Abstract
Background and Purpose
Published outcomes of pelvic lymph node dissection (PLND) during robot-assisted laparoscopic prostatectomy (RALP) demonstrate significant variability. The purpose of the study was to compare PLND outcomes in patients at risk for lymph node involvement (LNI) who were undergoing radical prostatectomy (RP) by different surgeons and surgical approaches.
Patients and Methods
Institutional policy initiated on January 1, 2010, mandated that all patients undergoing RP receive a standardized PLND with inclusion of the hypogastric region when predicted risk of LNI was ≥2%. We analyzed the outcomes of consecutive patients meeting these criteria from January 1 to September 1, 2010 by surgeons and surgical approach. All patients underwent RP; surgical approach (open radical retropubic [ORP], laparoscopic [LRP], RALP) was selected by the consulting surgeon. Differences in lymph node yield (LNY) between surgeons and surgical approaches were compared using multivariable linear regression with adjustment for clinical stage, biopsy Gleason grade, prostate-specific antigen (PSA) level, and age.
Results
Of 330 patients (126 ORP, 78 LRP, 126 RALP), 323 (98%) underwent PLND. There were no significant differences in characteristics between approaches, but the nomogram probability of LNI was slightly greater for ORP than RALP (P=0.04). LNY was high (18 nodes) by all approaches; more nodes were removed by ORP and LRP (median 20, 19, respectively) than RALP (16) after adjusting for stage, grade, PSA level, and age (P=0.015). Rates of LNI were high (14%) with no difference between approaches when adjusted for nomogram probability of LNI (P=0.15). Variation in median LNY among individual surgeons was considerable for all three approaches (11–28) (P=0.005) and was much greater than the variability by approach.
Conclusions
PLND, including hypogastric nodal packet, can be performed by any surgical approach, with slightly different yields but similar pathologic outcomes. Individual surgeon commitment to PLND may be more important than approach.
Introduction
Radical prostatectomy (RP) can be performed using a variety of approaches, but the field has drastically changed since the 2001 introduction of the robot-assisted laparoscopic prostatectomy (RALP) using the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA).1 This technique has rapidly gained popularity, and recent data suggest that it is the technique most commonly used for performing RP in the United States.2 Its rapid rise as the de facto “procedure of choice” occurred despite no randomized trials that compared RALP with more established surgical approaches. Because such studies are unlikely to be performed, it is incumbent on the oncologic community to scrutinize each aspect of the RALP procedure to demonstrate equivalency. While many reports have focused on the rapid recovery and decreased blood loss associated with RALP, less attention has been paid to certain critical oncologic outcomes, particularly with respect to pelvic lymph node dissection (PLND).
PLND is a controversial aspect of RP.3 Increased use of prostate-specific antigen (PSA) tests and routine screening for prostate cancer has led to a stage migration resulting in more patients with organ-confined disease. This downward trend in tumor stage has been accompanied by a decrease in lymph node involvement (LNI).4,5 Prediction models have been created to assess the risk of LNI and help guide practitioners in making more informed decisions.6 The combination of stage migration and the use of predictive tools have resulted in a declining trend in the use of concurrent PLND at the time of RP.7–9
At Memorial Sloan-Kettering Cancer Center (MSKCC), PLND is currently performed in patients with a ≥2% risk of LNI using a uniform template that includes the hypogastric nodal region regardless of surgical approach. In this study, we compare the outcomes of PLND using this uniform template in a contemporaneous cohort of patients with ≥2% risk of LNI who were undergoing RP through open radical retropubic (ORP), laparoscopic (LRP), or robot-assisted laparoscopic prostatectomy (RALP).
Patients and Methods
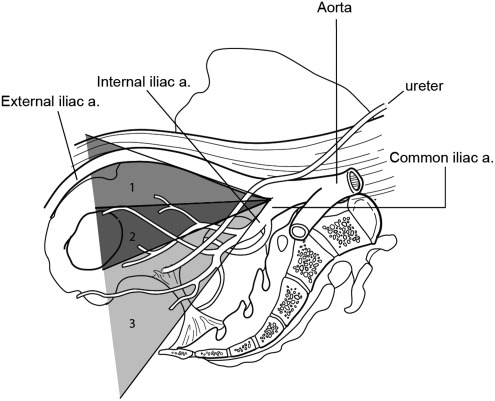
At MSKCC, indications and templates for PLND at RP had varied based on surgeon preferences for many years. To create a greater degree of uniformity, a departmental agreement was reached on January 1, 2010. It was determined that all patients with a ≥2% risk of LNI will undergo a PLND with a template that includes, at minimum, the external iliac, obturator, and hypogastric (internal iliac) lymph node packets (Fig. 1), in compliance with the most recent guidelines of the National Comprehensive Cancer Network.10,11 The preoperative nomogram used to calculate LNI in this study was based on more than 7000 patients, performed at six institutions, with clinically localized prostate cancer.6 Adherence to standardized patient selection and anatomic template are intended to be maintained regardless of surgical technique ORP, LRP, and RALP.
FIG. 1.
All patients with a ≥2% risk of lymph node involvement received at minimum a pelvic lymph node dissection with a template that included, at minimum, the external iliac, obturator, and hypogastric (internal iliac) lymph node packets—corresponding with zones 1, 2, and 3 in the Figure.
Institutional Review Board approval was obtained to perform a retrospective review of consecutive patients undergoing primary RP at MSKCC from January 1, 2010, to September 1, 2010. Patients with a history of radiation treatment or a <2% nomogram calculated risk of LNI were excluded from this analysis. Postoperative charts were reviewed for complications attributable to PLND.
Lymph node specimens were routinely submitted en bloc in left and right packages regardless of technique. Only one surgeon who was performing the open surgical approach only routinely further subdivided left and right packets into external iliac, obturator, and hypogastric packets. After the lymph nodes were grossly examined, they were placed into 10% neutral buffered formalin. Within 4 to 8 hours, they were loaded onto processors in which they were fixed in two cycles of formalin (10% neutral buffered) for 2 hours for each cycle (4 hour total). After being embedded in paraffin, slides were then stained with hematoxylin and eosin and examined microscopically. All processing of the lymph nodes was performed in the same hospital by the same team of technicians, and all pathologic review was performed by the same team of dedicated genitourinary pathologists.
All surgeons performing RP at MSKCC are trained urologic oncologists with extensive experience in performing RP. Differences in lymph node yield (LNY) between surgeons and approaches were compared using multivariable linear regression, adjusted for clinical stage (T1c, T2a, T2b+), biopsy Gleason grade (6, 7, 8+), PSA level, and age. To explore whether there were differences in LNYs between surgeons, independent of surgical approach, analysis was conducted by individual surgeon. To limit surgical experience as a potential confounder, surgeons with five or fewer total RPs over the study period, regardless of approach, were excluded from this subanalysis, leaving seven surgeons. All statistical analyses were conducted using STATA 11.0 (StataCorp, College Station, TX).
Results
Over the study period, 330 patients underwent primary RP for localized prostate cancer with nomogram risk of LNI ≥2% by 10 different urologic oncologists at MSKCC. Clinical and oncologic characteristics of the cohort, by surgical approach, are given in Table 1. Oncologic characteristics were very similar between groups; however, predicted probability of LNI was greater for ORP compared with RALP (P=0.04). The surgical approach for each patient was determined by the operating surgeon in conjunction with the patient's wishes.
Table 1.
Baseline Characteristics of the Sample
| Open: n=126 | Laparoscopic: n=78 | Robotic: n=136 | |
|---|---|---|---|
| Median age (IQR) | 61.6 (55.9, 65.3) | 62.1 (56.1, 68.2) | 63.3 (57.6, 67.7) |
| Median PSA at diagnosis (IQR) | 6.10 (4.50, 8.81) | 5.75 (4.66, 9.00) | 5.90 (4.60, 7.60) |
| Clinical stage, n (%): | |||
| T1 | 77 (61%) | 50 (64%) | 79 (63%) |
| T2a | 13 (10%) | 16 (21%) | 24 (19%) |
| T2b+ | 36 (29%) | 12 (15%) | 23 (18%) |
| Biopsy Gleason score, n (%) | |||
| 6 | 4 (3%) | 4 (5%) | 5 (4%) |
| 7 | 88 (70%) | 53 (68%) | 98 (78%) |
| 8 | 19 (15%) | 14 (18%) | 12 (10%) |
| Positive surgical margins, n (%) | 24 (19%) | 9 (12%) | 21 (17%) |
| Seminal vesicle invasion, n(%) | 15 (12%) | 8 (10%) | 13 (10%) |
| Extraprostatic extension, n (%) | 59 (47%) | 43 (55%) | 57 (45%) |
| Median predicted risk of LNI (IQR) | 2.87 (2.30, 5.11) | 2.76 (2.31, 4.88) | 2.60 (2.36, 3.98) |
IQR=interquartile range; PSA=prostate-specific antigen; LNI=lymph node involvement.
Table 2 shows rates of PLND, LNY, and number of positive nodes for each surgical approach. Rates of PLND were high among all modalities, with 98% (323/330) of the total cohort undergoing PLND. Seven patients with a calculated risk of LNI ≥2% failed to receive a PLND for unclear reasons. Among the patients undergoing PLND, the yield was highest for open RP (20 nodes) and lowest for robot-assisted RP (16 nodes). Although small, differences between groups were statistically significant after adjustment for clinical stage, biopsy Gleason grade, PSA level, and age (P=0.015). Rates of LNI were high regardless of surgical approach (ORP 17% [22/126], LRP 8% [6/77], RALP 13% [16/120]) and showed no statistically significant difference between groups (P=0.3, comparison of proportions by exact test), even after adjusting for nomogram predicted risk of LNI (P=0.15).
Table 2.
Characteristics of Patients with A ≥2% Probability of Lymph Node Involvement
| Total | Open | Laparoscopic | Robotic | |
|---|---|---|---|---|
| Number of patients | N=330 | n=126 | n=78 | n=126 |
| Patients receiving lymph node dissection, n (%) | 323 (98%) | 126 (100%) | 77 (99%) | 120 (95%) |
| Median number lymph nodes removed (IQR)a | 18.0 (13.0, 24.0) | 20.0 (14.0, 26.0) | 19.0 (13.0, 25.0) | 16.0 (11.0, 21.0) |
| Patients with positive lymph nodes, n (%)a | 44 (14%) | 22 (17%) | 6 (8%) | 16 (13%) |
| 0 | 279 (86%) | 104 (83%) | 71 (92%) | 104 (87%) |
| 1 | 24 (7%) | 11 (9%) | 4 (5%) | 9 (8%) |
| 2 | 6 (2%) | 3 (2%) | 1 (1%) | 2 (2%) |
| 3+ | 14 (4%) | 8 (6%) | 1 (1%) | 5 (4%) |
In those who underwent lymph node dissection.
Rates of symptomatic lymphocele were low in the ORP group (1/126, 1%), as well as in the LRP group (2/77, 3%) and the RALP group (4/120, 3%). There were no injuries to the obturator nerve or other complications that could be directly attributable to PLND. LNY was analyzed by individual surgeon and approach (Table 3). After adjusting for clinical covariates and surgical approach, there were significant differences between surgeons as to yield (P<0.001). Large variation in LNY was demonstrated between surgeons regardless of approach. Median LNY among surgeons using the robotic approach varied from 11 to 28, while median yields from both the open and laparoscopic approaches varied from 12 to 22.
Table 3.
Lymph Node Yield by Surgeon and Surgical Approach
| |
Surgical approach |
|||||
|---|---|---|---|---|---|---|
| |
Open RP |
Laparoscopic RP |
Robot-assisted RP |
|||
| Surgeon | Number performed | Median lymph node yield (IQR) | Number performed | Median lymph node yield (IQR) | Number performed | Median lymph node yield (IQR) |
| 1 | 5 | 15 (9, 20) | 9 | 12 (6, 14) | 0 | |
| 2 | 1 | 12 (12, 12) | 0 | 13 | 28 (18, 36) | |
| 3 | 69 | 18 (14, 23) | 0 | 8 | 11 (6, 14) | |
| 4 | 0 | 35 | 19 (13, 24) | 0 | ||
| 5 | 0 | 0 | 84 | 16.5 (12, 21) | ||
| 6 | 48 | 22 (15.5, 29) | 0 | 0 | ||
| 7 | 0 | 33 | 22 (17, 26) | 10 | 13 (9, 17) | |
Only surgeons who performed more than five radical prostatectomies (by any approach) over the study period are included in this table. Within that group, only patients with a high risk (≥2%) of lymph node involvement by preoperative nomogram, who had lymph node dissection performed, are included.
RP=radical prostatectomy; IQR=interquartile range.
Discussion
RALP is rapidly becoming the most common method of performing RP.2 Accumulating evidence suggests that PLND is being performed less frequently during RALP than might be anticipated or appropriate. Recent studies have repeatedly demonstrated that patients undergoing minimally invasive RP are significantly less likely to receive PLND than patients undergoing ORP.12,13 In addition, it is noteworthy that many of the largest series on RALP provide incomplete or no data regarding PLND, suggesting a lack of focus on this important oncologic aspect.14,15 In part, this may be because PLND during RALP has yet to be convincingly demonstrated in an equivalent fashion to PLND during ORP or LRP. To address this issue, we compared outcomes for a concurrent cohort of men with similar risk of LNI undergoing PLND performed with similar templates by three different surgical techniques.
We noted only modest differences in median nodal yields between surgical techniques (ORP 20, LRP 19, RALP 16) when using a standardized PLND template. These small but statistically significant differences resulted in no detectable difference in the rate of LNI between surgical techniques. Overall LNY was high in this series with a median yield of 18 nodes across all techniques, and lymph node positivity was similarly high at 14% (44/323).
The average LNY in this study was higher than in previously reported series, regardless of technique.16–18 We attribute the high LNY and the high rate of LNI in this study both to the relatively high risk of the cohort, limited to patients with a nomogram probability of LNI of ≥2%, and a meticulous PLND dissection that encompasses all the nodal tissue around the external iliac and obturator nerve as well as the hypogastric vessels.16–18 Because the most common method of collecting lymph nodes, regardless of approach, was to group lymph nodes from each side of the pelvis together and send them as single specimens, the specific contribution of the hypogastric PLND cannot be determined in this study. Dissections that include the hypogastric region remove greater numbers of lymph nodes, however.16,19 Up to 50% of LNI is in the hypogastric landing site, and in 20% of cases, it is isolated to this region and would have been missed had dissection not included this area.17,20,21 The high rates of LNI we observed, 14% overall, suggest the importance of including the hypogastric region in any PLND, and the lack of significant difference of LNI between techniques, even after accounting for differences in risk of LNI, suggests that adequate PLND can be performed regardless of approach.
There were few complications directly attributable to PLND, and there were no neural or vascular injuries that occurred as a result of PLND. Lymphoceles were infrequent in all groups and only detected when they were symptomatic and needed intervention. Minor variations between groups are likely because of the small number of events.
The impact of individual surgeons, their experience, and caseload have been demonstrated to impact various aspects of RP.22,23 Thus, we hypothesized that a similar effect might be seen with PLND, and to test this theory, we analyzed the median LNY by surgical approach and by surgeon. In an attempt to limit surgeon experience as a potential confounder, we limited this analysis to the surgeons who had performed the majority of the prostatectomies over the period of the study.
Wide variations existed in median LNY between surgeons, independent of surgical approach, and the variation between surgeons (ORP and LRP 12–22, RALP 11–28) was much greater than the variation between surgical approaches (ORP 20, LRP 19, RALP 16). This suggests that any difference in yield because of surgical approach may be overshadowed by variation between individual surgeons. While the numbers are small and accurate comparisons are difficult to make for those surgeons who performed more than one approach, variations in median LNY were also apparent but not in any consistent pattern favoring a given approach. These data emphasize the notion that an individual surgeon's commitment to perform PLND may be more important than the approach used to perform the dissection.
It is our experience that a thorough extended PLND takes on the order of 30 to 45 minutes, regardless of surgical approach. Overall operative time was recorded in all cases, but times to perform specific aspects of the case, such as PLND, were not specifically documented. We attempted to determine the additional time needed for PLND by comparing the time of cases without PLND to those with PLND; however, because of the limited number and distribution of cases without PLND, this was not an accurate comparison. In addition, other factors, such as degree of nerve sparing or trainee involvement, may influence the variability of the overall time of the operation more than PLND. Future studies that attempt to determine the additional time needed to perform PLND during RP should specifically record time needed for PLND.
Previous studies that compared PLND during open and robot-assisted RP have had various limitations. Zorn and associates24 demonstrated the feasibility of PLND during robot-assisted RP, but used a template that excluded the hypogastric region. In this study, PLND was performed in only 26% of patients undergoing RALP; the authors compared these outcomes with an historic ORP series at the same institution and found LNY was smaller with RALP (12.5 vs 15; P<0.01). The authors did not compare rates of LNI. Improving on this study design, Cooperberg and colleagues18 compared PLND in RALP and ORP over a concurrent period. A greater proportion of patients undergoing PLND were in the ORP group compared with the RALP group (47.8% vs 31.8%; P<0.01). PLND during ORP resulted in a greater mean yield (14.4 nodes vs 9.3 nodes; P<0.01) and a higher rate of LNI (7.3% vs 1.1%, P=0.02). Truesdale and coworkers25 compared the results of ORP vs RALP PLND performed over the same period and found only a borderline significant difference in LNY between ORP and RALP (7.49 nodes vs 6.35 nodes; P=0.06). When analysis was restricted to those in which PLND included the hypogastric region, however, no difference in yield was found between groups (P=0.27). LNI rates were significantly greater in the open group (8.8%) than in the RALP group (1%; P=0.009). More recent robotic series have demonstrated the feasibility of PLND with inclusion of the hypogastric nodal packets but have not compared these results with those for ORP or LRP.26,27
As the urologic community moves away from open surgery and toward robotics, fewer patients, regardless of risk, are receiving PLND.12,13 While the exact causes for these changes are not known—and are likely multifactorial—it is suggestive that this may be at least in part because of the adoption of new technology. The proximity of the nodal packets to the iliac vessels and the restricted movement of the bulky robotic arms may deter surgeons from performing this portion of the procedure as they progress through their learning curve and adoption of this technique. Our findings demonstrate that when surgeons are committed to performing a thorough PLND with inclusion of the hypogastric packet, they can achieve nodal yields and rates of LNI that rival more mature surgical approaches.
Our study has several strengths, including using a uniform template for PLND, consistent inclusion of the hypogastric packet, consistent indications for PLND, similar oncologic risk of patients across surgical approaches, and the concurrent period of analysis. In addition, all RPs were performed at a single center with a single group of highly specialized urologic pathologists who evaluated the specimens.
This study was limited by its retrospective nature and the inherent methodologic flaws consistent with any such design. Seven patients with ≥2% (but <3%) risk of LNI had PLND omitted for unclear reasons. While the minimal PLND template included external, obturator, and internal nodal packets, there was no limitation on the maximal template. Thus, at surgeon discretion in patients with a greater risk of LNI, surgeons extended their template beyond this “minimal” template, and this may have introduced bias. One surgeon routinely packeted the lymph nodes; this resulted in a lack of uniformity in the collection of these specimens. The nomogram used to predict risk of LNI was based on multi-institutional data from an earlier cohort who did not require a uniform, extended PLND in all patients and thus its applicability to this group may have some limitations. In addition, because of short follow-up, we have limited our analysis to pathologic outcomes of PLND and have not reported clinical outcomes, which are necessary to establish true equivalency.
Conclusions
This study demonstrates that PLND using a standardized template with inclusion of the hypogastric region can be consistently accomplished during RALP and results in yield and rates of LNI similar to those observed with the open or laparoscopic approaches. Our data demonstrate wide variation in nodal yield regardless of surgical approach and suggest that individual surgeon or commitment to PLND may be more important than approach. In the future, continued emphasis must be placed on performing PLND with inclusion of the hypogastric region during RP by all surgical approaches in all prostate cancer patients with ≥2% risk of LNI as presently recommended by NCCN guidelines.
Abbreviations Used
- LNI
lymph node involvement
- LNY
lymph node yield
- LRP
laparoscopic radical prostatectomy
- MSKCC
Memorial Sloan-Kettering Cancer Center
- ORP
open radical retropubic prostatectomy
- PLND
pelvic lymph node dissection
- PSA
prostate-specific antigen
- RALP
robot-assisted laparoscopic prostatectomy
- RP
radical prostatectomy
Acknowledgment
Supported by the Sidney Kimmel Center for Prostate and Urologic Cancers, by funds provided by David H. Koch through the Prostate Cancer Foundation, and by the National Cancer Institute (NCI) (U54CA137788 and U54CA132378).
Disclosure Statement
No competing financial interests exist.
References
- 1.Binder J. Kramer W. Robotically-assisted laparoscopic radical prostatectomy. BJU Int. 2001;87:408–410. doi: 10.1046/j.1464-410x.2001.00115.x. [DOI] [PubMed] [Google Scholar]
- 2.Orvieto MA. Patel VR. Evolution of robot-assisted radical prostatectomy. Scand J Surg. 2009;98:76–88. doi: 10.1177/145749690909800203. [DOI] [PubMed] [Google Scholar]
- 3.Briganti A. Blute ML. Eastham JH, et al. Pelvic lymph node dissection in prostate cancer. Eur Urol. 2009;55:1251–1265. doi: 10.1016/j.eururo.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 4.DiMarco DS. Zincke H. Sebo TJ, et al. The extent of lymphadenectomy for pTXNO prostate cancer does not affect prostate cancer outcome in the prostate specific antigen era. J Urol. 2005;173:1121–1125. doi: 10.1097/01.ju.0000155533.93528.4c. [DOI] [PubMed] [Google Scholar]
- 5.Bluestein DL. Bostwick DG. Bergstralh EJ. Oesterling JE. Eliminating the need for bilateral pelvic lymphadenectomy in select patients with prostate cancer. J Urol. 1994;151:1315–1320. doi: 10.1016/s0022-5347(17)35239-4. [DOI] [PubMed] [Google Scholar]
- 6.Cagiannos I. Karakiewicz P. Eastham JA, et al. A preoperative nomogram identifying decreased risk of positive pelvic lymph nodes in patients with prostate cancer. J Urol. 2003;170:1798–1803. doi: 10.1097/01.ju.0000091805.98960.13. [DOI] [PubMed] [Google Scholar]
- 7.Joslyn SA. Konety BR. Impact of extent of lymphadenectomy on survival after radical prostatectomy for prostate cancer. Urology. 2006;68:121–125. doi: 10.1016/j.urology.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 8.Gil-Vernet JM. Prostate cancer: Anatomical and surgical considerations. Br J Urol. 1996;78:161–168. doi: 10.1046/j.1464-410x.1996.00841.x. [DOI] [PubMed] [Google Scholar]
- 9.Kawakami J. Meng MV. Sadetsky N, et al. Changing patterns of pelvic lymphadenectomy for prostate cancer: Results from CaPSURE. J Urol. 2006;176:1382–1386. doi: 10.1016/j.juro.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2010;8:145. doi: 10.6004/jnccn.2010.0010. [DOI] [PubMed] [Google Scholar]
- 11.Mohler J. Bahnson RR. Boston B, et al. NCCN clinical practice guidelines in oncology: Prostate cancer. J Natl Compr Canc Netw. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 12.Hu JC. Prasad SM. Gu X, et al. Determinants of performing radical prostatectomy pelvic lymph node dissection and the number of lymph nodes removed in elderly men. Urology. 2011;77:402–406. doi: 10.1016/j.urology.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Feifer AH. Elkin EB. Lowrance WT, et al. Temporal trends and predictors of pelvic lymph node dissection in open or minimally invasive radical prostatectomy. Cancer. 2011;117:3933–3942. doi: 10.1002/cncr.25981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badani KK. Kaul S. Menon M. Evolution of robotic radical prostatectomy: Assessment after 2766 procedures. Cancer. 2007;110:1951–1958. doi: 10.1002/cncr.23027. [DOI] [PubMed] [Google Scholar]
- 15.Silberstein JL. Derweesh IH. Kane CJ. Lymph node dissection during robot-assisted radical prostatectomy: Where do we stand? Prostate Cancer Prostatic Dis. 2009;12:227–232. doi: 10.1038/pcan.2009.17. [DOI] [PubMed] [Google Scholar]
- 16.Allaf ME. Palapattu GS. Trock BJ, et al. Anatomical extent of lymph node dissection: Impact on men with clinically localized prostate cancer. J Urol. 2004;172:1840–1844. doi: 10.1097/01.ju.0000140912.45821.1d. [DOI] [PubMed] [Google Scholar]
- 17.Stone NN. Stock RG. Unger P. Laparoscopic pelvic lymph node dissection for prostate cancer: Comparison of the extended and modified techniques. J Urol. 1997;158:1891–1894. doi: 10.1016/s0022-5347(01)64161-2. [DOI] [PubMed] [Google Scholar]
- 18.Cooperberg MR. Kane CJ. Cowan JE. Carroll PR. Adequacy of lymphadenectomy among men undergoing robot-assisted laparoscopic radical prostatectomy. BJU Int. 2010;105:88–92. doi: 10.1111/j.1464-410X.2009.08699.x. [DOI] [PubMed] [Google Scholar]
- 19.Heidenreich A. Ohlmann CH. Polyakov S. Anatomical extent of pelvic lymphadenectomy in patients undergoing radical prostatectomy. Eur Urol. 2007;52:29–37. doi: 10.1016/j.eururo.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Briganti A. Chun FK. Salonia A, et al. Critical assessment of ideal nodal yield at pelvic lymphadenectomy to accurately diagnose prostate cancer nodal metastasis in patients undergoing radical retropubic prostatectomy. Urology. 2007;69:147–151. doi: 10.1016/j.urology.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Bader P. Burkhard FC. Markwalder R. Studer UE. Is a limited lymph node dissection an adequate staging procedure for prostate cancer? J Urol. 2002;168:514–518. doi: 10.1016/s0022-5347(05)64670-8. [DOI] [PubMed] [Google Scholar]
- 22.Vickers AJ. Bianco FJ. Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007;99:1171–1177. doi: 10.1093/jnci/djm060. [DOI] [PubMed] [Google Scholar]
- 23.Vickers A. Savage C. Bianco F, et al. Cancer control and functional outcomes after radical prostatectomy as markers of surgical quality: Analysis of heterogeneity between surgeons at a single cancer center. Eur Urol. 2011;59:317–322. doi: 10.1016/j.eururo.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zorn KC. Katz MH. Bernstein A, et al. Pelvic lymphadenectomy during robot-assisted radical prostatectomy: Assessing nodal yield, perioperative outcomes, and complications. Urology. 2009;74:296–302. doi: 10.1016/j.urology.2009.01.077. [DOI] [PubMed] [Google Scholar]
- 25.Truesdale MD. Lee DJ. Cheetham PJ, et al. Assessment of lymph node yield after pelvic lymph node dissection in men with prostate cancer: A comparison between robot-assisted radical prostatectomy and open radical prostatectomy in the modern era. J Endourol. 2010;24:1055–1060. doi: 10.1089/end.2010.0128. [DOI] [PubMed] [Google Scholar]
- 26.Feicke A. Baumgartner M. Talimi S, et al. Robotic-assisted laparoscopic extended pelvic lymph node dissection for prostate cancer: Surgical technique and experience with the first 99 cases. Eur Urol. 2009;55:876–883. doi: 10.1016/j.eururo.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Yee DS. Katz DJ. Godoy G, et al. Extended pelvic lymph node dissection in robotic-assisted radical prostatectomy: Surgical technique and initial experience. Urology. 2010;75:1199–1204. doi: 10.1016/j.urology.2009.06.103. [DOI] [PubMed] [Google Scholar]