Abstract
Context
Burkitt lymphoma (BL) is endemic in Uganda and because of the high incidence, diagnosis is often presumed during clinical care and epidemiological studies.
Objective
To assess the accuracy of the clinical and the local pathology diagnosis of BL as assessed by an outside pathology review diagnosis, and to understand the limitations on histopathology practice in a resource-constrained setting at one hospital in Uganda.
Design
Clinically presumed pediatric (< 15 years) BL cases with biopsies and pathology reports, from 1993 - 2007 were identified at St. Mary’s Hospital, Lacor (Gulu, Uganda). Local histopathology procedures, hematoxylyn and eosin stained tissue sections and formalin-fixed paraffin-embedded blocks were reviewed onsite by an outside pathologist, followed by outside study that included tissue microarray (TMA) immunohistochemistry and in situ hybridization.
Results
Local pathology laboratory procedures were inconsistent and suboptimal, especially for tissue fixation. There were 88 clinically presumed BL cases. 63 could be reviewed by outside pathology (25 cases of lost blocks or no remaining tumor) and showed a clinical diagnostic accuracy of 75% (47 confirmed out of 63), with a possible range of 62-85%, depending on the actual diagnosis of the 25 non-evaluable cases. There were 64 BL diagnosed by local pathology. 45 could be reviewed by outside pathology (19 cases of lost blocks or no remaining tumor), and showed a local pathology diagnostic accuracy of 82% (37 confirmed out of 45), with a possible range of 58%- 88%, depending on the actual diagnosis of the 19 non-evaluable cases. Non BL diagnoses included other non-Hodgkin lymphomas, Hodgkin’s lymphoma and benign infectious lymphadenopathy.
Conclusions
Accuracy of clinical diagnosis of Burkitt lymphoma was reduced by inclusion of other diseases with similar clinical presentations. Local pathology, using morphology alone, only marginally improved clinical accuracy and often could not support outside pathology review due to inadequate laboratory procedures. There is an urgent need to improve local pathology prior to conducting high quality clinical and epidemiological studies.
Keywords: Burkitt lymphoma, resource constrained pathology, Africa, cancer, Epstein-Barr virus, malaria
Introduction
Accurate and reliable histopathology diagnosis is critical for confirmation of significant diseases, disease classification, disease registration, and clinical and epidemiological studies, especially of cancer. Fewer than 50% of cancers in Africa are diagnosed by histopathology1 which undermines patient care, cancer registration, epidemiology and translational research. Lack of developed histopathology is especially limiting for non-Hodgkin’s lymphomas (NHL), a heterogeneous group of lymphoid malignancies that requires careful morphologic evaluation, immunophenotyping and genetic tests for accurate classification.2
Burkitt lymphoma (BL) is an aggressive NHL subtype that is endemic in regions of sub-Saharan Africa. Because of its common presentation and characteristic involvement of jaw or abdominal organs, BL diagnosis in Africa is often clinically presumptive supplemented only by aspiration cytology.3 As part of a plan to conduct a case-control study of BL in East Africa, we reviewed the epidemiology4 and the accuracy of case diagnosis by assessing local histotechnology practices, histopathology, archived formalin-fixed paraffin-embedded tissue (FFPET) integrity, block storage and the contribution of outside histopathology review in a BL endemic region of Uganda. We assessed whether local pathology diagnosis and outside pathology review could contribute significantly to the accuracy of final case study diagnosis and, thereby, boost investigator confidence in epidemiological studies of BL conducted in resource constrained settings.
Methods
Population, sample selection, construction of tissue microarray, and lymphoma subtype analysis
Clinically presumed pediatric (< 15 years) BL cases from 1993 - 2007 were identified from patient records at St. Mary’s Hospital (Lacor, Gulu, Uganda) and along with available pathology reports were entered into an epidemiology database. Mean age for boys and girls was compared using the Student’s t-test and differences in proportion for categorical variables were tested using the Chi-Squared test. Two-sided P-values < .05 were considered statistically significant.
Histology laboratory accession logs were used to secure hematoxylin and eosin (H&E) stained diagnostic tissue glass slides, FFPET blocks accession numbers, and dates. These were retrieved from storage, if available, and reviewed.4
Histology laboratory procedures were discussed and actual laboratory practices were observed over several days. Observations included gross tissue cutting, fixation, processing, paraffin embedding, sectioning and staining. Tissue cores (1 mm) for tissue microarray (TMAs) construction were cut from the deeply embedded tissues within these blocks and placed into prepared recipient paraffin blocks using a portable Quick-Ray System instrument (Unitma, Seoul, Korea).
At the USA reference facility, The Ohio State University (OSU) Comprehensive Cancer Center Pathology Core Research Laboratory (Columbus, OH, USA), thin sections (4 μm thick) of the TMA were cut and stained with H&E and monoclonal antibodies by immunohistochemistry (IHC) against CD3, CD15, CD20, CD30, CD34, CD43, CD44, CD45, CD45RO, CD68, CD79a, CD138, Bcl-2, Bcl-6, IgM, Ki67, MUM-1, TdT (DAKO, Carpinteria, CA, USA), CD5, and CD10 (Novocastra, Bannockburn, IL, USA); in situ hybridization (ISH) EBV, kappa (κ) and lambda (λ) light chains and dual color c-MYC gene break apart probe fluorescent in situ hybridization (FISH) FISHba c-MYC t(8;14) (Abbot/Vysis, Downer’s Grove, IL, USA).
For the limited objectives of this pilot evaluation, TMA slides were interpreted by one general pathologist and one hematopathologist for final NHL sub-grouping. The reactivity for IHC and ISH stains were scored qualitatively for tumor cells as negative or positive, except for the Ki67 that was scored as percent of tumor cells staining positive. The World Health Organization (WHO) classification of tumors of heamatopoetic and lymphoid tissues 2 was used for final NHL sub-grouping.
Results
The local pathology laboratory employed inconsistent and suboptimal histology techniques, especially for tissue fixation. There were no written laboratory procedures but technicians reported that formalin (10%) for tissue fixation was mixed from a container of aqueous formaldehyde; the final solution was not buffered. The surgery suite was directly across a walkway from the histopathology laboratory so that specimen transport and delayed processing were not problems. However, technicians reported leaving tissues in formalin for days waiting for a “batch” to process. Tissue was observed as it was processed by hand, mounted on wooden blocks, sectioned using a microtome, melted, gang embedded in a common container and then cut into small individual blocks with a surface attached label for storage. This method required reheating and provided ample opportunity for label transfer between blocks. Paraffin blocks were stored in plastic bags and labels were loosely attached to some blocks and missing on a few others. The stored FFPET blocks were small without supporting cassettes, and they could not be accurately matched to the original diagnostic H&E stained tissue section because the cut surface on the block was lost when the block was re-embedded. Accidental mislabeling during re-embedding could not be easily detected. Some older blocks were hard and brittle and did not core easily; some shattered. Many tissue biopsies were very small and some had been sectioned to near exhaustion. Diagnostic slides were reviewed but tissue preparation and staining were suboptimal for differential diagnosis.
Eighty-eight clinically presumed cases, 49 (56%) from boys, 37 (42%) from girls, and two lacking information on sex were identified. Among 50 cases with known age, the mean age at diagnosis was 7 years, and males were younger than females (6.7 vs. 8.5 years, P = .03). There were 47 cases confirmed as BL. A tumor was classified as BL when it met the morphologic criteria, i.e., a diffuse pattern of medium sized lymphoma cells with multiple distinct nucleoli, deeply basophilic cytoplasm with a “starry sky” pattern, was positive for at least one B cell and one germinal center B cell biomarker, was Bcl-2 and CD44 negative, was c-MYC positive or had no c-MYC signal by FISH testing and had a >90% proliferation index (Ki67) or indications of poor Ki67 staining (Figure 1A-1E). 7 cases were classified as B cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma (DLBCL) and BL. These tumors did not meet the typical morphologic criteria for BL, i.e., were characterized by pleomorphic cell populations of medium sized cells mixed with larger cells, a less distinct “starry sky” pattern, had greater Bcl-2 expression, and a lower proliferation index (Ki67).
Figure 1.
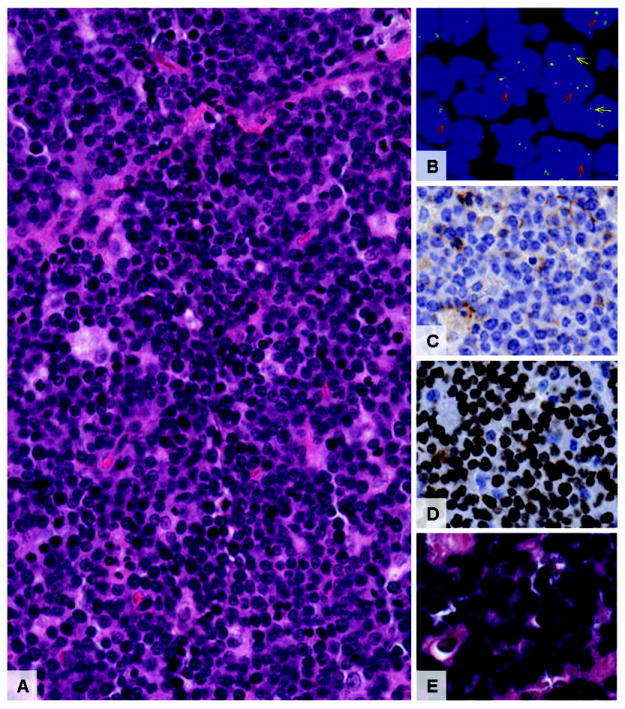
Micrographs (20X) of typical BL tissue morphology (hematoxylin & eosin, Figure 1A), positive dual color c-MYC gene break apart probe fluorescent in situ hybridization (FISHba, Figure 1B) with red arrows indicating a translocation and yellow arrows indicating normal gene, negative CD44 immunohistochemistry (IHC) (Figure 1C), high % Ki67 IHC (Figure 1D) and high % positive EBER in situ hybridization (ISH) (Figure 1E).
Immunohistochemistry markers among the 47 cases of BL and 7 cases of B-cell lymphoma, unclassifiable, varied in intensity and integrity of antigen by FFPET block age (1993 - 1999 vs. 2000 – 2007, Table 1), and likely individually by the length of exposure to formalin (Figure 2A-2L). Thus, positivity for CD10 (17% vs. 42%, P = .04), Ki-67 > 90% (24% vs. 65%, P = .004) and c-MYC FISH nuclear localization (3% vs. 50%, P < .001) was significantly lower in older compared to recent tissue samples. Positivity for CD20, CD79a, Bcl-6, IgM, κ, λ, and EBER was variable in occurrence and intensity, but was unrelated to block age (Table 1).
Table 1.
Immunological characteristics of Burkitt and B cell lymphoma, unclassifiable (based on outside review diagnosis), by period.
| Characteristic | 1993-1999 | 2000-2007 | P-value | |||
|---|---|---|---|---|---|---|
| # | (%) | # | (%) | |||
| CD20* | Negative | 4 | (13) | 3 | (13) | .98 |
| Positive | 26 | (87) | 20 | (87) | ||
|
| ||||||
| CD79a | Negative | 11 | (37) | 5 | (21) | .21 |
| Positive | 19 | (63) | 19 | (79) | ||
|
| ||||||
| CD10 | Negative | 25 | (83) | 14 | (58) | .04 |
| Positive | 5 | (17) | 10 | (42) | ||
|
| ||||||
| Bcl-6 | Negative | 14 | (47) | 9 | (38) | .50 |
| Positive | 16 | (53) | 15 | (63) | ||
|
| ||||||
| Ki-67* | <90% | 19 | (76) | 8 | (35) | .004 |
| ≥90% | 6 | (24) | 15 | (65) | ||
|
| ||||||
| c-MYC | Negative | 29 | (97) | 12 | (50) | <.001 |
| Positive | 1 | (3) | 12 | (50) | ||
|
| ||||||
| IGM | Negative | 13 | (43) | 11 | (46) | .85 |
| Positive | 17 | (57) | 13 | (54) | ||
|
| ||||||
| Kappa (κ) | Negative | 21 | (70) | 18 | (75) | .68 |
| Positive | 9 | (30) | 6 | (25) | ||
|
| ||||||
| Lambda (λ) | Negative | 21 | (70) | 20 | (83) | .26 |
| Positive | 9 | (30) | 4 | (17) | ||
|
| ||||||
| EBER | Negative | 5 | (17) | 5 | (21) | .70 |
| Positive | 25 | (83) | 19 | (79) | ||
Total is <54 because fewer cases were evaluable for these features.
Figure 2.
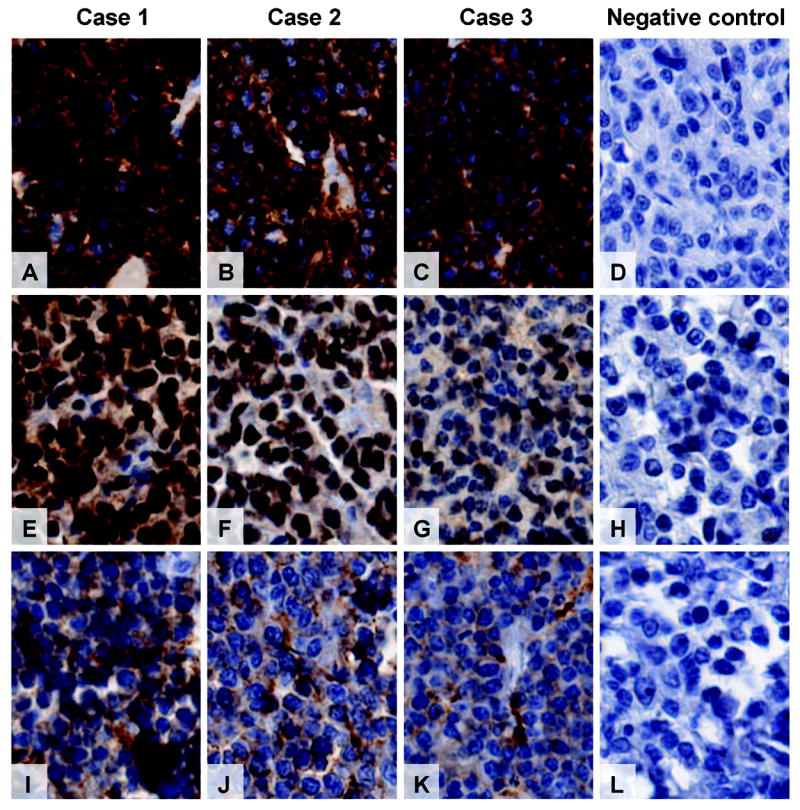
Micrographs (20X) showing BL tissues from cases 1 – 3 (and negative controls) with variable strength of antigen/antibody intensity immunohistochemistry (IHC) for CD20, Bcl-6 and CD10. CD20 (Figure 2A-2D) was the most intense and least variable, Bcl-6 (Figure 2E-2H) was the most variable in intensity and CD10 (Figure 2I-2L) was less intense but less variable. The negative controls are from adjacent CD20, Bcl-6 and CD10 negative lymphomas within the same TMA section and stained at the same time as the BL tissues.
TMA core data was sufficient for lymphoma subtyping in 63 of the 88 (72%) cases, including 45 cases diagnosed by local pathology. It was insufficient in 25 of 88 (28%) cases, due to lost blocks or no remaining tumor in the block, including 19 diagnosed by local pathology as BL. Case diagnosis by local histopathology and outside histopathology review is shown in Table 2. BL diagnosis by local pathology using morphology alone was supported by outside review pathology in 37 of 45 cases (82%, 95% confidence interval [95% CI] 68%-92%). Inaccuracy of local histopathology resulted from the inability to morphologically differentiate other malignant lymphomas with similar clinical presentations to that of BL, including B-cell lymphoma, unclassifiable (6 cases), marginal lymphoma (1 case), and lymphocytic predominant Hodgkin’s lymphoma (1 case).
Table 2.
Local pathology lymphoma diagnosis vs. outside pathology review of 88 clinical Burkitt lymphoma cases
| Outside review pathologist diagnosis | Local pathologist diagnosis | Total | |||
|---|---|---|---|---|---|
| BL | Missing pathology report* | NHL other | Hodgkin’s disease | ||
| BL | 37 | 6 | 3 | 1 | 47 |
| NHL other | |||||
| B-cell lymphoma, unclassifiable | 6 | - | - | 1 | 7 |
| Marginal zone lymphoma | 1 | - | - | - | 1 |
| Lymphoblastic lymphoma | - | - | 1 | - | 1 |
| Follicular lymphoma | - | - | 1 | - | 1 |
| Hodgkin’s disease | 1 | - | - | - | 1 |
| Lymphadenopathy HHV8 positive | - | - | 1 | - | 1 |
| Benign adenopathy | - | - | - | 4 | 4 |
| Lost block / no tumor | 19 | - | 4 | 2 | 25 |
| Total | 64 | 6 | 10 | 8 | 88 |
| Total excluding lost block / no tumor | 45 | 6 | 6 | 6 | 63 |
BL: Burkitt lymphoma; NHL: non-Hodgkin lymphoma; HHV8: human herpes virus 8.
Presumptive BL hospital case records indicated a biopsy but did not contain a pathology report for that biopsy.
Subtotals are in italics.
At best, if all the non-evaluable 19 cases diagnosed by local pathology as BL had been confirmed, the accuracy rate would have been 88% (56/64). At worst, if none had been confirmed, the accuracy rate would have been 58% (37/64). Thus the possible range of correct local pathology diagnosis is from 58%-88%.
By comparison, 63 clinically diagnosed BL were available for outside review and 47 were confirmed, an accuracy rate of 75% (47/63). At best, if all the non-evaluable 25 cases of clinically diagnosed BL had been confirmed as BL, the clinical accuracy rate would have been 82% (72/88). At worst, if none had been confirmed, the accuracy rate would have been 53% (47/88). Thus the possible range of correct clinical diagnosis is from 53-82%.
Discussion
The clinical presumptive diagnosis of BL was accurate for 75% of cases, with a possible range of 53-82%, depending on the actual diagnosis of the 25 non-evaluable cases. Local clinicians have a high expectation of BL when children present with jaw, neck and abdominal tumours. Extra nodal and splenic marginal zone lymphoma in a pediatric age patient would be indistinguishable clinically from BL. The local pathology diagnosis was accurate for 82% of cases reviewed outside, with a possible range of 58-88%, depending on the actual diagnosis of the 19 non-evaluable cases. The small size of the biopsies and inadequate tissue preservation and processing resulted in suboptimal H&E tissue sections for the local pathologist to interpret. Most pathologists in resource-constrained settings have not received specific training in the differential characteristics of cell size and tissue patterns of NHL including those with anatomic presentations similar to BL in children. Hodgkin’s lymphoma is common and also presents in children2. With lymphocyte predominant Hodgkin’s disease, the pathologist may have only a small biopsy showing a monotonous field of lymphocytes where typical Reed Sternberg cells may not be readily seen. The same technical limitations to slide quality would also compromise the potential contribution of telepathology consultations using virtual slides5.
Accuracy of clinical diagnosis of BL could not be determined for the entire patient population by outside review, because of poor tissue quality, lost paraffin blocks and lack of remaining tumor in some TMA cores. Antigens commonly used to confirm the BL subgroup, such as Ki67, Bcl-6 and CD10, were adversely affected by tissue processing as was the demonstration of the classic c-MYC translocation by FISH.
High-quality epidemiological studies suffer when histopathology does not support diagnostic accuracy.6 For example, one study conducted in Uganda7 and another in Malawi8 that included cases diagnosed clinically or by local histopathology (about 50% of all cases) reported significant positive associations of HIV with BL (OR 7.5 - 12.4). However, when the Ugandan data were re-analyzed using only cases confirmed by local or outside review pathology, the association was no longer statistically significant (OR 2.1),9 casting doubt on the validity of the previously reported significant association. In that study as well, outside review failed to confirm 9% of cases and could not subtype 24% of cases, similar to our experience. Campidelli and colleagues10 could evaluate only 20% of 600 pre-selected BL FFPET blocks, despite repeated efforts. Similarly, only 67% of 49 patients in the first clinical treatment trial for AIDS-related non-Hodgkin lymphoma in East Africa had tissue available for pathology review and only 51% of these lymphomas could be immunophenotyped.11 Given the variable proportion of pre-selected lymphoid tissues that can be successfully evaluated by outside review (13%12 to 80%13), such review pathology will have only limited success improving the accuracy of local histopathology.
The histopathologist is the custodian of diagnostic pathology,14 but local histology laboratory processes in resource constrained settings are rarely directly supervised by a pathologist or laboratory scientist. Uganda has only about 18 registered pathologists for a population of 30 million.15 While numerous tissue-based disease discoveries were made in Uganda in the 1960’s,16-18 when the number of pathologists was certainly lower than now, recent local pathology expertise has been shaped in relative isolation from international experience and laboratory practice standardization. This is reflected in the low quality of histopathology noted in our study and others,10, 12, 13 and the lack of written standardized procedures that are taken for granted in developed countries.19
This local laboratory had received a large donation of cassettes and moulds to support paraffin block production, but these supplies were not accompanied by written instructions for their use or training for the technicians. They remained unused, occupying precious storage space, while wooden blocks continued to be used for mounting paraffin tissue blocks for sectioning. Benediktsson and co-workers15 reported similar failures to use donated equipment and supplies, which although well received and treated with respect, were not used because they were not linked to local capacity building. During our assessment, the use of cassettes and moulds was discussed as a potential solution to mislabeling (switching labels). With the support of the hospital, the technicians were given basic hands on training and began using the cassettes.
Our results identify an urgent need to improve the quality of histopathology diagnosis and the quality of FFPET for molecular studies in Africa. We suggest that groups requiring high-quality histopathology should establish and support “histopathology core laboratories” to meet this need. The recent increased funding for international collaborative research in resource-constrained settings,20 including at Lacor Hospital,21 increases the feasibility of our suggestion. Histopathology core laboratories could provide stable, structured training and mentoring programs to meet the immediate need for quality histopathology diagnosis and high-quality FFPET for molecular studies in Africa. Histopathology core laboratories could provide a stable, structured training and mentoring program in pathology, where technicians, pathologists, and surgeons can “learn while doing”, and provide new opportunities for discovery, disease biomarker research and validation.22 Histopathology embedded within collaborative research could support the introduction of “best practices” into histopathology; provide resource-constrained countries with access to high-level consultation and training during research and ultimately improve patient care. A similar approach has been used to introduce field epidemiology and laboratory training programs (FELTPs) to support public health programs for disease surveillance in resource-constrained countries by the Centers for Disease Control and Prevention and other international groups.23 Financial support for “histopathology core laboratories” could come from collaborative epidemiological or clinical research studies utilizing these services,22 or from public health or clinical treatment trials programs, such as those associated with AIDS or cervical cancer.
To summarize, BL clinical diagnosis accuracy is compromised by the presence of other malignant lymphomas and lymphadenopathies with similar clinical presentations. The diagnostic accuracy of clinical BL was only marginally improved by local pathology. Local histotechnology practices limited successful outside pathology review as the remedy to local pathology diagnostic inaccuracy. For the same reason the potential of telepathology consultation using virtual slides is compromised. These findings highlight an urgent need to improve and standardize local pathology as a critical first step in planning or conducting high-quality clinical and epidemiological studies.
Acknowledgments
Grant support: We thank Mr. David Nohle of The Ohio State University AIDS and Cancer Specimen Resource for comments on the manuscript. We also thank the reviewers for their comments, which greatly improved the clarity of our manuscript. This work was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services (Bethesda, MD, USA) and with an Extramural Research Program grant to NCI Infections and Epidemiology Branch (Bethesda, MD, USA) and The Ohio State University Comprehensive Cancer Center Research Enhancement and Assistance Program (REAP 200925). The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the United States Department of Health and Human Services or participating entities.
References
- 1.Parkin DM, Wabinga H, Nambooze S. Completeness in an African cancer registry. Cancer Causes Control. 2001;12(2):147–152. doi: 10.1023/a:1008966225984. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC; Lyon: 2008. [Google Scholar]
- 3.Wright DH. Cytology and histochemistry of the Burkitt lymphoma. Br J Cancer. 1963;17(1):50–55. doi: 10.1038/bjc.1963.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogwang MD, Bhatia K, Biggar RJ, Mbulaiteye SM. Incidence and geographic distribution of endemic Burkitt lymphoma in northern Uganda revisited. Int J Cancer. 2008;123(11):2658–2663. doi: 10.1002/ijc.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brauchli K, O’mahony D, Banach L, Oberholzer M. iPath - a Telemedicine Platform to Support Health Providers in Low Resource Settings. Stud Health Technol Inform. 2005;114:11–17. [PubMed] [Google Scholar]
- 6.Mbulaiteye SM, Parkin DM, Rabkin CS. Epidemiology of AIDS-related malignancies an international perspective. Hematol Oncol Clin North Am. 2003;17(3):673–696. doi: 10.1016/s0889-8588(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 7.Newton R, Ziegler J, Beral V, et al. A case-control study of human immunodeficiency virus infection and cancer in adults and children residing in Kampala, Uganda. Int J Cancer. 2001;92(5):622–627. doi: 10.1002/1097-0215(20010601)92:5<622::aid-ijc1256>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.Mutalima N, Molyneux E, Jaffe H, et al. Associations between Burkitt lymphoma among children in Malawi and infection with HIV, EBV and malaria: results from a case-control study. PLoS ONE. 2008;3(6):e2505. doi: 10.1371/journal.pone.0002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkin DM, Garcia-Giannoli H, Raphael M, et al. Non-Hodgkin lymphoma in Uganda: a case-control study. AIDS. 2000;14(18):2929–2936. doi: 10.1097/00002030-200012220-00015. [DOI] [PubMed] [Google Scholar]
- 10.Campidelli C, Gazzola A, Vitone F, Pileri SA, Tumwine L. HIV infection and c-MYC status in endemic Burkitt lymphoma. Hum Pathol. 2008;39(9):1408–1409. doi: 10.1016/j.humpath.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Mwanda WO, Orem J, Fu P, et al. Dose-Modified Oral Chemotherapy in the Treatment of AIDS-Related Non-Hodgkin’s Lymphoma in East Africa. J Clin Oncol. 2009;27(21):3480–3488. doi: 10.1200/JCO.2008.18.7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engels EA, Mbulaiteye SM, Othieno E, et al. Kaposi sarcoma-associated herpesvirus in non-Hodgkin lymphoma and reactive lymphadenopathy in Uganda. Hum Pathol. 2007;38(2):308–314. doi: 10.1016/j.humpath.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Tumwine LK, Campidelli C, Righi S, Neda S, Byarugaba W, Pileri SA. B-cell non-Hodgkin lymphomas in Uganda: an immunohistochemical appraisal on tissue microarray. Hum Pathol. 2008;39(6):817–823. doi: 10.1016/j.humpath.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 14.Nathwani BN, Sasu SJ, Ahsanuddin AN, Hernandez AM, Drachenberg MR. The critical role of histology in an era of genomics and proteomics: a commentary and reflection. Adv Anat Pathol. 2007;14(6):375–400. doi: 10.1097/PAP.0b013e318159479d. [DOI] [PubMed] [Google Scholar]
- 15.Benediktsson H, Whitelaw J, Roy I. Pathology services in developing countries: a challenge. Arch Pathol Lab Med. 2007;131(11):1636–1639. doi: 10.5858/2007-131-1636-PSIDCA. [DOI] [PubMed] [Google Scholar]
- 16.Hutt MS, Burkitt D. Geographical distribution of cancer in East Africa: a new clinicopathological approach. Br Med J. 1965;2(5464):719–722. doi: 10.1136/bmj.2.5464.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies JN, Burkitt DP, Dodge OG. Salivary-Gland Tumors in Uganda. Cancer. 1964;17:1310–1322. doi: 10.1002/1097-0142(196410)17:10<1310::aid-cncr2820171014>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Davies JN. Epidemiology of cancer in Africa. Some aspects of the cancer situation in Uganda. Proc R Soc Med. 1963;56(7):532–535. [PMC free article] [PubMed] [Google Scholar]
- 19.Prento P, Lyon H. Commercial formalin substitutes for histopathology. Biotech Histochem. 1997;72(5):273–282. doi: 10.3109/10520299709082252. [DOI] [PubMed] [Google Scholar]
- 20.Whitworth JA, Kokwaro G, Kinyanjui S, et al. Strengthening capacity for health research in Africa. Lancet. 2008;372(9649):1590–1593. doi: 10.1016/S0140-6736(08)61660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabiani M, Accorsi S, Aleni R, et al. Estimating HIV prevalence and the impact of HIV/AIDS on a Ugandan hospital by combining serosurvey data and hospital discharge records. J Acquir Immune Defic Syndr. 2003;34(11):62–66. doi: 10.1097/00126334-200309010-00009. [DOI] [PubMed] [Google Scholar]
- 22.Orem J, Otieno MW, Banura C, et al. Capacity building for the clinical investigation of AIDS malignancy in East Africa. Cancer Detect Prev. 2005;29(2):133–145. doi: 10.1016/j.cdp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Nsubuga P, White M, Fontaine R, Simone P. Training programmes for field epidemiology. Lancet. 2008;371(9613):630–631. doi: 10.1016/S0140-6736(08)60281-0. [DOI] [PubMed] [Google Scholar]


