Abstract
Objective
To report the long-term outcomes of 1218 organs transplanted from donation after cardiac death (DCD) donors from January 1980 through December 2008.
Methods
One-thousand two-hundred-eighteen organs were transplanted into 1137 recipients from 577 DCD donors. This includes 1038 kidneys (RTX), 87 livers (LTX), 72 pancreas (PTX), and 21 DCD lungs. The outcomes were compared with 3470 RTX, 1157 LTX, 903 PTX, and 409 lung transplants from donors after brain death (DBD).
Results
Both patient and graft survival is comparable between DBD and DCD transplant recipients for kidney, pancreas, and lung after 1, 3, and 10 years. Our findings reveal a significant difference for patient and graft survival of DCD livers at each of these time points. In contrast to the overall kidney transplant experience, the most recent 16-year period (n = 396 DCD and 1,937 DBD) revealed no difference in patient and graft survival, rejection rates, or surgical complications but delayed graft function was higher (44.7% vs 22.0%; P < .001). In DCD LTX, biliary complications (51% vs 33.4%; P < .01) and retransplantation for ischemic cholangiopathy (13.9% vs 0.2%; P < .01) were increased. PTX recipients had no difference in surgical complications, rejection, and hemoglobin A1c levels. Surgical complications were equivalent between DCD and DBD lung recipients.
Conclusion
This series represents the largest single center experience with more than 1000 DCD transplants and given the critical demand for organs, demonstrates successful kidney, pancreas, liver, and lung allografts from DCD donors. (Surgery 2011;150:692-702.)
Collaborative efforts in the transplant community as well as mandates from governmental agencies to increase organ donation are attempting to find solutions for the disparity between the availability of organs for transplant and the need. Centers for Medicare and Medicaid Services, Joint Commission on Accreditation of Healthcare Organizations (JCAHO), and United Network for Organ Sharing (UNOS) encouraged increasing donation after cardiac death (DCD).1 Despite these initiatives, the number of organs obtainable for transplant has declined in recent years.2
Over a 5-year period (2003–2008), the number of DCD donors has tripled nationwide; moreover, a growing portion of the total donors is from donation after cardiac death. The rate of DCD donors, which was 4% in 2003 increased to 11% by 2008.3 The rates of DCD donation at the University of Wisconsin organ procurement organization have been consistent over this period with DCD donors comprising 17–30% of total organ donors. In addition, recently available data demonstrates that organ recovery from DCD donors increased 24% between 2006 and 2007, compared with a decline of 2% for standard criteria donors (SCD).4
The increase in DCD donors has contributed to a utilization of additional younger donors, augmented total donors in the United States over the past decade, and although still not meeting the need of the numbers on transplant waiting lists, may have contributed to a stabilization of waitlist deaths.5 The purpose of this large single center study of the long-term outcomes of donation after cardiac death organs is meant to add to the data supporting DCD donation as a means to enhance the donor pool as well as reveal clinical challenges created by DCD allografts.
MATERIALS AND METHODS
Data collection
The University of Wisconsin Institutional Review Board approved this study. From January 1, 1980, through December 31, 2008, 1218 organs were transplanted into 1137 recipients from 577 DCD donors. This includes 1038 kidneys (RTX), 87 livers (LTX), 72 pancreas (PTX), and 21 DCD lungs. The outcomes were compared with 3470 RTX, 1157 LTX, 903 PTX, and 409 lung transplants from donors after brain death (DBD). While criteria for machine perfusion of DCD and DBD kidneys was the same, 97.1% of DCD and 89.2% of DBD kidneys were machine perfused (P < .01). This was due to a higher percentage of DBD kidneys with less than 6 hours of anticipated preservation time being cold stored. Solitary kidney transplants were divided into 2 periods, Era 1 from 1980–1992 (N = 1306) and Era 2 from 1993–2008 (N = 2333).
Donor selection
Our institution has a long history of using kidneys from controlled DCD donors.6 In 1993, the program expanded to transplantation of extrarenal organs, such as the liver, simultaneous pancreas-kidney, isolated pancreas, and lung.7 The age for donors for DCD livers ranged from 3 to 64 years and for DCD pancreata from 3 to 60 years. Selection for potential donors for transplantation is not any different between DCD and DBD and has been published elsewhere.8 Donor characteristics for DCD lungs have been published9 and include a mean donor age of 30.4 ± 14.6 years, a predominance of male donors (66.7%), and a median length of 4 days of mechanical ventilation before withdrawal of support.
Recipient selection
Recipient selection was the same for DBD and DCD organs with the exception of liver transplantation where patients with previous liver transplants or multiple abdominal surgeries were avoided if possible in order to limit cold ischemia times.
Organ procurement
Our techniques for DCD procurement have been established in many publications.6-11 Briefly, withdrawal of life support occurred in the operating room for extra-renal DCD donors in nearly all cases. Preapproval for femoral arterial and venous isolation or cannulation was included in the consent for DCD donation. Heparin, 10,000–30,000 units as well as 10–20 mg of phentolamine were given while the patient was fully supported to prevent hepatic and renal arterial vasospasm. The patient’s physician of record withdrew life support and determined cessation of cardiopulmonary function. It was at the discretion of this physician whether declaration of death required electrocardiographic silence or 5 minutes after the onset of pulseless electrical activity (PEA). The majority of physicians utilized PEA and a 5-minute observation period as recommended by the Institute of Medicine and a National Conference on DCD Donation.12,13 Variable periods of hypotension and hypoxia occur prior to the declaration of death.
After the declaration of death, 1.5–3.0 L of cold University of Wisconsin (UW) solution was infused into the femoral cannula while a median sternotomy and midline abdominal incision was made. The circulation of cold flush was vented at the right atrium after incision or at the femoral venous cannula prior to incision. For retrieval of DCD lungs, the donor was reintubated and reventilated by means of hand bagging after suctioning. Four liters of preservation solution is infused in situ through the main pulmonary artery, and 2 L of retrograde flush is infused through the pulmonary veins. Two donors were perfused with Perfadex (Vitrolife, Göteborg, Sweden) as the preservation solution. The intra-abdominal organs were removed en bloc and placed in UW solution at 48C for storage. The back table separation of these organs required an additional 1–1.5 hours dissection and included further cold flush through the portal vein as well as orifices of the celiac, superior mesenteric, and renal arteries. The gallbladder and common bile duct were irrigated with UW solution at the back table prior to cold storage for travel.
The current acceptance criteria of warm ischemia time (WIT) for DCD lung retrieval was up to 60 minutes which resulted in a mean WIT of 30 ± 17 minutes with an outlier of 93 minutes. The WIT for DCD donor livers ranged from 4 to 48 minutes with a mean of 20.8 ± 9.4 minutes. The mean is similar for DCD pancreata recovered at a WIT of 20.8 ± 10.9 minutes with the maximum at 64 minutes. Between 1980–92 the average warm time for DCD kidneys was 18.8 minutes in comparison with 27.5 minutes in the 1993–2008 era. The maximum allowable time from extubation to cold perfusion on DCD donors was 2 hours with each organ having different WIT thresholds. A recent analysis has shown that 90% of DCD donors that expire do so in less than 1 hour. Those that expire in greater than 1 hour have a mean WIT of 87 minutes (range, 61–130).
Our experience with DCD donors reveals that 80% will progress to donation after support is withdrawn within a 2-hour period. Arrangements are always made for the continued care of the patient prior to withdrawal of support should they not progress in the 2 hour time frame. Families are usually disappointed that donation could not occur, but are appreciative of efforts to honor their loved one’s wishes to become a donor.
Immunosuppression
There is no adjustment of immunosuppression protocols based on DCD versus DBD. The initial immunosuppression protocol at our center consisted of quadruple sequential treatment with azathioprine (AZA), prednisone, cyclosporine A (CSA/Sandimmune, Novartis, East Hanover, NJ), and antibody induction [1993–1996: murine antihuman CD3 monoclonal antibody OKT3 (Muromonab, Ortho, Raritan, NJ); 1996– 1997: antithymocyte globulin (ATGAM, Upjohn, Kalamazoo, MI); 1998–present, anti–IL-2 receptor monoclonal antibody basiliximab (Simulect, Novartis, East Hanover, NJ)]. Prednisone 2 mg/kg/day was given on day 0 and tapered over several months. AZA 2.5 mg/kg/day was given on day 0 if white blood cell count exceeds 3000/mm3. CSA 10 mg/kg/day was given when creatinine fell below 3 and adjusted to maintain a level of 200–300 ng/mL by high-pressure liquid chromatography. In 1995, tacrolimus (Prograf, Astellas US, Deerfield, IL) replaced CSA and was given orally 2 mg twice daily to maintain levels of 8–20 ng/mL. In 1995, mycophenolate mofetil (MMF; CellCept, Roche, Nutley, NJ) replaced AZA and was given orally 1.5–3 g/day with the dose lowered if recipients experienced significant gastrointestinal symptoms or leukopenia. From November 2003 to December 2006, alemtuzumab (Campath-1H; Genzyme, Cambridge, MA) was used for induction, replacing IL-2 receptor blockade using an initial dose of 30 mg intraoperatively followed by a 30-mg dose on postoperative day 1.
The current regimen includes induction with basiliximab 20 mg IV on days 0 and 3 for 2 doses for the kidney and kidney-pancreas transplants with maintenance doses of MMF, prednisone, and tacrolimus. Highly sensitized patients undergo induction with rabbit anti-thymocyte globulin (Thymoglobulin, Genzyme, Cambridge, MA) with possible additional treatments of postoperative plasma exchange and intravenous immunoglobulin. The immunosuppressive protocol for liver transplants consists primarily of tacrolimus, mycophenolate mofetil, and prednisone. The current immunosuppression regimen for lung transplants include basiliximab for induction therapy and tacrolimus, mycophenolate mofetil, and prednisone as maintenance therapy.
Definitions
Controlled donation after cardiac death is defined as donors with irreversible catastrophic brain injury or end-stage neuromuscular disease followed by planned withdrawal of life-sustaining treatment. Delayed graft function (DGF) was defined as need for hemodialysis within the first week post-transplantation. All kidney transplant rejection episodes were biopsy-proven. Biopsies were performed in the setting of allograft dysfunction (increase in serum creatinine +0.2 mg/dL on 2 successive measurements). For DCD procurements, WIT was measured from withdrawal of ventilator support to initiation of cold perfusion via femoral cannulas on the aorta, and not at the time of cross clamping. Total kidney preservation time was measured from the time of aortic cross-clamping to reperfusion of the kidney in the recipient and includes both cold storage and machine pulsatile perfusion time. Pancreas cold storage time was measured from cross-clamping the donor aorta to the time of reperfusion of the pancreas in the recipient. Post-transplant diabetes mellitus (PTDM) was diagnosed based on criteria outlined by the International PTDM Consensus Guideline.14,15 Patients with a fasting plasma glucose level ≥126 mg/dl, confirmed on a subsequent day or requiring prolonged (30-day) treatment with insulin or oral hypoglycemic agents, were identified as having PTDM---a minor modification from FDA guidelines established for diagnosis. Patients with 2 consecutive hemoglobin A1c (HbA1c) levels >6%, as recommended by the ADA, were considered to have PTDM. Biliary complications consisted of ischemic cholangiopathy (defined as non-anastomotic biliary strictures with a patent hepatic artery,) common bile duct (CBD) leak, CBD anastomotic stricture, the presence of bile duct stones, casts, or sludge, or abscess or biloma formation.16
Statistical analysis
All statistical analyses were performed using SAS statistical software version 9.1, SAS Institute Inc. (Cary, NC). Continuous variables are summarized as mean and standard deviations (mean ± SD). Percentages are used to summarize categorical variables. Survival estimates are based on the methods of Kaplan and Meier and compared between groups using a log-rank test. P values < .05 were considered significant.
RESULTS
Between 1980 and 2008, we performed transplants using 1218 allografts from DCD donors. In the same time period we transplanted 4786 organs from DBD donors. This served as our comparison group.
Kidney
In our retrospective review, 965 DCD and 2674 DBD kidney allografts were identified for the 1980–2008 period. The 2 eras were marked by the introduction of extrarenal DCD transplants in 1993; Tables I–III.
Table I.
Eras
| Era | DBD | DCD |
|---|---|---|
| 1980–1992 | 737 | 569 |
| 1993–2008 | 1937 | 396 |
| Totals | 2674 | 965 |
DBD, Donation after brain death; DCD, donation after cardiac death.
Table III.
Gender
| Gender | DBD | DCD |
|---|---|---|
| Male | 1606 | 587 |
| Female | 1068 | 378 |
Patient and graft survival
Patient survival between all DBD and DCD kidney transplants done between 1980 and 2008 reach a significant difference. At 1, 3, and 10 years the patient survival among the DBD and DCD groups are: 95.2%, 88.4%, 60.7%, and 92.3%, 84.6%, 59.7%, respectively (P = .02, Fig 1). Kidney allograft survival at the 1-, 3-, and 10-year time points is 87.6%, 76.9%, and 43.6% for DBD, and 78.2%, 68.5%, 41.6% (P = .0004, Fig 2).
Fig 1.

Kaplan Meier curve of patient survival in renal transplant recipients by donor type (DBD vs DCD). At 1, 3, and l0 years, graft survival among DBD and DCD groups are: 87.6%, 43.6%, 76.8% and 78.1%, 41.6%, 68.1%, respectively.
Fig 2.
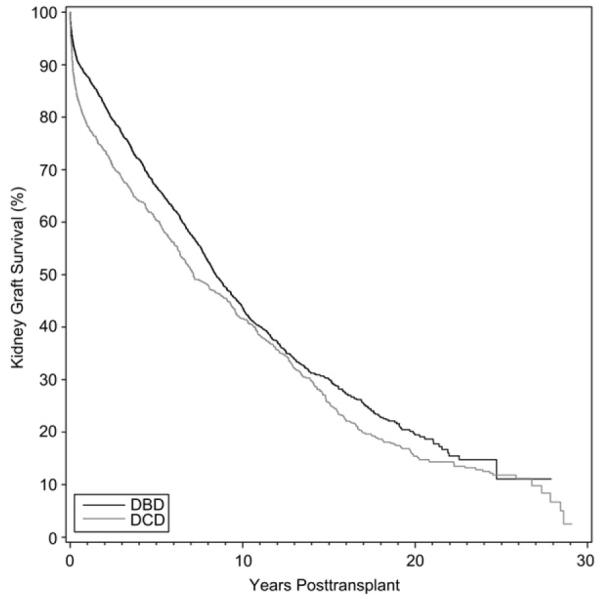
Kidney graft survival among recipients from DBD and DCD donors.
Further division by era of patient and graft survival are shown in Tables IV and V.
Table IV.
Patient survival between eras for DBD and DCD kidneys
| Patient survival (%) |
DBD |
DCD |
||||
|---|---|---|---|---|---|---|
| 1 year | 3 year | 10 year | 1 year | 3 year | 10 year | |
| 1980–1992* | 95 | 88.8 | 56.8 | 91.8 | 83.9 | 60.1 |
| 1993–2008† | 95.3 | 88.4 | 63.1 | 93.3 | 86.0 | 58.6 |
P = .58.
P = .06.
Table V.
Graft survival between eras for DBD and DCD kidneys
| Graft survival (%) |
DBD |
DCD |
||||
|---|---|---|---|---|---|---|
| 1 year | 3 year | 10 year | 1 year | 3 year | 10 year | |
| 1980–1992* | 83.1 | 73.9 | 39.8 | 72.4 | 62.7 | 38.1 |
| 1993–2008† | 89.4 | 77.9 | 45.8 | 86.6 | 77.0 | 47.3 |
P = .04.
P = 0.47.
For the second era, from 1993–2008 there was no difference in patient or graft survival when recipients of DCD renal transplants were compared with DBD transplant recipients.
Machine perfusion
Overall, about 97.1% of DCD kidneys were machine perfused as opposed to 89.23% in the DBD group. Further divided by eras, between 1980–92, 96.7% of DBD donor kidneys were perfused in comparison with 99.1% in the DCD group. In the more recent era (1993–2008), the rate of machine perfusion was a bit lower---86.3% in the DBD group and 94.1% in the DCD group.
Complications (1994–2008)
The overall DGF rate among DCD kidneys was higher and our data showed the DGF rate to be 35.7% in the DCD group vs 20.3% in the DBD group (P ≤ .0001). When comparing between eras, the DGF rate in fact worsened in both the DCD and DBD groups in the more recent time period. The DGF rate in DCD donor kidney in Era 1 and Era 2 were 29.53% and 44.7%, respectively (P ≤ .0001). In comparison, the rate of DGF in the DBD group between the eras was 15.74% (Era 1) and 22.04% (Era 2). This likely reflects utilization of older donors and donors with additional comorbidities. For the entire study period, the rate of rejection was significantly lower for recipients of DCD kidney transplants. However, when broken down by era, the rate of decreased rejection in DCD kidney recipients approaches but does not reach significance (P = .07).
Free of acute cellular rejection (overall)
Surgical complications remained comparable between the DCD and DBD kidneys. At 1 year, 94.2% of patients who received DBD kidneys were free of lymphoceles compared with 92% for the DCD group (P = .23); Table VI.
Table VI.
Free of acute cellular rejection (overall)
| 1 year | 3 year | 10 year | |
|---|---|---|---|
| DBD | 61.7 % | 57.1% | 51.3% |
| DCD | 48.3% | 44.7% | 39.1% |
P ≤ .0001.
At 12 months, 95.3% of recipients in the DBD group were free of renal artery stenosis in comparison with 96.4% in the DCD group (P = .81). The renal artery thrombosis rate was similarly low. At 6 months, 98.5% in the DBD group and 99.1% in the DCD group were free of renal artery thrombosis (P = .28). Specifically looking at the transplanted ureter, the results were encouraging and the complication rate was low. In the DBD group at 1 year, 96.9% of recipients and 95.4% at 5 years were free of ureteral strictures. In the DCD group, at 1.5 years 97.3% of recipients and 95.9% at 5 years were free of ureteral strictures; Table VII.
Table VII.
Free of acute cellular rejection between eras for DBD and DCD kidneys
| DBD |
DCD |
|||||
|---|---|---|---|---|---|---|
| Free of ACR (%) | 1year | 3 year | 10 year | 1 year | 3 year | 10 year |
| 1980–1992* | 49.6 | 45.7 | 40.7 | 33.4 | 30.0 | 25.8 |
| 1993–2008† | 66.3 | 61.4 | 55.4 | 69.8 | 66.5 | 58.3 |
P ≤ .0001.
P = .07.
Liver
In the time period between 1993 and 2008, 1157 livers were transplanted from brain dead donors, 87 livers were used from DCD donors. Demographics: Tables VIII and IX.
Table VIII.
Demographics for liver recipients
| Gender | DBD | DCD |
|---|---|---|
| Male | 703 | 54 |
| Female | 454 | 33 |
Table IX.
Donor liver characteristics
| Donor type | DBD* | DCD* |
|---|---|---|
| Age at transplant (year) | 47.5 ± 16.1 | 50.5 ± 13.08 |
| Donor age (year) | 36.4 ± 17.9 | 35.7 ± 13.3 |
| Cold time (hours) | 8.31 ± 2.55 | 7.21 ± 2.34 |
Mean ± SD.
For the DCD livers, the average warm ischemia time during procurement was noted to be 20.8 minutes (SD ± 9.37) with a range of 4–48 minutes.
A significant difference was seen in patient survival between the DBD and DCD groups. (P < .01, Fig 3, Table X).
Fig 3.

Patient survival liver allograft survival in recipients by donor type (DBD vs DCD).
Table X.
Patient survival in DBD and DCD liver transplants
| Patient survival | 1 year | 3 year | 10 year |
|---|---|---|---|
| DBD | 90.9 | 85.2 | 66.5 |
| DCD | 84 | 71.8 | 54.4 |
P = .0003.
There is also a statistically significant difference in graft survival between DBD and DCD livers (P < .01); Table XI. The Kaplan-Meier graph for graft survival in DCD liver transplant recipients is shown in Fig 4.
Table XI.
Graft survival in DBD and DCD liver transplants
| Graft survival (%) | 1 year | 3 year | 10 year |
|---|---|---|---|
| DBD | 86.2 | 79.7 | 60.2 |
| DCD | 69.4 | 59.6 | 42.9 |
P ≤ .0001.
Fig 4.

Liver graft survival in recipients by donor type (DBD vs DCD).
Recipients of livers from DCD donors had a higher biliary complication rate over DBD donors. At 1 year, DCD donors had a biliary complication rate of 51.1% vs 33.4% (P < .0001). The overall retransplant rate for ischemic cholangiopathy in all patients at our institution is at 1%. But, when we divide it by donor type, recipients with DCD donor livers do worse. The retransplantation rate for the DCD group is 13.9% vs 0.2% for the DBD livers (P < .01).
Pancreas
Between 1993 and 2008, we transplanted a total of 975 pancreata: 903 were from brain dead donors and 72 from DCD donors. Among these, 812 were simultaneous pancreas-kidney transplants and 163 were isolated pancreas transplants. The DCD group included 68 pancreata transplanted as SPKs and 4 as isolated pancreas transplants (Fig 5).
Fig 5.
Patient survival among SPK and PAN by donor type (DBD vs DCD).
Demographics
Pancreas graft survival for all patients were noted to be 87.1% at 1 year, 80.7% at 3 years, and 60.7% at 10 years, Table XII. When further divided by donor type (DCD vs DBD), it was comparable. At 1, 3, and 10 years, graft survival between the DBD and DCD groups were 87.4%, 80.9%, and 60.7% vs 83.1%, 77.8%, and 62.6% respectively (P = .99, Fig 6). Rate of acute cellular pancreas rejection was 27% at 10 years in the DBD group, compared with 20% for the DCD cohort (P = .63).
Table XII.
Donor pancreas characteristics
| Donor type | DBD* | DCD* |
|---|---|---|
| Age at transplant (year) | 39.2 ± 7.12 | 39.4 ± 7.01 |
| Peak PRA** (%) | 4.0 ± 10.5 | 3.6 ± 11.5 |
| Donor age (years) | 30.3 ± 13.4 | 31.9 ± 12.2 |
| Cold storage (hours) | 15.5 ± 4.84 | 14.2 ± 5.65 |
| Warm time (minutes) | N/A | 20.7 ± 10.94 |
Mean ± SD.
Panel reactive antibody
Fig 6.

Pancreas graft survival in recipients by donor type (DBD vs DCD).
Patient survival was also comparable between the 2 groups. For the DBD group, at 1, 3, and 10 years patient survival was noted to be 97.2%, 94.2%, and 78.8%, respectively. Patient survival was similar in the DCD group: 94%, 92.6%, 87% at 1, 3, and 10 years, respectively (P = .84).
Fasting blood glucose and HbA1c levels were nearly identical between the 2 groups in patients with functioning transplant pancreata. Incidence of PTDM was also comparable between the 2 groups and was calculated around 9% for both groups, including those with technical failure or graft failure. The mean HbA1C in pancreas recipients was 5.43 ± 0.75% with DBD allografts and 5.63 ± 0.57% at 1 year posttransplant. Patients from both the donor groups had equal chance at maintaining good glycemic control. Also, at the end of 1 year, 92.5% of patients who received DBD pancreata remained free of hypoglycemic agents. This was in comparison with 93.3% of the patients in the DCD group.
Vessel thrombosis rates were also low and comparable between both groups. Overall rate for thrombosis at 1 year was noted to be 6%. At the end of 1 year, 94.4% from the DBD group were free of thrombosis. This was in comparison with 89.2% in the DCD group (P = .10).
Pancreas-related surgical complications were studied in the DBD and DCD groups. Rates of pancreatic enzyme leak, transplant pancreatitis, pseudocyst formation, abdominal abscess formation, and pancreatic necrosis are minimal in both groups, with a similar number of occurrences in each cohort.
Lungs
Between 1993 and 2008, a total of 409 lungs were transplanted from DBD donors and 21 from DCD donors. Graft survival was noted to be lower but without reaching significance for the DCD group. At 1 year and 3 years, graft survival for the DBD and DCD groups were 81.8% and 68.3%, respectively, vs 69.2% and 60.6%, respectively (P = .59, Fig 7). Overall patient survival was more comparable. At the end of 1 and 3 years, respectively, 85.4% and 72.4% of patients were alive from the DBD group in comparison with 80% and 68.5%, respectively, from the DCD group (P = .87). Airway complications requiring intervention was 27.8% (n = 5) for DCD recipients versus 12.8% (n = 28) in those who received DBD organs (P = .08). Primary dysfunction was not significantly different between the 2 groups (P = .59). The rate of freedom from Bronchiolitis obliterans syndrome in DBD at 1 and 3 years was, respectively, 93.7% and 75.2% compared with 80.4% and 80.4%, respectively, for the DCD group (P = .59).
Fig 7.
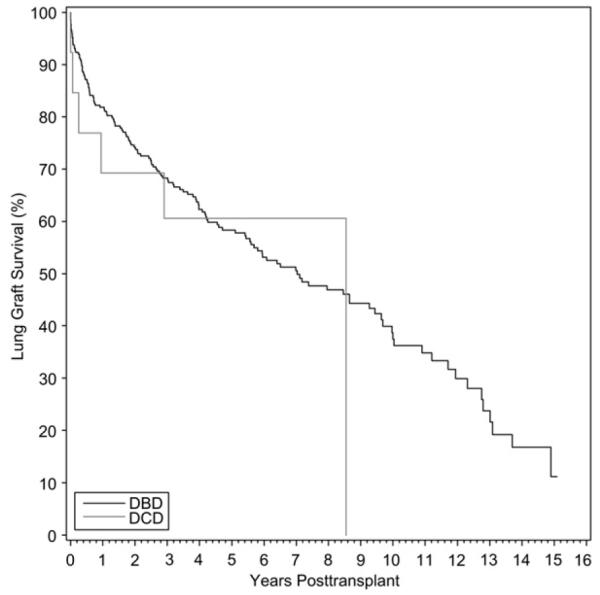
Lung graft survival in recipients by donor type (DBD vs DCD).
DISCUSSION
Our large cohort of 1137 recipients of transplants recovered from controlled DCD donors provides 10-year follow-up data to further confirm the value of donation after cardiac death in alle-viating the organ shortage crisis. The long-term patient and graft survival data in kidney, pancreas, and lung transplants from DCD donors promotes the idea that non-heart beating donors are on par with SCD transplants in keeping patients off transplant waiting lists with functioning grafts. The success of extrarenal allografts from DCD donors has encouraged investigation into the possibility of even DCD heart transplants.17
Overall, we did see a trend reaching significance in both patient and graft survival between DBD and DCD kidney recipients from 1980 to 2008, with DCD allografts fairing worse. This finding was not maintained when the kidney groups were separated into Era 1 and 2. Not only was the overall kidney graft survival significantly improved in Era 2, but there was no longer a difference in DBD and DCD kidney allograft outcomes. One may hypothesize that as the transplant community has found better ways to monitor and treat kidney transplant patients, in doing so has also erased the different outcomes of DBD and DCD allografts.
Our study also reveals an undeniable difference in outcomes of transplanted livers from DCD donors compared with the SCD counterparts. In patient and graft survival, as well as rates for biliary complications and retransplants for ischemic cholangiopathy, there is a definite disadvantage in allografts from DCD donors. However, this needs to be viewed from the perspective of the consequences of not receiving a liver transplant. There are certainly single center studies that have findings more encouraging for DCD liver allografts,18,19 but it is more commonly reported as clinical reality that DCD liver transplants have poorer outcomes than DBD allografts.2,20-23
More recent discussion about DCD liver transplants have included whether there is a particular subset population of patients with cirrhosis that may uniquely benefit from DCD allografts. Patients with hepatocellular carcinoma outside of Milan criteria or patients with MELD >30 are groupings that have been proposed.22,23 There appears to be emerging data that the benefits of DCD liver transplant occur in patients with MELD scores greater than 18 or 20. Identifying donor characteristics from DCD that contribute to inferior outcomes may further clarify how best to utilize DCD liver transplantation.
One may argue that since there is an underutilization of DBD pancreata for transplant nationwide, the promotion of DCD pancreata as allografts is unwarranted. As our institutional experience also demonstrates a survival benefit for SPK recipients compared with uremic type 1 diabetic patients undergoing living donor kidney transplantation (differences only appreciated beyond 10 years post-transplant), we could potentially extrapolate that SPK recipients from DCD donors would have the same survival advantage when compared with LDK or DDK. A larger data-base and longer follow-up are needed to prove this theory.
Limitations
Warm ischemia time has been defined as the duration of cardiopulmonary cessation to in situ cold perfusion in multiple publications.2,3,8,9,11,24,25 But documenting the duration of systolic blood pressure below 50 mmHg after extubation as well as oxygen saturations in the DCD donors may add insight into better criteria for selection of donors. It may change the viability of an organ considerably if electrocardiographic silence is a requirement of the physician administering end-of-life care at DCD procurement to declare death.
In conclusion, this series represents a nearly 30-year experience with more than 1000 DCD transplants. The critical demand for organs requires a commitment to expand the donor pool and this experience demonstrates successful kidney, pancreas, liver, and lung allografts from DCD donors.
DISCUSSION
Dr Darrell Campbell (Ann Arbor, MI)
I remember when this really first started off, we thought, “Well, okay, we can do this maybe in the perfect donor.” We tried kidneys, and that seemed to work okay. And then some brave soul, I think it was probably Tony, said “We can try this in liver transplants.” And that seemed to offer something. And then pancreas, and now lungs.
So it has been a very rapid evolution, but it is not without a tremendous amount of work. But I think you should be very proud of this effort because it has saved a lot of lives, and that is what we are all trying to do.
So with that evolution, there are some questions I have. And I tried to pick a few questions that I thought would be of interest to the nontransplanters in the group, also.
That trigger time when most of the patients that you have were taken to the operating room intubated, and then you extubate the patient and expect them to die. But sometimes they do not die when you are there, their heart continues to beat, and much longer than we thought it would, and there are some agonal respirations or something.
So my question is, What do you do in that circumstance? What percentage of the time do you have to say, “Well, this just is not going to work. Take them back to the floor.” That, in itself, is a terrible experience for the family, obviously. We do not want that to happen. But what are the criteria that you would use to say either too much time has passed or this is just too much ischemia, we cannot tolerate that, we are not going to do it.
Then the second question has something to do with the liver transplants. You said that the overall results are not as good for liver transplantation. And for the patient who is dying on the list, that is not an issue, because anything is better than nothing. So it is not a question of whether they would take it or not. But for the patient who is stable on the list, my question is, when that call comes at 2:00 in the morning, what is the informed consent that you do or do not engage in with that patient to say, “Well, we have a liver. You are not dying right at the moment, but this liver is not going to work as well as from a brain-dead donor.” I think that is an important question. I would be interested to know how you handle that.
And then the question is, because we do know that the results are not quite as good in the liver transplant, do you try to steer those donors to lower Model for End-Stage Liver Disease (MELD) patients, for instance. Again, for the nontransplanters, that would be imply a healthier patient who is in better shape. Is that a strategy that we should embark upon?
And I have 1 more because I know it is hard to remember all these questions. The final question is, I know you can either cold store these kidneys when we take them out or we can pump them. And the University of Wisconsin has traditionally pumped the kidneys, put them on a pump, and pumped preservation solution through them. When you do that, you get data about flow. And my question then is, when you pump your kidneys from a donation after cardiac death (DCD) donor, are there flow characteristics that would say to you, well, this just is not going to work, we’ll discard these kidneys?
Dr Anthony D’Alessandro (Madison, WI)
We did develop a predictive tool. Although it is not perfect, it is a scoring system that is basically a respiratory assessment drive that we previously published. And based on this scoring tool, it is 80% predictive that a patient will, within 2 hours, expire. And we have also looked at the ones that we decided not to proceed on. And we miss about 20% of the donors, if you will, if we do not go on those.
And so every time we do a DCD donor, there is a discussion with the family, the physicians, the nursing staff, as to what the disposition of the patient will be afterward, because that patient does need to be cared for until they do expire, which is usually several hours later at most.
What we do find, which is somewhat different, I think, than what you referred to, is that families are quite appreciative. In fact, when the patient does not expire within that time frame, they are somewhat disappointed that there was not something that could come out of this tragedy that they could donate. So they do appreciate the opportunity and the effort that was made to see if their loved one could donate.
There have been a couple of other tools that are validated. There are some United Network for Organ Sharing (UNOS) criteria that look at not only respiratory drive but a number of other factors. But still, none of them are 100% predictive. So there are situations, as you mentioned, that, when we do a DCD donor, that patient will not expire within that time frame.
And we have a time frame that we pick that, beyond that, there is too much hypotension, hypoxia to make those donor organs usable.
As regards your question about informed consent, although informed consent is not required by any organization or UNOS for the utilization of DCD organs, because our data show a difference in outcomes, we have instituted, I believe for the last 3 years, an informed consent regarding biliary complications, and all the complications related to transplantation for DCD organs, that is discussed at the time of evaluation, and not at the time when we call a patient in the middle of the night.
And we find that is a very useful tool to discuss these types of livers. And what you mentioned is true, the results are not as good, but we are trying to determine not getting a liver transplant. What is the cost of that? And will a liver come in time before this patient expires on the waiting list?
And that gets to the next question regarding the MELD score and using them in lower MELD scores. There are emerging data, and our data as well, that suggest that you should not use these organs in patients with low MELD scores, that they may have time to wait for a higher quality liver to come along. And in fact, in our institution, we do not use the DCD livers now for patients unless their MELD is >18.
We always are stuck trying to put an organ, say, that is of lesser quality into a sicker patient, which has a much higher MELD score because we know that the outcome of the sicker patients is not as good, and the outcome of a marginal organ is not as good. So you combine them, you may not get a great result, but what you are doing is comparing that with whether that patient survives or not. I think that is important. So we do not use those in lower MELD scores.
As regards cold storage versus machine perfusion, as you know, we have been perfusing forever. I do not think we have ever stopped perfusing kidney transplants. And we do look at the flow characteristics. And interestingly enough, we discard very few of the organs that go on the pump. And usually we look at a resistance >0.4. Definitely >0.5, we would discard those. It also depends on how the kidney looks on the pump, whether it still looks modeled or not, well-perfused in those first couple of hours after we have put it on.
But by and large, I think the way we select our potential donors and the organs we recover, we have a low discard rate off the pump.
Dr Scott Gruber (Detroit, MI)
The vast majority of both the DCD and the DBD kidneys were machine perfused, although there significantly were less than the DBD group. What were your criteria for machine perfusion. Or, stated differently, which kidneys were not perfused? And do you think this difference positively affected the outcomes of the DCD group?
Second, did you expand the maximum warm ischemic time that was permissible for procurement of the DCD organs over the course of the study?
Third, was donor age significantly lower in the DCD group, both overall and in both of the 2 eras? If so, do you see this as a major contributor to outcomes?
Can much of the success of DCD simply be due to use of organs from younger donors with less other negative factors, such as comorbidities and pressers and so on?
Fourth, given the negative outcomes from your group, as well as the Northwestern group and others, regarding the use of DCD livers, what do you think is the future for transplantation of these organs? With these small numbers that you have, I think it will be difficult to determine how best to select out the appropriate donor and recipient combination for these high-risk transplants.
And then, fifth, was there a comparable proportion of SPK versus solitary pancreas transplants in the 2 groups? I think this could have had influenced your outcomes.
Finally, even though the difference did not reach significance, perhaps because of small numbers, the thrombosis rate of 11% is somewhat high in the DCD pancreas group, particularly for your program. Do you have any explanation for this, particularly in the absence of a difference in the incidence of transplant pancreatitis and acute rejection that might have contributed to the thrombosis?
Dr Anthony D’Alessandro
With regard to your first question on machine perfusion, the default is that all kidneys get put on the machine. And it is only because either the operating room is ready when we return, or there is vascular reconstruction that needs to be done, that we do not perfuse the kidneys. So there is no real intent to either perfuse or not perfuse any of the kidneys, although there has been some discussion in some groups because of costs whether we should just cold store standard criteria donors versus machine perfuse the other, more marginal, ECD/DCD donors, even though the delayed graft function rate has always been shown to be less with your machine perfuse.
We are actually going the other way in terms of warm ischemic time. We are decreasing it, particularly in liver transplantation. We would prefer that warm ischemic time to be 20 minutes, no longer than 30 minutes, based on our current definition. We are looking at sats and blood pressures and when they fall below certain levels to see if that may be more predictive.
The vast majority of kidneys were recovered in <1 hour, so we are still continuing with 1 hour for kidneys. We go to 2 hours, but there have only been a handful of cases beyond 1 hour that we have used.
There was no difference in donor age statistically between the DCD or the DBD groups that we saw.
I have not looked in the earlier experience. There tended to be, in the earlier experience, probably younger donors, as I recall how we went for donors in the earlier years. But later on, it has become the opposite direction. We have actually gone for more older donors than younger donors because of the changing donor population.
With regards to the liver and the negative outcomes, we continue to modify and vary our protocols to see. And hopefully, we can perhaps look at some of the techniques that we are using, modify those. And I think this requires some study in the laboratory to determine whether there are microthrombi that are developing, whether there is some other mechanism that is resulting in this ischemia, or are there other ways to prevent this ischemia in the donor before reperfusion.
There were no isolated pancreases in this series. All the pancreases were simultaneous kidney–pancreas transplantation. And I agree with you. The thrombosis rate, although numerically higher in the DCD pancreas, it did not achieve significance, but it did raise our concern that perhaps there is a higher thrombosis rate, although it is about 6%, I believe, in the DBD donors, not statistically different. But it does concern us that there may be something there that we have not really flushed out yet.
Table II.
All DCD/DBD demographics for donor and recipient
| DCD (mean ± SD) |
DBD (mean ± SD) |
|
|---|---|---|
| Age at transplant (years) | 44.76 ± 13.2 | 47.6 ± 13.4 |
| Duration on waitlist (months) |
14.6 ± 10.7 | 14.9 ± 11.6 |
| Peak PRA (%) | 10.15 ± 21.0 | 14.7 ± 25.1 |
| Donor age (years) | ||
| Combined eras | 36.3 ± 15.9 | 37.2 ± 17.4 |
| Era 1 (1980–1992)* | 29.8 ± 14.2 | 28.7 ± 13.7 |
| Era 2 (1993–2008)* | 45.5 ± 13.7 | 40.7 ± 17.6 |
| Machine perfused | 97.1% | 89.23% |
P ≤ .001 between eras.
Acknowledgments
Supported by grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources (NCRR), National Institutes of Health (NIH) (D.P.F. and J.D.M.). Accepted for publication July 11, 2011.
REFERENCES
- 1.Skaro AI, Jay CL, Baker TB, Wang E, Pasricha S, Lyuksemburg V, et al. The impact of ischemic cholangiopathy in liver transplantation using donors after cardiac death: the untold story. Surgery. 2009;146:543–52. doi: 10.1016/j.surg.2009.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein AS, Messersmith EE, Ratner LE, Kochik R, Baliga PK, Ojo AO. Organ donation and utilization in the United States, 1999-2008. Am J Transplant. 2010;10:973–86. doi: 10.1111/j.1600-6143.2009.03008.x. [DOI] [PubMed] [Google Scholar]
- 3.Scientific Registry of Transplant Recipients data reports on July 15. 2009 Available at: http://www.srtr.org.
- 4.Tuttle-Newhall JE, Krishnan SM, Levy MF, McBride V, Orlowski JP, Sung RS. Organ donation and utilization in the United States: 1998-2007. Am J Transplant. 2009;9:879–93. doi: 10.1111/j.1600-6143.2009.02565.x. [DOI] [PubMed] [Google Scholar]
- 5.Pomfret EA, Sung RS, Allan J, Kinkhabwala M, Melancon JK, Roberts JP. Solving the organ shortage crisis: the 7th annual American Society of Transplant Surgeons’ State-of-the-Art Winter Symposium. Am J Transplant. 2008;8:745–52. doi: 10.1111/j.1600-6143.2007.02146.x. [DOI] [PubMed] [Google Scholar]
- 6.Cooper JT, Chin LT, Krieger NR, Fernandez LA, Foley DP, Becker YT, et al. Donation after cardiac death: the university of wisconsin experience with renal transplantation. Am J Transplant. 2004;4:1490–4. doi: 10.1111/j.1600-6143.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 7.D’Alessandro AM, Fernandez LA, Chin LT, Shames BD, Turgeon NA, Scott DL, et al. Donation after cardiac death: the University of Wisconsin experience. Ann Transplant. 2004;9:68–71. [PubMed] [Google Scholar]
- 8.Fernandez LA, Di Carlo A, Odorico JS, Leverson GE, Shames BD, Becker YT, et al. Simultaneous pancreas-kidney transplantation from donation after cardiac death: successful long-term outcomes. Ann Surg. 2005;242:716–23. doi: 10.1097/01.sla.0000186175.84788.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Oliveira NC, Osaki S, Maloney JD, Meyer KC, Kohmoto T, D’Alessandro AM, et al. Lung transplantation with donation after cardiac death donors: long-term follow-up in a single center. J Thorac Cardiovasc Surg. 2010;139:1306–15. doi: 10.1016/j.jtcvs.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 10.D’Alessandro AM, Hoffmann RM, Knechtle SJ, Eckhoff DE, Love RB, Kalayoglu M, et al. Successful extrarenal transplantation from non-heart-beating donors. Transplantation. 1995;59:977–82. doi: 10.1097/00007890-199504150-00009. [DOI] [PubMed] [Google Scholar]
- 11.Foley DP, Fernandez LA, Leverson G, Chin LT, Krieger N, Cooper JT, et al. Donation after cardiac death: the University of Wisconsin experience with liver transplantation. Ann Surg. 2005;242:724–31. doi: 10.1097/01.sla.0000186178.07110.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernat JL, D’Alessandro AM, Port FK, Bleck TP, Heard SO, Medina J, et al. Report of a National Conference on Donation after cardiac death. Am J Transplant. 2006;6:281–91. doi: 10.1111/j.1600-6143.2005.01194.x. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine . Non-heart-beating organ transplantation: Practice and protocols. National Academy Press; Washington, DC: 2000. p. 174. [PubMed] [Google Scholar]
- 14.Davidson J, Wilkinson A, Dantal J, Dotta F, Haller H, Hernandez D, et al. New-onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation. 2003;75(10 Suppl):SS3–S24. doi: 10.1097/01.TP.0000069952.49242.3E. [DOI] [PubMed] [Google Scholar]
- 15.Davidson JA, Wilkinson A. International Expert Panel on New-Onset Diabetes after Transplantation. New-Onset Diabetes After Transplantation 2003 International Consensus Guidelines: an endocrinologist’s view. Diabetes Care. 2004;27:805–12. doi: 10.2337/diacare.27.3.805. [DOI] [PubMed] [Google Scholar]
- 16.Foley D, Fernandez LA, Leverson G, Anderson M, Mezrich J, Sollinger HW, et al. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long term outcomes from a single center. Ann Surg. 2011;253:817–25. doi: 10.1097/SLA.0b013e3182104784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osaki S, Anderson JE, Johnson MR, Edwards NM, Kohmoto T. The potential of cardiac allografts from donors after cardiac death at the University of Wisconsin Organ Procurement Organization. Eur J Cardiothorac Surg. 2010;37:74–9. doi: 10.1016/j.ejcts.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Chan EY, Olson LC, Kisthard JA, Perkins JD, Bakthavatsalam R, Halldorson JB, et al. Ischemic cholangiopathy following liver transplantation from donation after cardiac death donors. Liver Transpl. 2008;14:604–10. doi: 10.1002/lt.21361. [DOI] [PubMed] [Google Scholar]
- 19.Fujita S, Mizuno S, Fujikawa T, Reed AI, Kim RD, Howard RJ, et al. Liver transplantation from donation after cardiac death: a single center experience. Transplantation. 2007;84:46–9. doi: 10.1097/01.tp.0000267424.88023.7b. [DOI] [PubMed] [Google Scholar]
- 20.Doshi MD, Hunsicker LG. Short- and long-term outcomes with the use of kidneys and livers donated after cardiac death. Am J Transplant. 2007;7:122–9. doi: 10.1111/j.1600-6143.2006.01587.x. [DOI] [PubMed] [Google Scholar]
- 21.Mateo R, Cho Y, Singh G, Stapfer M, Donovan J, Kahn J, et al. Risk factors for graft survival after liver transplantation from donation after cardiac death donors: an analysis of OPTN/UNOS data. Am J Transplant. 2006;6:791–6. doi: 10.1111/j.1600-6143.2006.01243.x. [DOI] [PubMed] [Google Scholar]
- 22.Merion RM, Pelletier SJ, Goodrich N, Englesbe MJ, Delmonico FL. Donation after cardiac death as a strategy to increase deceased donor liver availability. Ann Surg. 2006;244:555–62. doi: 10.1097/01.sla.0000239006.33633.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selck FW, Grossman EB, Ratner LE, Renz JF. Utilization, outcomes, and retransplantation of liver allografts from donation after cardiac death: implications for further expansion of the deceased-donor pool. Ann Surg. 2008;248:599–607. doi: 10.1097/SLA.0b013e31818a080e. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto K, Eghtesad B, Gunasekaran G, Fujiki M, Uso TD, Quintini C, et al. Use of tissue plasminogen activator in liver transplantation from donation after cardiac death donors. Am J Transplant. 2010;10:2665–72. doi: 10.1111/j.1600-6143.2010.03337.x. [DOI] [PubMed] [Google Scholar]
- 25.Mathur AK, Heimbach J, Steffick DE, Sonnenday CJ, Goodrich NP, Merion RM. Donation after cardiac death liver transplantation: predictors of outcome. Am J Transplant. 2010;10:2512–9. doi: 10.1111/j.1600-6143.2010.03293.x. [DOI] [PubMed] [Google Scholar]


