Abstract
Carbon monoxide (CO) is a physiological messenger with diverse functions in the kidney, including controlling afferent arteriole (Af-Art) tone both directly and via tubuloglomerular feedback (TGF). We have reported that CO attenuates TGF, but the mechanisms underlying this effect remain unknown. We hypothesized that CO, acting via cGMP, cGMP-dependent protein kinase (PKG), and cGMP-stimulated phosphodiesterase-2 (PDE2), reduces cAMP in the macula densa, leading to TGF attenuation. In vitro, microdissected rabbit Af-Arts and their attached macula densa were simultaneously perfused. TGF was measured as the decrease in Af-Art diameter elicited by switching macula densa NaCl from 10 to 80 mM. Adding a CO-releasing molecule (CORM-3, 5×10−5mol/L) to the macula densa blunted TGF from 3.3±0.3 to 2.0±0.3 µm (P<0.001). The guanylate cyclase inhibitor LY-83583 (10−6mol/L) enhanced TGF (5.8±0.6 µm; P<0.001 vs. control) and prevented the effect of CORM-3 on TGF (LY-83583 + CORM-3, 5.5±0.3 µm). Similarly, the PKG inhibitor KT-5823 (2×10−6mol/L) enhanced TGF and prevented the effect of CORM-3 on TGF (KT-5823, 6.0±0.7 µm; KT-5823 + CORM-3, 5.9±0.8 µm). However, the PDE2 inhibitor BAY-60-7550 (10−6mol/L) did not prevent the effect of CORM-3 on TGF (BAY-60-7550, 4.07±0.31 µm; BAY-60-7550 + CORM-3, 1.84±0.31 µm, P<0.001). Finally, the degradation-resistant cAMP analog dibutyryl-cAMP (db-cAMP, 10−3mol/L) prevented the attenuation of TGF by CORM-3 (db-cAMP, 4.6±0.5 µm; db-cAMP + CORM-3, 5.0±0.6 µm). We conclude that CO attenuates TGF by reducing cAMP via a cGMP-dependent pathway mediated by PKG, rather than PDE2. Our results will lead to a better understanding of the mechanisms that control the renal microcirculation.
Keywords: cGMP, afferent arteriole, TGF, macula densa, cAMP
Introduction
The regulation of the renal microcirculation is influenced by many factors, including circulating hormones, sympathetic nervous system, and intrinsic autoregulatory mechanisms, most of which converge on the afferent arteriole (Af-Art), the main regulator of renal blood flow. Among the autoregulatory mechanisms, tubuloglomerular feedback (TGF) is a well established feedback mechanism, initiated by an increase in NaCl reabsorption via the Na-K-2Cl cotransporter type 2 (NKCC2) at the macula densa. The macula densa, which is in close contact with the Af-Art, then releases ATP, which is hydrolized to adenosine and causes Af-Art constriction. Understanding the mechanisms of TGF regulation is essential to better understand the regulation of renal vascular resistance, glomerular filtration rate (GFR) and renal function.
Carbon monoxide (CO) is a low molecular weight gas that shares similar properties with another low molecular weight gas, namely nitric oxide (NO). CO, like NO, is generated under physiological conditions1. The synthetic enzymes that produce CO, heme oxygenase-1 (HO-1) and heme oxygenase-2 (HO-2), are widely expressed in the kidney, both in vascular and tubular structures, thus CO is endogenously produced in the kidney2. Furthermore, we have recently shown that both HO isoforms are expressed in the macula densa3. Both NO and CO exert important roles in regulation of vascular tone and blood pressure4, and we have recently reported that CO in the macula densa, just like NO, attenuates TGF in vitro and in vivo3,5. However, to our knowledge there is no information addressing the mechanism of action of CO in the macula densa, and in fact, little is known about the signaling downstream from CO in renal epithelial cells. In the vasculature, the effects of CO are mainly mediated by activation of soluble guanylate cyclase and increases in cGMP6. It is reasonable to postulate that cGMP is involved in the attenuation of TGF by CO, since we have previously shown that in the macula densa cGMP attenuates TGF7.
The primary detection mechanism of TGF appears to be uptake of NaCl by means of the NKCC2, located in the apical membrane of macula densa cells. It is well known that NaCl absorption by the thick ascending limb can be increased by various hormones and autacoids that increase cAMP production8. cAMP then stimulates exocytic insertion of NKCC2 in the apical membrane via cAMP-dependent protein kinase (PKA)9 and NKCC2-mediated NaCl transport10. Similarly, it may be that in the macula densa cAMP production influences TGF by acting on NKCC2. Furthermore, it may be that cGMP acts to decrease cAMP, either via cGMP-dependent protein kinase (PKG), as shown in the cortical collecting duct11, or via cGMP-stimulated (type 2) cyclic nucleotide phosphodiesterase (PDE2), as shown in the thick ascending limb12. However, whether cAMP enhances TGF and whether it is involved in the mechanism by which CO attenuates TGF remain unknown.
Here we hypothesized that CO in the macula densa attenuates TGF by reducing cAMP via activation of PKG and PDE2. To address this hypothesis, we studied the effect of CO on TGF before and during inhibition of soluble guanylate cyclase, PKG, and PDE2, and in the absence and presence of a cell-permeant stable cAMP analog in the macula densa. For this we used a technique developed by us consisting of simultaneous perfusion of a microdissected Af-Art and its attached macula densa. This preparation has the advantage that it allows us to control pressure in the Af-Art and luminal fluid composition at the macula densa as well as to obtain real-time images of the Af-Art, without the confounding effects of hemodynamic or hormonal influences.
Methods
New Zealand White rabbits weighing 1.5–2 Kg (Covance, Battle Creek, MI) were given standard chow (Harlan Laboratories, Indianapolis, IN) and tap water ad libitum. Rabbits were anesthetized ketamine (50 mg/kg, i.m.), xylazine (10 mg/kg, i.m.), and pentobarbital (25 mg/kg i.v.). Kidneys were quickly harvested, sliced along their corticomedullary axis, and cooled to 4°C. All protocols were approved by Henry Ford Health System’s Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Af-Arts with the macula densa attached were microdissected and microperfused as previously described13. Briefly, a single superficial Af-Art was isolated from each rabbit with its intact glomerulus and adherent tubular segments consisting of the terminal portion of the thick ascending limb, macula densa, and early distal tubule. Microdissection was performed by using fine forceps, at 4–6°C, and accomplished within an hour of harvesting the kidney. Samples were transferred to a 37°C chamber mounted on an inverted microscope where both the Af-Art and the end of either the distal tubule or thick ascending limb were cannulated with an array of concentric glass pipettes as described previously13. This system allows us to simultaneously perfuse the Af-Art and macula densa and introduce chemicals by exchanging the perfusion solution in a few seconds, while keeping the holding and perfusion pipettes in place. It also keeps a constant pressure on the Af-Art perfusion (maintained at 60 mmHg) and a constant flow at the macula densa (maintained at 20 nL/min), which is within the range of physiological flow rates14. Another advantage of this system is that it makes it highly unlikely for any drugs present in the effluent of the tubular perfusate to diffuse through the bath and act directly in the Af-Art, since the exchange rate of bath perfusate is 1 ml/min, thus effluents from the macula densa are immediately diluted 50,000 times and washed out before they can act on other structures13.
Experimental protocols
A 30-min equilibration period was allowed before taking any measurements. Protocols consisted of four consecutive TGF responses induced by switching macula densa NaCl from 10 to 80 mM. The first TGF response was used as a control, while subsequent TGF responses were induced in the presence of various pharmacological probes administered in the macula densa perfusate. Images of the Af-Art were acquired five minutes after the introduction of drugs at 5-s intervals and; three individual measurements of Af-Art diameter were taken at the site of maximum constriction and ± 5 µm around it. The following five experimental groups were studied:
Time controls: 1a, four consecutive TGF responses with no drugs added. 1b, first TGF, control; second and third TGF, CORM-3 (5×10−5mol/L).
Role of soluble guanylate cyclase in the attenuation of TGF by CO: first TGF, control; second TGF, CORM-3, third TGF, LY-83583 (10−6mol/L), fourth TGF, CORM-3+LY-83583.
Role of PKG in the attenuation of TGF by CO: first TGF, control; second TGF, CORM-3 (5×10−5mol/L), third TGF, KT-5823 (2×10−6mol/L), fourth TGF, CORM-3+KT-5823.
Role of PDE2 in the attenuation of TGF by CO: first TGF, control; second TGF, CORM-3 (5×10−5mol/L), third TGF, BAY-60-7550 (10−6mol/L), fourth TGF, CORM-3+BAY-60-7550.
Role of cAMP in the attenuation of TGF by CO: first TGF, control; second TGF, CORM-3 (5×10−5mol/L), third TGF, Dibutyryl-cAMP (db-cAMP, 10−3mol/L), fourth TGF, CORM-3+db-cAMP.
Chemicals and solutions
Ru(CO)3Cl, known as CORM-3, a CO-releasing molecule (synthesized by J. R. Falck, Dallas, TX), was used as a CO donor. This was freshly prepared before the experiments by dissolving the compound in distilled water. LY-83583, a soluble guanylate cyclase inhibitor, was purchased from Enzo Life Sciences (Farmingdale, NY). KT-5823, an inhibitor of PKG, and BAY-60-7550, a PDE2 inhibitor, were purchased from Cayman Chemical (Ann Arbor, MI). Db-cAMP, a cell-permeable analogue of cAMP, was purchased from Tocris Bioscience (Minneapolis, MN).
Statistics
Paired t-tests were used to compare the TGF responses, defined as the decrease in Af-Art diameter induced by switching macula densa NaCl from 10 to 80 mM. Hochberg’s step-up procedure was used to adjust the P values for multiple comparisons. Values are expressed as mean ± SEM and a P < 0.05 was considered significant.
Results
1. Time control
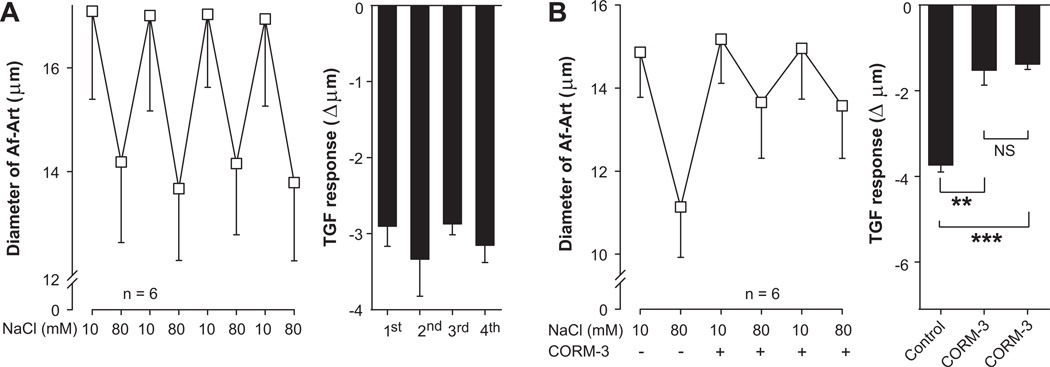
We first tested whether TGF responses are stable and reproducible with time. Four consecutive TGF responses were induced by increasing the macula densa perfusate NaCl from 10 to 80 mM. The first TGF response decreased Af-Art diameter by 2.9 ± 0.3 µm (from 17.1 ± 1.7 to 14.2 ± 1.6 µm, n = 6), subsequent TGF responses were 3.3 ± 0.5, 2.9 ± 0.1, and 3.2 ± 0.2 µm (Fig 1a). Thus, all four consecutive TGF responses were not significantly different, indicating reproducibility. We also tested the effect of the CO-releasing molecule CORM-3 (5×10−5mol/L) when repeatedly added to the macula densa perfusate. The first (control) TGF response decreased Af-Art diameter by 3.7 ± 0.2 µm (from 14.9 ± 1.1 to 11.1 ± 1.2 µm, n = 6). The second and third TGF responses (in the presence of CORM-3) were attenuated to 1.5 ± 0.3 µm (P < 0.01 vs. control) and 1.4 ± 0.1 µm (P < 0.001 vs. control), respectively (Fig 1b). The two TGF responses performed in the presence of CORM-3 were not different from each other. These data suggest that CO attenuates TGF and that the effect of CORM-3 on TGF is reproducible over time, i.e. does not suffer from tachyphylaxis.
Figure 1.
A, TGF was induced by switching luminal NaCl in the macula densa from 10 to 80 mM four consecutive times. TGF responses were reproducible, indicating stability of the preparation. B, Effect of CORM-3 (5×10−5mol/L) when applied two consecutive times on TGF. CORM-3 attenuated TGF in a reproducible manner. ** P < 0.01, *** P < 0.001.
2. Role of soluble guanylate cyclase in the attenuation of TGF by CO
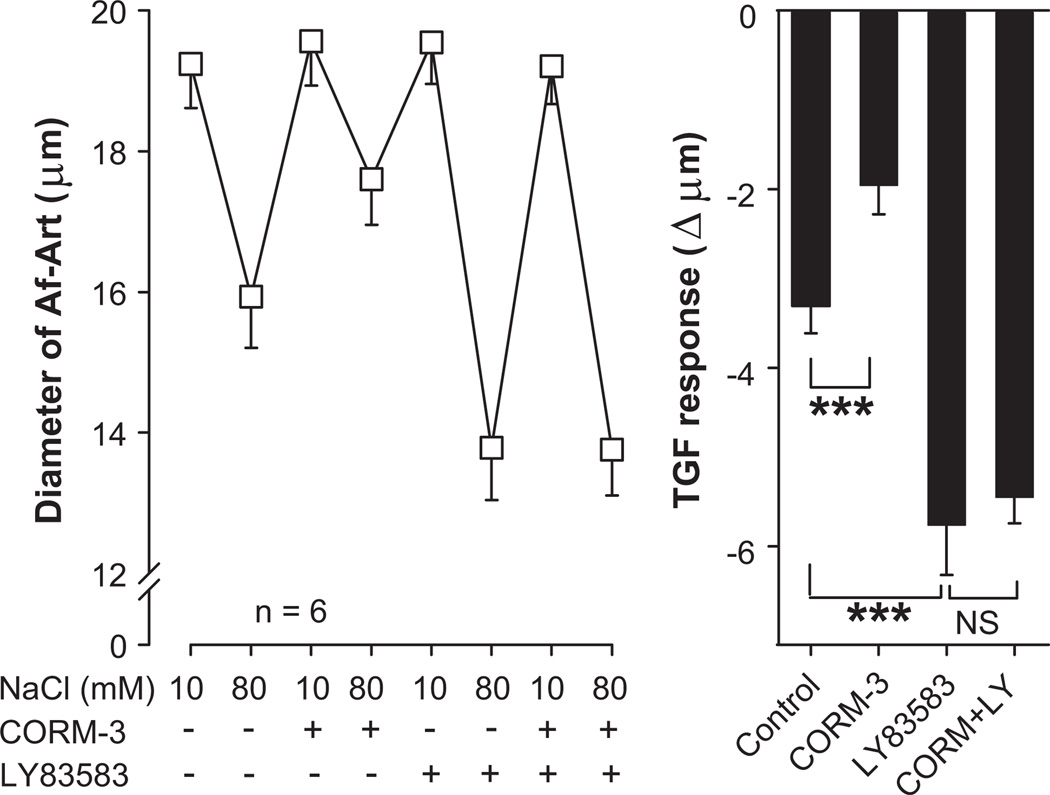
We then tested whether activation of the soluble guanylate cyclase/cGMP cascade is involved in the attenuation of TGF by CO. Control TGF decreased Af-Art diameter by 3.3 ± 0.3 µm (from 19.2 ± 0.6 to 15.9 ± 0.7 µm, n = 6). The CO-releasing molecule CORM-3 (5×10−5mol/L) added to the macula densa perfusate attenuated TGF to 2.0 ± 0.3 µm (P < 0.001 vs. control). The soluble guanylate cyclase inhibitor LY-83583 (10−6mol/L) added to the macula densa perfusate enhanced TGF to 5.8 ± 0.6 µm (P < 0.001 vs. control). CORM-3 failed to attenuate TGF in the presence of LY-83583, as TGF in the presence of both drugs was 5.5 ± 0.3 µm (P = NS vs. LY-83583 alone, Fig 2). These data suggest that soluble guanylate cyclase inhibition potentiates TGF, and that the attenuation of TGF by CO is mediated by activation of the soluble guanylate cyclase/cGMP system.
Figure 2.
Effect of the guanylate cyclase inhibitor LY-83583 (10−6mol/L) added to the macula densa on the attenuation of TGF by CORM-3 (5×10−5mol/L). CORM-3 did not attenuate TGF in the presence of LY-83583. *** P < 0.001.
3. Role of PKG in the attenuation of TGF by CO
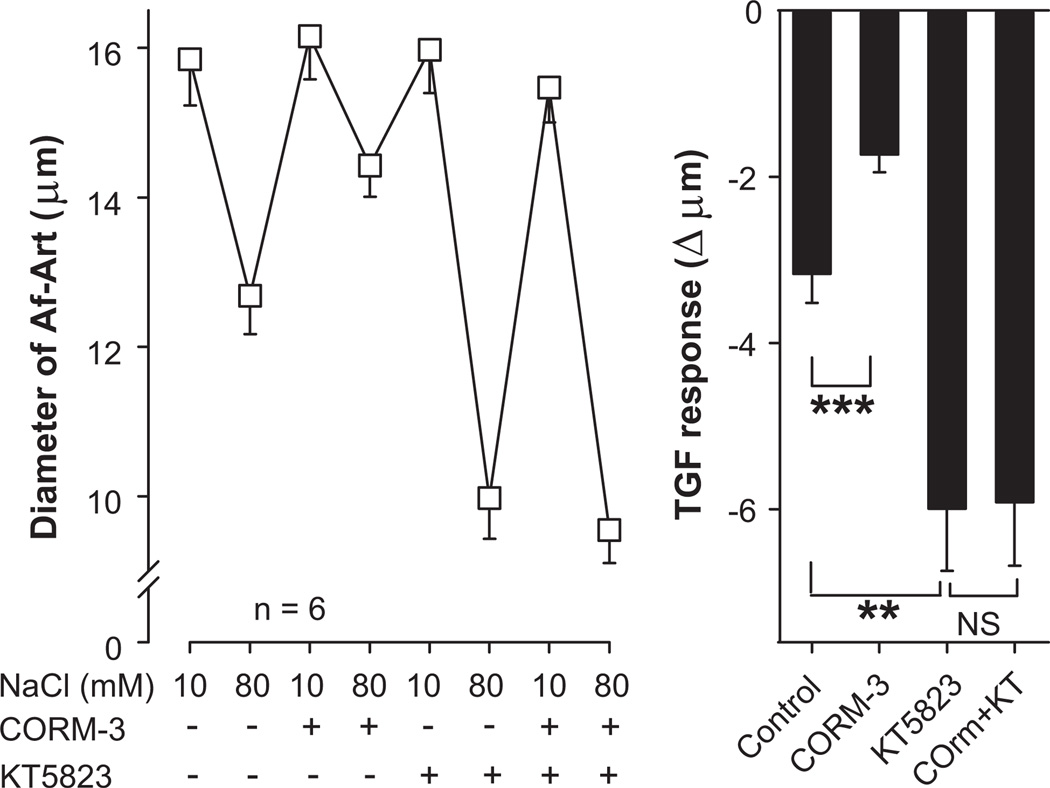
Most of the effects of cGMP in the nephron are mediated by activation of either PKG7,15 or PDE216. We first tested whether PKG is involved in the attenuation of TGF by CO. Control TGF decreased Af-Art diameter by 3.2 ± 0.3 µm (from 15.8 ± 0.6 to 12.7 ± 0.5 µm, n = 6). CORM-3 added to the macula densa perfusate attenuated TGF to 1.7 ± 0.2 µm (P < 0.001 vs. control). The PKG inhibitor KT-5823 (2×10−6mol/L) added to the macula densa perfusate enhanced TGF to 6.0 ± 0.7 µm (P < 0.01 vs. control). CORM-3 failed to attenuate TGF in the presence of KT-5823, as TGF in the presence of both drugs was 5.9 ± 0.8 µm (P = NS vs. KT-5823 alone, Fig 3). These data suggest that the attenuation of TGF by CO is mediated by PKG.
Figure 3.
Effect of the protein kinase G inhibitor KT-5823 (2×10−6mol/L) added to the macula densa on the attenuation of TGF by CORM-3 (5×10−5mol/L). CORM-3 did not attenuate TGF in the presence of KT-5823. ** P < 0.01.
4. Role of PDE2 in the attenuation of TGF by CO
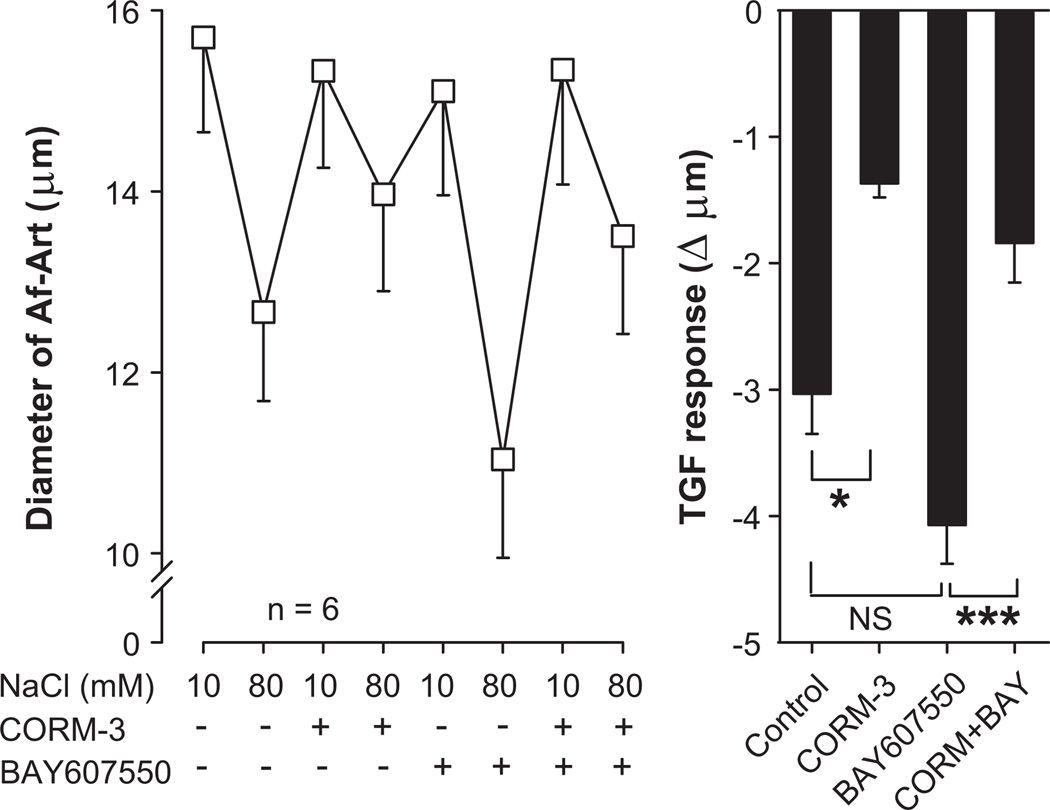
We then tested whether PDE2 is involved in the attenuation of TGF by CO. Control TGF decreased Af-Art diameter by 3.0 ± 0.3 µm (from 15.7 ± 1 to 12.7 ± 1 µm, n = 6). CORM-3 added to the macula densa perfusate attenuated TGF to 1.4 ± 0.1 µm (P < 0.05 vs. control). The PDE2 inhibitor BAY-60-7550 (10−6mol/L) added to the macula densa perfusate TGF enhanced TGF to 4.1 ± 0.3 µm (although without reaching statistical significance, P = 0.08 vs. control). BAY-60-7550 did not prevent CORM-3 from attenuating TGF, as TGF in the presence of both drugs was 1.8 ± 0.3 µm (P < 0.001 vs. BAY-60-7550 alone, Fig 4). These data suggest that the attenuation of TGF by CO is not mediated by PDE2.
Figure 4.
Effect of the phosphodiesterase type 2 inhibitor BAY-60-7550 (10−6mol/L) added to the macula densa on the attenuation of TGF by CORM-3 (5×10−5mol/L). BAY-60-7550 did not prevent the attenuation of TGF by CORM-3. ** P < 0.01, *** P < 0.001.
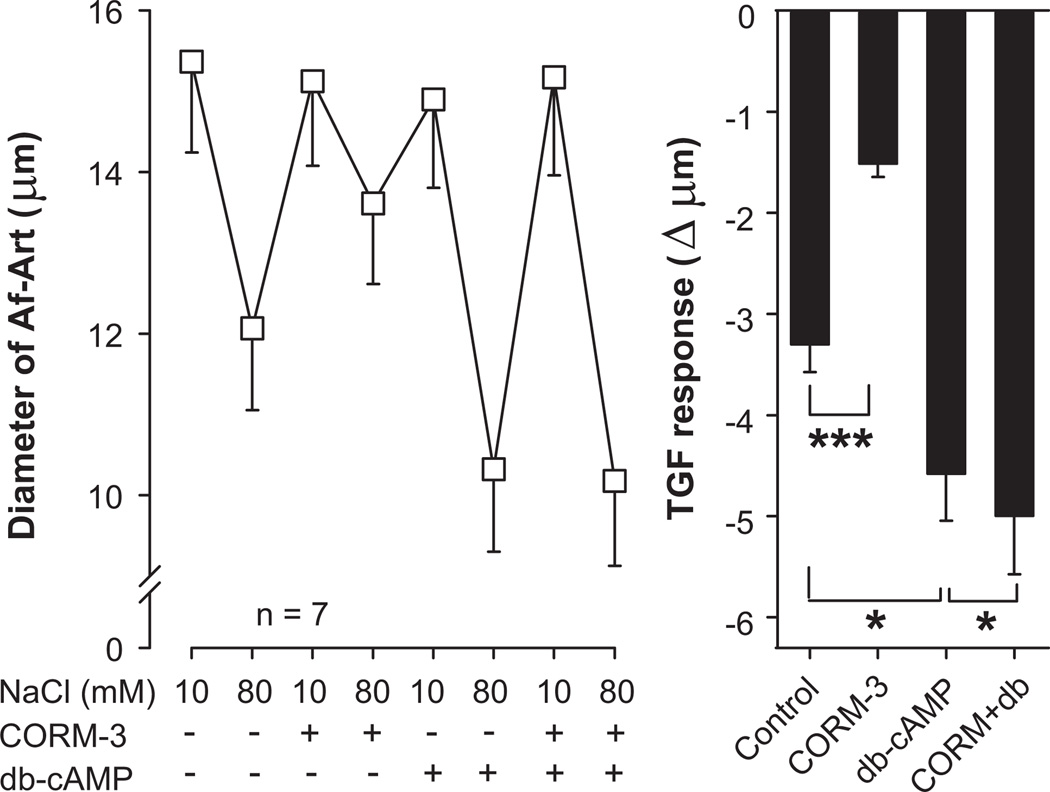
5. Role of cAMP in the attenuation of TGF by CO
Finally, we investigated the role of cAMP in TGF and in the attenuation of TGF by CO. Control TGF decreased Af-Art diameter by 3.3 ± 0.3 µm (from 15.4 ± 1.1 to 12.1 ± 1 µm, n = 7). CORM-3 added to the macula densa perfusate attenuated TGF to 1.5 ± 0.1 µm (P < 0.001 vs. control). The PDE-resistant, cell permeant cAMP analog db-cAMP (10−3mol/L) added to the macula densa perfusate enhanced TGF to 4.6 ± 0.5 µm (P < 0.05 vs. control). CORM-3 failed to attenuate TGF in the presence of db-cAMP, as TGF in the presence of both drugs was 5.0 ± 0.6 µm (P < 0.05 vs. db-cAMP alone, Fig 5). These data suggest that cAMP potentiates TGF and that the attenuation of TGF by CO is mediated by cAMP.
Figure 5.
Effect of the cAMP analog dibutyryl cAMP (db-cAMP, 10−3mol/L) added to the macula densa on the attenuation of TGF by CORM-3 (5×10−5mol/L). CORM-3 did not attenuate TGF in the presence of db-cAMP. * P < 0.05, *** P < 0.001.
Discussion
We report here that a CO donor attenuates TGF and that blocking either soluble guanylate cyclase or PKG, but not PDE2, can block this effect. Furthermore, preventing a decrease in cAMP by loading the macula densa with a cAMP analog can also block the attenuation of TGF by CO. This data support our hypothesis that CO in the macula densa attenuates TGF by reducing cAMP via cGMP/PKG pathway.
We previously reported that the HO system attenuates TGF, because inhibiting tubular HOs with SnMP potentiated TGF, and adding exogenous CO to the macula densa perfusate attenuated TGF. Here we report on the mechanisms underlying the attenuation of TGF by CO. The signaling of CO in renal tubular epithelial cells has not been extensively studied. The soluble guanylyl cyclase/cGMP pathway mediates the protective effect of CO against cisplatin-induced toxicity in a proximal tubule cell line17, but not the antioxidant effects of CO in a mouse thick ascending limb cell line18. Our data clearly demonstrate that when soluble guanylate cyclase in the macula densa is inhibited, the effect of CO on TGF is prevented. This indicates that most of the effect of CO is due to activation of the soluble guanylate cyclase/cGMP system.
Because CO has been shown to cause vasodilation when applied directly to the Af-Art19,20, it could be argued that the attenuation of TGF we observed is due to diffusion of CO from the macula densa perfusate to the Af-Art. As explained in the method section, this is highly unlikely due to the dilutional effect of the bath perfusion. Furthermore, our data suggest this is not the case, since the attenuation of TGF by CO was completely blocked by adding the soluble guanylate cyclase inhibitor LY-83583 to the macula densa perfusate; and we have previously shown that LY-83583 in the macula densa perfusate does not diffuse to the vascular compartment, since it did not alter the vasodilator effect of acetylcholine on the Af-Art (NO/soluble guanylate cyclase/cGMP/dependent vasodilator)7. Taken together, these data indicate that CO, similarly to NO7,21, acts locally in the macula densa, by increasing cGMP.
Since inhibiting guanylate cyclase blocks the effect of both NO and CO on the TGF response, and since it has been reported that in the vasculature CO could induce the release of NO19,20, it could be argued that CO is acting via NO. However, we have previously shown that CORM-3 attenuates TGF even in the presence of the nitric oxide synthase 1 (NOS1) inhibitor 7-NI, and that inhibiting endogenous CO synthesis with the HO inhibitor SnMP potentiates TGF even in the presence of the of the NOS inhibitor L-NAME5.
In the nephron, the effects of cGMP are mediated mainly by activation of PKG7,15 or PDE216 and PDE2. PKGs are serine/threonine kinases encoded by two genes, type I and type II. PKG I is located predominantly in the cytoplasm and PKG II is anchored to the plasma membrane by N-terminal myristoylation22. We have previously shown that in the macula densa cGMP attenuates TGF while blocking PKG potentiates TGF7. To test whether PKG is involved in CO-induced attenuation of TGF, we used KT-5823, an inhibitor of PKG. We found that inhibition of PKG prevented the attenuation of TGF by CORM-3. To our knowledge, this is the first study to demonstrate that the attenuation of TGF by CO in the macula densa is a PKG-mediated process.
PDE is a family of enzymes that hydrolyzed cGMP and cAMP23. PDE2 is unique in being markedly stimulated by cGMP to degrade cAMP24. It has been shown that in the thick ascending limb cGMP activates PDE2 which in turn reduces cAMP and NKCC2-dependent Na entry16. To test whether PDE2 is also involved in the attenuation of TGF by CO, we added the PDE2 inhibitor, BAY-60-7550 to the macula densa perfusate. We found that PDE2 inhibition did not prevent the attenuation of TGF by CORM-3. Taken together, these data indicate that CO in macula densa attenuates TGF via a cGMP-dependent pathway mediated by PKG, rather than PDE2.
Various factors that stimulate cAMP, such as arginine vasopressin, parathyroid hormone and β-adrenergic agonists, are known to stimulate NaCl absorption in the thick ascending limb8. Furthermore, cAMP is well known to increase NKCC2-dependent apical NaCl entry16,25–27, likely by stimulating PKA, which phosphorylates NKCC228 and increases exocytic insertion of NKCC2 in the apical membrane9. If also true for the macula densa, this effect would be expected to potentiate TGF. Thus cAMP and cGMP appear to have opposing actions on TGF. Moreover, cGMP, acting via PKG, inhibits adenylyl cyclase in the collecting duct, and this cascade results in a decrease in intracellular cAMP11. To test whether the attenuation of TGF by CO is mediated by a decrease in cAMP, we loaded the macula densa with a stable cAMP analogue, db-cAMP. We found that db-cAMP enhanced TGF and prevented the attenuation of TGF by CORM-3. These data demonstrate that intracellular cAMP modulates TGF, and that decreases in cAMP participate in the attenuation of TGF by CO.
The treatments that prevented the attenuation of TGF by exogenous CO in our study, namely inhibition of guanylate cyclase or PKG, and db-cAMP loading, also potentiated TGF when used by themselves. This may be due to blockade of endogenous CO. Our previous studies suggest that CO is endogenously produced in the macula densa, as we showed that both isoforms of HO are expressed in the macula densa and that blocking CO production with a HO inhibitor potentiates TGF3,5. In addition, because NO shares at least part of the same signaling pathway with CO, it is possible that the potentiation of TGF seen with inhibitiors of guanylate cyclase and PKG was partly due to inhibition of NO signaling.
Our finding that db-cAMP potentiates TGF is in apparent contradiction with two previous in vivo micropuncture studies showing that db-cAMP inhibited TGF29,30. However, a direct vasodilatory effect of db-cAMP due to diffusion to the Af-Art cannot be excluded in in vivo studies, particularly since they only observed inhibition of TGF at high concentrations of db-cAMP, 10 times higher than the one we used. Also in one study perfusion was applied to the late proximal tubule, thus going through the thick ascending limb before reaching the macula densa, and because cAMP stimulates Na reabsorption in the thick ascending limb, it is likely that Na delivery to the macula densa was decreased, thus counteracting the direct effect of cAMP on TGF.
The HO system as a whole is antioxidant, as it transforms a pro-oxidant compound (free heme) into an antioxidant compound (biliverdin/bilirubin)1, and we have shown that biliverdin attenuates TGF by acting as an antioxidant3. However, the effect of CO on oxidative stress is less clear. It has been reported that CO increases the generation O2− from the mitochondria31, but inhibits the generation of O2− from NADPH oxidase-1 (NOX-1)32. Current evidence suggests that the attenuation of TGF by CO is not due to an antioxidant effect. This assertion is based on two previously published experiments 1) the attenuation of TGF by tempol was completely absent in the presence of the NOS1 inhibitor 7-nitroindazole33, i.e. it is NO-dependent; and 2) the attenuation of TGF by CO is not affected whatsoever by 7-nitroindazole5, i.e. it is NO-independent. Furthermore, NOX-1, the enzyme associated with an antioxidant effect of CO is not expressed in the macula densa34.
Perspectives
In summary, we found that CO in the macula densa attenuates TGF by reducing cAMP via a cGMP-dependent pathway mediated by PKG, rather than PDE2. Taken together with our previous studies3,5, these data show that CO acts similarly, although independently from, NO. Thus, it is possible that, like NO, CO by inhibiting TGF favors Af-Art dilation, leading to increases in RBF, GFR, and Na excretion. Considerable evidence indicates that CO is antihypertensive and natriuretic35, our experiments suggest that part of those actions may be due to attenuation of TGF. Furthermore, because oxidative stress and Ang II are known inducers of HO-136, but decrease NO in the macula densa33,37, it is possible that the relative contribution of CO to TGF attenuation becomes greater in conditions with increased Ang II and/or oxidative stress.
Novelty and Significance.
1. What Is New?
In the present project, we focused on the effects of carbon monoxide. Although carbon monoxide is considered a poison at high concentrations, it also acts as a hormone when produced in small amounts. This is the first report of how carbon monoxide affects tubuloglomerular feedback, a mechanism that controls the renal microcirculation.
2. What Is Relevant?
The kidney plays a key role in high blood pressure (hypertension). Kidney diseases cause hypertension, and hypertension can in turn damage the kidney. Defective regulation of the renal microcirculation is believed to be a central issue in this problem by causing hypertension and contributing to the renal damage seen in hypertension and diabetes. However, the understanding of the mechanisms that control the renal microcirculation remains incomplete.
Acknowledgments
Sources of funding
This study was supported by National Institutes of Health P01 Grant HL-090550 to J. L. Garvin 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict(s) of Interest/Disclosure(s) None.
References
- 1.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 2.da Silva J-L, Zand BA, Yang LM, Sabaawy HE, Lianos E, Abraham NG. Heme oxygenase isoform-specific expression and distribution in the rat kidney. Kidney Int. 2001;59:1448–1457. doi: 10.1046/j.1523-1755.2001.0590041448.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Garvin JL, D'Ambrosio MA, Falck JR, Leung P, Liu R, Ren Y, Carretero OA. Heme oxygenase metabolites inhibit tubuloglomerular feedback in vivo. Am J Physiol Heart Circ Physiol. 2011;300:H1320–H1326. doi: 10.1152/ajpheart.01118.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang F, Kaide J-I, Wei Y, Jiang H, Yu C, Balazy M, Abraham NG, Wang W, Nasjletti A. Carbon monoxide produced by isolated arterioles attenuates pressure-induced vasoconstriction. Am J Physiol Heart Circ Physiol. 2001;281:H350–H358. doi: 10.1152/ajpheart.2001.281.1.H350. [DOI] [PubMed] [Google Scholar]
- 5.Ren Y, D'Ambrosio MA, Wang H, Liu R, Garvin JL, Carretero OA. Heme oxygenase metabolites inhibit tubuloglomerular feedback (TGF) Am J Physiol Renal Physiol. 2008;295:F1207–F1212. doi: 10.1152/ajprenal.90243.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 7.Ren Y, Garvin JL, Carretero OA. Role of macula densa nitric oxide and cGMP in the regulation of tubuloglomerular feedback. Kidney Int. 2000;58:2053–2060. doi: 10.1111/j.1523-1755.2000.00377.x. [DOI] [PubMed] [Google Scholar]
- 8.Greger R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev. 1985;65:760–797. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- 9.Caceres PS, Ares GR, Ortiz PA. cAMP stimulates apical exocytosis of the renal Na(+)-K(+)-2Cl(−) cotransporter NKCC2 in the thick ascending limb: role of protein kinase A. J Biol Chem. 2009;284:24965–24971. doi: 10.1074/jbc.M109.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molony DA, Reeves WB, Andreoli TE. Na+:K+:2Cl− cotransport and the thick ascending limb. Kidney Int. 1989;36:418–426. doi: 10.1038/ki.1989.211. [DOI] [PubMed] [Google Scholar]
- 11.Garcia NH, Stoos BA, Carretero OA, Garvin JL. Mechanism of the nitric oxide-induced blockade of collecting duct water permeability. Hypertension. 1996;27:679–683. doi: 10.1161/01.hyp.27.3.679. [DOI] [PubMed] [Google Scholar]
- 12.Herrera M, Ortiz PA, Garvin JL. Regulation of thick ascending limb transport: role of nitric oxide. Am J Physiol Renal Physiol. 2006;290:F1279–F1284. doi: 10.1152/ajprenal.00465.2005. [DOI] [PubMed] [Google Scholar]
- 13.Ito S, Carretero OA. An in vitro approach to the study of macula densa-mediated glomerular hemodynamics. Kidney Int. 1990;38:1206–1210. doi: 10.1038/ki.1990.335. [DOI] [PubMed] [Google Scholar]
- 14.Good DW, Wright FS. Luminal influences on potassium secretion: sodium concentration and fluid flow rate. Am J Physiol. 1979;236:F192–F205. doi: 10.1152/ajprenal.1979.236.2.F192. [DOI] [PubMed] [Google Scholar]
- 15.Hong NJ, Garvin JL. Nitric oxide reduces flow-induced superoxide production via cGMP-dependent protein kinase in thick ascending limbs. Am J Physiol Renal Physiol. 2009;296:F1061–F1066. doi: 10.1152/ajprenal.90707.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ortiz PA, Garvin JL. NO Inhibits NaCl absorption by rat thick ascending limb through activation of cGMP-stimulated phosphodiesterase. Hypertension. 2001;37:467–471. doi: 10.1161/01.hyp.37.2.467. [DOI] [PubMed] [Google Scholar]
- 17.Tayem Y, Johnson TR, Mann BE, Green CJ, Motterlini R. Protection against cisplatin-induced nephrotoxicity by a carbon monoxide-releasing molecule. Am J Physiol Renal Physiol. 2006;290:F789–F794. doi: 10.1152/ajprenal.00363.2005. [DOI] [PubMed] [Google Scholar]
- 18.Kelsen S, Patel BJ, Parker LB, Vera T, Rimoldi JM, Gadepalli RS, Drummond HA, Stec DE. Heme oxygenase attenuates angiotensin II-mediated superoxide production in cultured mouse thick ascending loop of Henle cells. Am J Physiol Renal Physiol. 2008;295:F1158–F1165. doi: 10.1152/ajprenal.00057.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorup C, Jones CL, Gross SS, Moore LC, Goligorsky MS. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am J Physiol. 1999;277:F882–F889. doi: 10.1152/ajprenal.1999.277.6.F882. [DOI] [PubMed] [Google Scholar]
- 20.Botros FT, Navar LG. Interaction between endogenously produced carbon monoxide and nitric oxide in regulation of renal afferent arterioles. Am J Physiol Heart Circ Physiol. 2006;291:H2772–H2778. doi: 10.1152/ajpheart.00528.2006. [DOI] [PubMed] [Google Scholar]
- 21.Wilcox CS, Deng X, Welch WJ. NO generation and action during changes in salt intake: roles of nNOS and macula densa. Am J Physiol. 1998;274:R1588–R1593. doi: 10.1152/ajpregu.1998.274.6.R1588. [DOI] [PubMed] [Google Scholar]
- 22.Feil R, Lohmann SM, de JH, Walter U, Hofmann F. Cyclic GMP-dependent protein kinases and the cardiovascular system: insights from genetically modified mice. Circ Res. 2003;93:907–916. doi: 10.1161/01.RES.0000100390.68771.CC. [DOI] [PubMed] [Google Scholar]
- 23.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100:309–327. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 24.Zaccolo M, Movsesian MA. cAMP and cGMP signaling cross-talk: role of phosphodiesterases and implications for cardiac pathophysiology. Circ Res. 2007;100:1569–1578. doi: 10.1161/CIRCRESAHA.106.144501. [DOI] [PubMed] [Google Scholar]
- 25.Hebert SC, Andreoli TE. Effects of antidiuretic hormone on cellular conductive pathways in mouse medullary thick ascending limbs of Henle: II. determinants of the ADH-mediated increases in transepithelial voltage and in net Cl-absorption. J Membr Biol. 1984;80:221–233. doi: 10.1007/BF01868440. [DOI] [PubMed] [Google Scholar]
- 26.Sun A, Grossman EB, Lombardi M, Hebert SC. Vasopressin alters the mechanism of apical Cl- entry from Na+:Cl− to Na+:K+:2Cl− cotransport in mouse medullary thick ascending limb. J Membr Biol. 1991;120:83–94. doi: 10.1007/BF01868594. [DOI] [PubMed] [Google Scholar]
- 27.Molony DA, Reeves WB, Hebert SC, Andreoli TE. ADH increases apical Na+, K+, 2Cl− entry in mouse medullary thick ascending limbs of Henle. Am J Physiol. 1987;252:F177–F187. doi: 10.1152/ajprenal.1987.252.1.F177. [DOI] [PubMed] [Google Scholar]
- 28.Gunaratne R, Braucht DW, Rinschen MM, Chou CL, Hoffert JD, Pisitkun T, Knepper MA. Quantitative phosphoproteomic analysis reveals cAMP/vasopressin-dependent signaling pathways in native renal thick ascending limb cells. Proc Natl Acad Sci U S A. 2010;107:15653–15658. doi: 10.1073/pnas.1007424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnermann J, Osswald H, Hermle M. Inhibitory effect of methylxanthines on feedback control of glomerular filtration rate in the rat kidney. Pflugers Arch. 1977;369:39–48. doi: 10.1007/BF00580808. [DOI] [PubMed] [Google Scholar]
- 30.Bell PD. Cyclic AMP-calcium interaction in the transmission of tubuloglomerular feedback signals. Kidney Int. 1985;28:728–732. doi: 10.1038/ki.1985.191. [DOI] [PubMed] [Google Scholar]
- 31.Taille C, El-Benna J, Lanone S, Boczkowski J, Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem. 2005;280:25350–25360. doi: 10.1074/jbc.M503512200. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez AI, Gangopadhyay A, Kelley EE, Pagano PJ, Zuckerbraun BS, Bauer PM. HO-1 and CO decrease platelet-derived growth factor-induced vascular smooth muscle cell migration via inhibition of Nox1. Arterioscler Thromb Vasc Biol. 2010;30:98–104. doi: 10.1161/ATVBAHA.109.197822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren Y, Carretero OA, Garvin JL. Mechanism by which superoxide potentiates tubuloglomerular feedback. Hypertension. 2002;39:624–628. doi: 10.1161/hy0202.103299. [DOI] [PubMed] [Google Scholar]
- 34.Zhang R, Harding P, Garvin JL, Juncos R, Peterson E, Juncos LA, Liu R. Isoforms and functions of NAD(P)H oxidase at the macula densa. Hypertension. 2009;53:556–563. doi: 10.1161/HYPERTENSIONAHA.108.124594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stec DE, Drummond HA, Vera T. Role of carbon monoxide in blood pressure regulation. Hypertension. 2008;51:597–604. doi: 10.1161/HYPERTENSIONAHA.107.097154. [DOI] [PubMed] [Google Scholar]
- 36.Li P, Jiang H, Yang L, Quan S, Dinocca S, Rodriguez F, Abraham NG, Nasjletti A. Angiotensin II induces carbon monoxide production in the perfused kidney: relationship to protein kinase C activation. Am J Physiol Renal Physiol. 2004;287:F914–F920. doi: 10.1152/ajprenal.00073.2004. [DOI] [PubMed] [Google Scholar]
- 37.Fu Y, Zhang R, Lu D, Liu H, Chandrashekar K, Juncos LA, Liu R. NOX2 is the primary source of angiotensin II-induced superoxide in the macula densa. Am J Physiol Regul Integr Comp Physiol. 2010;298:R707–R712. doi: 10.1152/ajpregu.00762.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]