Abstract
Background
Inflammatory cytokines dysregulate microvascular function, yet how cytokines affect lymphatic endothelial cells (LEC) are unclear.
Methods and Results
We examined effects of TNF-α, IL-1β, and IFN-γ on LEC proliferation, endothelial cell adhesion molecule (ECAM) expression, capillary formation, and barrier changes in murine (SV-LEC) and human LECs (HMEC-1a).
Results
All cytokines induced ICAM-1, VCAM-1, MAdCAM-1, and E-selectin in SV-LECs; TNF-α, IL-1β and IFN-γ induced ECAMs (but not MAdCAM-1) in HMEC-1a. IL-1β increased, while IFN-γ and TNF-α reduced SV-LEC proliferation. While TNF-α induced, IFN-γ decreased, and IL-1β did not show any effect on HMEC-1a proliferation. TNF-α, IL-1β, and IFN-γ each reduced capillary formation in SV-LEC and in HMEC-1a. TNF-α and IL-1β reduced barrier in SV-LEC and HMEC-1a; IFN-γ did not affect SV-LEC barrier, but enhanced HMEC-1a barrier. Inflammatory cytokines alter LEC growth, activation and barrier function in vitro and may disturb lymphatic clearance increasing tissue edema in vivo.
Conclusion
Therapies that maintain or restore lymphatic function (including cytokines blockade), may represent important strategies for limiting inflammation.
Introduction
Angiogenesis regulated by growth factor, hormone, and environmental signaling is active during development,1 and is now also recognized as an important event in inflammation. Angiogenesis is associated with blood vessels of an ‘immature,’ often ‘inflammatory,’ phenotype exhibiting increased leukocyte/platelet rolling, arrest, and extravasation, endothelial cell adhesion molecule (ECAM) expression,2,3 and solute permeability and participates in the etiology of chronic inflammatory diseases (e.g., IBD, arthritis, lupus, and diabetes, and models of these conditions).4,5 Angiogenesis links with inflammation have been better defined over the past few decades; similar discoveries of lymphatic-specific biomarkers and growth factors now permit similar investigations in lymphangiogenesis.
Lymphatic endothelial cells (LEC) are functionally, biochemically, and developmentally distinct from blood endothelial cells (BEC).6 In ‘lymphangiogenesis,’ new lymphatic capillaries can be generated by sprouting, migration, and proliferation from pre-existing LEC or recruitment of LEC progenitors.7–10 Changes in lymphatic function and proliferation alter interstitial fluid absorption and homeostasis, leukocyte and tumor cell traffic in tumor metastasis, lymphedema, and inflammation.11–13 After development, lymphangiogenesis14,15 is active during tissue repair, regeneration, and inflammation.16 Although BEC and LEC may respond to equivalent physiological levels of cytokines and exhibit similar responses in survival, migration, and proliferation, unregulated or supraphysiological increases in cytokines may initiate or drive inflammation-associated pathological events. Lymphangiogenesis17,18 might however have different effects on inflammation as compared to angiogenesis. For example, ECAMs expression on inflamed venules facilitate leukocyte extravasation into tissues.19,20 ECAM induction on 1° lymphatics might support the transfer of leukocytes and antigen-presentation cells to lymph nodes.21
Mediators that induce and regulate lymphatics are not yet defined, but an improved understanding of the role(s) played by modulation of lymphatic endothelial integrity could reduce inflammatory edema produced by LEC dysfunction. For example, TNF-α can induce BEC inflammation and edema, and TNF-α suppression enhances lymphangiogenesis.22 Although cytokines such as TNF-α participate in inflammatory angiogenesis in several clinical conditions,23–25 cytokines may also regulate lymphatic responses, and play roles in different phases of inflammation that are not yet well understood. To overcome limitations encountered with in vivo lymphangiogenesis models, we used human and mouse LECs to study lymphatic responses to inflammatory cytokines. We found that Th1 cytokines modulate ECAM expression, proliferation, capillary formation and barrier in mouse SV-LECs and human HMEC-1a. LEC, like BEC, are dysregulated by inflammatory cytokines and suggest that cytokine-mediated LEC dysfunction exacerbates inflammation, especially in disorders such as Crohns' disease and ulcerative colitis.
Materials and Methods
Cell culture
Our described mouse (SV-LEC) and human (HMEC-1a) cell lines were cultured as reported previously.26,27
Proliferation
TNF-α, IL-1β, and IFN-γ were purchased from Thermo/Fisher (Waltham, MA). 10% confluent cultures were treated with test agents in medium assaying at time points up to 72 h. LEC growth in response to cytokines was assessed by MTT assay.28 Cells without cytokines were used as 100% ‘control’ level of cell proliferation with N = 8 replicates.
LEC capillary tube formation assay
Capillary formation on Matrigel (BD Biosciences, San Jose, CA)was used to examine LEC responses to cytokines.29 Confluent LEC were incubated with cytokines in 10% DMEM for 72 h, loaded with calcein 2-AM (2 μg/ml, 30’) trypsinized, and plated onto Matrigel for 4 h. Capillaries were quantitated as described29 by counting the number of lymphatic capillaries from central focal planar digital micrographs.
ECAM expression in LEC cells
ECAM expression was assessed as described.26 LEC were treated with TNF-α, IL-1β, or IFN-γ for 24 h, and incubated with respective anti-ECAM antibody, followed by HRP-tagged 2° antibody. ECAM expression was analyzed and expressed as the % maximum (TNF-α induced) level of absorbance at 450 nm. All protocols were performed at least n = 4 replicates.
LEC trans-endothelial electrical resistance (TEER) in response to cytokines
LEC barrier was measured by TEER. LECs were cultured on 8 μm transwells and electrical resistance changes developed LEC monolayers in response to cytokines were measured 0–72 h using an Epithelial Volt Ohm Meter (WPI, Sevenage, United Kingdom).
Statistics
Data were analyzed for statistical differences using Instat (Graphpad Software, La Jolla, CA) for one-way ANOVA with Bonferroni post-testing.
Results
Effects of cytokines on lymphatic endothelial cell proliferation
SV-LEC
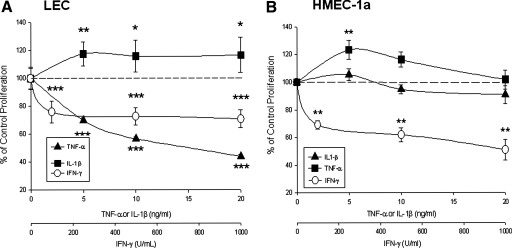
At 72 h, TNF-α and IFN-γ dose-dependently inhibited, while IL-1β dose-dependently increased proliferation. At 72 h, TNF-α decreased proliferation to 70 ± 2% of control at 5 ng/ml (***p < 0.001), to 57 ± 2% of control at 10 ng/ml (***p < 0.001), and to 44 ± 2% of control at 20 ng/ml (***p < 0.001) concentrations. We found all concentrations of IFN-γ (100, 500, and 1000 U/ml) decreased LEC proliferation. IFN-γ decreased proliferation to 76 ± 2% of control at 100 U/ml (***p < 0.001), to 73 ± 2% of control at 500 U/ml (***p < 0.001), and to 71 ± 2% of control at 1000 U/ml (***p < 0.001). IL-1β increased proliferation 18% over control at 5 ng/ml (118 ± 3% of control, *p < 0.05), 16% over control at 10 ng/ml (116 ± 4% of control, *p < 0.05), and 14% over control at 20 ng/ml (114 ± 3%, *p < 0.05) (Fig. 1).
FIG. 1.
SV-LEC proliferation and cytokines. (A) TNF-α, IFN-γ inhibited, IL-1β stimulated LEC proliferation (72 h). TNF-α, (5–20 ng/ml), IFN-γ (100–1000 U/ml) decreased LEC proliferation. IL-1β increased LEC proliferation: IL-1β, (5- 20 ng/ml). (B) HMEC-1a proliferation and cytokines: IFN-γ (100–1000 U/ml) dose dependently decreased HMEC-1a proliferation. IL-1β did not affect HMEC-1a proliferation. At 72 h, 5 ng/ml TNF-α increased HMEC-1a proliferation; (10 and 20 ng/ml:not significant).
HMEC-1a
At 72 h, IFN-γ dose dependently decreased lymphatic proliferation whereas IL-1β did not alter LEC proliferation. Interestingly, TNF-α significantly increased cell proliferation at low concentration; this effect was not seen at higher concentrations. At 72 h, TNF-α increased proliferation to 123 ± 6.80% of control at 5 ng/ml (**p < 0.01), to 116 ± 5.30% (ns) at 10 ng/ml, and to 101.74 ± 6.81% (ns) at 20 ng/ml concentrations. IL-1β had no effect on human LEC proliferation. At 72 h, IL-1β did not affect proliferation (105.45 ± 4.41% of control at 5 ng/ml (ns), 94.75 ± 3.24% of control at 10 ng/ml, and 90.87 ± 6.45% at 10 ng/ml). IFN-γ significantly decreased LEC proliferation at all concentrations. At 72 h, IFN-γ decreased proliferation to 69.04 ± 3.13% of control at 100 U/ml (**p < 0.01), to 61.78 ± 5.19% to that of control at 500 U/ml (**p < 0.01) and almost to half, (50.9 ± 7.63% at 1000 U/ml **p < 0.01) (Fig. 1).
Cytokine-induced LEC adhesion molecule expression
Previous studies show that cytokines induce LEC ICAM-1 and VCAM-1 in LEC in vivo21 at concentrations and kinetics reported to mobilize ECAMs in BEC.3,26 We therefore determined ECAM expression in LEC.
SV-LEC
ICAM-1 surface expression
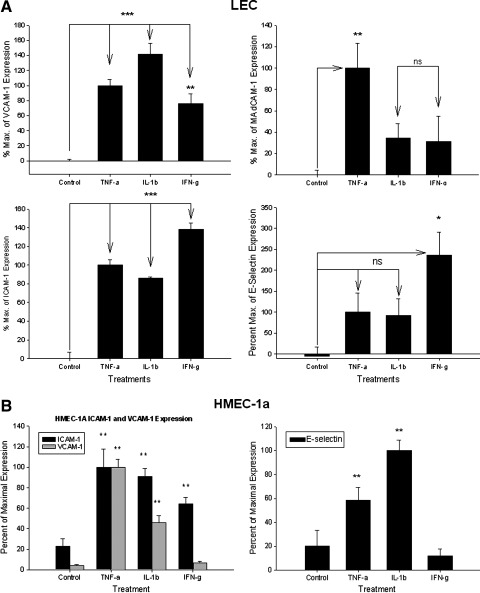
TNF-α (20 ng/ml) induced ICAM-1 expression (i.e.,100 ± 6% ‘maximum’ ICAM-1 expression, ***p < 0.001); IL-1β (10 ng/ml) induced ICAM-1 expression to 86 ± 1% of expression, (***p < 0.001); IFN-γ (1000 U/ml) induced ICAM-1 expression to 138 ± 7% maximum of TNF-α induced levels of ICAM-1 expression, (***p < 0.001) (Fig. 2a).
FIG. 2.
(A) ECAM expression in SV-LEC. ICAM-1. TNF-α (20 ng/ml) induced ICAM-1 over untreated controls; IL-1β (10 ng/ml) and IFN-γ (1000 U/ml) induced ICAM-1. VCAM-1. TNF-α (20 ng/ml) and IL-1β (10 ng/ml) and IFN-γ (1000 U/ml) induced VCAM-1. MAdCAM-1. TNF-α (20 ng/ml) significantly induced MAdCAM-1 expression. Neither IL-1β, nor IFN-γ induced MAdCAM-1. E-Selectin. Only IFN-γ (1000 U/ml) induced E-selectin on LEC. TNF-α (20 ng/ml) or IL-1β (10 ng/ml) slightly, (n.s.) increased E-selectin. (B) HMEC-1a ECAM expression. ICAM-1. TNF-α (20 ng/ml), IL-1β (10 ng/ml), and IFN-γ (1000 U/ml) each induced ICAM-1. VCAM-1. TNF-α (20 ng/ml), IL-1β (10 ng/ml) but not IFN-γ (1000 U/ml) induced VCAM-1. E-selectin. IL-1β and TNF-α induced E-Selectin on HMEC-1a cells.
VCAM-1 surface expression
TNF-α (20 ng/ml) induced VCAM-1 expression to 100 ± 8% maximum, (***p < 0.001); IL-1β (10 ng/ml) significantly induced VCAM-1 expression (141 ± 15% maximum, ***p < 0.001); IFN-γ (1000 U/ml) induced VCAM-1 expression (75 ± 14% maximum, **p < 0.01) (Fig. 2a).
MAdCAM-1 surface expression
TNF-α (20 ng/ml) induced MAdCAM-1 expression [set as 100 ± 23% (i.e., ‘maximum’), **p < 0.01]; IL-1β (10 ng/ml) did not induce MAdCAM-1 (34 ± 13% maximum, n.s.; IFN-γ (1000 U/ml) did not induce MAdCAM-1 expression (31 ± 24% maximum, ns) (Fig. 2a).
E-Selectin surface expression
Neither TNF-α (20 ng/ml) (100 ± 46% maximum) or IL-1β (10 ng/ml) significantly affected E-selectin expression (92 ± 40% maximum). Only IFN-γ (1000 U/ml) induced E-selectin expression on LEC (to 235 ± 55% maximum, *p < 0.05) (Fig. 2a).
HMEC-1a
ICAM-1 expression
TNF-α (20 ng/ml) induced ICAM-1 (100 ± 17.7% maximum, **p < 0.01). IL-1β (10 ng/ml) also induced ICAM-1 (90.68 ± 7.92% maximum, **p < 0.01); IFN-γ (1000 U/ml) induced ICAM-1 (but to only 64.34 ± 6.16% maximum, **p < 0.01) (Fig. 2b).
VCAM-1 expression
TNF-α (20 ng/ml) induced VCAM-1 expression (100 ± 7.94% maximum, **p < 0.01); IL-1β (10 ng/ml) also induced VCAM-1 (46.17 ± 6.77% maximum, **p < 0.01); IFN-γ (1000 U/ml) did not alter expression of VCAM-1 on HMEC-1a, and was different from its effect seen on SV-LEC (6.79 ± 1.64% maximum of VCAM-1) (Fig. 2b).
E-selectin expression
IL-1β showed the greatest induction of E-selectin on HMEC-1a cells. TNF-α (20 ng/ml) significantly induced E-Sel expression (58.75 ± 10.32% maximum, **p < 0.01); IL-1β (10 ng/ml) significantly induced E-Sel (100 ± 8.77% maximum, **p < 0.01); IFN-γ (1000 U/ml) did not significantly affect the expression of E-Selectin on HMEC-1a cells, also different from its effect seen on SV-LEC (only 11.91 ± 5.93% maximum, p > 0.05) (Fig. 2b).
MAdCAM-1 expression
No expression of surface MAdCAM-1 expression was observed in response to any of these cytokines on HMEC-1a (data not shown). Neither VEGF-C nor D (100 ng/ml, 24 h) increased ECAMs expression on human or mouse LEC (data not shown)
Effect of cytokines on LEC capillary formation. Cytokines reduce SV-LEC capillary formation
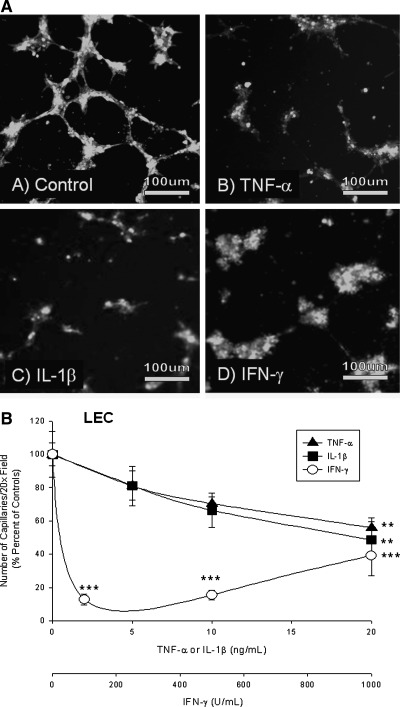
TNF-α and IL-1β significantly reduced LEC capillaries only at higher concentrations, while IFN-γ reduced capillary formation at all concentrations. TNF-α decreased LEC lymphatic capillaries by 19% at 5 ng/ml to 81 ± 12% of control (100%), (not sig.), by 30% 10 ng/ml (to 70 ± 4% of control (100%), n.s.), and 44% at 20 ng/ml to 56 ± 6% of control (100%, **p < 0.01). IL-1β decreased the number of SV-LEC LEC capillaries by 19% (5 ng/ml) to 81 ± 9% of control, (n.s.), 34% at 10 ng/ml to 66 ± 10% of control (100%), (n.s.), and 51% at 20 ng/ml (to 49 ± 11% of control, **p < 0.01). IFN-γ decreased the number of lymphatic capillaries by 87% at 100 U/ml (to 13 ± 3% of control, ***p < 0.001), 85% at 500 U/ml (to 15 ± 3% of control (100%), ***p < 0.001), and 61% at 1000 U/ml (to 39 ± 12% of control, ***p < 0.001) (Figs. 3a and 3b).
FIG. 3.
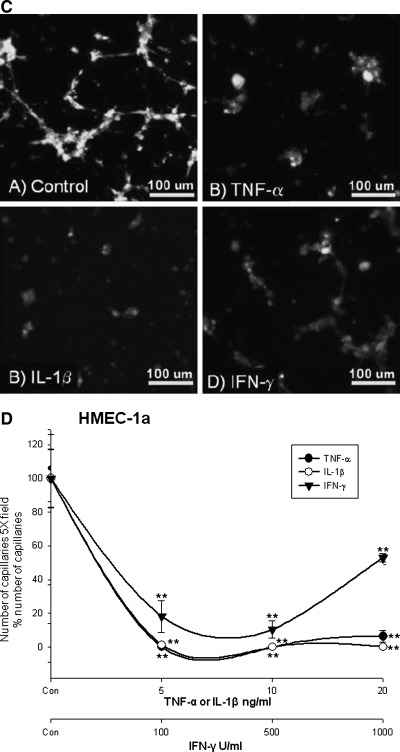
(A) Cytokines reduce SV-LEC capillary tube formation. TNF-α and IL-1β, (not IFN-γ) reduced numbers of lymphatic capillaries. TNF-α, IL-1β, and IFN-γ significantly decreased the number of lymphatic capillaries (all concentrations). (B) Micrographs of SV-LEC capillary tube formation. (C) HMEC-1a. TNF-α and IL-1β completely inhibited HMEC-1a tube formation. IFN- γ partly decreased lymphatic tube formation but was less effective than TNF-α/IL-1β. (D) Micrographs of HMEC-1a capillary tube formation.
Cytokines reduce HMEC-1a lymphatic capillary tube formation
HMEC-1a were analyzed by fluorescent activated cell sorting to check D2-40 expression. 78.6% of HMEC-1a were D2-40+. D2-40+ cells from HMEC-1a were sorted to obtain ∼98.1% pure D2-40+ cells. The D2-40+ cells obtained were used for performing capillary tube formation assay on Matrigel. All concentrations of TNF-α, IL-1β, and IFN-γ tested decreased LEC capillaries. However IFN- γ at higher concentration increased LEC capillary numbers compared to TNF-α and IL-1β (still less than controls). The rank order of lymphatic capillary inhibition was found to be TNF-α ∼ = IL-1β < IFN-γ. TNF-α completely inhibited HMEC-1a capillary formation (at 5 ng, 10 ng, and 20 ng/ml) at 72 h. IL-1β showed the same dose-effect as TNF-α. However IFN- γ at 100 U/ml decreased LEC capillary formation by 16.13 ± 9.31% (**p < 0.01), at 500 U/ml to 8.91 ± 5.16% (**p < 0.01) and at 1000 U/ml to 52.97 ± 2.58 % (**p < 0.01) vs. controls (Figs. 3c and 3d).
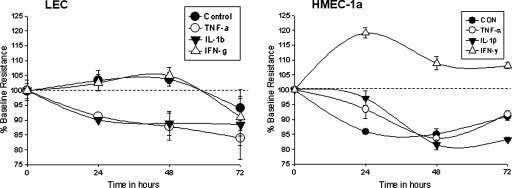
Trans endothelial electrical resistance (TEER)
SV-LEC
Control
Control TEER of SV-LEC remained stable without changes in resistance throughout the study (0 h = 100%, 24 h = 103.5 ± 3.51%, 48 h = 103.9 ± 2.23%, 72 h = 94.22 ± 3.97%).
TNF-α
20 ng/ml time-dependently decreased barrier in SV-LEC monolayers (0 h = 100%, 24 h = 91.3 ± 7.5% **p < 0.01, 48 h = 87.8 ± 4.6% **p < 0.01, 72h = 84 ± 2.48 **p < 0.01) (Fig. 4).
FIG. 4.
SV-LEC and HMEC-1a barrier. SV-LEC TEER of control cells was constant throughout the study. TNF-α and IL-1β time-dependently decreased SV-LEC TEER. IFN-γ did not alter TEER. HMEC-1a TEER. Control TEER of control cells decreased slightly over 72 h. TNF-α: TNF-α decreased TEER at 48–72 h. IL-1β: No significant alterations in HMEC-1a TEER with IL-1b (10 ng/ml). IFN-γ: IFN-γ significantly increased HMEC-1a TEER at 24–72 h.
IL-1β
IL-1β 10 ng/ml time-dependently decreased barrier in SV-LEC monolayers (0 h = 100%, 24 h = 90.1 ± 0.8 **p < 0.01, 48 h = 88.9 ± 4% **p < 0.01, 72 h = 88.5 ± 11.8 **p < 0.01) (Fig. 4).
IFN-γ
IFN-γ did not alter SV-LEC barrier compared with control over 72 h (0 h = 100%, 24 h = 102.5 ± 2.73%, 48 h = 105 ± 2.73% and 72 h = 91 ± 6.3%) (Fig. 4).
HMEC-1a
Control
Untreated HMEC-1a were used as controls. Barrier in control cells decreased significantly from 24 h to 72 h (0 h = 100%, 24 h = 85.95 ± 0.55 **p < 0.01, 48 h = 85.1 ± 1.72 **p < 0.01, 72 h = 91.03 ± 1.26 **p < 0.01) (Fig. 4).
TNF-α
TNF-α at 20 ng/ml did not alter the HMEC-1a barrier at 24 h. Barrier decreased at 48–72 h (0 h = 100%, 24 h = 93.45 ± 3.11, 48 h = 83.6 ± 1.15% **p < 0.01, 72 h = 91.74 ± 1.28% *p < 0.05) (Fig. 4).
IL-1β
No change in HMEC-1a barrier was observed with IL-1β (10 ng/ml treatment) at 24 h. Barrier decreased slightly (n.s.) from 48 h to 72h (0 h = 100%, 24 h =97.17 ± 2.38%, 48 h = 81.5 ± 1.63%, 72 h = 83.22 ± 0.77%) (Fig. 4).
IFN-γ
IFN-γ increased HMEC-1a barrier at all time points vs. controls or other treatments (0 h = 100%, 24 h = 119.07 ± 1.73% **p < 0.01, 48 h = 108.93 ± 2.25% *p < 0.05, and 72 h = 108.07 ± 1.04% *p < 0.05) (Fig. 4).
Discussion
The diverse physiological functions of BEC and LEC are regulated by several classes of vasoactive mediators. Lymphangiogenesis is regulated by signaling mechanisms, including VEGFs,6 cytokines,30,31 and environmental cues.32 Although studies demonstrate expansion of lymphatics within inflamed tissues,17 whether lymphangiogenesis during inflammation is beneficial or exacerbating is controversial. Besides lymphatic vessel density, characteristics of lymphatic vessels may be affected by amplification of cytokines at inflammatory sites. So far, few studies have considered how cytokine effects on LEC proliferation, capillary tube formation, and ECAM expression might influence inflammation.
Angiogenesis and lymphangiogenesis are complex processes modulated by immune cells, complex networks of cytokines, and VEGFs. Franchi et al. report inflammatory cytokines play a pivotal role in angiogenesis, proliferation, and ECAM expression.33,34 In BEC, TNF-α and IL-1β are angiogenic and upregulate ICAM-1 and VCAM-1. TNF-α has been reported to decrease while IL-1β increases proliferation. IFN-γ inhibits proliferation but upregulates ICAM-1 and VCAM-1.35,36 VEGF-A is itself an inflammatory cytokine that induces ICAM-1 in BEC.37 VEGF-A supports endothelial survival, proliferation, migration, capillary genesis and permeability, and inflammatory cell recruitment to BEC; VEGF-A also modulates LEC proliferation.38 VEGF-C released by platelets, monocytes, macrophages, and BEC,39 also regulates BEC proliferation, vascular permeability,40 capillary formation,41 inflammatory-activation,42 and leukocyte binding to BEC.37 VEGF-C and VEGF-D also play dominant roles in BEC and LEC survival, proliferation, migration, capillary formation in vivo and in vitro;43 their effects in inflammation are less well characterized.
Cytokines modulate lymphatic endothelial cell proliferation
VEGFs modulate BEC and LEC proliferation during development and disease.44 While TNF-α, IL-1β, and IFN-γ modulate BEC proliferation, capillary formation, and ECAM expression, effects of cytokines on LEC are not fully understood. We found that TNF-α, IL-1β, and IFN-γ dose-dependently influence mouse and human LEC. Previously TNF-α has been reported to reduce LEC proliferation.22,29 Interestingly TNF-α inhibited proliferation only at low (5 ng/ml) concentrations in human LEC; higher TNF-α levels did not inhibit growth. IL-1β released by leukocytes, mast cells, and BEC2 positively regulates BEC survival, proliferation, and angiogenesis in vivo. We found a similar effect in mouse LEC where proliferation was increased by IL-1β at 5–20 ng/ml. Human LEC did proliferate in response to IL-1β. IFN-α/IFN-γ inhibit mouse and human LEC proliferation and might be ‘anti-lymphangiogenic’.25 Consistent with those findings, we observed all concentrations of IFN-γ decreased human and mouse LEC proliferation.
Cytokine-mediated ECAM surface expression in SV-LEC and HMEC-1a cells
Inflammatory cytokines are central mediators of inflammation and potently induce adhesion molecules on BEC and LEC in vitro.25,45–49 While BEC upregulate ICAM-1, VCAM-1, MAdCAM-1, and E-selectin in response to inflammatory cytokines and VEGF-A,37 less information is available about the expression of these adhesion molecules on stimulated LEC. In human umbilical vein endothelial cells, VEGF-A and TNF-α synergistically enhance E-selectin expression, which recruits neutrophils to activated endothelium at sites of inflammation.50 While blood vascular ECAM expression brings leukocytes to sites of inflammation, lymphatic vascular ECAM expression may retain leukocyte populations at sites of inflammation or facilitate entry into lymphatics, potentially maintaining/resolving inflammation by regulating the exit of these leukocytes from inflamed tissues. An improved understanding of the cooperation between adhesive systems on both sides of the vascular system may enhance our ability to coordinate and regulate the entry and exit of leukocyte to control inflammation.
Johnson et al. reported that inflammatory cytokines upregulate LEC ICAM-1, VCAM-1, and E-selectin, and the chemokines MCP-1, RANTES, and MIP-3α.21,36 That study found high ICAM-1 and VCAM-1 induction in response to TNF-α (followed by IL-1β and IFN-γ. Similar studies using human neonatal LEC also found TNF-α dose-dependently increased VCAM-1 and ICAM-1.51 Furthermore TNF-α induces VCAM-1 and ICAM-1 expression on both BEC and LEC, but at a quantitatively lower level in LEC. Sawa et al. reported that lymphocyte transmigration across lymphatics may not depend on cytokines52 and that cytokines may influence other LEC behaviors independent of lymphocyte trafficking. Other studies have shown that TNF-α induces expression of ICAM-1, VCAM-1, MAdCAM-1, and E-selectin in the SVEC4-10 endothelial cell line isolated from murine (C3H/Hej) axillary lymph nodes, which represent high endothelial venular endothelial cells.53–55
We compared differences between ECAMs induction by cytokines in mouse and human LEC. In mouse LEC, ICAM-1, VCAM-1 and E-selectin were all potently induced by TNF-α, IL-1β, and IFN-γ. These responses did not show the rank order potency reported by Johnson et al.21 and may reflect differences in the anatomic origin of these cells (skin vs. mesentery), species or in vitro culture conditions. Only TNF-α (not IL-1β nor IFN-γ) induced MAdCAM-1 in mouse LEC. Human LEC showed different ECAM induction with cytokines. TNF-α and IL-1β increased ICAM-1, VCAM-1, and E-Sel in mouse LEC. IFN-γ induced ICAM-1, but neither VCAM-1 nor E-Sel. Moreover we could not induce MAdCAM-1 with any of cytokines (TNF-α, IL-1β, and IFN-γ) on human LEC. MAdCAM-1 is strongly expressed by high endothelial venules, gut, liver, and brain BEC, particularly after TNF-α exposure45 and plays central roles in T-cell immune responses. In mouse colon endothelial cells, MAdCAM-1 expression is increased by TNF-α but not IL-1β, or IFN-γ.46 Although MAdCAM-1 was weakly expressed on control mouse LEC, it was increased by TNF-α.46
Cytokines decrease LEC capillary formation
VEGF and cytokines (e.g., IL-18) can be both ‘angiogenic’ and inflammatory.37,56 Despite the decreased cell proliferation in mouse LEC with TNF-α, low TNF-α concentrations did not affect capillary formation, but higher TNF-α concentrations suppressed LEC capillary formation. IL-1β has been reported to increase angiogenesis;57 our present study found a significant decrease in capillary formation with IL-1β at 10 and 20 ng/ml, (no effect was seen at low levels). Conversely, IFN-γ has been reported as an ‘anti-angiogenic’ factor.33,35,58 We also found that IFN-γ potently reduced capillary tubes in mouse LEC. The rank order of cytokines disturbing LEC capillaries was IFN-γ > IL-1β > TNF-α. Similar cytokines effects were found in human LEC. While TNF-α and IL-1β reduced capillary tube formation, IFN-γ did not. Recently, Eph receptor-ephrin signaling has been linked to lymphatic remodeling;59,60 whether or how cytokines modulate Eph-ephrin signaling to affect lymphangiogenesis needs further investigation. Although inflammation is clearly associated with both increased lymphangiogenesis and elevated tissue cytokines, our results suggest that TNF-α, IL-1β, and IFN-γ may not be potent lymphangiogenic factors in vitro. We have previously reported that inflammation in experimental colitis is associated with a robust increase in lymphangiogenesis (and angiogenesis).61 Our findings clearly show that these cytokines inhibit lymphatic proliferation and capillary formation, and disturb many lymphatic properties leading to an inflamed phenotype. Therefore an inflamed lymphatic phenotype in vivo likely reflects complex synergic/antagonistic interactions between inflammatory mediators and growth factors that need to be identified and characterized.
Cytokines differentially affect lymphatic TEER
Cytokines such as TNF-α, IL-1β, and IFN-γ disrupt BEC integrity, leading to loss of solute barrier. Inflammation also increases lymphatic drainage; lymphangitis may aggravate clinical and experimental inflammation.62–65 BEC and LEC differential responses to cytokines might also reflect anatomical or species differences.66,67 Disturbances in LEC barrier, especially in 1° lymphatics could increase tissue edema. We found TNF-α and IL-1β decreased mouse and human LEC barrier at 24 h with a rank order in mouse of TNF-α ∼ = IL-1β > IFN-γ; in human LEC the order was: TNF > IL-1β > IFN-γ. IFN-γ did not affect mouse LEC barrier but increased human LEC. In vivo, lymphatic flow is driven by smooth muscle contraction, unidirectional valves, respiratory and skeletal muscle action. At high interstitial pressures, LEC capillaries are pulled open by anchoring emilin-1 and fibrillin filaments, facilitating drainage of fluid, macromolecules, and cells. Cytokine disturbances in clearance could intensify inflammatory disorders (e.g., IBD). It is unknown how LEC barrier changes interact with lymphatic contractility to govern fluid drainage to lymph nodes, but suggest that cytokine dysregulation of LEC barrier will exacerbate inflammation, both by maintenance of an inflamed LEC phenotype and disturbances in tissue architecture.
Conclusions
We describe several alterations in LEC in response to TNF-α, IL-1β, and IFN-γ. LEC ECAM expression to enhance trans-LEC immune cell trafficking or retention may modulate duration or intensity of inflammation. Reduced capillary formation by cytokines suggests that they are antilymphangiogenic at high physiological levels, exception of IFN-γ which decreased capillaries in mouse but has modestly increased in human LEC. Cytokines also dysregulate LEC barrier, leading to diminished fluid clearance and edema. These changes might disturb lymphangion pumping, and combined with altered LEC barrier could severely impair resolution of inflammation.68–71 Our results clearly show cytokines disturb LEC functions in both mouse and human LEC in vitro with differential responses of these two cell types to cytokines.
Author Disclosure Statement
The authors have no conflicts of interest or financial ties to disclose.
References
- 1.Adams RH. Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 2.Ali H. Haribabu B. Richardson RM. Snyderman R. Mechanisms of inflammation and leukocyte activation. Med Clin North Am. 1997;81:1–28. doi: 10.1016/s0025-7125(05)70503-4. [DOI] [PubMed] [Google Scholar]
- 3.Granger DN. Kubes P. The microcirculation and inflammation: Modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol. 1994;55:662–675. [PubMed] [Google Scholar]
- 4.Connor TJ. Harkin A. Kelly JP. Leonard BE. Olfactory bulbectomy provokes a suppression of interleukin-1beta and tumour necrosis factor-alpha production in response to an in vivo challenge with lipopolysaccharide: Effect of chronic desipramine treatment. Neuroimmunomodulation. 2000;7:27–35. doi: 10.1159/000026417. [DOI] [PubMed] [Google Scholar]
- 5.Shigematsu T. Specian RD. Wolf RE. Grisham MB. Granger DN. MAdCAM mediates lymphocyte-endothelial cell adhesion in a murine model of chronic colitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1309–G1315. doi: 10.1152/ajpgi.2001.281.5.G1309. [DOI] [PubMed] [Google Scholar]
- 6.Alitalo K. Tammela T. Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 7.Makinen T. Norrmen C. Petrova TV. Molecular mechanisms of lymphatic vascular development. Cell Mol Life Sci. 2007;64:1915–1929. doi: 10.1007/s00018-007-7040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He Y. Rajantie I. Ilmonen M, et al. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004;64:3737–3740. doi: 10.1158/0008-5472.CAN-04-0088. [DOI] [PubMed] [Google Scholar]
- 9.Kerjaschki D. Huttary N. Raab I, et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12:230–234. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- 10.Butler MG. Isogai S. Weinstein BM. Lymphatic development. Birth defects. Res C Embryo Today. 2009;87:222–231. doi: 10.1002/bdrc.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stacker SA. Williams RA. Achen MG. Lymphangiogenic growth factors as markers of tumor metastasis. APMIS. 2004;112:539–549. doi: 10.1111/j.1600-0463.2004.apm11207-0812.x. [DOI] [PubMed] [Google Scholar]
- 12.Ji RC. Lymphatic endothelial cells, lymphedematous lymphangiogenesis, and molecular control of edema formation. Lymphat Res Biol. 2008;6:123–137. doi: 10.1089/lrb.2008.1005. [DOI] [PubMed] [Google Scholar]
- 13.Schoppmann SF. Lymphangiogenesis, inflammation and metastasis. Anticancer Res. 2005;25:4503–4511. [PubMed] [Google Scholar]
- 14.Cueni LN. Detmar M. The lymphatic system in health and disease. Lymphat Res Biol. 2008;6:109–122. doi: 10.1089/lrb.2008.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karpanen T. Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol. 2008;3:367–397. doi: 10.1146/annurev.pathmechdis.3.121806.151515. [DOI] [PubMed] [Google Scholar]
- 16.Mouta C. Heroult M. Inflammatory triggers of lymphangiogenesis. Lymphat Res Biol. 2003;1:201–218. doi: 10.1089/153968503768330247. [DOI] [PubMed] [Google Scholar]
- 17.Geleff S. Schoppmann SF. Oberhuber G. Increase in podoplanin-expressing intestinal lymphatic vessels in inflammatory bowel disease. Virchows Arch. 2003;442:231–237. doi: 10.1007/s00428-002-0744-4. [DOI] [PubMed] [Google Scholar]
- 18.Fogt F. Pascha TL. Zhang PJ. Gausas RE. Rahemtulla A. Zimmerman RL. Proliferation of D2-40-expressing intestinal lymphatic vessels in the lamina propria in inflammatory bowel disease. Int J Mol Med. 2004;13:211–214. [PubMed] [Google Scholar]
- 19.Stokes KY. Clanton EC. Bowles KS, et al. The role of T-lymphocytes in hypercholesterolemia-induced leukocyte-endothelial interactions. Microcirculation. 2002;9:407–417. doi: 10.1038/sj.mn.7800148. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter AC. Alexander JS. Endothelial PKC delta activation attenuates neutrophil transendothelial migration. Inflamm Res. 2008;57:216–229. doi: 10.1007/s00011-007-7031-4. [DOI] [PubMed] [Google Scholar]
- 21.Johnson LA. Clasper S. Holt AP, et al. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203:2763–2777. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polzer K. D.Baeten D. A.Soleiman A, et al. Tumour necrosis factor blockade increases lymphangiogenesis in murine and human arthritic joints. Ann Rheum Dis. 2008;67:1610–1616. doi: 10.1136/ard.2007.083394. [DOI] [PubMed] [Google Scholar]
- 23.Rosell A. Arai K. Lok J, et al. Interleukin-1beta augments angiogenic responses of murine endothelial progenitor cells in vitro. J Cereb Blood Flow Metab. 2009;29:933–943. doi: 10.1038/jcbfm.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thapa D. Lee JS. Park MA, et al. Inhibitory effects of clotrimazole on TNF-alpha-induced adhesion molecule expression and angiogenesis. Arch Pharm Res. 2009;32:593–603. doi: 10.1007/s12272-009-1416-6. [DOI] [PubMed] [Google Scholar]
- 25.Shao X. Liu C. Influence of IFN- alpha and IFN- gamma on lymphangiogenesis. J Interferon. Cytokine Res. 2006;26:568–574. doi: 10.1089/jir.2006.26.568. [DOI] [PubMed] [Google Scholar]
- 26.Ando T. Jordan P. Joh T, et al. Isolation and characterization of a novel mouse lymphatic endothelial cell line: SV-LEC. Lymphat Res Biol. 2005;3:105–115. doi: 10.1089/lrb.2005.3.105. [DOI] [PubMed] [Google Scholar]
- 27.Cho WG. Albuquerque RJ. Kleinman ME, et al. Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth. Proc Natl Acad Sci USA. 2009;106:7137–7142. doi: 10.1073/pnas.0812317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carpenter A. Rassam A. Jennings MH, et al. Effects of ammonium tetrathiomolybdate, an oncolytic/angiolytic drug on the viability and proliferation of endothelial and tumor cells. Inflamm Res. 2007;56:515–519. doi: 10.1007/s00011-007-7025-2. [DOI] [PubMed] [Google Scholar]
- 29.Wells SR. Jennings MH. Rome C. Hadjivassiliou V. Papas KA. Alexander JS. Alpha-, gamma- and delta-tocopherols reduce inflammatory angiogenesis in human microvascular endothelial cells. J Nutr Biochem. 2010;21:589–597. doi: 10.1016/j.jnutbio.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Jackson DG. Biology of the lymphatic marker LYVE-1 and applications in research into lymphatic trafficking and lymphangiogenesis. APMIS. 2004;112:526–538. doi: 10.1111/j.1600-0463.2004.apm11207-0811.x. [DOI] [PubMed] [Google Scholar]
- 31.Jackson DG. Lymphatic markers, tumour lymphangiogenesis and lymph node metastasis. Cancer Treat Res. 2007;135:39–53. doi: 10.1007/978-0-387-69219-7_4. [DOI] [PubMed] [Google Scholar]
- 32.Goldman J. Rutkowski JM. Shields JD, et al. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J. 2007;21:1003–1012. doi: 10.1096/fj.06-6656com. [DOI] [PubMed] [Google Scholar]
- 33.Naldini A. Pucci A. Bernini C. Carraro F. Regulation of angiogenesis by Th1- and Th2-type cytokines. Curr Pharmaceut Design. 2003;9:511–519. doi: 10.2174/1381612033391423. [DOI] [PubMed] [Google Scholar]
- 34.Franchi A. Massi D. Santucci M, et al. Inducible nitric oxide synthase activity correlates with lymphangiogenesis and vascular endothelial growth factor-C expression in head and neck squamous cell carcinoma. J Pathol. 2006;208:439–445. doi: 10.1002/path.1892. [DOI] [PubMed] [Google Scholar]
- 35.Naldini A. Carraro F. Role of inflammatory mediators in angiogenesis. Curr Drug Targets Inflamm Allergy. 2005;4:3–8. doi: 10.2174/1568010053622830. [DOI] [PubMed] [Google Scholar]
- 36.Parr MB. Parr EL. Interferon-gamma up-regulates intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 and recruits lymphocytes into the vagina of immune mice challenged with herpes simplex virus-2. Immunology. 2000;99:540–545. doi: 10.1046/j.1365-2567.2000.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goebel S. Huang M. Davis WC, et al. VEGF-A stimulation of leukocyte adhesion to colonic microvascular endothelium: Implications for inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2006;290:G648–G654. doi: 10.1152/ajpgi.00466.2005. [DOI] [PubMed] [Google Scholar]
- 38.Bjorndahl MA. Cao R. Burton JB, et al. Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res. 2005;65:9261–9268. doi: 10.1158/0008-5472.CAN-04-2345. [DOI] [PubMed] [Google Scholar]
- 39.Abdel–Malak NA. Harfouche R. Hussain SN. Transcriptome of angiopoietin 1-activated human umbilical vein endothelial cells. Endothelium. 2007;14:285–302. doi: 10.1080/10623320701678268. [DOI] [PubMed] [Google Scholar]
- 40.Brkovic A. Sirois MG. Vascular permeability induced by VEGF family members in vivo: Role of endogenous PAF and NO synthesis. J Cell Biochem. 2007;100:727–737. doi: 10.1002/jcb.21124. [DOI] [PubMed] [Google Scholar]
- 41.Lohela M. Saaristo A. Veikkola T. Alitalo K. Lymphangiogenic growth factors, receptors and therapies. Thromb Haemost. 2003;90:167–184. doi: 10.1160/TH03-04-0200. [DOI] [PubMed] [Google Scholar]
- 42.Krysiak O. Bretschneider A. Zhong E, et al. Soluble vascular endothelial growth factor receptor-1 (sFLT-1) mediates downregulation of FLT-1 and prevents activated neutrophils from women with preeclampsia from additional migration by VEGF. Circ Res. 2005;97:1253–1261. doi: 10.1161/01.RES.0000194324.29363.82. [DOI] [PubMed] [Google Scholar]
- 43.Song M. Yang H. Yao S, et al. A critical role of vascular endothelial growth factor D in zebrafish embryonic vasculogenesis and angiogenesis. Biochem Biophys Res Commun. 2007;357:924–930. doi: 10.1016/j.bbrc.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 44.Ji RC. Eshita Y. Kato S. Investigation of intratumoural and peritumoural lymphatics expressed by podoplanin and LYVE-1 in the hybridoma-induced tumours. Int J Exp Pathol. 2007;88:257–270. doi: 10.1111/j.1365-2613.2007.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ando T. Langley RR. Wang Y, et al. Inflammatory cytokines induce MAdCAM-1 in murine hepatic endothelial cells and mediate alpha-4 beta-7 integrin dependent lymphocyte endothelial adhesion in vitro. BMC Physiol. 2007;7:10. doi: 10.1186/1472-6793-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ando T. Jordan P. Wang Y, et al. MAdCAM-1 expression and regulation in murine colonic endothelial cells in vitro. Inflamm Bowel Dis. 2005;11:258–264. doi: 10.1097/01.mib.0000160807.53858.1c. [DOI] [PubMed] [Google Scholar]
- 47.Oshima T. Jordan P. Grisham MB, et al. TNF-alpha induced endothelial MAdCAM-1 expression is regulated by exogenous, not endogenous nitric oxide. BMC Gastroenterol. 2001;1:5. doi: 10.1186/1471-230X-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oshima T. Pavlick KP. Laroux FS, et al. Regulation and distribution of MAdCAM-1 in endothelial cells in vitro. Am J Physiol Cell Physiol. 2001;281:1096–C1105. doi: 10.1152/ajpcell.2001.281.4.C1096. [DOI] [PubMed] [Google Scholar]
- 49.Oshima T. Laroux FS. Coe LL, et al. Interferon-gamma and interleukin-10 reciprocally regulate endothelial junction integrity and barrier function. Microvasc Res. 2001;61:130–143. doi: 10.1006/mvre.2000.2288. [DOI] [PubMed] [Google Scholar]
- 50.Stannard AK. Khurana R. Evans IM. Sofra V. Holmes DI. Zachary I. Vascular endothelial growth factor synergistically enhances induction of E-selectin by tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 2007;27:494–502. doi: 10.1161/01.ATV.0000255309.38699.6c. [DOI] [PubMed] [Google Scholar]
- 51.Sawa Y. Sugimoto Y. Ueki T. Ishikawa H. Sato A. Nagato T. Yoshida S. Effects of TNF-alpha on leukocyte adhesion molecule expressions in cultured human lymphatic endothelium. J Histochem Cytochem. 2007;55:721–733. doi: 10.1369/jhc.6A7171.2007. [DOI] [PubMed] [Google Scholar]
- 52.Sawa Y. Ueki T. Hata M, et al. LPS-induced IL-6, IL-8, VCAM-1, and ICAM-1 expression in human lymphatic endothelium. J Histochem Cytochem. 2008;56:97–109. doi: 10.1369/jhc.7A7299.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sasaki M. Mathis JM. Jennings MH, et al. Reversal of experimental colitis disease activity in mice following administration of an adenoviral IL-10 vector. J Inflamm (Lond) 2005;2:13. doi: 10.1186/1476-9255-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sasaki M. Jordan P. Joh T, et al. Melatonin reduces TNF-a induced expression of MAdCAM-1 via inhibition of NF-kappaB. BMC Gastroenterol. 2002;2:9. doi: 10.1186/1471-230X-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sasaki M. Ostanin D. Elrod JW, et al. TNF-alpha -induced endothelial cell adhesion molecule expression is cytochrome P-450 monooxygenase dependent. Am J Physiol Cell Physiol. 2003;284:C422–C428. doi: 10.1152/ajpcell.00271.2002. [DOI] [PubMed] [Google Scholar]
- 56.Park CC. Morel JC. Amin MA, et al. Evidence of IL-18 as a novel angiogenic mediator. J Immunol. 2001;167:1644–1653. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- 57.Voronov E. Shouval DS. Krelin Y, et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kommineni VK. Nagineni CN. William A. Detrick B. Hooks JJ. IFN-gamma acts as anti-angiogenic cytokine in the human cornea by regulating the expression of VEGF-A and sVEGF-R1. Biochem Biophys Res Commun. 2008;374:479–484. doi: 10.1016/j.bbrc.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Makinen T. RAdams RH. Bailey J, et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pfaff D. Fiedler U. Augustin HG. Emerging roles of the angiopoietin-Tie and the ephrin-Eph systems as regulators of cell trafficking. J Leukoc Biol. 2006;80:719–726. doi: 10.1189/jlb.1105652. [DOI] [PubMed] [Google Scholar]
- 61.Ganta VC. Cromer W. Mills GL, et al. Angiopoietin-2 in experimental colitis. Inflamm Bowel Dis. 2010;16:1029–1039. doi: 10.1002/ibd.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kajiya K. Huggenberger R. Drinnenberg I. Ma B. Detmar M. Nitric oxide mediates lymphatic vessel activation via soluble guanylate cyclase alpha1beta1-impact on inflammation. FASEB J. 2008;22:530–537. doi: 10.1096/fj.07-8873com. [DOI] [PubMed] [Google Scholar]
- 63.Middel P. Reich K. Polzien F, et al. Interleukin 16 expression and phenotype of interleukin 16 producing cells in Crohn's disease. Gut. 2001;49:795–803. doi: 10.1136/gut.49.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Middel P. Thelen P. Blaschke S, et al. Expression of the T-cell chemoattractant chemokine lymphotactin in Crohn's disease. Am J Pathol. 2001;159:1751–1761. doi: 10.1016/S0002-9440(10)63022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ryan TJ. Microcirculation in psoriasis: Blood vessels, lymphatics and tissue fluid. Pharmacol Ther. 1980;10:27–64. doi: 10.1016/0163-7258(80)90008-x. [DOI] [PubMed] [Google Scholar]
- 66.Gerli R. Alessandrini C. Initial lymph vessels of the skin and elastic fibres form an integral morphofunctional structure. Ital J Anat Embryol. 1995;100:579–587. [PubMed] [Google Scholar]
- 67.Gerli R. Solito R. Weber E. Agliano M. Specific adhesion molecules bind anchoring filaments and endothelial cells in human skin initial lymphatics. Lymphology. 2000;33:148–157. [PubMed] [Google Scholar]
- 68.Hanley CA. Elias RM. Movat HZ. Johnston MG. Suppression of fluid pumping in isolated bovine mesenteric lymphatics by interleukin-1: Interaction with prostaglandin E2. Microvasc Res. 1989;37:218–229. doi: 10.1016/0026-2862(89)90039-3. [DOI] [PubMed] [Google Scholar]
- 69.Wu TF. MacNaughton WK. der Weid PY. Lymphatic vessel contractile activity and intestinal inflammation. Mem Inst Oswaldo Cruz. 2005;100:107–110. doi: 10.1590/s0074-02762005000900018. [DOI] [PubMed] [Google Scholar]
- 70.Muthuchamy M. Zawieja D. Molecular regulation of lymphatic contractility. Ann NY Acad Sci. 2008;1131:89–99. doi: 10.1196/annals.1413.008. [DOI] [PubMed] [Google Scholar]
- 71.der Weid PY. Zawieja DC. Lymphatic smooth muscle: The motor unit of lymph drainage. Int J Biochem Cell Biol. 2004;36:1147–1153. doi: 10.1016/j.biocel.2003.12.008. [DOI] [PubMed] [Google Scholar]