romanoski CE, Lee S, Kim MJ, Ingram-Drake L, Plaisier CL, Yordanova R, Tilford C, Guan B, He A, Gargalovic PS, Kirchgessner TG, Berliner JA, Lusis AJ. Systems genetics analysis of gene-by-environment interactions in human cells. Am J Hum Genet. 2010;86:399–410. doi: 10.1016/j.ajhg.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai CF, Seshadri V, Huang K, Shao JS, Cai J, Vattikuti R, Schumacher A, Loewy AP, Denhardt DT, Rittling SR, Towler DA. An osteopontin-nadph oxidase signaling cascade promotes pro-matrix metalloproteinase 9 activation in aortic mesenchymal cells. Circ Res. 2006;98:1479–1489. doi: 10.1161/01.RES.0000227550.00426.60. [DOI] [PubMed] [Google Scholar]
- 35.Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (adamts) superfamily: Functions and mechanisms. The Journal of biological chemistry. 2009;284:31493–31497. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurohara K, Komatsu K, Kurisaki T, Masuda A, Irie N, Asano M, Sudo K, Nabeshima Y, Iwakura Y, Sehara-Fujisawa A. Essential roles of meltrin beta (adam19) in heart development. Developmental biology. 2004;267:14–28. doi: 10.1016/j.ydbio.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 37.Chesneau V, Becherer JD, Zheng Y, Erdjument-Bromage H, Tempst P, Blobel CP. Catalytic properties of adam19. The Journal of biological chemistry. 2003;278:22331–22340. doi: 10.1074/jbc.M302781200. [DOI] [PubMed] [Google Scholar]
- 38.Bochkov VN, Philippova M, Oskolkova O, Kadl A, Furnkranz A, Karabeg E, Afonyushkin T, Gruber F, Breuss J, Minchenko A, Mechtcheriakova D, Hohensinner P, Rychli K, Wojta J, Resink T, Erne P, Binder BR, Leitinger N. Oxidized phospholipids stimulate angiogenesis via autocrine mechanisms, implicating a novel role for lipid oxidation in the evolution of atherosclerotic lesions. Circ Res. 2006;99:900–908. doi: 10.1161/01.RES.0000245485.04489.ee. [DOI] [PubMed] [Google Scholar]
- 39.Murphy G, Nagase H. Progress in matrix metalloproteinase research. Mol Aspects Med. 2008;29:290–308. doi: 10.1016/j.mam.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.