Abstract
The application of external biophysical signals is one approach to tissue engineering that is explored less often than more traditional additions of exogenous biochemical and chemical factors to direct cell and tissue outcomes. The study of bioelectromagnetism and the field of electrotherapeutics have evolved over the years, and we review biocompatible electric stimulation devices and their successful application to tissue growth. Specifically, information on capacitively coupled alternating current, inductively coupled alternating current, and direct current devices is described. Cell and tissue responses from the application of these devices, including two- and three-dimensional in vitro studies and in vivo studies, are reviewed with regard to cell proliferation, adhesion, differentiation, morphology, and migration and tissue function. The current understanding of cellular mechanisms related to electric stimulation is detailed. The advantages of electric stimulation are compared with those pf other techniques, and areas in which electric fields are used as an adjuvant therapy for healing and regeneration are discussed.
Introduction
In the United States, there is a annual need for more than 110,000 organs, including kidney, liver, pancreas, and heart valves, and fewer than 2% of organ donors available to fill these needs. Wait times range from months to years and depend several factors, including blood type, age, and ethnicity.1 In 1981, Bell and Ehrlich published one of the first studies describing the development of tissue engineered skin constructs2 using fibroblasts and a collagen mesh seeded with epidermal cells as a living skin replacement. Over the past 30 years, engineered acellular and cellular scaffold constructs (biomaterial matrices plus cells) originating from autographic, allographic, and xenographic sources have been studied for the purpose of meeting donor needs. Today, tissue engineering is a multidisciplinary field using engineering and life science practices for restoring, maintaining, and improving tissue function that has been lost because of injury, disease, or aging.3 Tissue engineering strategies to improve and restore tissue function include pharmacologic applications, protein integration including hormones and growth factors, mechanical and electric stimuli, biochemical cocktails, and the incorporation of natural and synthetic biomaterials.
Although scientists have hypothesized about and identified the presence of bioelectric signals for longer than 300 years, the application of electric stimuli has not been fully exploited for restoring tissue function. Electric fields have several potential advantages over alternative approaches, including the absence of toxic chemicals, the absence of immunogenic responses in the host tissue, and less expensive applications than growth factors and many chemical applications. Furthermore, many devices used for the application of electric fields employ simple equipment designed with a basic theoretical understanding of electromagnetism. Devices that incorporate electrodes into tissues work well for repeatedly treating the same specific area depending on size in culture or in vivo. Electrodes can also be used for monitoring functionality (such as cardiac or neuron action potentials) at that spot. Finally, electric field techniques require little cell handling and processing of tissue engineered constructs.
This review focuses on electric field device design and tissue response to electric field stimulation for the purpose of engineering functional tissues. Tissue responses that are reviewed include differentiation, proliferation, morphology, adhesion, migration and function. Underlying cellular mechanisms involved in the responses in vitro and in vivo are discussed to the extent understood. All studies reviewed outline tissue responses to frequencies that are nonradiative or nonionizing radiative. Excluded from this review are cellular techniques commonly found in molecular biology assays that employ electric field gradients or electric shock, such as electrophoresis and electroporation. Finally, electric field techniques used for scaffold design or surface modifications, including electrospinning or bioelectrospray, are not reviewed, nor is the application of electrically conductive polymers in the field of tissue engineering. For reviews of these topics, the reader is referred to recent publications.4–10
Electric Properties in Native Tissues and Organisms
Cells and tissues can be characterized according to their electric properties, including resting membrane potential, ionic current flow, resistance, capacitance, permittivity, and conductivity (Table 1). These electric properties vary according to tissue type, tissue health, and tissue age and are present in developing, normal, and wounded tissues and organisms (Fig. 1).11,12 Measurements of these properties provide information regarding cell concentration, moisture content, fat content, cell orientation, disease presence, and even time of tissue death.12 In one example, fibroblast-seeded tissue constructs had capacitance measurements that varied linearly between 0.4 and 2 pF, depending upon cell number.12 In another example, conductivity and permittivity were reported to be greater in malignant tissues than in normal tissues, with the extent of difference depending on tissue type.13,14 Thus, electric properties may also be used as a biomarker for malignancy.14–16
Table 1.
Electrical Properties of Cells and Tissues
| Electrical characteristic | Definition | Unit | Symbol | Biological range |
|---|---|---|---|---|
| Voltage | Potential energy per unit charge | Volt (W A−1) | V | −10–−100 mV |
| Resistance | The opposition of electric current | Ohm (V A−1) | Ω | 0.1–1 kΩ |
| Current | Charge transfer per unit time | Amp (C S−1) | A | nA–mA |
| Capacitance | The ability to store charge | Farad (C V−1) | F | 1 nF–1 pF |
| Permittivity | The extent that an electric field affects a material | Farads/meter (F m−1) | ɛ | 10–10,000 |
| Conductivity | The ability to conduct current | Siemens (S m−1) | σ | 1mσ–1 μσ |
FIG. 1.
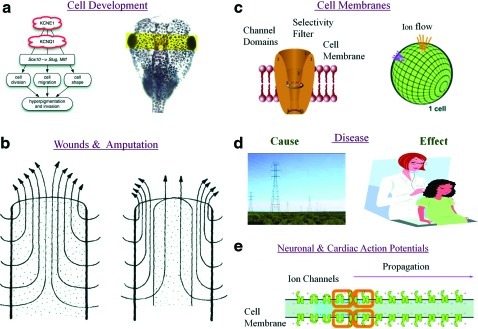
Electric fields are innate in organisms. (a) During cell development, ion channel expression regulates phenotype. For example, overexpression of potassium voltage-gated channel subfamily E member 1 (KCNE1) induces hyperpigmentation in frog embryos.127 Current flow is in the μA range. Voltage potential is in the mV range. (b) Electric currents radiate outward, in the μA range, perpendicular to wounds and amputations in newts and frog digits.17,18 (c) Ion channels create voltage gradients. Voltages in the mV range are measured across the intercellular and extracellular space in cells. Different potentials are measured per cell and per membrane. This voltage is defined as membrane potentials in excitable and nonexcitable cells. Electric field strength across a cell's membrane is on the order of 1 million V/m because the distance across the cell membrane is in the nm range. (d) Exposure to high-strength electromagnetic fields in the kV range may cause disease or earlier disease onset, whereas changes in a tissue's permittivity or conductivity may be a biomarker of disease.14 (e) Voltage potentials are detected in the mV range in the intercellular space and in the μV range in the extracellular space of cardiac and neuronal tissues. Ionic current propagation, detected in the mV range, is the method of physical signal transduction in excitable tissues. Images taken from.17,127–129 Images reprinted with permission. ©2002 from Molecular Biology of the Cell, 4th Ed, Alberts et al. Reproduced by permission of Garland Science/Taylor and Francis LLC. Copyright 2008 National Academy of Sciences. Color images available online at www.liebertonline.com/teb
Electric currents are known to be an important regulator of embryonic development because their presence emerges as early as the first cell division. For example, Xenopus laevis exhibits an inwardly positive current, generated from an influx of Na+ ions across the ectoderm during the embryonic stage.17 Inwardly positive currents in X. laevis can be detected from the blastopore beginning at stage 11 of embryonic development, with the largest current density of 115 μA/cm2 detected at stage 22. Canceling out an inwardly positive current flow, by driving biophysical stimuli into the X. laevis embryo, results in developmental abnormalities such as delayed head development, the absence of eye structure, neural tube closures at the anterior end, and skin pigment changes.17
The electric currents present in injured animals and organisms play a role in healing wounds and amputated digits. More specifically, electric currents are detected exiting wounds and stumps in frog and newt limbs,18 with the strongest currents radiating outward along the edge of the wound.19 The hypothesis is that large electric currents near the edge of the wound direct epithelial cell migration into this area. For example, in a wounded rat cornea, electric current densities of 4.2 μA/cm2 are detected along the edge of the wound, compared with 2.5 μA/cm2 in the wound center19 To increase healing and regeneration, outward currents are coaxed from the stump, by applying outward currents, whereas applying inward currents reduces regeneration.18 In studies observing the regenerative capacity of amputated tails in the lizard Eublepharis and gecko Pachydactylus, it has been noted that a copious peripheral nerve supply is necessary for complete tail repair. The explanation as to why peripheral nerve endings are key for complete regeneration is that peripheral nerve endings provide an outward current supply to the end of the wounded digit.20–22
Electric field strengths in wounds vary according to organism. For example, the electric field strength in mammalian skin wounds are 150 mV/mm, whereas the electric field strength in corneal epidermal wounds ranges up to 40 mV/mm.23 Current strengths are also time-dependent during the healing process. In newts, the largest outward currents of 10 to 100 μA/cm2 occur approximately 1 week fter amputation, whereas, they range from 0.2 to 1.4 μA/cm2 directly after amputation.18
In summary, tissues can be characterized according to an array of electric properties, including voltage, current, resistance, capacitance, permittivity, and conductivity. These electric properties are detected in developing, wounded, normal, and diseased tissues. By introducing external electric stimuli into tissues, the course of development and healing is altered. The concept of applying an external electric stimulus to modulate tissues has engendered the hypothesis that applying electric fields improves the function of engineered tissues.
Electric Field Device Designs
Because induced electric currents and applied electric fields are known to alter development in animals such as X. laevis and improve healing in wounded frog and newt limbs, the same strategy has been used to enhance positive outcomes in tissue engineered constructs in vitro and in vivo. Electric fields are present surrounding electric currents in native organisms and tissue. Defined in SI units as volts per meter (V/m) or Newtons per coulomb (N/C), electric fields exert forces on other charged particles and can be described as vector fields with magnitude- and charge-dependent direction. Electric fields emanate outward from positive charges and radiate inward toward negative charges. For enhancing the quality of engineered tissues, such as proliferation, adhesion, differentiation, morphology, migration, and function, cells are exposed to electric fields using one of several stimulation devices. These devices have been designed several ways, with a few common features. First, all devices contain a cell chamber that provides a biocompatible, sterile environment for holding cells and tissue constructs. For daily, long-term exposure, when the cells' temperature might decrease, the chamber or the entire device is designed with dimensions that are incubator compatible.24 Second, devices are designed as closed circuit systems and provide physiologically viable strengths that are on the order of 10−3 V and 10−6 A. Finally, stimulation chambers can be designed to be protected from external unwanted electric sources. The use of a faraday cage surrounding the device ensures that external electromagnetic fields do not interfere with cell and tissue studies.25 Devices can be designed to apply direct current (DC) or alternating current (AC) fields. In vitro DC systems adopt a salt bridge setup, whereas AC systems use capacitively coupled (CC) or inductively coupled (IC) designs. Chamber designs have been built for two- and three-dimensional (2D and 3D) in vitro studies and adapted for in vivo work.
Direct current in vitro device designs
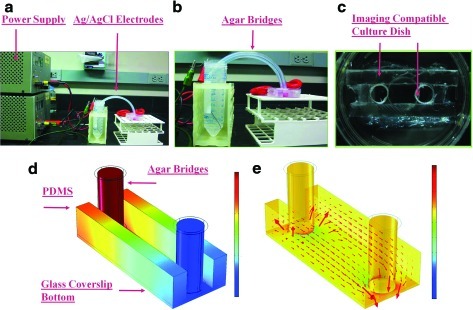
DC systems provide a uniform continuous electric field to engineered tissues. DC device designs are mainly used in vitro to align cells, observe cell migration across the surface or into engineered constructs, and enhance cell differentiation (Table 2). In vitro DC systems are constructed using a salt bridge design for the purpose of preventing nonreversible faradaic, cytotoxic reactions, such as hydrolysis, from occurring in the medium next to the cells.26,27 Salt bridges are electrochemical cells that work like batteries, transferring electric current to ionic current through agar bridges through a set of oxidation-reduction redox reactions. Redox reactions occur at the electrodes. An electrode attached to the positive terminal is oxidized (known as electron loss) while an electrode connected to the negative terminal is reduced (known as electron gain), forming an anode and a cathode, respectively. In vitro salt bridge designs consist of a DC power supply, two electrodes, and two agar bridges connected to a cell chamber or coverslip (Fig. 2a–c).28–31 Voltage supplies must be large, at least 70 V, to overcome the salt bridge's large resistance, which mainly derives from the agar bridges.30 Silver/silver chloride electrodes are the most common electrode because they are biocompatible, robust, used in laboratory electrophysiology studies, and employed in clinical devices.30,31 They are typically submersed in saline, connecting the power supply and the agar bridges. Cell chamber or coverslip dimensions are small, in the millimeter range, to reduce surface area and maximize current density to the tissue, as justified through Ohm's Law in conductive medium (E=J*ρ; where E is the electric field in V/m; J is the current density in A/m2; and ρ is the local resistivity in ohm meters).29
Table 2.
Tissue Responses to Direct Current and Alternating Current Fields
| Tissue response to applied direct current fields | Cells and tissue type | References | Tissue response to applied alternating current fields | Cells and tissue type | References |
|---|---|---|---|---|---|
| Morphology (orientration) | Cardiac, human adipose-derived stem cells, rat mesenchymal stem cells, human skin cells | 26,28,29,32 | Functionality | Neuron (rat), muscle (skeletal and cardiac) | 38,42,82–84 |
| Migration | Corneal epithelial cells, adipose-derived stromal cells, vascular endothelial cells, keratinocytes, fibroblasts (mouse embryo and human) | 31,70–77 | Differentiation | Human mesenchymal stem cells, bovine cartilage, SAOS-2 cells (human sarcoma osteogenic), mouse osteoblasts (MC3T3-E1) | 13,34,37,51,55 |
| Differentiation | Human dermal fibroblasts, human mesenchymal stem cells, bovine chondrocytes | 30,78,79 | Proliferation | Primary rat osteoblasts, human bone marrow stem cells, human osteoblasts (SAOS-2), primary bone (rabbit) | 33,43,47,58,66,81 |
| Morphology | Human condrocytes, Jurkat cells, SAOS-2 | 35,36,85 | |||
| Adhesion | Fibroblasts (rat tendon and human), rat bone marrow osteoprogenitors, rat bone marrow mesenchymal stem cells | 29,40 |
FIG. 2.
In vitro direct current (DC) stimulation device. (a) An example of a DC chamber design using a salt bridge setup. Materials for this design include a power supply, silver/silver chloride electrodes, agar bridges, and an imaging-compatible cell culture chamber. (b) Electric current transfers to ionic current via the silver/silver chloride electrodes and agar bridges in phosphate buffered saline (PBS). This image displays the cell culture dish with agar bridges and silver chloride electrodes in 50-mL conical tubes containing PBS. (c) An imaging-compatible chamber containing a 150-μm-thick coverslip bottom. The cover slip bottom is used for imaging after electric field application. (d) The distribution of the applied voltage through the chamber. Quantitative values are not shown because the applied voltage is user dependent. (e) The field direction and distribution throughout the DC chamber are noted by the red arrows. Field direction and strength is constant throughout the chamber, as noted by arrow direction, arrow size, and color. Electric field distributions and strengths are calculated through finite element analysis software, such as COMSOL Multiphysics. Ionic current supplied over the cells in is the mA range.30 Color images available online at www.liebertonline.com/teb
As energy is transferred to the cell chamber from the power supply, increases in chamber temperature, known as Joule heating, may occur in the tissue engineered constructs. Increases in heat can be described and calculated mathematically as; 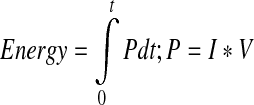 ; where energy is defined in joules, P is defined as power in watts, t is defined as time in seconds, I is defined as current supplied in amperes, and V is defined as voltage supplied in volts. From this equation, the amount of energy in joules may be converted to the heat unit calories. For monitoring temperature increases, a type K thermocouple wire can be used under the center chamber well.30 There has not been extensive research examining the effects of joule heating from electric stimulation on tissue engineered constructs in the static and low frequency ranges (0–300 Hz).
; where energy is defined in joules, P is defined as power in watts, t is defined as time in seconds, I is defined as current supplied in amperes, and V is defined as voltage supplied in volts. From this equation, the amount of energy in joules may be converted to the heat unit calories. For monitoring temperature increases, a type K thermocouple wire can be used under the center chamber well.30 There has not been extensive research examining the effects of joule heating from electric stimulation on tissue engineered constructs in the static and low frequency ranges (0–300 Hz).
DC devices have been used for studying the effects of electric fields over a wide range of cell types and tissues, including cardiac cells, stem cells (human adipose derived and mesenchymal), epithelial cells, keratinocytes, and fibroblasts (mouse and human) (Table 2). The extent of changes in cell morphology, alignment, and migration have been cell-type dependent. For example, one study demonstrated that fibroblasts reorient perpendicular to the electric field at 0.1 V/mm, whereas keratinocytes reorient at 0.4 V/cm.32
AC in Vitro Device Designs
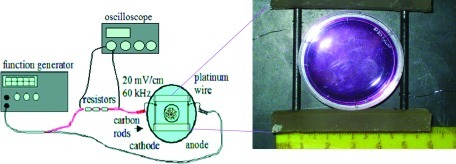
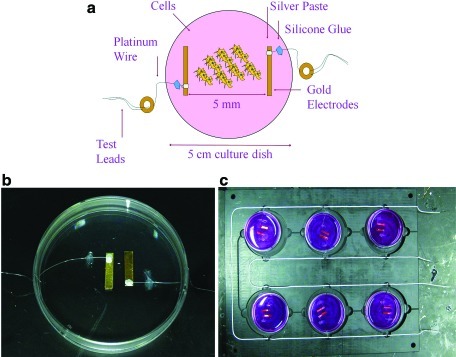
AC devices provide a bidirectional electric field and are categorized as CC (Fig. 3) or IC (Fig. 4) models. CC and IC AC devices have mainly been studied for increasing pulsatile contraction in cardiac or skeletal muscle cells, communication within neural networks, enhanced differentiation, and enhanced proliferation (Table 2). For CC and IC chambers, components include a function or waveform generator,33–41 which supplies low-strength voltage (1–10 Vpeak to peak) and a wide range of frequency signals (0.2 Hz–2 MHz) having sine, square, or triangle pulse shapes with varying pulse duration. They also include noncorrosive electrodes, such as carbon, platinum, gold, titanium, and stainless steel alloys,42–46 a cell culture dish, and an optional amplifier.38,40,42,47 For applying complicated stimulation regimens, data acquisition boards interfaced with an instrument driver and application software, such as LabVIEW, can replace the function generator.42 For higher voltage or frequency ranges, high-end stimulators, such as those from Grass Technologies, are used.26
FIG. 3.
In vitro capacitively coupled (CC) alternating current (AC) device. The AC device is powered by a function generator. Three resistors are placed in series with the device for the purpose of verifying that the correct signal strength is applied to the cell–tissue construct during each treatment. Voltage measurements are taken using an oscilloscope across the resistors (or may be taken across the carbon rods).37 Biomed Central is the original publisher of the drawing. Color images available online at www.liebertonline.com/teb
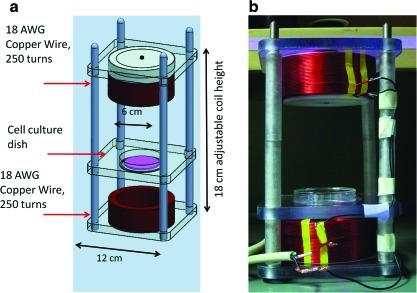
FIG. 4.
In vitro inductively coupled (IC) AC device. (a) For more complicated designs, devices are created using modeling software, such as SolidWorks, and then sent out to a fabrication facility. In this design, the distance between the top and bottom coil is adjustable for the purpose of increasing or decreasing the field strength applied to the tissues. (b) In this device, the cell culture dish is placed within the center of the device between each coil. In many studies, cells are given overnight (or up to 24 hours) to attach to the scaffold or the bottom of the dish before electric stimulation. Color images available online at www.liebertonline.com/teb
CC devices generate an electric field by storing charge on two parallel electrode plates. One electrode is positively charged, and the other stores a negative charge, forming an electric field between them. The field strength is inversely proportional to the distance between the electrodes.48 The electrodes are modeled as capacitors to calculate the electric field strength reaching the cells. For an even field distribution between the electrodes, a straight electrode edge is required. Before stimulation, the electrodes are placed on opposite sides of the tissue sample.42,49,50 AC devices are defined as CC when electrodes are not in direct ohmic contact with the cell culture medium. In the case that electrodes are in direct contact with the cell–tissue construct or the tissue culture medium, the AC device functions by transferring electric current to ionic current at the electrode–electrolyte interface. Ionic current is then conducted through the culture medium over the engineered cell constructs (Fig. 5a, 5b). Cell culture medium has a conductivity of approximately 10 ms/cm11 and is an important factor when predicting the electric field penetration into tissue. Because of relative device simplicity and low cost, AC chambers can be scaled up easily for stimulating multiple constructs (Fig. 5c).24
FIG. 5.
(a) In vitro AC device. (b) For direct AC stimulation, electrodes are placed inside the device chamber with the cells or tissue. The choice of electrode material depends on characteristics such as charge transfer, corrosiveness, biocompatibility, and cost.46,131 Before the experiment, electric measurements are taken inside the culture dish to verify that the desired current strength reaches the cells. Electric measurements may also be taken across each chamber for verifying desired voltages. Large resistances at each node may decrease voltage potential and overall charge delivered to the cells. (c) Group of six AC chambers. Color images available online at www.liebertonline.com/teb
AC devices have largely been constructed for studies on contracting skeletal and cardiac tissue.24,37–39,42,51 In one study, engineered muscle tissue was constructed from mouse C2C12 myoblasts on an acceulularized mouse extensor digitorum longus muscle.42 Results revealed that differentiated skeletal myotubes were able to contract longitudinally at 17.3±1.2 μN after 40V, 40 Hz electric stimulation.42 In a second study, AC devices were built for stimulating 3D muscle tissue constucts. Myoblasts were grown on 3D polyglycolic acid mesh scaffolds and exposed to cardiac-like current fluxes of 1.54mA, 564 mV/cm. Although proliferation doubled by day 14, there was not a difference in differentiation between stimulated samples and controls.24
Several studies examining the effects of CC AC fields on bone cells and cartilage explants have been performed.13,52–54 These studies use parameters of 60 kHz, 20 mV/cm with varying duty cycles. Results revealed greater extracellular matrix production, aggrecan and collagen gene expression, osteoinductive bone morphogenetic protein (-2, -4, -5, -6, -7), and alkaline phosphatase (ALP) activitity,13,52–54 suggesting the usefullness of electric fields for preserving matrix production in the presence of osteoarthritis.
The principal component of an IC device is the conducting coil.47,55–58 IC devices work by driving current down the coiled wire, generating a magnetic field through the center of the coil, where the sample is located. The magnetic field strength is proportional to the current strength in the coil, as described in the Biot-Savart Law.48 IC devices typically have a coil diameter on the order of decimeters and contain hundreds of coil turns to reach magnetic field strengths of 10−3 T in magnitude.59 IC setups are frequently used for delivering pulsed electromagnetic fields (PEMF) to tissue engineered constructs.43,47,55,60,61
IC AC setups have been self-constructed and mainly used in bone and cartilage tissue studies.34,43,47,55,58,61,62 Typically, IC AC studies have been performed using extremely low frequency fields, which have been effective at altering cell proliferation and differentiation markers. For example, one study seeded osteoblasts onto poly(DL-lactic-co-glycolic acid) (PLGA) scaffolds and then exposed the tissue engineered constructs to a 7.5-Hz quasirectangular waveform with 0.13-, 0.24-, or 0.32-mT fields. Assays revealed greater cell proliferation in osteoblasts exposed to 0.13-mT field strengths. A 0.32-mT field decreased cell proliferation but enhanced ALP activity.47 In a second study, rat osteoblasts exposed to a 48-Hz, 1.55-mT IC AC field increased proliferation after 24 hours of exposure.60 ALP activity was enhanced in the same cells after 48 hours of exposure.
DC in vivo device designs
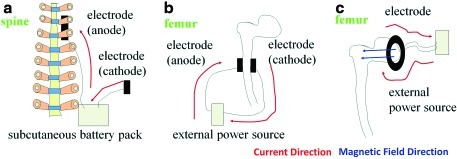
In vivo DC models consist of a cathode, anode, battery pack, and wire (Fig. 6a). For clinical application, the anode is implanted surgically in contact with the wound, and the cathode is placed in the nearby soft tissue.63 The battery pack is connected through the wire and typically placed subcutaneously between the anode and cathode. In some cases, in vivo systems include a potentiometer to account for varying resistance between animals. Depending on tissue resistance, the potentiometer is adjusted before stimulation so that the same current strength is delivered during each treatment. During treatments, electricly generated ions from the anode penetrate into the surrounding tissue.
FIG. 6.
In vivo electric stimulation devices. (a) DC design, (b) AC design, (c) AC design. Electric current directions are noted by red arrows. Magnetic field directions are marked by blue arrows. Color images available online at www.liebertonline.com/teb
AC in vivo device designs
AC in vivo devices operate under the same theoretical principles as in vitro devices, although they are modified for an in vivo environment.64 For in vivo AC setups, electrodes are placed next to each side of the wounded area (Fig. 6b). One or two electrodes have been used for unipolar or bipolar applications, stimulating one or both sides of the tissues. Electrode size determines the area treated.65 For example, point electrodes affect only a small area, whereas large electrodes provide stimuli to long bones, covering a much larger area. Other types of AC setups employ a ring or coil around or next to the wounded area (Fig. 6c). Mechanistically, electric current is transferred to ionic current across the electrode–electrolyte interface. In vivo, AC devices have been applied across biomaterial constructs implanted in the thoracic muscle,66 although in most cases, electric field stimulation is used during tissue development in vitro. In vivo AC devices are commonly used for healing bone fractures that do not heal, failed fusions, and pseudoarthritis.67
In summary, device options for applying electric fields to engineered tissues include DC setups, AC CC devices, AC IC devices, and general AC pulsed devices. Devices have been designed for in vitro and in vivo applications.
Tissue Response After Electric Field Application
Studies have demonstrated that electric stimulation alters several properties of engineered tissues, including cell differentiation, proliferation, morphology, adhesion, migration, and function, with different stimulation parameters affecting different sets of tissue characteristics. For example, whereas uniform DC field stimulation typically directs cell orientation, alters cell morphology, and directs cell migration, AC fields enhance cell differentiation and increase tissue function (Table 2). In the next section, we will first review tissue responses from DC field stimulation and then review responses from AC field stimulation.
Tissue Responses to a DC Electric Field
Morphology
Cells exhibit changes in morphology such as elongation, reorientation, and alignment perpendicular to the applied DC electric field. Morphological changes in response to applied DC fields are typically observed within the first 24 hours of exposure.26,28,29,32 For example, human adipose tissue–derived stem cells and human epicardial fat–derived stem cells were elongated and aligned perpendicular to the electric field within 4 hours of exposure to a 6-V/cm electric field.28 In a second example, NIH3T3 cells that originated from a mouse embryonic cell line elongated after 3 hours of exposure to 6-V/cm field.26 The response time for morphological changes to occur depends on the field strength. For example, human skin fibroblasts aligned over 24 hours using a 0.1 V/mm field, but when a higher-strength field of 0.4 V/mm was applied, cells aligned within 3 hours.32 Finally, the strength at which electric fields alter cell morphology, cell elongation, and cell alignment depends on the cell type. For example, whereas fibroblasts aligned and elongated perpendicular to a 7-V/cm stimulus for 60 minutes, mesenchymal stem cells did not show reorientation.29 Controlling cell morphology, such as elongation, orientation, and alignment, in cell–tissue constructs is important for engineering the corneal stroma layer,68 connecting nerve ends after peripheral nerve injuries channels,69 and aligning cardiac muscle cells.26
Migration
Aside from morphology, cell migration, also defined as galvanotaxis, is another well-known effect of exposure to a DC field. In response to DC field stimulation, the direction of migration depends on cell type.70 For example, whereas human keratinocytes and mouse embryonic fibroblasts migrate toward the cathode, human granulocytes, rabbit corneal endothelial cells, and human vascular endothelial cells migrate toward the anode.71–75 Cell migration is important in the field of tissue engineering for several applications, including cell infiltration into scaffolds and integration with host tissue. For example, human fibroblasts migrated through a 3D 0.58-mg/mL collagen gel after application of a 0.1-V/cm field.76 In another example, collagen sheets of human and bovine epithelial cells migrated to the cathode at a rate of 15 μm per hour during exposure to a 150-mV/mm field.31,77
Differentiation
There have been some publications establishing increased messenger RNA (mRNA) transcript expression, protein synthesis, and differentiation in response to a DC electric field. For example, adult human dermal fibroblasts exposed to a 100-mV/mm field for 1 hour increased 164 gene transcripts compared to unstimulated cells. Seventy-three of the 164 transcripts increased more than 1.4 times.30 In another example, osteogenic differentiating human mesenchymal stem cells (hMSCs) were exposed to a 0.1-V/cm field 30 minutes per day for 10 days. In these studies, ALP was significantly greater than in the control group. Calcium mineralization was also greater in the treated group.78 Nevertheless, not all articles have reported enhanced differentiation effects in response to a DC field. For example, in one article, chondrocytes were isolated from 18- to 24-month old cows, seeded on agarose constructs, and then exposed to 4-mA/cm2 current density for 6 hours. There were no significant differences in mRNA aggrecan expression or mRNA collagen type II expression from controls.79
Tissue Responses to an AC Electric Field
Differentiation
Several articles have reported that applied electric fields enhance levels of gene expression in bone, connective, and muscular cells undergoing differentiation.13,30,34,37,51,55,78,79 For example, levels of ALP, an early marker of bone differentiation, are hugher in cells undergoing bone differentiation when treated with pulsed electromagnetic fields.13,34,37,55 During osteogenic stem cell differentiation, hMSCs exposed to 60 kHz, 20 mV/cm for 40 minutes daily demonstrated greater expression of ALP and type I collagen than nonstimulated controls.37 In 3D tissue constructs, cells from the human sarcoma osteogenic cell line (SAOS-2) were seeded on polyurethane scaffolds and exposed to a 75±2-Hz, 5±1-mV, 2±0.2-mT pulsed electromagnetic field. Results indicated increases in extracellular matrix deposition of decorin, osteocalcin, osteopontin, type I collagen, and type III collagen 1.3, 12.2, 12.1, 10.0, and 10.5 fold, respectively.55 Gene expression levels and effects of cell differentiation from applied electric fields may be age dependent.34 For example, it was shown that 15-Hz, 7-mT PEMF treatment on osteoblasts during differentiation increased bone-like tissue, whereas a 15-Hz, 7-mT PEMF treatment on osteoblast differentiation during the mineralization stage decreased bone formation.34 In cartilage explants, a quintupling of proteoglycan and a doubling of collagen was found in samples exposed to 20-mV/cm, 60-kHz electric field stimulation in vitro.13
Electric fields have also been reported to increase genes in cardiomyocytes. In one tissue engineering study, neonatal rat ventricular myocytes seeded on collagen sponges using Matrigel were exposed to 2-ms 5-V/cm, 1-Hz rectangular pulses continuously for 5 days.51 Constructs were evaluated using Western blots, polymerase chain reaction, and immunohistochemistry. Increased gene expression included major histocompatibility complex, connexin 43, creatine kinase-MM, cardiac troponin I, sarcomeric alpha-actin, beta isoforms of myosin heavy chain, and beta-integrin.51
Proliferation
Proliferation plays an important role in metabolism and bone growth. The field of regenerative medicine has demonstrated enhanced cell proliferation after exposure to electric field treatments. For example osteoblasts on PLGA scaffolds revealed greater proliferation, up to 39% on day 6 in culture and up to 14% on day 12, after exposure to a 300-ms quasirectangular pulses with a repetition rate of 7.5 Hz.47 In these experiments, electric field stimulation was applied for 2 hours per day.47 In another example, SAOS-2 human osteoblasts seeded on titanium fiber mesh scaffolds proliferated twice as much in the presence of a 75-Hz electromagnetic field as unexposed scaffolds.33 In another article, hMSCs increased in cell number 20% to 60% in the exponential growth phase during electric field stimulation. Specifically, PEMF stimulation lead to more stem cells in the G2/M cell cycle phase in the first 6 to 12 hours, with more hMSCS in the G0/G1 phase between 18 and 24 hours.58 In vivo, decalcified bone matrix was implanted along the thoracic musculature in rats and exposed to 1 to 10-mV/cm, 10 - 20 μA/cm2 for 8 hours per day. At this field strength, there was no difference in DNA content between the exposed and control groups.80 In another in vivo study, a porous, coated titanium implant was placed in the humeri of Japanese rabbits. Bone growth was promoted after 14 days of exposure to a 10-Hz, 2-G magnetic field with a 25-μs pulse. Exposure depended on the length of stimulation. Several factors, including implant design, material, pore size, and implant adhesion to bone, may affect bone ingrowth by electromagnetic stimulation.81
Adhesion
Cell adhesion plays an important role in cell behavior and in cultivating an optimal microenvironment for engineering 2D and 3D tissues in culture. The application of electromagnetic fields may alter attachment properties. For example, hMSC exposed to an externally applied DC field showed strong 3D adhesion to collagen gels after exposure to a 7-V/cm electric field.29 Cell adhesion is important in morphogenesis and organogenesis, including anchorage, osteogenic cell differentiation, proliferation, and cell–cell signaling, although not all reports have shown that low-frequency AC and DC fields have increased attachment, and some field applications have shown the opposite. For example, electric fields of 60 and 1,000 Hz applied to rat tendon fibroblasts and rat bone marrow osteoprogenitor cells caused extensive detachment of preattached cells and prevented cell attachment to substrates.40
Functionality
Many function studies focus on contracting cardiac muscle cells, contracting skeletal muscle cells, and neuronal communication.42 In cardiac muscle, the amplitude contractions of tissue constructs were 7 times as high in 5-V/cm, 1-Hz, 2-ms rectangular-pulse pulsatile electric field, than nonstimulated controls.51 Electric field stimulation in one layer of cardiomyocytes can synchronize beating in an adjacent muscle sheet.82 Greater contraction in cultured beating myotubes on collagen dishes exposed to 5-Hz electric stimulation has been reported.38 In vivo, electric stimulation can affect short- and long-term peripheral nerve regeneration and increase systolic and diastolic filling in the heart.83,84
Morphology
Electric fields have been used to alter tissue and cell shape, generating multilayer tissues, cell aggregates, and shaped cells. Multilayer tissues and cell aggregates are important in the field of tissue engineering for the development of 3D tissue constructs and the study of cellular microenvironments.36,85 Morphological changes depend on electrode size, applied voltage, and field distribution.36,85 For example, in a pulsed electromagnetic field, chondrocytes changed from satellite to spindle to spherical shapes within 6 hours of 100-Hz, 0.0039-mV/cm electric field exposure, whereas 500-Hz frequencies did not have any effect.56
Infection Prevention in Engineered Scaffolds
Tissue engineered constructs may become contaminated because of a lack of proper aseptic technique, nonsterilized tools, or a nonsterilized work environment. Lost experiments due to bacterial, viral, or fungal contamination wastes valuable research time, delays results, and is costly to the laboratory. To address this concern, the use of electric stimulation has been researched to reduce bacteria levels in engineered scaffolds. Collagen gels seeded with Escherichia coli NCTC 9001 cells and treated with a range of pulsed electric fields with 0 to 100 pulses at 1-μS widths and a strength of 24 kV/cm inactivated up to 1.5 log10 colony forming units/ml. Higher field strengths and a greater number of pulses were more lethal to E. coli. Complete inactivation of bacteria depended on contamination levels.50
In summary, electric fields can control differentiation, proliferation, morphology, adhesion, migration, function, and infection. DC tissue stimulation is generally useful for altering cell orientation, morphology, and migration, and AC is generally useful for tissue differentiation, proliferation, function, and morphology, with an emphasis on function and differentiation. Finally, although many studies have been completed in vitro, other will be needed in vivo.
Mechanisms
Electric fields alter ionic currents and ion distribution in the extracellular space, altering membrane potential.86 Electric fields activate a cascade of signaling pathways that upregulate transcription and translation levels,87–90 and electric fields exert an electric force on cells' membranes,91 but identifying the underlying mechanisms correlating field exposure to a defined tissue response remains difficult.90 For example, mechanisms that initiate cellular responses vary according to the applied frequency or electric field strength.11 DC and AC chambers may influence different mechanisms for altering cell fate. Furthermore, the activation of different signaling cascades may yield different cellular responses, specifically with regard to differentiation, morphology, adhesion, migration, and proliferation. Finally, the application of electric fields may increase temperature in culture medium in vitro or raise temperature levels in the surrounding tissue in vivo,11 complicating the differentiation between field effects and stress responses related to temperature.
During electric field exposure, cell membrane potential is changed.92 In nonexcitable cells, cell membranes near the anode hyperpolarize, whereas those near to the cathode depolarize.92,93 During electric field treatment, the density and distribution of ion channels and receptors may reorganize.56 Changes in ion channels, gap junctions, ligand binding, and membrane protein density may affect signaling cascades and alter downstream processes.28,56 In osteoblasts, electromagnetic fields may interfere with hormone receptor interactions, such as parathyroid hormone (PTH), on the cell surface.40,94 For example, osteoblast-like mouse cells showed less ability to produce cyclic adenosine monophosphate in response to PTH in the presence of an electric field.40,94
The explanation for better osteoblast healing through electric fields may involve voltage-gated calcium channel activation.87–90 During field application, an increase in Ca2+ influx was noted in bone and skeletal muscle.95,96 Osteoblast stimulation by pulsed electromagnetic field increased Ca2+ influx, increasing downstream factors such as prostaglandin E2, insulin receptor substrate-1, and TGF-β.97 Electric stimulation may also increase cytosolic calcium oscillations in osteoblasts that resemble the Ca2+ oscillation pattern in differentiated osteoblasts.78,88 By blocking Ca2+ channels, using blockers such as verapamil and W-7, during field application, effects in TGF-β and proliferation are not observed,87 yet piecing together a complete mechanistic pathway remains to be achieved.
It is not clear whether some cells detect electric field activity at the membrane bilayer or whether electromagnetic fields are able to penetrate through the membrane walls directly, activating electrons in DNA cells. Changes in stress response transcription factors from applied electric and electromagnetic fields may also regulate or upregulate transcription factors or stress factors such as heat shock protein 70.98
Electric fields may exert a force that alters free ion concentration.82 Changes in free ion concentration will result in changes in cell morphology and upregulation in differentiation. For example, force fields may be an explanation for contraction in such cells as fibroblastic and myoblastic cell types or for changes in chondrocyte morphology.
In summary, cellular mechanisms remain unclear. If they could be further elucidated, more-systematic and -predictable outcomes could be envisioned.
Electric fields as an adjuvant therapy
The field of tissue engineering involves collaborative efforts between scientists, engineers, and surgeons to identify solutions for tissue repair and regeneration. Thus, the combination of biophysical strategies with traditional approaches, such as mechanical stimulation, biomaterial surface modification, and protein addition, is a logical next step.
External forces such as cyclical stretch and pulsatile flow improve 3D tissue organization and extracellular matrix deposition.99 Therefore, a logical step is to combine biomechanical strategies with bioelectric stimulation. Several groups have designed and used bioreactors that provide electric and mechanical stimulation for engineering cell tissue constructs.43 An electromechanical bioreactor provided a 300-ms quasirectangular pulse for 2 hours per day over 18 days to an osteoblast-seeded porous PLGA scaffold. Electromechanical stimulation was sufficient for regulating osteoblast proliferation and differentiation. In addition to bone cells, PEMF mechanical bioreactors have been used on cardiac cells.47 Collagen gels seeded with cardiomyocytes were studied in electromechanical bioreactors in vitro to mimic cyclic strains observed in vivo.100 Preliminary findings demonstrated that the cardiomyocytes were able to live in the electromechanical chamber. For scaling up production and translation into the clinic, multiple challenges must be addressed. Chamber designs need to be customizable, externally activated, sterile, cost effective, able to hold multiple tissue constructs, and able to deliver stimulation under tightly controlled conditions.99,101
Other biophysical approaches that use a combined therapy approach include the optimization of biomaterial surfaces, such as surface microstructure and chemistry,55,102 and biophysical stimulation to optimize tissue response. Corneal epithelial cells were seeded on quartz patterned surfaces with grooves at 1, 2, and 4 μm and depths ranging from 40 to 1100 nm at 150 mV/mm for 3 hours.102 Results showed cell alignment orthogonal to the electric field. Effects were synergistic when using biophysical and bioelectric strategies.102
Several studies combined electric fields and proteins or growth factors to control cell reorientation and migration. In one neuronal regeneration study, the addition of 50 or 100 ng/mL of brain-derived growth factor, neurotrophin 3, or neurotrophin 4 and an applied 100-mV/mm electric field for 5 hours resulted in an orientation of a neuron population that was 4 times as great (-36±9° vs -9±5°) as with an electric field alone. Furthermore, the addition of brain-derived neurotropic factor lowered the electric field strength needed to reorient the neurons towards the cathode.103 The migration of cultured bovine corneal epithelial cells with the addition of epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), or TGF-β1 has been assessed. EGF resulted in cell migration that was 4 times as greater, up to 12 to 16 μm/h in a 100-mV/mm DC field, as with cells that did not have EGF present. The combination of growth factors, specifically EGF+TGF-β1 and EGF+bFGF+TGF-β1, had an additive effect. In other studies, electric fields have shown the ability of cells to reorient and migrate in an electric field, although their orientation and migration ability is serum dependent.31,104 Finally, growth factors have been shown to promote stem cell differentiation in the presence of electric fields. For example, the combination of PEMF and bone morphogenetic protein-2 revealed synergistic effects regarding higher levels of ALP, osteocalcin, TGF-β1, and prostaglandin E2.105,106
The evolution of electrotherapeutics toward current clinical devices
Although research in the field of tissue engineering is less than half a century old, the field of electrotherapeutics has been evolving for longer than 300 years. Although several papers divide the discussion of the field's rich evolution107,108 equally between the 17th century and the present day, our discussion focuses on the development of clinical devices in the 20th and 21st centuries.
In the 1900s, numerous clinical electric devices were patented, and the Food and Drug Administration (FDA) approved many, many of which are still in use today. For example, in 1952, the first fully implantable cardiac pacemaker was tested in humans.109–111 In the late 1970s, the FDA approved several noninvasive and implantable electric bone growth stimulators for healing nonunions and spinal fusions.37,112–114 Specifically, noninvasive pulsed electromagnetic stimulators by Orthofix, including the Cervical-Stim and Spinal-Stim, were used to improve rates of bone fusion.115,116 Stimulators by Orthofix such as the Physio-Stim, which is used for healing long and small bone nonunions, demonstrated success rates of up to 88% after application of at least hours per day.117–118 Bone stimulators have been reported to be applied daily over several weeks.119 By the mid 1980s, patents were filed on electrodes for growing bone.120,121 In 1997, deep brain stimulators, consisting of an electrode and pacemaker, were first approved for the treatment of tremors. Applications for deep brain stimulation were extended to Parkinson's disease for reducing or eliminating the need for antitremor medications. Tthe FDA has approved Activa Deep Brain Stimulators (Medtronic).122 Implantable pulse generators, such as Activa, generally last 4 to 5 years, although generator time depends on battery life, which depends on factors such as pulse amplitude and pulse duration.123 Finally, in 2002, devices similar to those providing transcutaneous electric nerve stimulation through applying low-intensity current were approved for healing chronic pressure ulcers, neuropathic diabetic ulcers, and venous leg ulcers.124
Clinical devices deliver DC or AC to tissues. Many clinical devices have been used for the healing of nonunions. DC devices have mainly been implantable and include the SpF – XL IIb Spinal Fusion Stimulator and the SpF PLUS-Mini Spinal Fusion Stimulator.125 AC devices have mainly been characterized as external and include products such as the EBI Bone Healing System, the Orthopak 2 Bone Growth Stimulator, and the SpinalPak II Spinal Fusion Stimulator.115,116 Implantable devices ensure patient adherence and are also useful when surgery is already needed.
Future Directions
The value of incorporating electric field stimulation for tissue engineering applications has been demonstrated, although much work is needed to optimize tissue responses for specific goals in research and in the clinic. Because electric field stimulation may have regions of linear response or nonlinear response and therapeutic “window effects,” well-defined characterization of such effects could provide improved options for directing and fine-tuning functional engineered tissues.126 This characterization would include quantifying effects over a range of voltage and current strengths, frequency ranges, pulse shapes and durations, and cell sources and lineages. Adjusting the biomaterial implant properties or scaffold design,62 in concert with the changes in electric fields, would be a next step.
Although it can be seen that some papers have used modeling and simulation software36,85 or have completed theoretical calculations56 to identify field strength that reaches the cells, this is not apparent in all papers. This inequity makes it difficult to compare studies and outcomes in the field. Chamber designs and field strength analysis would help standardize models and results. In particular, modeling tools would be useful for examining electric field strengths and electric field distribution across the surface and inside different tissue shapes and tissue densities. In addition, modeling software would allow for a quicker, more-accurate analysis of optimal field strengths to target the “therapeutic window” inside tissues. Modeling software would also allow provide information on optimal electrode placement. Ultimately these tools would aid in making better choices regarding parameters (voltage, frequency, current, waveform shape), leading to better experiment outcomes, better functional tissues, and lower costs.
Electrode optimization and the development of unique electrode designs will also be important as the field moves ahead, particularly in considering size, spacing, and material compositions to optimize distributions of fields and local biological responses. Innovative techniques for transferring charge across cells and tissue will become of key importance. More specifically, conductive polymers (although not discussed in this review) will gain in popularity as one method of charge transfer. Electrode incorporation in flexible biomaterials (conductive and nonconductive) holds promise for making secure contact with tissue and for allowing charge transfer within the treatment area. A second method os charge transfer may be using chemicals for altering ionic currents and membrane potentials that activate ion channels and receptors. All of these studies remain in the early stages and suggest that this is an important and ripe field for investigators to explore. The potential effect is immense, with influence expected at fundamental biological levels as well as in translational medicine.
Acknowledgments
This paper was supported by the Tissue Engineering Resource Center (TERC) through National Institutes of Health (NIH) grant P41EB002520 from the National Institute of Biomedical Imaging and Bioengineering and NIH grant R01 AR005593 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases,. The authors would like to thank Lisa R. Yanushefski for assistance in researching clinical electric stimulation devices and the history of electrotherapeutics, William G. Curless for assistance with the IC electric field device design, Amanda L. Baryshyan for the design of the 6-well AC chamber, and the Tufts Machine Shop for fabrication of the IC AC device and 6-well AC chamber.
Disclosure Statement
No competing financial interests exist.
References
- 1.Tsai M. Li W. Tuan R. Chang W. Modulation of osteogenesis in human mesenchymal stem cells by a pulsed electromagnetic field stimulation. J Orthop Res. 2009;27:1169. doi: 10.1002/jor.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell E. Ehrlich H. Buttle D. Nakatsuji T. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science. 1981;211:1052. doi: 10.1126/science.7008197. [DOI] [PubMed] [Google Scholar]
- 3.Langer R. Vacanti J. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 4.Odenwalder P. Irvine S. McEwan J. Jayasinghe S. Bio-electrosprays: a novel electrified jetting methodology for the safe handling, deployment of primary living organisms. Biotechnol J. 2007;2:622. doi: 10.1002/biot.200700031. [DOI] [PubMed] [Google Scholar]
- 5.Bhardwaj N. Kundu S. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;28:325. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Fedorov V. Nikolski V. Efimov I. Effect of electroporation on cardiac electrophysiology. Methods Mol Biol. 2008;423:433. doi: 10.1007/978-1-59745-194-9_34. [DOI] [PubMed] [Google Scholar]
- 7.Guiseppi-Elie A. Electroconductive hydrogels: synthesis, characterization and biomedical applications. Biomaterials. 2010;31:2701. doi: 10.1016/j.biomaterials.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 8.Li M. Guterman-Tretter E. Guo Y. MacDiarmid A. Lelkes P. Yuan X. Yuan X. Sheng J. Li H. Song C. Yen W. Research progresses on electroactive and electrically conductive polymers for tissue engineering scaffolds. Zhongguo Yi Xue Ke Xue Yuan Bao. 2006;28:845. [PubMed] [Google Scholar]
- 9.Brody J. Kern S. History and Principles of conductive media for standard DNA electrophoresis. Anal Biochem. 2004;333:1. doi: 10.1016/j.ab.2004.05.054. [DOI] [PubMed] [Google Scholar]
- 10.Vesterberg O. History of electrophoretic methods. J Chromatogr. 1989;480:3. doi: 10.1016/s0021-9673(01)84276-x. [DOI] [PubMed] [Google Scholar]
- 11.Polk C. Postow E. Handbook of Biological Effects of Electromagnetic Fields. Boca Raton, FL: CRC Press; 1996. p. 618. [Google Scholar]
- 12.Markx G.H. The use of electric fields in tissue engineering. Organogenesis. 2008;4:11. doi: 10.4161/org.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brighton C. Wang W. Clark C. Up-regulation of matrix in bovine articular cartilage explants by electric fields. Biochem Biophys Res Commun. 2006;342:556. doi: 10.1016/j.bbrc.2006.01.171. [DOI] [PubMed] [Google Scholar]
- 14.Joines W. Zhang Y. Li C. Jirtle R. The measured electrical properties of normal, malignant human tissues from 50 to 900MHz. Medical Physics. 1994;21:547. doi: 10.1118/1.597312. [DOI] [PubMed] [Google Scholar]
- 15.Hoyt K. Castaneda B. Zhang M. Nigwekar P. Di Sant'agnese P. Joseph J. Strang J. Rubens D. Parker K. Tissue elasticity properties as biomarkers for prostate cancer. Cancer Biomark. 2008;4:213. doi: 10.3233/cbm-2008-44-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halter R. Schned A. Heaney J. Hartov A. Schutz S. Paulsen K. Electrical impedance spectroscopy of benign and malignant prostatic tissues. U Urol. 2008;179:1580. doi: 10.1016/j.juro.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 17.Hotary K. Robinson K. Endogenous electrical currents and voltage gradients in Xenopus embryos and the consequences of their disruption. Devel Biol. 1994;166:789. doi: 10.1006/dbio.1994.1357. [DOI] [PubMed] [Google Scholar]
- 18.Borgens R. Vanable J. J. Jaffe L. Role of subdermal current shunts in the failure of frogs to regenerate. J Exp Zool. 1979;209:49. doi: 10.1002/jez.1402090106. [DOI] [PubMed] [Google Scholar]
- 19.Zhao M. Electric fields in wound healing - an overriding signal that directs cell migration. Sem Cell Devel Biol. 2009;20:674. doi: 10.1016/j.semcdb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Whimster I. Nerve supply as a stimulator of the growth of tissues including skin. II. Animal evidence. Clin Exp Dermatol. 1978;3:389. doi: 10.1111/j.1365-2230.1978.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 21.Thornton C. Amphibian limb regeneration and its relation to nerves. Am Zoologist. 1970;10:113. doi: 10.1093/icb/10.2.113. [DOI] [PubMed] [Google Scholar]
- 22.Becker R. The bioelectric factors in amphibian-limb regeneration. J Bone Joint Surg Am. 1961;43-A:643. [PubMed] [Google Scholar]
- 23.Nuccitelli R. Endogenous electric fields in embryos during development, regeneration and wound healing. Radiat Prot Dosimetry. 2003;106:375. doi: 10.1093/oxfordjournals.rpd.a006375. [DOI] [PubMed] [Google Scholar]
- 24.Pedrotty D. Koh J. Davis B. Taylor D. Niklason L. Engineering skeletal myoblasts: roles of three-dimensional culture and electrical stimulation. Am J Physiol Heart Circ. 2004;288:H1620. doi: 10.1152/ajpheart.00610.2003. [DOI] [PubMed] [Google Scholar]
- 25.General Books LLC. Electrostatics: Andre-Marie Ampere, Electric Charge, Electric Potential, Electric Field, Faraday Cage, Triboelectric Effect. New York: 2010. [Google Scholar]
- 26.Tandon N. Cannizzaro C. Chao P. Maidhof R. Marsano A. Au H. Radisic M. Vunjak-Novakovic G. Electrical stimulation systems for cardiac tissue engineering. Nat Protoc. 2009;4:155. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greatbatch W. Metal electrodes in bioengineering. CRC Critical Reviews in Bioengineering. Crit Rev Bioeng. 1981;5:1. [PubMed] [Google Scholar]
- 28.Tandon N. Goh B. Marsano A. Chao P. Montouri-Sorrentino C. Gimble J. Vunjak-Novakovic G. Alignment and elongation of human-derived stem cells in response to direct-current electrical stimulation. Conf Proc IEEE Eng Med Biol Soc. 2009;1:6517. doi: 10.1109/IEMBS.2009.5333142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun S. Titushkin I. Cho M. Regulation of mesenchymal stem cell adhesion and orientation in 3D collagen scaffold by electrical stimulus. Bioelectrochemistry. 2006;69:133. doi: 10.1016/j.bioelechem.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Jennings J. Chen D. Feldman D. Transcriptional response of demal fibroblasts in direct current electric fields. Bioelectromagnetics. 2008;29:394. doi: 10.1002/bem.20408. [DOI] [PubMed] [Google Scholar]
- 31.Zhao M. Agius-Fernandez A. Forrester J. McCaig C. Directed migration of corneal epithelial sheets in physiological electric fields. Invest Opthalmol Vis Sci. 1996;37:2548. [PubMed] [Google Scholar]
- 32.Dube J. Methot S. Moulin V. Goulet D. Bourdage M. Auger F. Germain L. External electric fields induce morphological changes on human skin cells cultured in vitro. New Delhi, India: XXVIIIth General Assembly of Radio Science (URSIGA); 2005. p. 4. [Google Scholar]
- 33.Fassina L. Visai L. De Angelis C. Benazzo F. Magenes G. Surface Modification of a porous polyurethane through a culture of human osteoblasts and an electromagnetic bioreactor. Tech Health Care. 2007;15:33. [PubMed] [Google Scholar]
- 34.Diniz P. Shomura K. Soejima K. Ito G. Effects of pulsed electomagnetic field (PEMF) stimulation on bone tissue like formation are dependent on the maturation stages of the osteoblasts. Bioelectromagnetics. 2002;23:398. doi: 10.1002/bem.10032. [DOI] [PubMed] [Google Scholar]
- 35.Sebastian A. Buckle A. Markx G. Tissue engineering with electric fields: Immobilization of mammalian cells in multilayer aggregates using dielectrophoresis. Biotechnol Bioeng. 2007;98:694. doi: 10.1002/bit.21416. [DOI] [PubMed] [Google Scholar]
- 36.Sebastian A. Venkatesh A. Markx G. Tissue Engineering with electric fields: Investigation of the shape of mammalian cell aggregates formed at interdigitated oppositely castellated electrodes. Electrophoresis. 2007;28:3821. doi: 10.1002/elps.200700019. [DOI] [PubMed] [Google Scholar]
- 37.Hronik-Tupaj M. Rice W. Cronin-Golomb M. Kaplan D. Georgakoudi I. Osteoblastic differentiation and stress response of human mesenchymal stem cells exposed to alternating current electric fields. Biomed Eng Online. 2011;10:9. doi: 10.1186/1475-925X-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kondo H. Hashimoto S. Yamasaki K. Ono K. Okada M. Fujisato T. Kobayashi H. Mochizuki S. Ohsuga M. Yoshiura M. Tsutsui H. Akazawa K. Kawai T. Uto S. Tsujita K. Yamada E. Movement of Cultured Myotube with Electrical Stimulation. WMSCI 2008: 12th World Multi-Conference on Systemics; Cybernetics and Informatics; 2008. p. 104. [Google Scholar]
- 39.Shimizu T. Yamato M. Akutsu T. Shibata T. Isoi Y. Kikuchi A. Umezu M. Okano T. Electrically communicating three-dimensional cardiac tissue mimic fabricated by layered cultured cardiomyocyte sheets. J Biomed Mater Res. 2002;60:110. doi: 10.1002/jbm.1284. [DOI] [PubMed] [Google Scholar]
- 40.Blumenthal N. Ricci J. Breger L. Zychlinsky A. Solomon H. Chen G. Dorfman R. Effects of low-intensity AC and/or DC electromagnetic fields on cell attachment and induction of apoptosis. Bioelectromagnetics. 1997;18:264. doi: 10.1002/(sici)1521-186x(1997)18:3<264::aid-bem10>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 41.George I. Geddis M. Lill Z. Lin H. Gomez T. Blank M. Oz M. Goodman R. Myocardial function improved by electromagnetic field induction of stress protein hsp70. J Cell Physiol. 2008;216:816. doi: 10.1002/jcp.21461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borschel G. Dennis R. Kuzon W.J. Contractile skeletal muscle tissue-engineered on an acellular scaffold. Plast Reconstr Surg. 2004;113:595. doi: 10.1097/01.PRS.0000101064.62289.2F. [DOI] [PubMed] [Google Scholar]
- 43.Fassina L. Visai L. Saino E. Cusella De Angelia C. Benazzo F. Magenes G. Surface modification of titanium fiber-mesh scaffolds through a culture of human SAOS-2 osteoblasts electromagnetically stimulated. IFMBE Proc. 2007;16:238. [Google Scholar]
- 44.Alvarez O. Mertz P. Smerbeck R. Eaglstein W. The healing of superficial skin wounds is stimulated by external electrical current. J Invest Dermatol. 1983;81:144. doi: 10.1111/1523-1747.ep12543498. [DOI] [PubMed] [Google Scholar]
- 45.Serena E. Flaibani M. Carnio S. Boldrin L. Vitiello L. De Coppi P. Elvassore N. Electrophysiologic stimulation improves myogenic potential of muscle precursor cells grown in a 3D collagen scaffold. Neurol Res. 2008;30:207. doi: 10.1179/174313208X281109. [DOI] [PubMed] [Google Scholar]
- 46.Tandon N. Cannizzaro C. Figallo E. Voldman J. Vunjak-Novakovic G. Characterization of electrical stimulation electrodes for cardiac tissue engineering. Conf Proc IEEE Eng Med Biol Soc. 2006;1-15:6409. doi: 10.1109/IEMBS.2006.259747. [DOI] [PubMed] [Google Scholar]
- 47.Tsai M. Chang W. Chang K. Hou R. Wu T. Pulsed electromagnetic fields affect osteoblast proliferation and differentiation in bone tissue engineering. Bioelectromagnetics. 2007;28:519. doi: 10.1002/bem.20336. [DOI] [PubMed] [Google Scholar]
- 48.Tipler P. Physics for Scientists and Engineers. New York: Worth Publishers; 1991. [Google Scholar]
- 49.Hartig M. Joos U. Wiesmann H. Capacitively coupled electric fields accelerate proliferation of osteoblast-like primary cells and increase bone extracellular formation in vitro. Eur Biophys J. 2000;2000:499. doi: 10.1007/s002490000100. [DOI] [PubMed] [Google Scholar]
- 50.Griffiths S. Smith S. MacGregor S. Anderson J. van der Walle C. Beveridge J. Helen Grant M. Pulsed electric field treatment as a potential method for microbial inactivation in scaffold materials for tissue engineering: the inactivation of bacteria in collagen gel. J Appl Microbiol. 2008;105:963. doi: 10.1111/j.1365-2672.2008.03829.x. [DOI] [PubMed] [Google Scholar]
- 51.Radisic M. Park H. Shing H. Consi T. Schoen F. Langer R. Freed L. Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101:18129. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z. Clark C. Brighton C. Up-regulation of bone morphogenetic proteins in cultured murine bone cells with use of specific electric fields. J Bone Joint Surg Am. 2006;88:1053. doi: 10.2106/JBJS.E.00443. [DOI] [PubMed] [Google Scholar]
- 53.Brighton C. Wang W. Clark C. The effect of electrical fields on gene and protein expression in human osteoarthritic cartilage explants. J Bone Joint Surg Am. 2008;90:833. doi: 10.2106/JBJS.F.01437. [DOI] [PubMed] [Google Scholar]
- 54.Wang W. Wang Z. Zhang G. Clark C. Brighton C. Up-regulation of chondrocyte matrix genes and products by electric fields. Clin Orthop Relat Res. 2004;427(Suppl):S163. doi: 10.1097/01.blo.0000143837.53434.5c. [DOI] [PubMed] [Google Scholar]
- 55.Fassina L. Visai L. Benazzo F. Bennedetti L. Calligaro A. De Angelis M. Farina A. Maliardi V. Margenes G. Effects of electromagnetic stimulation on calcified matrix production by SAOS-2 cells over a polyurethane porous scaffold. Tissue Eng. 2006;12:1985. doi: 10.1089/ten.2006.12.1985. [DOI] [PubMed] [Google Scholar]
- 56.Jahns M. Lou E. Durdle N. Bagnall K. Raso J. Cinats D. Barley R. Cinats J. Jomha N. The effect of pulsed electromagnetic fields on chondrocyte morphology. Med Biol Eng Comput. 2007;45:917. doi: 10.1007/s11517-007-0216-8. [DOI] [PubMed] [Google Scholar]
- 57.Yang W. Huo X. Song T. Effects of extremely low-frequency-pulsed electromagnetic field on different-derived osteoblast-like cells. Electromagn Biol Med. 2008;27:298. doi: 10.1080/15368370802289604. [DOI] [PubMed] [Google Scholar]
- 58.Sun L. Hsieh D. Yu T. Chiu H. Lu S. Luo G. Kuo T. Lee O. Chiou T. Effect of pulsed electromagnetic field on the proliferation and differentiation potential of human bone marrow mesenchymal stem cells. Bioelectromagnetics. 2009;30:251. doi: 10.1002/bem.20472. [DOI] [PubMed] [Google Scholar]
- 59.Tipler , editor. Physics for Scientists and Engineers. New York: Worth Publishers; 1991. [Google Scholar]
- 60.Yang W. Huo X. Song T. Effects of extremely low-frequency-pulsed electromagnetic field on different-derived osteoblast-like cells. Electromagn Biol Med. 2008;27:298. doi: 10.1080/15368370802289604. [DOI] [PubMed] [Google Scholar]
- 61.Jahns M. Lou E. Durdle N. Bagnall K. Raso J. Cinats D. Barley R. Cinats J. Jomha N. The effect of pulsed electromagnetic fields on chondrocyte morphology. Med Biol Eng Comput. 2007;45:917. doi: 10.1007/s11517-007-0216-8. [DOI] [PubMed] [Google Scholar]
- 62.Ijiri K. Matsunaga S. Fukuyama K. Maeda S. Sakou T. Kitano M. Senba I. The effect of pulsing electromagnetic field on bone ingrowth into a porous coated implant. Anticancer Res. 1996;16:2853. [PubMed] [Google Scholar]
- 63.Aaron R. Ciombor D. Deborah M. Simon B. Treatment of nonunions with eelctric and electromagnetic fields. Clin Orthop. 2004;419:21. doi: 10.1097/00003086-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 64.Udina E. Furey M. Busch S. Silver J. Gordon T. Fouad K. Electrical stimulation of intact peripheral sensory axons in rats promotes outgrowth of their central projections. Exp Neurol. 2008;210:238. doi: 10.1016/j.expneurol.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Livshitz L. Mizrahi J. Einziger P. Interaction of array of finite electrodes with layered biological tissue: effect of electrode size and configuration. IEEE Trans Neural Syst Rehabil Eng. 2001;9:355. doi: 10.1109/7333.1000115. [DOI] [PubMed] [Google Scholar]
- 66.Aaron R. Ciombor D. Acceleration of experimental endochondral ossification by biophysical stimulation of the progenitor cell pool. J Orthop Res. 1996;14:582. doi: 10.1002/jor.1100140412. [DOI] [PubMed] [Google Scholar]
- 67.Biomet. EBI Bone Healing System [on-line] 2009. http://www.biomet.com/osteobiologics/products.cfm?pdid=7&majcid=15&prodid=142. [May;2011 ]. http://www.biomet.com/osteobiologics/products.cfm?pdid=7&majcid=15&prodid=142
- 68.Gil E. Park S. Marchant J. Omenetto F. Kaplan D. Response of human corneal fibroblasts on silk film surface patterns. Macromol Biosci. 2010;10:664. doi: 10.1002/mabi.200900452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Madduri S. Papaloizos M. Gander B. Trophically and topographically functionalized silk fibroin nerve conduits for guided peripheral nerve regeneration. Biomaterials. 2010;31:2323. doi: 10.1016/j.biomaterials.2009.11.073. [DOI] [PubMed] [Google Scholar]
- 70.Hammerick K. Longaker M. Prinz F. In vitro effects of direct current electric fields on adipose-derived stromal cells. Biochem Biophys Res Commun. 200;397:2. doi: 10.1016/j.bbrc.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li X. Kolega J. Effects of direct current electric fields on cell migration and actin filament distribution in bovine vascular endothelial cells. J Vasc Res. 2002;39:391. doi: 10.1159/000064517. [DOI] [PubMed] [Google Scholar]
- 72.Sheridan D. Isseroff R. Imposition of a physiologic DC electric field alters the migratory response of human keratinocytes on extracellular matrix molecules. J Invest Dermatol. 1996;106:642. doi: 10.1111/1523-1747.ep12345456. [DOI] [PubMed] [Google Scholar]
- 73.Onuma E. Hui S. A calcium requirement for electric field-induced cell shape changes and preferential orientation. Cell Calcium. 1985;6:281. doi: 10.1016/0143-4160(85)90012-0. [DOI] [PubMed] [Google Scholar]
- 74.Chang P. Sulik G. Soong H. Galvanotropic and galvanotactic responses of corneal endothelial cells. J Formos Med Assoc. 1996;95:623. [PubMed] [Google Scholar]
- 75.Zhao M. Bai H. Wang E. Electrical stimulation directly induces preangiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci. 2004;117:397. doi: 10.1242/jcs.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun S. Wise J. Cho M. Human fibroblast migration in three-dimensional collagen gel in response to noninvasive electrical stimulus. I. Characterization of induced three-dimensional cell movement. Tissue Eng. 2004;10:1548. doi: 10.1089/ten.2004.10.1548. [DOI] [PubMed] [Google Scholar]
- 77.Zhao M. McCaig C. Agius-Fernandez A. Forrester J. Araki-Sasaki K. Human corneal epithelial cells reorient and migrate cathodally in a small applied electric field. Curr Eye Res. 1997;16:973. doi: 10.1076/ceyr.16.10.973.9014. [DOI] [PubMed] [Google Scholar]
- 78.Sun S. Liu Y. Lipsky S. Cho M. Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells. FASEB J. 2007;21:1472. doi: 10.1096/fj.06-7153com. [DOI] [PubMed] [Google Scholar]
- 79.Akanji O. Lee D. Bader D. The effects of direct current stimulation on isolated chrondrocytes seeded in 3D agarose constructs. Biorheology. 2008;45:229. [PubMed] [Google Scholar]
- 80.Aaron R. Ciombor D. Acceleration of experimental endochondral ossification by biophysical stimulation of the progenitor cell pool. J Orthop Res. 1996;14:582. doi: 10.1002/jor.1100140412. [DOI] [PubMed] [Google Scholar]
- 81.Ijiri K. Matsunaga S. Fukuyama K. Maeda S. Sakou T. Kitano M. Senba I. The effect of pulsing electromagnetic field on bone ingrowth into a porous coated implant. Anticancer Res. 1996;16:2853. [PubMed] [Google Scholar]
- 82.Shimizu T. Yamato M. Akutsu T. Shibata T. Isoi Y. Kikuchi A. Umezu M. Okano T. Electrically communicating three-dimensional cardiac tissue mimic fabricated by layered cultured cardiomyocyte sheets. J Biomed Mater Res. 2002;60:110. doi: 10.1002/jbm.1284. [DOI] [PubMed] [Google Scholar]
- 83.George I. Geddis M. Lill Z. Lin H. Gomez T. Blank M. Oz M. Goodman R. Myocardial function improved by electromagnetic field induction of stress protein hsp70. J Cell Physiolog. 2008;216:816. doi: 10.1002/jcp.21461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ito H. Bassett C. Effect of weak pulsing electromagnetic fields on neural regeneration in the rat, clinical orthopaedics and related research. 1983. p. 283. [PubMed]
- 85.Sebastian A. Buckle A. Markx G. Tissue engineering with electric fields: Immobilization of mammalian cells in multilayer aggregates using dielectrophoresis. Biotechnol Bioeng. 2007;98:694. doi: 10.1002/bit.21416. [DOI] [PubMed] [Google Scholar]
- 86.McLeod K. Microelectrode measurements of low frequency electric field effects in cells and tissues. Bioelectromagnetics. 1992;(Suppl 1):161. doi: 10.1002/bem.2250130716. [DOI] [PubMed] [Google Scholar]
- 87.Zhuang H. Wang W. Seldes R. Tahernia D. Fan H. Brighton C. Electrical stimulation induces level of TGF-B1 mRNA in osteoblastic cells by a mechanism involving calcium/calmodulin pathway. Biochem Biophys Res Commun. 1997;237:225. doi: 10.1006/bbrc.1997.7118. [DOI] [PubMed] [Google Scholar]
- 88.Brighton C. Wang W. Seldes R. Zhang G. Pollack S. Signal transduction in electrically stimulated bone cells. J Bone Joint Surg Am. 2001;83A:1514. doi: 10.2106/00004623-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 89.Cho M. Marler J. Thatte H. Golan D. Control of calcium entry in human fibroblasts by frequency-dependent electricall stimulation. Frontiers in Bioscience. 2002;7:A1. doi: 10.2741/A733. [DOI] [PubMed] [Google Scholar]
- 90.Aaron R. Boyan B. Ciombor D. Schwartz Z. Simon B. Stimulation of growth factor synthesis by electric and electromagnetic fields. Clin Orthop. 2004;419:30. doi: 10.1097/00003086-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 91.Panagopoulos D. Karabarbounis A. Margaritis L. Mechanism for action of electromagnetic fields on cells. Biochem Biophys Res Commun. 2002;298:95. doi: 10.1016/s0006-291x(02)02393-8. [DOI] [PubMed] [Google Scholar]
- 92.Gross D. Loew L. Webb W. Optical imaging of cell membrane potential changes induced by applied electric fields. Biophys J. 1986;50:339. doi: 10.1016/S0006-3495(86)83467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sauer H. Rahimi G. Hescheler J. Wartenberg M. Effects of electrical fields on cardiomyocyte differentiation of embryonic stem cells. J Cell Biochem. 1999;75:710. doi: 10.1002/(sici)1097-4644(19991215)75:4<710::aid-jcb16>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 94.Luben R. Cain C. Chen M. Rosen D. Adey W. Effects of electromagnetic stimuli on bone and bone cells in vitro: inhibition of responses to parathyroid hormone by low-energy low-frequency fields. Proc Natl Acad Sci U S A. 1982;79:4180. doi: 10.1073/pnas.79.13.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spadaro J. Bergstrom W. In vivo and in vitro effects of a pulsed electromagnetic field on net calcium flux in rat calvarial bone. Calcif Tissue Int. 2002;70:496. doi: 10.1007/s00223-001-1001-6. [DOI] [PubMed] [Google Scholar]
- 96.Launikonis B. Stephenson D. Friedrich O. Rapid Ca2+ flux through the transverse tubular membrane, activated by individual action potentials in mammalian skeletal muscle. J Physiol. 2009;587:2299. doi: 10.1113/jphysiol.2009.168682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brighton C. Wang W. Seldes R. Zhang G. Pollack S. Signal transduction in electrically stimulated bone cells. J Bone Joint Surg Am. 2001;83A:1514. doi: 10.2106/00004623-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 98.Goodman R. Blank M. Insights into electromagnetic interaction mechanisms. J Cell Physiol. 2002;192:16. doi: 10.1002/jcp.10098. [DOI] [PubMed] [Google Scholar]
- 99.Mantero S. Sadr N. Riboldi S. Lorenzoni S. Montevecchi F. A new electro-mechanical bioreactor for soft tissue engineering. J Appl Biomater Biomech. 2007;5:107. [PubMed] [Google Scholar]
- 100.Feng Z. Matsumoto T. Nomura Y. Nakamura T. An electro-tensile bioreactor for 3-D culturing of cardiomyocytes. A bioreactor system that stimulates the mycocardium's electrical and mechanical response in vivo. IEEE Eng Med Biol Mag. 2005;24:73. doi: 10.1109/memb.2005.1463399. [DOI] [PubMed] [Google Scholar]
- 101.Butler D. Hunter S. Chokalingham K. Cordray M. Shearn J. Juncosa-Melvin N. Nirmalanandham S. Jain A. Using functional tissue engineering and bioreactors to mechanically stimulated tissue-engineered constructs. Tissue Eng Part A. 2009;15:741. doi: 10.1089/ten.tea.2008.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rajnicek A. Foubister L. McCaig C. Alignment of corneal and lens epithelial cells by cooperative effects of substratum topography and DC electric fields. Biomaterials. 2008;29:2082. doi: 10.1016/j.biomaterials.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 103.McCaig C. Sangster L. Stewart R. Neurotrophins enhance electric field-directed growth cone guidance and directed nerve branching. Dev Dyn. 2000;217:229. doi: 10.1002/(SICI)1097-0177(200003)217:3<299::AID-DVDY8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 104.Zhao M. Agius-Fernandez A. Forrester J. McCaig C. Orientation and directed migration of cultured corneal epithelial cells in small electric fields are serum dependent. J Cell Sci. 1996;109:1405. doi: 10.1242/jcs.109.6.1405. [DOI] [PubMed] [Google Scholar]
- 105.Schwartz Z. Simon B. Duran M. Barabino G. Chaudhri R. Boyan B. Pulsed electromagnetic fields enhance BMP-2 dependent osteoblastic differentiation of human mesenchyamal stem cells. J Orthop Res. 2008;26:1250. doi: 10.1002/jor.20591. [DOI] [PubMed] [Google Scholar]
- 106.Selvamurugan N. Kwok S. Vasilov A. Jefcoat S. Partridge N. Effects of BMP-2 and pulsed electromagnetic field (PEMF) on rat primary osteoblastic cell proliferation and gene expression. J Orthop Res. 2007;25:1213. doi: 10.1002/jor.20409. [DOI] [PubMed] [Google Scholar]
- 107.Basford J. A historical perspective of the popular use of electric and magnetic therapy. Arch Phys Med Rehabil. 2001;82:1261. doi: 10.1053/apmr.2001.25905. [DOI] [PubMed] [Google Scholar]
- 108.Piccolino M. Luigi Galvani and Animal electricity: two centuries after the foundation of electrophysiology. Trends Neurosci. 1997;20:443. doi: 10.1016/s0166-2236(97)01101-6. [DOI] [PubMed] [Google Scholar]
- 109.Greatbatch W. Chardack W. A transistorized implantable pacemaker for the correction of complete atrioventricular block. Surgery. 1960;48:643. [PubMed] [Google Scholar]
- 110.Greatbatch W. History [on-line] 2010. http://www.greatbatch.com/history.aspx?s=about. [May;2011 ]. http://www.greatbatch.com/history.aspx?s=about
- 111.Greatbatch W. Implantable Cardiac Pacemaker, USA Patent US003036274. 1962.
- 112.Biomet. SpinalPakII Spine Fusion Stimulator [on-line] 2009. http://www.biomet.com/osteobiologics/products.cfm?pdid=7&majcid=16&prodid=162. [May;2011 ]. http://www.biomet.com/osteobiologics/products.cfm?pdid=7&majcid=16&prodid=162
- 113.Biomet. Osteogen Dual Lead Bone Growth Stimulator [on-line] 2009. http://www.biomet.com/osteobiologics/products.cfm?pdid=7&majcid=15&prodid=144. [May;2011 ]. http://www.biomet.com/osteobiologics/products.cfm?pdid=7&majcid=15&prodid=144
- 114.Biomet. Osteogen Bone Growth Stimulator [on-line] 2009. http://www.biomet.com/osteobiologics/products.cfm?pdid=7&majcid=15&prodid=143. [May;2011 ]. http://www.biomet.com/osteobiologics/products.cfm?pdid=7&majcid=15&prodid=143
- 115.Foley K.T. Mroz T.E. Arnold P.M. Chandler H.C. Dixon R.A. Girasole G.J. Renkens J.L. Riew K.D. Sasso R.C. Smith R.C. Tang H. Wecht D.A. Whiting D.M. Randomized, prospective controlled clinical trial of pulsed electromagnetic field stimulation for cervical fusion. Spine J. 2008;8:436. doi: 10.1016/j.spinee.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 116.Orthofix. Spinal-Stim Summary of Safety and Effectiveness. P850007/S6. February 7, 1990. One-year postoperative pre-marketing data from randomized double-blind placebo-controlled clinical trial. 2010. http://www.orthofix.com/products/spine_spinalstim.asp. [Nov;2011 ]. http://www.orthofix.com/products/spine_spinalstim.asp
- 117.Physio-Stim Bone Growth Stimulator, U.S.A. ed: Orthofix, Inc. 2009. http://www.orthofix.com/products/physio.stim_biobonegrowth.asp?cid=42 http://www.orthofix.com/products/physio.stim_biobonegrowth.asp?cid=42
- 118. 1986, Physio-Stim PMA 850007-02/21/86, Supplement - 12/23/86.
- 119.Midura R. Ibiwoye M. Powell K. Sakai Y. Doehrin T. Grabiner M. Patterson T. Zborowski M. Wolfman A. Pulsed electromnagnetic field treatments enhance the healing of fibular osteotomies. J Orthop Res. 2005;23:1035. doi: 10.1016/j.orthres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 120.Brighton C. Black J. Redla W. Implantable bone growth stimulator 1985. . Patent no. 4549547.
- 121.Brighton C. Methods for non-invasive electrical stimulation of epiphyseal plate growth. 1984. Patent no. 4467809.
- 122.Medtronic. Questions and Answers - DBS Therapy [on-line] 2010. http://www.medtronic.com/your-health/essential-tremor/therapy/questions-and-answers/index.htm. [May;2011 ]. http://www.medtronic.com/your-health/essential-tremor/therapy/questions-and-answers/index.htm
- 123.Ondo W. Meilak C. Vuong K. Predictors of battery life for the activa soletra 7426 neurostimulator. Parkinsonism Relat Disord. 2007;13:240. doi: 10.1016/j.parkreldis.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 124.Balakatounis K. Angoules A. Low-intensity electrical stimulation in wound healing: review of the efficacy of externally applied currents resembling the current of injury. Eplasty. 2008;8:283. [PMC free article] [PubMed] [Google Scholar]
- 125.Biomet. Implantable Stimulation [on-line] 2011. http://www.biomet.com/boneHealing/products.cfm?pdid=10&majcid=51. [May;2011 ]. http://www.biomet.com/boneHealing/products.cfm?pdid=10&majcid=51
- 126.Wei L. Goodman R. Henderson A. Changes in levels of c-myc and histone H2B following exposure of cells to low-frequency sinusoidal electromagnetic fields: evidence for a window effect. Bioelectro-magnetics. 1990;11:269. doi: 10.1002/bem.2250110403. [DOI] [PubMed] [Google Scholar]
- 127.Morokuma J. Blackiston D. Adams D. Seebohm G. Trimmer B. Levin M. Modulation of potassium channel function confers a hyperproliferative invasive phenotype on embryonic stem cells. Proc Acad Sci USA. 2008;105:16608. doi: 10.1073/pnas.0808328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Levin M. Large-scale biophysics: ion flows and regeneration. Trends Cell Biol. 2007;17:261. doi: 10.1016/j.tcb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 129.Alberts B. Lewis J. Johnson A. Lewis J. Raff M. Molecular Biology of the Cell. New York: Taylor and Francis, Inc.; 2002. [Google Scholar]
- 130.Cannizzaro C. Tandon N. Figallo E. Park H. Gerecht S. Radisic M. Elvassore N. Vunjak-Novakovic G. Practical aspects of cardiac tissue engineering with electrical stimulation. Methods Mol Med. 2007;140:291. doi: 10.1007/978-1-59745-443-8_16. [DOI] [PubMed] [Google Scholar]