DMP1, highly expressed in osteocytes, is involved in local effects on bone mineralization as well as systemic phosphate metabolism. DMP1 protein is localized in the lacunar and canalicular walls of osteocytes and the gene responds rapidly to mechanical stimulation1–3. Here we propose that one major role of DMP1 is in maintaining integrity and stiffness of the lacunar and canalicular walls. The global knock-out of the DMP1 gene results in mice that have canalicular walls which are irregular in shape and cell processes which are almost twice the normal diameter (McKee, Harris and Feng, unpublished).
We previously have shown that an 8 to 10kb 5′ flanking region plus intron 1 of the DMP1 cis-regulatory region contains control regions for both osteocyte specificity and response to mechanical loading. In fact this DNA region of the DMP1 gene can and has been used to target other genes to the osteocyte with some degree of specificity2–4. However, our more recent comparisons of human/mouse and human/dog/opossum/rat conserved nucleotide sequences (CNS) suggest the cis-regulatory system for the DMP1 gene is more complex. We have identified 200 to 500 nucleotide CNS from −20kb of the transcription start site throughout the transcription unit and in the 20kb 3′ flanking region. Therefore, we are building a DMP1 BAC construct that will have all of these CNS and potential enhancer modules of the DMP1 gene driving a GFP reporter. In parallel, we have constructed a database of all transcription factors that bind within the DMP1 CNS and cross-indexed this set of binding sites with osteocyte selective transcription factors, identified in primary mouse osteocytes as well as other classes of transcription factors. We then constructed a small computational model for testing which regions of the DMP1 gene give the gene its osteocyte specificity and what regions are more likely responsible for the mechanical load responses.
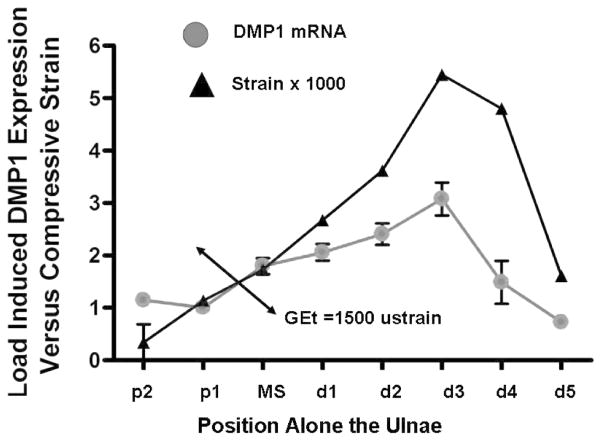
DMP1 expression, specifically in osteocytes of the mouse ulna, increases 2- to 3-fold at 24 hours after a single 2.4N load for 30 seconds at 2 Hz. We constructed a finite element model (FEA) of the mouse ulna and demonstrated a direct statistically significant correlation between DMP1 expression and computed tissue strains. This correlation shows that DMP1 expression in osteocytes is highest in areas of maximal compressive and tensile strains in cortical bone, in both the longitudinal and cross-sectional axes. DMP1 is an early mechanical response gene as its expression is first elevated at 1hour after loading and continues to increase up to 24hours. Using a 3-D model relating the 3-dimensional strain field with DMP1 expression, the gene expression threshold (GEt) for DMP1 is approximately 1500 μstrain, as shown in Figure 1.
Figure 1.
Load-induced DMP1 Expression (24 hours) correlates with compressive strain determined from finite element model. The GEt or gene expression threshold (minimum strain to activate DMP1) is 1,500 microstrain.
Using information on the phenotype of the DMP1 knock-out mice where DMP1 appears necessary for normal lacunar and canalicular structure, we have proposed a model relating DMP1 expression to mechanical load and therefore to maintenance of mineral quality. Dr. Dan Nicolella, SWRI, San Antonio, TX, suggested several years ago, that under normal load conditions, the canaliculi and lacunae in the DMP1-null mice, because of their lack of stiffness and compromised structure, would amplify any load signals resulting in the osteocyte in this animal being overstimulated. One would predict that other osteocyte load responsive genes, such as MEPE and osteopontin, would be increased in the DMP1-null mouse and indeed we have data that both osteopontin and MEPE are overexpressed in the DMP1 KO model under normal cage activity.
The MEPE gene, which produces MEPE protein, is also highly responsive to mechanical signals5. MEPE protein is most likely cleaved by a cathepsin-like protease to a highly phosphorylated C-terminal ASARM peptide in osteocytes. We know that overproduction of MEPE and the ASARM peptide are associated with hypophosphatemic and osteomalacia, phenotype very similar to the DMP1 knock-out mouse2. Excess MEPE leads to overproduction of ASARM peptide, which further increases osteomalacia, degradation of mineral quality, and also inhibits phosphate resorption in the kidney (Rowe PS, personnel communication). This phenomena leads to hypophosphatemia similar to what is observed in the DMP1 KO mouse. We also suggest in this model that the normal function of the ASARM peptide derived from the load responsive MEPE gene is in reducing local mineral content in the perilacunar region around osteocytes6. MEPE protein and the ASARM peptide are highly concentrated just outside the lacunae of the osteocyte in a ring corresponding to this less mineralized region of the osteocyte5. This less mineralized bone region around each osteocyte may serve as an important strain amplifier7. In our model, the canaliculi would have greater load-induced DMP1 protein lining the walls and in the perilacunar matrix. DMP1 could serve as a potential signaling molecule through its RGD domain, regulating the level of strain perceived by the osteocyte. MEPE through altering the mineral content of the perilacunar space could be indirectly modifying strain perceived by the osteocyte. It was also suggested at the Sun Valley meeting by Dr. David Burr, Indiana University, that local demineralization around osteocyte lacunae could alter local calcium and phosphate concentrations of the osteocyte after mechanical loading. These changes in calcium and phosphate at the local level could in turn alter gene expression locally in the osteocyte.
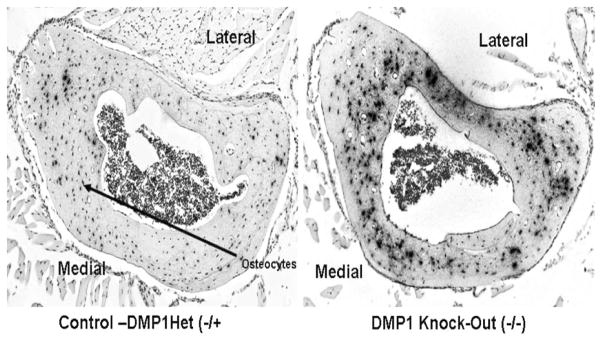
Figure 2.
MEPE mRNA expression in osteocytes is 6-fold higher in the DMP1 knock-out compared to the control mice.
Footnotes
Dr. McKee has funding and a consultancy with Enobia Pharma. All other authors have no conflict of interest.
References
- 1.Gluhak-Heinrich T, Bonewald LF, MacDougall M, Harris SE, Pavlin D. Mechanical loading stimulates dentin matrix protein 1 (DMP1) in osteocytes in vivo. J Bone Miner Res. 2003;18:807–817. doi: 10.1359/jbmr.2003.18.5.807. [DOI] [PubMed] [Google Scholar]
- 2.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang W, Lu Y, Kalajzic I, Guo D, Harris MA, Gluhak-Heinrich J, Kotha S, Bonewald LF, Feng JQ, Rowe DW, Turner CH, Robling AG, Harris SE. Dentin matrix protein 1 gene cis regulation: use in osteocytes to characterize local responses to mechanical loading in vitro and in vivo. J Biol Chem. 2005;280:20680–20690. doi: 10.1074/jbc.M500104200. [DOI] [PubMed] [Google Scholar]
- 4.Kalajzic I, Braut D, Guo D, Jiang X, Kronenberg MS, Mina M, Harris MA, Harris SE, Rowe DW. Dentin matrix protein 1 expression during osteoblastic differentiation, generation of an osteocyte GFP-transgene. Bone. 2004;35:74–82. doi: 10.1016/j.bone.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Gluhak-Heinrich J, Pavlin D, Yang Y, MacDougall M, Harris SE. MEPE expression in osteocytes during orthodontic tooth movement. Arch Oral Biol. 2007;52:684–690. doi: 10.1016/j.archoralbio.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling J, Miller M, Moravits D, Lankford J, Bonewald L, Nicolella D. Microstructural compositional changes associated with osteocyte lacunae detected using Raman imaging. J Bone Miner Res. 2005;20:S149–S150. [Google Scholar]
- 7.Bonivtch AR, Bonewald LF, Nicolella DP. Tissue strain amplification at the osteocyte lacuna: a microstructural finite element analysis. J Biomech. 2007;40:2199–2206. doi: 10.1016/j.jbiomech.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]