Abstract
Tobacco addiction requires activation by nicotine of a variety of central nicotinic acetylcholine receptors (nAChRs). In animals, both nAChR antagonists and immunization against nicotine can reduce nAChR activation by nicotine and block a variety of addiction-relevant behaviors. However, clinical use of nAChR antagonists for smoking cessation is limited by dose-related side effects, and immunization does not reliably produce sufficient antibody levels in smokers to enhance smoking cessation rates. Combining these approaches may be one way of addressing the limitations of each while enhancing overall efficacy. This study examined the individual and combined effects of passive immunization with the monoclonal nicotine-specific antibody Nic311 and the nicotinic receptor antagonist mecamylamine (MEC) on nicotine’s discriminative stimulus effects. Rats were trained to discriminate 0.4 mg/kg nicotine from saline using a two-lever operant discrimination procedure. Antagonism of nicotine discrimination by Nic311 (160 mg/kg i.v.) and ascending doses of MEC (0.03, 0.1, 0.3, and 1.0 mg/kg s.c.) was assessed across four consecutive daily 2-min extinction test sessions using a 2 × 2 design. Nic311 alone produced a 24-48% reduction in % nicotine-lever responding (%NLR) across all four test sessions. MEC produced a dose-dependent decrease in %NLR, with no effect at the two lowest doses and 80-93% attenuation at the two highest doses. Nic311 combined with MEC significantly suppressed %NLR at every MEC dose (85-92% reduction across all four test sessions). Very low doses of MEC that were ineffective alone completely blocked nicotine discrimination when combined with Nic311. These data demonstrate that nicotine-specific antibodies and MEC can work synergistically to suppress the subjective effects of nicotine and suggest that low doses of MEC may significantly enhance the efficacy of immunotherapy.
Keywords: nicotine, drug discrimination, mecamylamine, monoclonal nicotine-specific antibodies, rat
1. Introduction
Nicotine is considered the principal constituent in tobacco responsible for initiating and maintaining tobacco addiction. It produces a constellation of neuropharmacological and behavioral effects that are similar to those produced by other drugs of abuse (Le Foll and Goldberg, 2006). These effects are mediated through nicotine’s activation and desensitization of a variety of nicotinic acetylcholine receptors (nAChR) in brain (Changeux, 2010; Picciotto et al., 2008). Several medications currently used or under development for treatment of tobacco addiction act by altering nAChR activation by nicotine (Lerman et al., 2007).
Administration of a nAChR antagonist disrupts nAChR activation and can reduce addiction-relevant CNS and behavioral effects of nicotine. Mecamylamine, a noncompetitive and largely nonselective nAChR antagonist, reduces the reinforcing and discriminative stimulus effects of nicotine or tobacco in animals and humans (Lerman et al., 2007; Smith and Stolerman, 2009). It is currently the only nAChR antagonist approved for use in humans, albeit as an antihypertension medication. It has facilitated smoking cessation in clinical trials when combined with nicotine replacement therapy (Rose et al., 1998; Rose et al., 1994). However, its clinical development has been hampered because of its peripheral side effects at effective doses (e.g., constipation, abdominal cramps, dizziness, Rose et al., 1998; Tennant et al., 1984). Preclinical development of other nAChR antagonists with efficacy similar to or better than mecamylamine, but reduced peripheral side effects, has been an important focus in medication development for tobacco addiction (Dwoskin et al., 2009; Papke et al., 2008; Wilkins et al., 2002).
Immunotherapy presents an alternative means of reducing activation of nAChRs by nicotine that is mechanistically distinct from the use of a receptor antagonist. Vaccination with a nicotine immunogen elicits production of nicotine-specific antibodies that selectively bind and sequester nicotine in blood and thereby reduce the level of free or unbound nicotine that can distribute into brain and activate nAChRs. There are several potential advantages of immunotherapy over other approved or experimental pharmacotherapies for nicotine addiction (LeSage et al., 2006b). First, immunotherapies target nicotine itself rather than the brain receptors mediating nicotine’s reinforcing effects and so do not block effects of endogenous acetylcholine. As such, nicotine vaccines do not have the central nervous system side effects associated with other types of medications. For this same reason, nicotine vaccines do not block peripheral nAChRs or produce the side effects that limit use of MEC. Second, reducing nicotine distribution to brain presumably decreases nicotine activation of all types of nAChRs, and therefore all of nicotine’s neuropharmacological effects in brain that are vital to maintaining tobacco addiction. This is difficult to accomplish with any one or combination of nAChR-targeted medications other than nicotine itself. Immunization has proven effective in reducing a variety of nicotine’s CNS and behavioral effects in preclinical studies (e.g., DA release, locomotor activity, nicotine self-administration, (Cornish et al., 2011; LeSage et al., 2006b; Moreno et al., 2010; Moreno and Janda, 2009; Roiko et al., 2009) and increasing abstinence in Phase II clinical trials (Escobar-Chávez et al., 2011; Hatsukami et al., 2011). However, efficacy in Phase II trials has been limited to individuals with the highest serum antibody concentrations (e.g. top 30%), and preliminary results from Phase III trials suggest no effect of vaccine on 16-week continuous abstinence rates at 52 weeks from the quit date (although post hoc analysis indicated antibody levels were positively correlated with abstinence rates, Fahim et al. 2011). The primary limitation of immunotherapy has been the modest and variable serum levels of antibody elicited by current vaccines.
Strategies are needed to address the limitations of nAChR antagonists and immunotherapy to improve their clinical potential. Although identifying improved nAChR antagonists and vaccines should be helpful for this purpose (e.g., Keyler et al., 2008; Moreno et al., 2010; Papke et al., 2008; Pravetoni et al., in press; Wooters et al., 2011), new “second-generation” medications have not yet entered clinical trials. Alternatively, combining current nAChR antagonist medications and vaccines might enhance their efficacy. Receptor-based and immunologic treatments are attractive complements because their mechanisms (pharmacodynamic versus pharmacokinetic, respectively) are distinct, yet they target the same process. Each interrupts nAChR activation at one of two critical and sequential steps toward receptor activation; nicotine distribution to the receptor (immunotherapy), and the extent of receptor binding (competitive antagonist) or activation once bound (noncompetitive antagonist). The goal of this approach would be to achieve a high degree of blockade and efficacy using sub-toxic doses of MEC and achievable antibody concentrations via immunization. As a result, vaccine efficacy might be enhanced, while side effects of nAChR antagonism are minimized.
The purpose of the present study was to examine the separate and combined effects of immunization with the monoclonal nicotine-specific antibody Nic311 and MEC on nicotine’s discriminative stimulus effects in rats. Immunization against nicotine can be achieved via vaccination or direct administration of antibodies (passive immunization). We chose the latter for this initial proof-of-principle study because, in contrast to vaccination, serum antibody concentrations can be precisely controlled and immediately achieved.
2. Materials and Methods
2.1. Animals
Twenty-three male Holtzman rats (Harlan, Indianapolis) weighing 300-350g at the start of the experiment were maintained with limited access to food (18 g/day rat chow) and unlimited access to water. Each rat was individually housed in a temperature- and humidity-controlled colony room under a reversed 12h light/dark cycle (lights off at 10:00 am). Animal husbandry and experimental protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation, and were in accordance with the 2011 National Research Council Guide for the Care and Use of Laboratory Animals (8th edition), and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003).
2.2. Apparatus
Experimental sessions occurred in sixteen identical operant-conditioning chambers (ENV-008, Med Associates Inc., St. Albans, VT). The front panel contained two response levers, a stimulus light over each response lever, and an aperture between the levers for delivery of 45-mg food pellets (PJAI-0045, Research Diets, New Brunswick, NJ). A house light was located on the back panel near the chamber ceiling to provide ambient illumination. Each chamber was enclosed in a sound-attenuating box equipped with an exhaust fan that provided masking noise.
2.3. Drugs
Nicotine bitartrate and mecamylamine (Sigma Chemical Co., St. Louis, MO) were dissolved in sterile saline. The pH of the nicotine solution was adjusted to 7.4 with dilute NaOH. All nicotine doses and concentrations are expressed as that of the base. MEC doses are expressed as that of the salt. The nicotine-specific monoclonal antibody Nic311 is an IgG1κ derived from mice immunized with the immunogen 3′-aminomethylnicotine conjugated to recombinant Pseudomonas exoprotein A, has a Kd for nicotine of 60 nM and <1% cross-reactivity with mecamylamine, nicotine metabolites or a variety of neurotransmitters including acetylcholine (Keyler et al., 2005). Nic311 was purified by protein G chromatography to ≥95% of total protein content with endotoxin levels of <0.2 EU/mg. Nic311 was diluted in 2 ml phosphate-buffered saline (concentration of approximately 30 mg/ml). The Nic311 dose of 160 mg/kg was selected based on pilot data indicating that it produces a partial attenuation of nicotine discrimination, allowing for detection of added effects by mecamylamine. Control IgG was human polyclonal IgG (Gammagard; Baxter Healthcare Corp., Westlake Village, CA) that does not bind nicotine or alter nicotine pharmacokinetics or behavior in rats (Cornish et al., 2011).
2.4. Nicotine Discrimination Training
The training procedures that were used have been described in detail elsewhere (LeSage et al., 2009). Briefly, rats were trained to discriminate nicotine alone (0.4 mg/kg, s.c.) from saline using a 2-lever discrimination procedure. Lever pressing was reinforced under a terminal variable-interval 15 sec schedule using 45-mg food pellets. Discrimination was assessed twice weekly (Tues and Fri) during 2-min extinction test sessions. Discrimination was considered stable when a) >80% responding occurred on the injection-appropriate lever during two consecutive saline and nicotine test sessions, b) >95% injection-appropriate responding occurred on six consecutive training sessions, and c) response rates (total responses/session) were stable (no trend across these four test sessions and six training sessions).
After stable discrimination performance was achieved, a nicotine generalization dose-effect function was determined, involving substitution of a range of nicotine doses (0.0, 0.05, 0.1, 0.2 and 0.4 mg/kg) during Tues and Fri test sessions. Doses were administered in a mixed sequence that was counterbalanced across subjects. At least two weeks after the acute dose-response determination and when performance was stable, four consecutive daily test sessions with the training dose were conducted Tues-Fri to assess the stability of discrimination of the training dose across repeated test sessions. This repeated-testing procedure allowed studying the initial time course of Nic311 effects (see below). Rats that failed to meet discrimination criteria (at least 80% responding on the nicotine lever) on any of these 4 consecutive tests were excluded from the study. Those that met criteria on every test session were implanted with a jugular catheter to allow i.v. administration of Nic311.
2.5. Catheter Implantation and Maintenance
Each rat was implanted with a chronic indwelling jugular catheter under intramuscular droperidol (2.0 mg/kg) and fentanyl (0.04 mg/kg) anesthesia according to our standard protocol (e.g., LeSage et al., 2002; LeSage et al., 2010). Rats were allowed to recover for at least four days after surgery, during which each rat received daily infusions of a glycerol/heparinized-saline solution (25% glycerol, 25 units/ml heparin) and antibiotic (rocephin, 5.25 mg, first two days post-op) into the jugular catheter. To help maintain catheter patency throughout the remainder of the experiment, catheters were flushed Monday through Thursday with the 25% glycerol/heparinized-saline solution, and “locked” on Fridays with 50% glycerol/heparinized-saline. Infusions of methohexital (0.1 ml, 50 mg/ml, IV) were administered occasionally to determine catheter patency (production of ataxia) if malfunctions were suspected. One rat in each group had a catheter-related problem that was easily fixed. One rat in the Control IgG + Saline group had its vascular-access harness replaced three days following antibody administration when it escaped from its harness. This had no effect on its discrimination performance. The other three rats developed a clot in their catheter several days prior to antibody administration, which was cleared and blood return was regained. Baseline performance was unaffected in these rats, but they were nonetheless allowed at least one additional week of baseline assessment before antibody administration. All rats had an intact and patent catheter at the time of antibody administration.
2.6. Antibody and Drug Assessment
Rats were randomly assigned to one of four groups according to a 2×2 design, with antibody (Control IgG or Nic311) and MEC pretreatment (Saline or MEC) as factors (N=6/group, except N=5 for Control IgG + Saline group). During this phase, rats were exposed to another four consecutive test sessions with the nicotine training dose as described above. Twenty hours before the first of these sessions (Monday afternoon), Nic311 (160 mg/kg) or control antibody was administered. Fifteen minutes before each subsequent test session on Tues – Fri, saline or MEC was injected s.c. 15 min prior to the session. For rats exposed to MEC, ascending doses of MEC (0.03, 0.1, 0.3, and 1.0 mg/kg) were administered across sessions in order to examine immunization effects on the MEC dose-response curve and to provide a positive control for maximum suppression of discrimination at the highest MEC dose. A between-subjects design was employed for antibody assessment because of the long half-life (approximately 1 week) of Nic311 (Roiko et al., 2009). A within-subjects design would have required at least five weeks (i.e., five half-lives) between conditions to allow sufficient clearance of antibody.
2.7 Statistical Analysis
The percentage of responding on the nicotine-appropriate lever (%NLR) and overall response rate (responses/second) during the 2-min extinction test sessions served as the primary dependent measures. To assure that nicotine discrimination was comparable across groups prior to Nic311 and MEC testing, nicotine generalization functions and performance during repeated testing prior to prior to Nic311 and MEC treatment were analyzed by mixed-factor ANOVA, followed by Bonferroni post-hoc tests comparing each dependent measure at a given nicotine dose to saline and between groups. Nic311 and MEC treatment effects were determined by comparing mean %NLR and response rate during each consecutive test session between groups using a mixed-factor ANOVA, followed by Bonferroni post-hoc tests. Full generalization was defined as %NLR greater than or equal to 80%, while partial generalization was defined as %NLR greater than or equal to 20% but less than 80%.
3. Results
3.1 Baseline Discrimination Performance
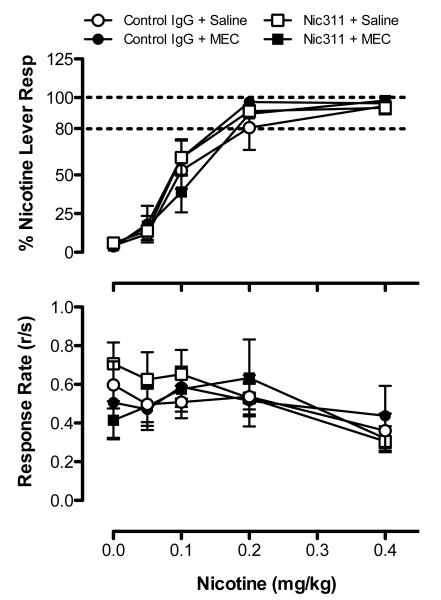
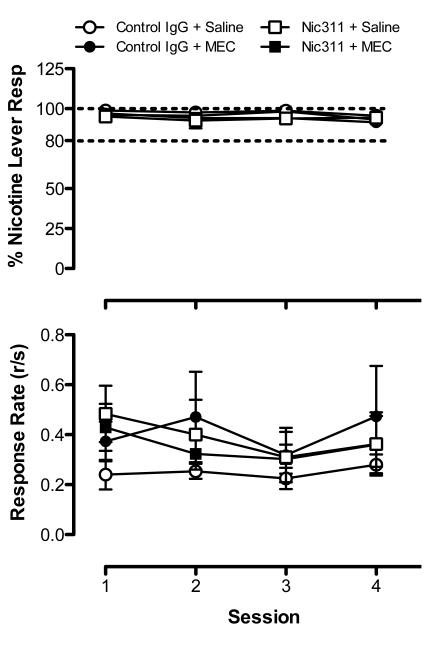
Figure 1 shows the dose-response curves for %NLR (top panel) and overall response rate (bottom panel) obtained prior to treatment with Nic311 and MEC. A main effect of dose on both %NLR (F=122.8, p<0.0001) and response rate (F=5.61, p<0.001) was observed, but no effect of group or a dose x group interaction for either measure. Figure 2 shows %NLR (top panel) and response rate (bottom panel) during repeated testing with the training dose. No main effects or interaction on either measure was observed. Discrimination performance remained stable across the four consecutive test sessions in all groups. Together, these findings demonstrate that discrimination performance was comparable between groups prior to assessing treatment effects.
Figure 1.
Baseline mean (±SEM) %NLR (top panel) and response rate (bottom panel) during nicotine substitution dose-response determinations in each treatment group prior to assessing Nic311 and MEC effects. Each point represents the mean of 5-6 rats. Dashed horizontal lines indicate criterion levels of performance for discrimination of the 0.4 mg/kg nicotine training dose.
Figure 2.
Baseline mean (±SEM) %NLR (top panel) and response rate (bottom panel) during consecutive daily test sessions with the 0.4 mg/kg nicotine training dose in each treatment group prior to assessing Nic311 and MEC effects. See Figure 1 for further details.
3.2 Treatment Effects
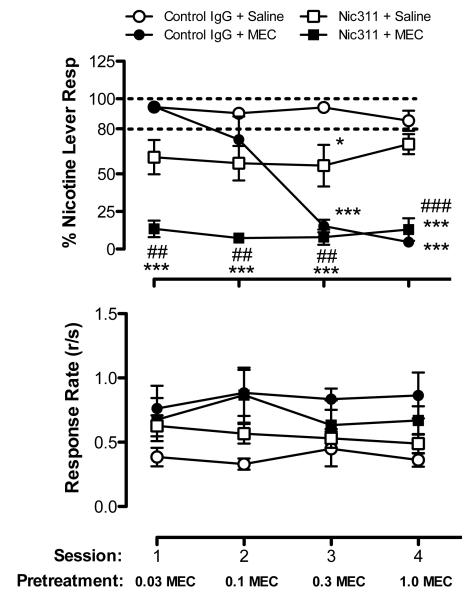
Figure 3 shows %NLR (top panel) and response rate (bottom panel) during repeated nicotine (0.4 mg/kg) test sessions in each treatment group during assessment of Nic311 and MEC effects. There was a significant main effect of treatment (F=34.17, p<0.0001), session (F=10.07. p<0.0001), and treatment x session interaction (F=9.41, p<0.0001) on %NLR. Control rats (Control IgG + Saline) exhibited stable nicotine discrimination and response rates across all test sessions. MEC alone (Control IgG + MEC) produced a dose-dependent decrease in %NLR (F=49.76, p<0.001). The two lower doses had no significant effect on %NLR, whereas the two higher doses significantly decreased %NLR compared to controls (t=7.66, p<0.0001, t=7.85, p<0.0001 for the 0.3 and 1.0 mg/kg doses, respectively). Nic311 alone (Nic311 + Saline) produced a significant partial reduction in %NLR (main effect F=15.05, p<0.01), with %NLR significantly lower on day 3 compared to controls (t=3.02, p<0.05). The combination of Nic311 + MEC markedly suppressed %NLR across all test sessions compared to controls (F=11.0, p<0.01). %NLR was significantly lower compared to Nic311 alone across all sessions (main effect F=31.07, p<0.001) and the two lower doses of MEC alone. Consequently, the potency of MEC in immunized rats was significantly higher than in rats treated with MEC alone. No differences in response rates were observed between groups on any test day.
Figure 3.
Mean (±SEM) %NLR (top panel) and response rate (bottom panel) during consecutive daily test sessions with the 0.4 mg/kg nicotine training dose in each treatment group following administration of Nic311, MEC, or both. Significantly different from Control IgG+Saline, *p<0.05, ***p<0.001. Significantly different from Nic311+Saline, ##p<0.01, ###p<0.001. See Figure 1 for further details.
4. Discussion
Despite its ability to attenuate the dependence-related effects of nicotine in both animals and humans, clinical development of mecamylamine for smoking cessation has been limited by adverse side effects. Similarly, despite efficacy in preclinical studies and early clinical trials, the effects of immunization with nicotine vaccines have been partial, owing to the limited and variable serum antibody levels that are achieved. The present study shows that combining immunization with nicotine-specific antibodies and MEC is more effective at blocking the discriminative stimulus (i.e. subjective) effects of nicotine than either treatment alone, and the enhancement of efficacy is synergistic. These data provide proof of principle that combining a nAChR antagonist with immunotherapy to block nAChR activation through complementary mechanisms is one potential means of addressing the limitations of each treatment and enhancing overall efficacy.
The present findings are consistent with previous studies showing that immunization can attenuate nicotine discrimination. Immunization with a peptide-based vaccine against nicotine attenuated nicotine discrimination in rats under a goal-tracking assay (Sanderson et al., 2003), and passive immunization with polyclonal nicotine-specific antibodies reduced nicotine discrimination in rats under an operant assay (Malin et al., 2002). The present findings are also consistent with the well-documented dose-dependent effects of MEC alone on nicotine discrimination (e.g., Hirschhorn and Rosecrans, 1974; Smith and Stolerman, 2009; Stolerman et al., 1984; Young and Glennon, 2002), which have generally shown attenuation of discrimination at doses of 0.3 mg/kg MEC and higher, but not at 0.1 mg/kg (Young and Glennon, 2002; Zakharova et al., 2005; Zaniewska et al., 2006). The marked effect of the 0.03 mg/kg MEC dose when combined with immunization is striking given that both 0.03 mg/kg and 0.1 mg/kg were ineffective when administered alone.
Nicotine vaccines reduce and slow nicotine distribution to brain and their efficacy is closely correlated with the extent of their effects on nicotine pharmacokinetics. The most important limitation of vaccination against nicotine in humans is that the mean antibody levels produced in serum are modest, lower than those produced in animals. This is likely due to reluctance to use stronger adjuvants such as Freund’s in humans, and more immunogenic routes that are often used in animals such as intraperitoneal injection. In addition, serum antibody levels vary greatly among individuals. Augmenting vaccination with a low and well-tolerated dose of MEC could address these low antibody levels by further reducing nAChR activation, and might be particularly useful for individuals who have a less robust response to vaccination.
In the present study, immunization was achieved by passive administration of nicotine-specific antibody rather than vaccination, the mode of immunization used clinically. While it remains to be determined whether a similar synergistic effect is achieved with vaccination, the effects of vaccination or passive immunization on nicotine pharmacokinetics are essentially identical and passive immunization has been frequently used as a surrogate for vaccination in preclinical development of immunotherapies. The antibody dose used here produces serum antibody concentrations higher than those generally associated with vaccination in rats (Keyler et al., 2005; LeSage et al., 2006b) and was chosen because pilot data suggested that lower doses of Nic311 alone had little or no effect on nicotine discrimination (data not shown). This may be due in part to the large single nicotine dose required to produce a discriminative stimulus, which in turn requires a high dose of antibody to antagonize it. However, lower serum antibody concentrations achieved by vaccination in rats have been able to attenuate other addiction-related behavioral effects of nicotine (e.g., nicotine self-administration, (LeSage et al., 2006a; Lindblom et al., 2002). Moreover, the synergistic nature of the interaction between Nic311 and MEC suggests that the effects of lower antibody concentrations achieved through vaccination are likely to also be augmented by MEC. Further studies over a range of Nic311 doses, with vaccination rather than passive immunization, and in behavioral paradigms other than nicotine discrimination will all be helpful in evaluating the clinical potential of this interaction.
This study demonstrates efficacy in blocking the early acute effects of a single, albeit large daily nicotine dose, which contrasts with the repeated self-administration of nicotine associated with tobacco addiction. Although not measured in this study, the serum nicotine concentrations associated with the 0.4 mg/kg nicotine training dose were likely higher (130-150 ng/ml) than the mid-day serum nicotine concentrations associated with nicotine self-administration in animals or smoking in humans (10-50 ng/ml, Benowitz et al., 2009; LeSage et al., 2003; LeSage et al., 2002; Pratt et al., 1983). By this measure the current protocol provided a rigorous test of treatment effects. Whether the effects observed here translate to a repeated dosing model needs to be specifically studied.
Immunization slows both the rate of nicotine distribution to brain and nicotine clearance (LeSage et al. 2006b). This effect could have altered the time course of nicotine discrimination in the present study. If nicotine discrimination was tested at longer intervals after nicotine administration, the difference between the immunized and non-immunized groups might have been smaller. In other words, immunization effects may be weaker at longer nicotine pretreatment intervals. A study examining immunization effects on the time course of nicotine discrimination is required to address this issue.
To our knowledge this is the first study to examine the stability of discrimination performance over several consecutive daily test sessions, which allowed study of the initial time course of antibody effects on nicotine discrimination. Although performance was clearly stable over four consecutive test sessions during baseline (Figure 2) and in the Control IgG + Saline group during the immunization phase (Figure 3), it is unclear to what extent this protocol may have induced response perseveration on the nicotine-appropriate lever. Use of consecutive test sessions that alternate between saline and nicotine would have prevented such perseveration. Nonetheless, any perseveration that may have occurred in the present study did not prevent antibody and mecamylamine from producing an orderly attenuation of discrimination.
The present study demonstrates an important interaction between immunization and a nAChR antagonist. Apart from suggesting the potential use of vaccination and low dose MEC in combination for smoking cessation, it introduces the possibility that immunization might serve more broadly to reduce the required dose, or enhance the efficacy, of medications other than nAChR antagonists. Many drugs that attenuate the behavioral effects of nicotine are more effective when the nicotine dose or intake is low (Levin et al., 2011; Mansbach et al., 2000; Markou et al., 2004; Paterson et al., 2004; Quarta et al., 2007; Stolerman et al., 1983). Viewed from this perspective, immunization could serve as a general means of reducing the dose of nicotine reaching and activating nAChRs, enhancing the efficacy of diverse other types of nicotine addiction medications. This could be particularly useful when a medication is found to be effective but also causes unwanted side effects, as is the case with MEC, a setting in which immunization could provide a dose-sparing effect. In this manner immunization could serve as a platform for medication development, enhancing medication efficacy or reducing the required dose and allowing medications to be used which would otherwise be dismissed because of their side effects. By the same reasoning it is possible that the combination of immunization + mecamylamine could serve as a platform for the addition of other medications. In this context, immunization + mecamylamine would provide nAChR blockade while other medications could supply additional desired actions (e.g., relieve withdrawal symptoms).
Highlights.
Effects of immunization and mecamylamine on nicotine discrimination were studied
Combination produced greater attenuation than immunization or mecamylamine alone
Enhancement of effect was synergistic
Acknowledgements
The authors thank Catherine Early and Danielle Burroughs for their technical assistance in conducting the study. Supported by NIH/NIDA grant DA-10714 (Pentel, PI) and a career development award from the Minneapolis Medical Research Foundation (Pentel, PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benowitz NL, Hukkanen J, Jacob P. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J-P. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci. 2010;11:389–401. doi: 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- Cornish KE, Harris AC, Lesage MG, Keyler DE, Burroughs D, Earley C, et al. Combined active and passive immunization against nicotine: Minimizing monoclonal antibody requirements using a target antibody concentration strategy. Int Immunopharmacol. 2011 doi: 10.1016/j.intimp.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwoskin LP, Smith AM, Wooters TE, Zhang Z, Crooks PA, Bardo MT. Nicotinic receptor-based therapeutics and candidates for smoking cessation. Biochem Pharmacol. 2009;78:732–43. doi: 10.1016/j.bcp.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Chávez JJ, Domínguez-Delgado CL, Rodríguez-Cruz IM. Targeting nicotine addiction: the possibility of a therapeutic vaccine. Drug Des Devel Ther. 2011;5:211–24. doi: 10.2147/DDDT.S10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim REF, Kessler PD, Fuller SA, Kalnik MW. Nicotine Vaccines. CNS and Neurological Disorders - Drug Targets. 2011;10:905–915. doi: 10.2174/187152711799219343. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, et al. Immunogenicity and Smoking-Cessation Outcomes for a Novel Nicotine Immunotherapeutic. Clin Pharmacol Ther. 2011;89:392–9. doi: 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn ID, Rosecrans JA. Studies on the time course and the effect of cholinergic and adrenergic receptor blockers on the stimulus effect of nicotine. Psychopharmacology (Berl) 1974;40:109–20. doi: 10.1007/BF00421360. [DOI] [PubMed] [Google Scholar]
- Keyler DE, Roiko SA, Benlhabib E, LeSage MG, Peter JVS, Stewart S, et al. Monoclonal nicotine-specific antibodies reduce nicotine distribution to brain in rats: dose- and affinity-response relationships. Drug Metab Dispos. 2005;33:1056–61. doi: 10.1124/dmd.105.004234. [DOI] [PubMed] [Google Scholar]
- Keyler DE, Roiko SA, Earley CA, Murtaugh MP, Pentel PR. Enhanced immunogenicity of a bivalent nicotine vaccine. Int Immunopharmacol. 2008;8:1589–94. doi: 10.1016/j.intimp.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology. 2006;184:367–81. doi: 10.1007/s00213-005-0155-8. [DOI] [PubMed] [Google Scholar]
- Lerman C, LeSage MG, Perkins KA, O'Malley SS, Siegel SJ, Benowitz NL, et al. Translational research in medication development for nicotine dependence. Nature reviews Drug discovery. 2007;6:746–62. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology (Berl) 2003;170:278–86. doi: 10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Hieda Y, Collins G, Burroughs D, Le C, et al. Effects of a nicotine conjugate vaccine on the acquisition and maintenance of nicotine self-administration in rats. Psychopharmacology (Berl) 2006a;184:409–16. doi: 10.1007/s00213-005-0027-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Pentel PR. Current status of immunologic approaches to treating tobacco dependence: vaccines and nicotine-specific antibodies. The AAPS journal. 2006b;8:E65–75. doi: 10.1208/aapsj080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, Pentel PR. Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacol Biochem Behav. 2002;72:279–89. doi: 10.1016/s0091-3057(01)00775-4. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Perry JL, Kotz CM, Shelley D, Corrigall WA. Nicotine self-administration in the rat: effects of hypocretin antagonists and changes in hypocretin mRNA. Psychopharmacology (Berl) 2010;209:203–12. doi: 10.1007/s00213-010-1792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Shelley D, Ross JT, Carroll FI, Corrigall WA. Effects of the nicotinic receptor partial agonists varenicline and cytisine on the discriminative stimulus effects of nicotine in rats. Pharmacol Biochem Behav. 2009;91:461–7. doi: 10.1016/j.pbb.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Slade S, Wells C, Petro A, Rose JE. D-cycloserine selectively decreases nicotine self-administration in rats with low baseline levels of response. Pharmacol Biochem Behav. 2011;98:210–4. doi: 10.1016/j.pbb.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom N, De Villiers SH, Kalayanov G, Gordon S, Johansson AM, Svensson TH. Active Immunization against Nicotine Prevents Reinstatement of Nicotine-Seeking Behavior in Rats. Respiration. 2002;69:254–60. doi: 10.1159/000063629. [DOI] [PubMed] [Google Scholar]
- Malin DH, Alvarado CL, Woodhouse KS, Karp H, Urdiales E, Lay D, et al. Passive immunization against nicotine attenuates nicotine discrimination. Life Sci. 2002;70:2793–8. doi: 10.1016/s0024-3205(02)01523-0. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Chambers LK, Rovetti CC. Effects of the competitive nicotinic antagonist erysodine on behavior occasioned or maintained by nicotine: comparison with mecamylamine. Psychopharmacology. 2000;148:234–42. doi: 10.1007/s002130050047. [DOI] [PubMed] [Google Scholar]
- Markou A, Paterson NE, Semenova S. Role of gamma-aminobutyric acid (GABA) and metabotropic glutamate receptors in nicotine reinforcement: potential pharmacotherapies for smoking cessation. Ann N Y Acad Sci. 2004;1025:491–503. doi: 10.1196/annals.1316.061. [DOI] [PubMed] [Google Scholar]
- Moreno AY, Azar MR, Warren NA, Dickerson TJ, Koob GF, Janda KD. A critical evaluation of a nicotine vaccine within a self-administration behavioral model. Mol Pharm. 2010;7:431–41. doi: 10.1021/mp900213u. [DOI] [PubMed] [Google Scholar]
- Moreno AY, Janda KD. Immunopharmacotherapy: vaccination strategies as a treatment for drug abuse and dependence. Pharmacol Biochem Behav. 2009;92:199–205. doi: 10.1016/j.pbb.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Dwoskin LP, Crooks PA, Zheng G, Zhang Z, McIntosh JM, et al. Extending the analysis of nicotinic receptor antagonists with the study of alpha6 nicotinic receptor subunit chimeras. Neuropharmacology. 2008;54:1189–200. doi: 10.1016/j.neuropharm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. The GABAB receptor agonists baclofen and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacology. 2004;172:179–86. doi: 10.1007/s00213-003-1637-1. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–42. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt JA, Stolerman IP, Garcha HS, Giardini V, Feyerabend C. Discriminative stimulus properties of nicotine: further evidence for mediation at a cholinergic receptor. Psychopharmacology. 1983;81:54–60. doi: 10.1007/BF00439274. [DOI] [PubMed] [Google Scholar]
- Pravetoni M, Keyler DE, Pidiparthi RR, Carroll FI, Runyon S, Murtaugh MP, et al. Structurally distinct nicotine immunogens elicit antibodies with non-overlapping specificity. Biochem Pharmacol. doi: 10.1016/j.bcp.2011.11.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta D, Naylor CG, Stolerman IP. The serotonin 2C receptor agonist Ro-60-0175 attenuates effects of nicotine in the five-choice serial reaction time task and in drug discrimination. Psychopharmacology (Berl) 2007;193:391–402. doi: 10.1007/s00213-007-0802-3. [DOI] [PubMed] [Google Scholar]
- Roiko SA, Harris AC, LeSage MG, Keyler DE, Pentel PR. Passive immunization with a nicotine-specific monoclonal antibody decreases brain nicotine levels but does not precipitate withdrawal in nicotine-dependent rats. Pharmacol Biochem Behav. 2009;93:105–11. doi: 10.1016/j.pbb.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC. Nicotine-mecamylamine treatment for smoking cessation: the role of pre-cessation therapy. Experimental and Clinical Psychopharmacology. 1998;6:331–43. doi: 10.1037//1064-1297.6.3.331. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Levin ED, Stein RM, Ripka GV. Mecamylamine combined with nicotine skin patch facilitates smoking cessation beyond nicotine patch treatment alone. Clin Pharmacol Ther. 1994;56:86–99. doi: 10.1038/clpt.1994.105. [DOI] [PubMed] [Google Scholar]
- Sanderson SD, Cheruku SR, Padmanilayam MP, Vennerstrom JL, Thiele GM, Palmatier MI, et al. Immunization to nicotine with a peptide-based vaccine composed of a conformationally biased agonist of C5a as a molecular adjuvant. Int Immunopharmacol. 2003;3:137–46. doi: 10.1016/s1567-5769(02)00260-6. [DOI] [PubMed] [Google Scholar]
- Smith JW, Stolerman IP. Recognising nicotine: the neurobiological basis of nicotine discrimination. Handb Exp Pharmacol. 2009:295–333. doi: 10.1007/978-3-540-69248-5_11. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Pratt JA, Kumar R. Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology. 1984;84:413–9. doi: 10.1007/BF00555223. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Pratt JA, Garcha HS, Giardini V, Kumar R. Nicotine cue in rats analysed with drugs acting on cholinergic and 5-hydroxytryptamine mechanisms. Neuropharmacology. 1983;22:1029–37. doi: 10.1016/0028-3908(83)90021-7. [DOI] [PubMed] [Google Scholar]
- Tennant FS, Jr., Tarver AL, Rawson RA. Clinical evaluation of mecamylamine for withdrawal from nicotine dependence. NIDA Res Monogr. 1984;49:239–46. [PubMed] [Google Scholar]
- Wilkins LH, Jr., Haubner A, Ayers JT, Crooks PA, Dwoskin LP. N-n-alkylnicotinium analogs, a novel class of nicotinic receptor antagonist: inhibition of S(-)-nicotine-evoked [(3)H]dopamine overflow from superfused rat striatal slices. J Pharmacol Exp Ther. 2002;301:1088–96. doi: 10.1124/jpet.301.3.1088. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Smith AM, Pivavarchyk M, Siripurapu KB, McIntosh JM, Zhang Z, et al. bPiDI: a novel selective α6β2* nicotinic receptor antagonist and preclinical candidate treatment for nicotine abuse. Br J Pharmacol. 2011;163:346–57. doi: 10.1111/j.1476-5381.2011.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R, Glennon RA. Nicotine and bupropion share a similar discriminative stimulus effect. Eur J Pharmacol. 2002;443:113–8. doi: 10.1016/s0014-2999(02)01554-6. [DOI] [PubMed] [Google Scholar]
- Zakharova ES, Danysz W, Bespalov AY. Drug discrimination analysis of NMDA receptor channel blockers as nicotinic receptor antagonists in rats. Psychopharmacology (Berl) 2005;179:128–35. doi: 10.1007/s00213-004-2067-4. [DOI] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Przegaliński E, Filip M. Evaluation of the role of nicotinic acetylcholine receptor subtypes and cannabinoid system in the discriminative stimulus effects of nicotine in rats. Eur J Pharmacol. 2006;540:96–106. doi: 10.1016/j.ejphar.2006.04.034. [DOI] [PubMed] [Google Scholar]