Abstract
A Membrane-bound Antiparallel Dimer of Rat Islet Amyloid Polypeptide IAPP, an amyloidogenic peptide linked to type 2 diabetes, forms a heterogeneous mixture of membrane-bound oligomers implicated in cytotoxicity and fibril formation. Structural characterization of these species has been a long-standing problem. Here, we examine the structure of a previously unrecognized antiparallel dimer of rat IAPP bound to anionic membrane Nanodiscs, using a novel combination of single-pair FRET and Rosetta model refinement.
Keywords: single-molecule studies, molecular modeling, amyloid, oligomerization, diabetes
Islet amyloid polypeptide (IAPP or amylin) is a 37-residue peptide hormone co-secreted with insulin by pancreatic β-cells. IAPP is natively unstructured, but gains α-helical structure on binding anionic membranes,[1] and forms β-sheet rich amyloid fibers during the progression of type II diabetes.[2] Membrane disruption by non-fibrillar α-helical oligomers of IAPP has been implicated in β-cell dysfunction and death;[3] anionic membranes also dramatically accelerate IAPP fibril formation,[4] possibly by favoring an oligomeric nucleating state. Membrane-bound states of IAPP are therefore of significant interest.[5] Lipid-bound monomeric IAPP has been structurally characterized at high resolution,[6, 7] but oligomers are dynamic, heterogeneous and transient, presenting a challenge for traditional structure determination approaches. Several groups have studied these oligomeric states,[8, 9] but fundamental questions about topology and stoichiometry remain.
We approached this problem using intermolecular single-pair Förster resonance energy transfer (spFRET) measurements to selectively study membrane-bound IAPP oligomers from a predominantly monomeric population. To avoid experimental complications due to fiber formation, we used the rat isoform of IAPP (rIAPP), which differs in 6 residues from the human isoform (hIAPP). Like hIAPP, rIAPP can permeabilize membranes[10] and causes cell toxicity,[11] but it does not form amyloid.[12] rIAPP was singly labeled with either a donor (Alexa 488) at one of five residues, or an acceptor (Atto 610) at residue 1 (Fig. 1). Measurements were made in a dilute mixture of labeled IAPP and DOPG Nanodiscs[13] as the particles diffused through a focused laser beam. The energy transfer efficiency (ETeff) was calculated for each transit of a fluorescent particle through the focal volume. Energy transfer only occurs when at least one donor and acceptor labeled rIAPP are bound to the same Nanodisc (Fig. S1c).
Figure 1.
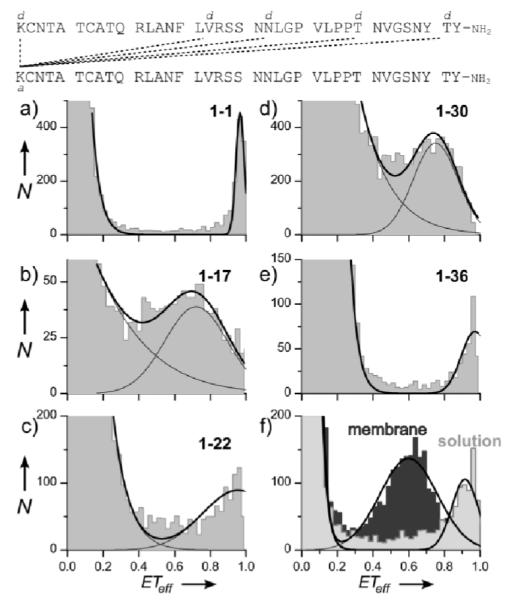
a-e. spFRET histograms collected from a mixture of rIAPP, separately labeled with donor (d) or acceptor (a) at the indicated positions, and Nanodiscs. A small fraction of rIAPP forms membrane-bound dimers, and the minor species peak ETeff reports on the dimer structure. f. SpFRET of rIAPP double-labeled at residues 1 and 36, in solution (light grey) and on membranes (dark grey). If IAPP formed a parallel membrane-bound dimer, the intermolecular ETeff value for the 1-36 residue pair (panel e) would more closely match the latter intramolecular value (~0.6).
Under our experimental conditions, we expect rIAPP to be largely monomeric (free in solution or membrane-bound) as reflected by the major peak centered at ETeff=0 seen in all histograms (Fig. 1a-e). A minor but significant population (~0.5% of events) with ETeff > 0.25 results from Nanodiscs containing at least one donor-acceptor rIAPP pair. Residue pairs 1-36 and 1-1 displayed the highest peak ETeff, followed by 1-22, 1-30 and 1-17. As an orthogonal probe of inter-rIAPP contacts, chemical crosslinking showed reactivity approximating the spFRET data (Fig. S1b); however, this ensemble assay required much higher protein and lipid concentrations than spFRET, and consequently reports on a more heterogenous population of oligomers. In the absence of Nanodiscs, the high ETeff population is not observed, consistent with the expectation that rIAPP is monomeric in solution at the low concentrations used here (Fig. S1d). Importantly, bacterially expressed IAPP provides a similar high ETeff population to synthesized peptide (Fig. S1e), indicating that these events do not represent a contaminating sub-population resulting from the peptide synthesis process. Several techniques including fluorescence, gel filtration chromatography and cross-linking suggest that an IAPP dimer is a favorable oligomeric state in solution and on membranes, where it is the dominant oligomer at low protein:lipid ratios.[4, 14] Doubling the amount of donor-labeled rIAPP increased the high ETeff population by a factor of two, as expected of a dimer rather than a higher-order oligomer (Fig. S1f). The fraction of high ETeff events (~0.5%) is significantly higher than would be expected if rIAPP were to bind Nanodiscs without self-association (~0.02%; see SI for details). Thus, while another highly-favored complex may exist, a dimer is both the statistically most probable oligomer at the low concentrations used for spFRET, and the simplest model consistent with the data.
Assuming that high ETeff peak positions report on a dimer structure, they show residue 1 of one monomer relatively close to residues 1 and 36 of the other, and far from 17 and 30, ruling out a parallel in-register coiled-coil. Given that the dye-linker moieties are relatively large compared to systems like IAPP dimers, a solely qualitative analysis of these data is inadequate. Therefore, we used Rosetta[15] to generate stable models of rIAPP dimers consistent with spFRET, as detailed in the SI. The flexible linkers attaching dyes to protein can add up to 15.7 Å (Alexa 488) and 10.1 Å (Atto 610) to ETeff-derived distances (Fig. S2), so each spFRET constraint was considered satisfied if the difference between inter-dye distance (calculated from ETeff and the Förster equation) and Cα-Cα distance is less than the sum of these average dye-Cα distances. Harmonic potentials were applied to distances outside these broad bounds.
While unconstrained refinement produced both parallel and antiparallel topologies of comparable energy, spFRET constraints strongly biased the search towards three similar conformations: dimers interacting via a short (~5 turns) antiparallel coiled-coil motif, with the flexible tails oriented so that the N- and C-termini of a given monomer are approximately equidistant from the N-terminus of the other monomer (Fig. 2). The low-energy models obtained by the constrained search are also compatible with NMR-derived secondary structure assignments of membrane-bound monomeric rIAPP.[16] SpFRET constraints were self-consistent and narrowed the search space progressively as additional constraints were applied. Importantly, randomized constraints failed to generate a stable structure (Fig. S3).
Figure 2.
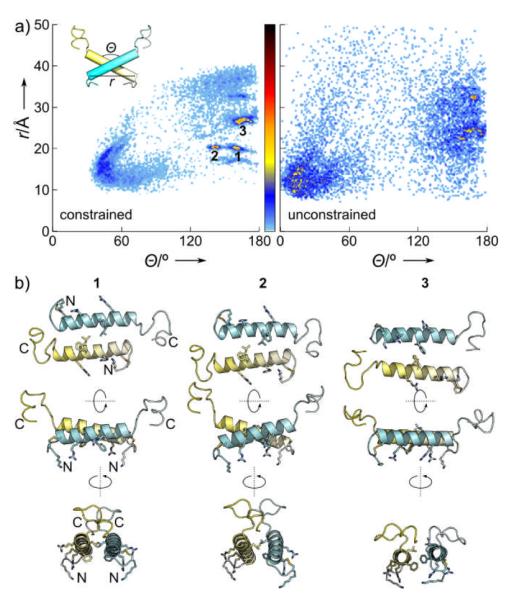
a. rIAPP dimer models generated by constrained (left) and unconstrained (right) docking. These are plotted in terms of distance between the N-terminal ends of the two helices r vs. interhelical angle θ. The unconstrained search produced parallel and antiparallel dimers with approximately equal probability, while the constrained search strongly favored 3 particular low-energy clusters . b. Detailed views of representatives from each cluster, perpendicular to (top) and in the plane of (middle, bottom) of the membrane. Sidechains proposed to participate in dimerization and membrane binding are shown as sticks.
Constrained dimer models have a dimer interface involving residues L12, F15, and L16. These display a very plausible lipid-binding interface even though the simulations did not include a membrane: residues A5, A8, L12, F15 and L23 form a central hydrophobic patch; charged residues K1, R11 and R18 are free to interact with anionic head-groups; and flexible C-terminal tails are exposed to solution. The resulting orientation in the plane of the membrane resembles that predicted (Fig. S4) by MCPep[17] as the expected binding mode of an amphipathic helical peptide like IAPP. The dimer interface corresponds well to self-assembly sequences suggested by others.[18] Compared to proposed models of human hIAPP and rIAPP dimers in the gas phase (in the absence of solvent or a membrane),[19] our results indicate increased helical structure and a stronger preference for antiparallel arrangements. An antiparallel dimer also differs from a crystal structure of IAPP fused to the 370-residue maltose-binding protein (MBP),[20] again in the absence of a membrane, which shows a helix-helix dimer with a similar hydrophobic interface but a 55° inter-helix angle. The differences between these various dimer models may reflect the crucial role that environment, and in particular membranes, can play in controlling IAPP conformation. We note that if IAPP has both a preferred membrane-binding face and a symmetric dimer interface, then purely geometric considerations require an antiparallel topology for a membrane-bound dimer. The spFRET-constrained models satisfy this requirement, whereas a parallel dimer would imply either an asymmetric dimer interface or different membrane-binding orientations for the two monomers.
hIAPP and rIAPP differ at 6 residues, one (position 18) located in the membrane-binding region and the other 5 in the flexible C-terminus. None form part of the dimer interface in the low-energy antiparallel models, and the structures of both membrane-bound monomers are known to be broadly similar.[1, 6] All of the favorable interactions involved in maintaining the rIAPP dimer are also available for similar hIAPP self-association, suggesting that hIAPP may also form an antiparallel helix-helix dimer, an especially intriguing possibility given that fibrils show a parallel arrangement.[7, 9] If so, then either the analogous antiparallel hIAPP dimer would represent a low-energy off-pathway species, or the amyloidogenic pathway must include a transition from a previously unanticipated antiparallel arrangement to a parallel one. Stabilization of an antiparallel dimer could, in either case, inhibit further oligomerization and subsequent pathology, building on previous small-molecule designs that deliberately target the α-helical surface of IAPP.[21]
SpFRET proved uniquely well-suited to dissecting the heterogeneity inherent in cooperative membrane binding by IAPP, by distinguishing membrane-bound dimers from the predominant monomeric species. SpFRET-constrained Rosetta calculations (to our knowledge, the first combined application of these powerful techniques) found a population of low-energy antiparallel helix-helix dimers that satisfy all spFRET restraints. These dimers appear competent for membrane binding, and agree with other reports on the IAPP homodimeric interaction interface. The models presented here are the most detailed yet proposed for a membrane-bound IAPP oligomer. Future studies will extend this methodology to hIAPP, and focus on subsequent states in the oligomerization pathway including those which disrupt membrane integrity.[10] We will also study the possible effects of more physiological, heterogeneous membrane compositions in modulating IAPP conformation. More generally, the combination of spFRET and high-resolution refinement may prove useful in the study of other unstructured, toxic amyloid-formers including amyloid-β, α-synuclein, tau and prion protein.[3, 22]
Experimental Section
Donor (Alexa 488) and acceptor (Atto 610) dyes were attached using click chemistry (to synthesized mutants bearing an alkyne at residues 17, 22, 30 or 36) or amine coupling to residue 1. Nanodiscs were prepared as previously described.[13] Confocal spFRET was performed on mixtures of 100 pM donor-labeled peptide, 500 pM acceptor-labeled peptide and 40 nM Nanodiscs, using a previously described setup.[13] Crosslinking was performed by click chemistry between IAPP azide-labeled at residue 1 and the four alkyne-bearing mutants. Constrained dimer model refinement was implemented in Rosetta as detailed in Supporting Information.
Supplementary Material
Acknowledgments
This work was funded by an American Heart Association Postdoctoral Fellowship to A.N. and NIH Grant GM-084391 to E.R. and A.D.M. The authors thank Dr. S.G. Sligar (UIUC) for the gift of MSP1D1; Y. Gofman of the Ben-Tal group (Tel Aviv University) for assistance with MCPep; Drs. J. Knight, T. Craggs and C.J. Wilson for critical reading; J. Dunn for expressed IAPP; the W.M. Keck Biotechnology Research Laboratory for peptide synthesis; and the Yale FAS High Performance Computing Center.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
Abhinav Nath, Molecular Biophysics & Biochemistry Yale University New Haven CT 06520-8114.
Andrew D. Miranker, Molecular Biophysics & Biochemistry Yale University New Haven CT 06520-8114.
Elizabeth Rhoades, Molecular Biophysics & Biochemistry Yale University New Haven CT 06520-8114.
References
- [1].Williamson J, Loria J, Miranker A. J Mol Biol. 2009;393:383–396. doi: 10.1016/j.jmb.2009.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Westermark P, Wernstedt C, Wilander E, Hayden D, O’Brien T, Johnson K. Proc Natl Acad Sci U S A. 1987;84:3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]a).Hebda J, Miranker A. Annu Rev Biophys. 2009;38:125–152. doi: 10.1146/annurev.biophys.050708.133622. [DOI] [PubMed] [Google Scholar]; b) Abedini A, Raleigh D. Protein Eng Des Sel. 2009;22:453–459. doi: 10.1093/protein/gzp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Knight J, Hebda J, Miranker A. Biochemistry. 2006;45:9496–9508. doi: 10.1021/bi060579z. [DOI] [PubMed] [Google Scholar]
- [5].Brender JR, Lee EL, Cavitt MA, Gafni A, Steel DG, Ramamoorthy A. J Am Chem Soc. 2008;130:6424–6429. doi: 10.1021/ja710484d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]a).Patil S, Xu S, Sheftic S, Alexandrescu A. J Biol Chem. 2009;284:11982–11991. doi: 10.1074/jbc.M809085200. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nanga R, Brender J, Xu J, Hartman K, Subramanian V, Ramamoorthy A. J Am Chem Soc. 2009;131:8252–8261. doi: 10.1021/ja9010095. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Nanga RP, Brender JR, Vivekanandan S, Ramamoorthy A. Biochim Biophys Acta. 2011;1808:2337–2342. doi: 10.1016/j.bbamem.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]a).Luca S, Yau W, Leapman R, Tycko R. Biochemistry. 2007;46:13505–13522. doi: 10.1021/bi701427q. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Jayasinghe S, Langen R. J Biol Chem. 2004;279:48420–48425. doi: 10.1074/jbc.M406853200. [DOI] [PubMed] [Google Scholar]
- [8]a).Mishra R, Geyer M, Winter R. Chembiochem. 2009;10:1769–1772. doi: 10.1002/cbic.200900237. [DOI] [PubMed] [Google Scholar]; b) Soong R, Brender J, Macdonald P, Ramamoorthy A. J Am Chem Soc. 2009;131:7079–7085. doi: 10.1021/ja900285z. [DOI] [PubMed] [Google Scholar]; c) Evers F, Jeworrek C, Tiemeyer S, Weise K, Sellin D, Paulus M, Struth B, Tolan M, Winter R. J Am Chem Soc. 2009;131:9516–9521. doi: 10.1021/ja8097417. [DOI] [PubMed] [Google Scholar]; d) Jha S, Sellin D, Seidel R, Winter R. J Mol Biol. 2009;389:907–920. doi: 10.1016/j.jmb.2009.04.077. [DOI] [PubMed] [Google Scholar]; e) Ling YL, Strasfeld DB, Shim SH, Raleigh DP, Zanni MT. J Phys Chem B. 2009;113:2498–2505. doi: 10.1021/jp810261x. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Kayed R, Bernhagen J, Greenfield N, Sweimeh K, Brunner H, Voelter W, Kapurniotu A. J Mol Biol. 1999;287:781–796. doi: 10.1006/jmbi.1999.2646. [DOI] [PubMed] [Google Scholar]
- [9].Fu L, Ma G, Yan E. J Am Chem Soc. 2010;132:5405–5412. doi: 10.1021/ja909546b. [DOI] [PubMed] [Google Scholar]
- [10].Last NB, Rhoades E, Miranker AD. Proc Natl Acad Sci U S A. 2011;108:9460–9465. doi: 10.1073/pnas.1102356108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Magzoub M, Miranker A. unpublished results.
- [12].Nishi M, Chan SJ, Nagamatsu S, Bell GI, Steiner DF. Proc Natl Acad Sci U S A. 1989;86:5738–5742. doi: 10.1073/pnas.86.15.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nath A, Trexler A, Koo P, Miranker A, Atkins W, Rhoades E. Methods Enzymol. 2010;472:89–117. doi: 10.1016/S0076-6879(10)72014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]a).Andreetto E, Yan L, Tatarek-Nossol M, Velkova A, Frank R, Kapurniotu A. Angew Chem Int Ed Engl. 2010;49:3081–3085. doi: 10.1002/anie.200904902. [DOI] [PubMed] [Google Scholar]; b) Yan L, Tatarek-Nossol M, Velkova A, Kazantzis A, Kapurniotu A. Proc Natl Acad Sci U S A. 2006;103:2046–2051. doi: 10.1073/pnas.0507471103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]a).Rohl C, Strauss C, Misura K, Baker D. Methods Enzymol. 2004;383:66–93. doi: 10.1016/S0076-6879(04)83004-0. [DOI] [PubMed] [Google Scholar]; b) Chaudhury S, Lyskov S, Gray J. Bioinformatics. 2010;26:689–691. doi: 10.1093/bioinformatics/btq007. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kaufmann K, Lemmon G, Deluca S, Sheehan J, Meiler J. Biochemistry. 2010;49:2987–2998. doi: 10.1021/bi902153g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Williamson J, Miranker A. Protein Sci. 2007;16:110–117. doi: 10.1110/ps.062486907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shental-Bechor D, Haliloglu T, Ben-Tal N. Biophys J. 2007;93:1858–1871. doi: 10.1529/biophysj.106.103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]a).Azriel R, Gazit E. J Biol Chem. 2001;276:34156–34161. doi: 10.1074/jbc.M102883200. [DOI] [PubMed] [Google Scholar]; b) Fox A, Snollaerts T, Casanova C. Errecart, Calciano A, Nogaj L, Moffet D. Biochemistry. 2010;49:7783–7789. doi: 10.1021/bi100337p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]a).Dupuis NF, Wu C, Shea JE, Bowers MT. J Am Chem Soc. 2009;131:18283–18292. doi: 10.1021/ja903814q. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dupuis NF, Wu C, Shea JE, Bowers MT. J Am Chem Soc. 2011;133:7240–7243. doi: 10.1021/ja1081537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wiltzius J, Sievers S, Sawaya M, Eisenberg D. Protein Sci. 2009;18:1521–1530. doi: 10.1002/pro.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hebda J, Saraogi I, Magzoub M, Hamilton A, Miranker A. Chem Biol. 2009;16:943–950. doi: 10.1016/j.chembiol.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]a).Elbaum-Garfinkle S, Ramlall T, Rhoades E. Biophys J. 2010;98:2722–2730. doi: 10.1016/j.bpj.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Morillas M, Swietnicki W, Gambetti P, Surewicz W. J Biol Chem. 1999;274:36859–36865. doi: 10.1074/jbc.274.52.36859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.