Abstract
Increasing evidence implicates hydrogen peroxide (H2O2) as an intra- and intercellular signaling molecule that can influence processes from embryonic development to cell death. Most research has focused on relatively slow signaling, on the order of minutes to days, via second messenger cascades. However, H2O2 can also mediate subsecond signaling via ion channel activation. This rapid signaling has been examined most thoroughly in the nigrostriatal dopamine (DA) pathway, which plays a key role in facilitating movement mediated by the basal ganglia. In DA neurons of the substantia nigra, endogenously generated H2O2 activates ATP-sensitive K+ (KATP) channels that inhibit DA neuron firing. In the striatum, H2O2 generated downstream from glutamatergic AMPA receptor activation in medium spiny neurons acts as a diffusible messenger that inhibits axonal DA release, also via KATP channels. The source of dynamically generated H2O2 is mitochondrial respiration; thus, H2O2 provides a novel link between activity and metabolism via KATP channels. Additional targets of H2O2 include transient receptor potential (TRP) channels. In contrast to the inhibitory effect of H2O2 acting via KATP channels, TRP channel activation is excitatory. This review describes emerging roles of H2O2 as a signaling agent in the nigrostriatal pathway and other basal ganglia neurons.
Introduction
Reactive oxygen species (ROS), including superoxide (•O2-), hydrogen peroxide (H2O2), and the hydroxyl radical (•OH), are often viewed as toxic waste from cellular oxidative metabolism. Over the past decade, however, accumulating evidence indicates that ROS are normal components of signaling pathways. In particular, H2O2 is implicated in physiological processes ranging from embryonic development to cell death (Sundaresan and others 1995; Nishida and others 2000; Rhee 2006; Rhee and others 2005; Stone and Yang 2006; Miller and others 2007b; D'Autreaux and Toledano 2007; Coffman and others 2009; Gerich and Funke 2009; Groeger and others 2009; Rigoulet and others 2010). In brain cells, H2O2 has been implicated as an intracellular regulator of neuronal activity, growth, and organelle function (Avshalumov and others 2005; Miller and others 2007b; Gerich and Funke 2009; Lee and Rice 2008), as well as a diffusible messenger for neuron-glia signaling (Atkins and Sweatt 1999) and inter-neuronal communication, including regulation of synaptic transmission and plasticity (Samanta and others 1998; Auerbach and Segal 1997; Klann and Thiels 1999; Nishida and others 2000; Nemoto and others 2000; Kamsler and Segal 2003; Avshalumov and others 2003; 2008).
In contrast to many ROS, H2O2 is neither a free radical nor an ion. These properties limit reactivity (Cohen 1994) and increase membrane permeability (Ramasarma 1982; Makino and others 2004; Bienart and others 2006, 2007; Adimora and others 2010), so that it is well-suited as both an intracellular signaling agent and as a diffusible messenger. Given its low reactivity, H2O2 does not readily mediate oxidative damage, unless exposed to free metal ions that can catalyze conversion of H2O2 to highly reactive •OH (Cohen 1994). The ability of H2O2 to diffuse away from a site of generation is a characteristic shared with other diffusible messengers, including nitric oxide (NO•), a free radical, and carbon monoxide (CO) (Dawson and Snyder 1994; Kiss and Vizi 2001). In the case of H2O2, however, cell-specific membrane permeability, as well as competing effects of the antioxidant network may govern its efflux and entry (Makino and others 2004; Bienart and others 2007; Adimora and others 2010; Mishina and others 2010).
The first evidence that ROS could regulate neurotransmission was Terry Pellmar's finding that exogenously applied H2O2 can suppress the amplitude of evoked population spikes in hippocampal slices, possibly by inhibiting transmitter release (Pellmar 1986 1987). The possibility of release inhibition was later tested directly in experiments to assess the effect of H2O2 on evoked dopamine (DA) release in striatal slices, monitored using carbon-fiber microelectrodes and fast-scan cyclic voltammetry (Chen and others 2001). Those studies not only confirmed that exogenously applied H2O2 could reversibly suppress axonal DA release, but also demonstrated for the first time that endogenously generated H2O2 could modulate transmitter release on a subsecond time scale. Subsequent work over the next decade examined where and how modulatory H2O2 acts in striatum to modulate DA release and demonstrated that H2O2 also modulates DA neuron activity and somatodendritic DA release in the substantia nigra pars compacta (SNc). Regulation of the nigrostriatal DA system is important because of the central role this pathway plays in the control of movement by the basal ganglia.
This review summarizes data showing that endogenous H2O2 is an intracellular signal that modulates the activity of individual DA neurons in the SNc and a diffusible messenger in striatum. The predominant effect of activity-dependent H2O2 on the nigrostriatal pathway is inhibitory, mediated by activation of ATP-sensitive K+ (KATP) channels. However, emerging evidence indicates that this dynamically generated modulator can also act at a specific class of transient receptor potential (TRP) channels to have an excitatory effect on non-DA neurons in the striatum and in the substantia nigra pars reticulata (SNr). Also discussed are consequences of false H2O2 signaling that can occur during mitochondrial dysfunction, mimicked by partial inhibition of mitochondrial complex I by rotenone. Under these conditions, activity-independent H2O2 generation can also lead to KATP channel opening and suppression of axonal DA release. Thus, elevated H2O2 levels can provide both true and false signals in the brain, and thereby mediate both physiological and pathophysiological signaling.
H2O2 generation, regulation, and functional concentration
H2O2 generation
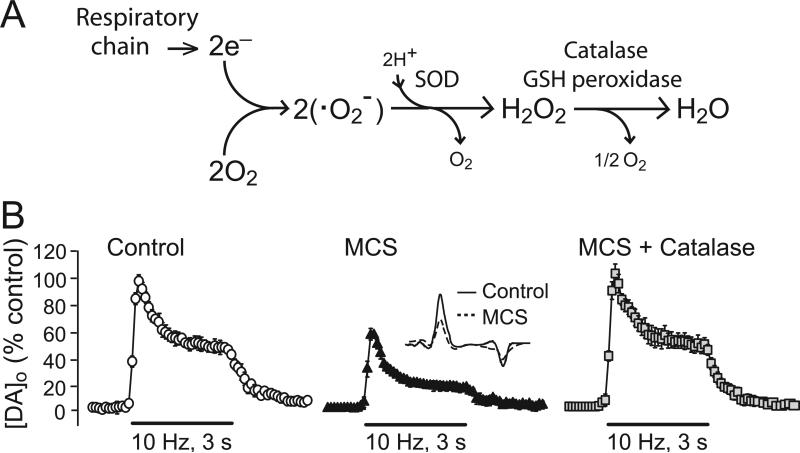
Cellular generation of ROS can occur from a variety of processes, the most ubiquitous of which is mitochondrial respiration (Boveris and Chance 1973; Kennedy and others 1992; Dugan and others 1995; Peuchen and others 1997; Liu and others 2002; Adam-Vizi, 2005; Bao and others 2009). Mitochondria produce •O2- from the single-electron reduction of molecular oxygen (Fig. 1A). Additional sources of •O2- include the enzymes NADPH oxidase (Barbior 1984; Lambeth 2004; Infanger and others 2006; Rhee 2006; Bedard and Krause 2007) and monoamine oxidase (MAO; Maker and others 1981; Azzaro and others 1985). Levels of •O2- are managed by mitochondrial and cytosolic forms of superoxide dismutase, which convert two molecules of •O2- to H2O2 and water (Fig. 1A). Production of •O2- and H2O2 is not insignificant: data from isolated brain mitochondria suggest that up to 5% of O2 consumed becomes H2O2 (Arnaiz and others 1999), although this may depend on cell-specific levels of mitochondrial uncoupling proteins and other factors (Liss and others 2005). Generation of H2O2 is also enhanced during periods of increased local activity, given the increased O2 consumption required to re-establish ion gradients.
Figure 1. Factors that regulate cellular H2O2 and consequences of manipulation of some of these on evoked striatal DA release.
A) Production of H2O2 by the mitochondrial respiratory chain and regulation by antioxidant enzymes. Mitochondria produce •O2- from the single-electron reduction of molecular oxygen; H2O2 is formed from •O2- by the action of superoxide dismutase (SOD) as well as by spontaneous dismutation. H2O2 levels are managed in part by peroxidase enzymes, GSH peroxidase and catalase. B) Modulation of striatal DA release evoked using 10 Hz, 30-pulse trains by manipulation of endogenous H2O2 availability in guinea-pig brain slices. Inhibition of GSH peroxidase by mercaptosuccinate (MCS; 1 mM) leads to suppression of evoked DA release, monitored with carbon-fiber microelectrodes and fast-scan cyclic voltammetry (inset shows DA voltammograms under control conditions and in MCS). Application of catalase (500 IU/mL) in the continued presence of MCS reverses H2O2-dependent DA release suppression. Data are means ± SEM (modified from Avshalumov et al. 2003; copyright Journal of Neuroscience, used with permission).
H2O2 regulation
Absolute intracellular H2O2 concentration, and thus the availability of H2O2 to act as a diffusible messenger, is determined by the balance between activity-dependent H2O2 generation and the competitive process of removal by antioxidant enzymes and interactive thiols. The major peroxidase enzymes are glutathione (GSH) peroxidase, which is cytosolic and mitochondrial (Stults and others 1977), and catalase, which is localized in intracellular peroxisomes (Cohen 1994; Peuchen and others 1997; Dringen and others 2005) (Fig. 1A). Regulation of H2O2 and its precursor •O2- by antioxidant enzymes is important because interaction of either H2O2 or •O2- with trace metal ions, like iron and copper, can produce the aggressive radical, •OH, which is managed by the low molecular weight antioxidants GSH and ascorbate (Cohen 1994). Additional regulation of H2O2 comes from thioredoxins and other cellular thiols, as well as peroxiredoxins, which are abundant but have lower catalytic efficacy than GSH peroxidase or catalase (Rhee and others 2001, 2005; Hofmann and others 2002; Adimora and others 2010; Mishina and others 2010).
A role for H2O2 as a neuromodulator, however, requires more subtle regulation by the antioxidant network usually considered. This network must be structured to allow levels of H2O2 (and other ROS) to reach functional concentrations intracellularly and at potentially distant targets, yet prevent oxidative stress (Avshalumov and others 2004). Key features of this permissive, yet protective environment include: 1) the predominance of ascorbate in neurons, which provides good •OH scavenging with little interference of H2O2 signaling; 2) the predominance of cytosolic GSH peroxidase in glia; and 3) sub-compartmentalization of catalase in peroxisomes in neurons and glia that help facilitate transient H2O2 elevation and subsequent escape from the compartment of generation (Cohen 1994; Desagher et al. 1996; Rice and Russo-Menna 1998; Avshalumov and Rice 2002; Rice 2000; Avshalumov and others 2004; Dringen and others 2005).
Functional concentrations of modulatory H2O2
At any given time, cellular levels of H2O2 reflect the balance among generation, metabolism, and H2O2 diffusion into and out of a cell. However, there is little consensus on the range of physiologically relevant intracellular H2O2 concentrations. Determination of absolute concentrations has been hindered by the characteristics of first-generation H2O2-sensitive fluorescent dyes, exemplified by dihydro-dichlorofluorescein (H2DCF), which becomes fluorescent dichlorofluorescein after oxidation by H2O2 in an irreversible reaction. Dye irreversibility precludes accurate calibration, because fluorescence intensity is proportional to the number of molecules activated throughout the monitoring period, rather than to concentration per se (Oyama and others 1994; Avshalumov and others 2007). Attempts to determine intracellular H2O2 levels have been complicated further by high cellular peroxidase activity, which rapidly depletes known concentrations of exogenous H2O2 (see Dringen and others 2005 for review). Nonetheless, upper limits from 100 nM to 1 μM H2O2 have been estimated experimentally using cultured cells or using mathematical models of contributing factors (Antunes and Cadenas 2000, 2001; Stone 2004; Adimora and others 2010). The upper limits determined experimentally are usually indicated by the onset of irreversible damage, with predictions of intracellular H2O2 levels that are 10-100-fold lower than the concentrations of H2O2 applied exogenously. Consistent with these predictions and the strong regulation by peroxidase activity they imply, endogenous peroxidase activity in guinea-pig hippocampal slices is sufficient to prevent irreversible physiological damage during exposure to exogenous H2O2 at a concentration of 1.5 mM for 15 min; however, damage is seen when either GSH peroxidase or catalase is inhibited pharmacologically before H2O2 application (Avshalumov and others 2004). Other studies have shown that mitochondrial function is not impaired by mM levels of H2O2 when the brain antioxidant network is intact (Gerich and others 2009). Strong H2O2 regulation is also seen in the steep concentration-response for hippocampal population spike inhibition, with no suppression with 1.2 mM H2O2, but maximal (80-90%) suppression with 1.5 mM (Avshalumov and others 2000). Based on models that predict intracellular H2O2 concentrations that are 10-100-fold lower than that applied exogenously, these data imply reversible efficacy at cellular levels of 15-150 μM.
Of course, these calculations do not directly assess the range of endogenous H2O2 fluctuations required for cell signaling. Comparisons of the effect of exogenously applied and endogenously elevated H2O2 do begin to address this, however. The concentration of exogenous H2O2 required for suppression of the hippocampal population spike, 1.5 mM, is also required to cause a reversible 30-40% suppression of DA release evoked by local electrical stimulation in guinea-pig striatal slices (Chen and others 2001; 2002). As in hippocampal slices, there is no evidence of oxidative damage in H2O2-exposed striatal slices; moreover, striatal DA content is unaltered, indicating that the observed decrease in evoked extracellular DA concentration ([DA]o) is not from oxidative loss of the releasable pool of DA (Chen and others 2001). Importantly, when endogenous H2O2 signaling in the striatum is amplified by inhibition of GSH peroxidase with mercaptosuccinate (MCS, 1 mM), a 30-40% decrease in evoked [DA]o is also seen (Chen and others 2002; Avshalumov and others 2003); this effect is reversible by exogenous catalase, confirming that it is H2O2 dependent (Fig. 1B). Amplification of endogenous H2O2 with MCS causes no change in striatal DA content (Avshalumov and others 2008). As discussed further below, MCS amplifies endogenous levels of H2O2 generated during this brief, 3 s stimulation, with suppression of evoked [DA]o seen within the first few hundred milliseconds of stimulation. This dynamic regulation is much faster than the slower processes of H2O2 generation involved in development, for example, which occur over a time frame of minutes to hours.
The similar degree of suppression of DA release with either exogenously added or endogenously elevated H2O2 on DA release implies that comparable H2O2 signaling is achieved under both conditions. These data further suggest that levels of H2O2 for physiologically relevant signaling without oxidative damage are also in the range of 15-150 μM. Fortunately, the question of functional H2O2 concentrations in the intra- and extracellular compartments of brain tissue may be resolved in the near future, given the advent of new tools for monitoring tissue levels of H2O2, including second generation imaging dyes and fluorescent protein-based probes (Miller and others 2007a; Meyer and Dick 2010; Srikun and others 2010; Heller and others 2010; Funke and others 2011), as well as real-time electrochemical methods (Sanford and others 2010; Li and others 2010).
H2O2 generation in SNc DA neurons and striatal medium spiny neurons
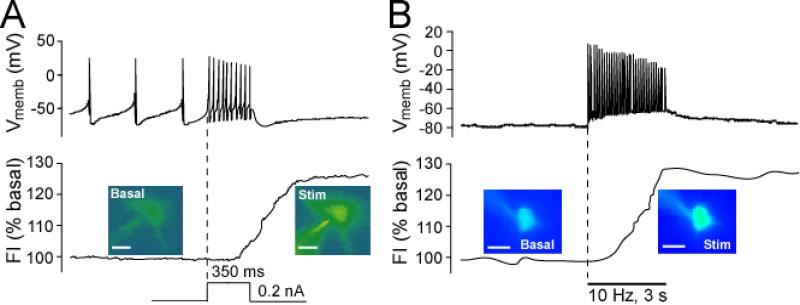
Despite the limitations of DCF fluorescence imaging for quantitative evaluation of absolute cellular H2O2 concentrations, this dye has been used to monitor relative basal and stimulated levels of H2O2. Basal DCF fluorescence is detected in all SNc DA neurons examined (Fig. 2A), indicating tonic, activity-dependent H2O2 generation during the spontaneous pacemaker firing these cells exhibit in brain slice preparations (Avshalumov and others 2005). Basal H2O2 levels can be amplified in a concentration-dependent manner by the GSH-peroxidase inhibitor, MCS (Avshalumov and others 2005). Moreover, in the absence of MCS, cellular H2O2 levels can be elevated dynamically when DA neuron firing rate is increased during depolarizing current injection: a 25-30% increase in DCF fluorescence is seen with an increase in mean spike frequency from ~3 Hz to ~32 Hz (Fig. 2A) (Avshalumov and others 2005). Both basal and elevated H2O2 levels have significant effects on the spontaneous firing of SNc DA neurons, as discussed further below.
Figure 2. Basal and activity-dependent H2O2 generation in SNc DA neurons and striatal MSNs.
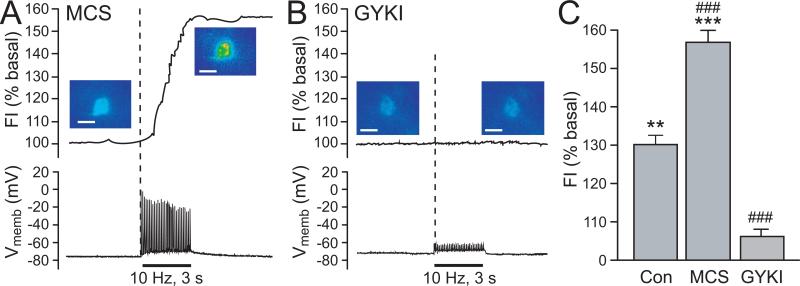
Representative examples of simultaneous current-clamp recordings of membrane voltage (Vmemb) and intracellular H2O2 indicated by changes in DCF fluorescence intensity (FI) in guinea-pig striatal or midbrain slices. The time course of stimulus-induced changes in DCF FI is shown with pseudocolor images recorded under basal conditions and at the end of stimulation (scale bar = 20 μm in DCF images). A) In all SNc DA neurons tested (n = 17), depolarizing current injection (0.2 nA, 350 ms) induced an increase in firing rate (upper panel) accompanied by elevated H2O2 levels (p < 0.01 vs. basal FI; lower panel). Dashed vertical line indicates onset of current injection (modified from Avshalumov and others 2005; copyright Journal of Neuroscience, used with permission). B) In all striatal MSNs tested (n = 11), local pulse-train stimulation (30 pulses, 10 Hz) generated a single action potential with each stimulus pulse (upper panel). In 7 of 11 MSNs, this activity was accompanied by a significant increase in DCF FI (p < 0.01 vs. basal) (lower panel) (modified from Avshalumov and others 2008; copyright American Physiological Society, used with permission).
In contrast to SNc DA neurons, which are spontaneously active in vitro, stratial medium spiny neurons (MSNs) are typically electrically silent in brain slice preparations. Nonetheless, under control conditions, basal DCF fluorescence is also seen in striatal MSNs, reflecting a basal H2O2 tone (Fig. 2B). During local electrical stimulation, each pulse of the stimulus train (30 pulses, 10 Hz) elicits a single action potential in MSNs (Fig. 2B). This stimulus paradigm also produces a ~30% increase in DCF fluorescence in a majority of MSNs (Avshalumov and others 2008) (Fig. 2B). Blockade of glutamatergic AMPA receptors (AMPARs) prevents stimulus-induced action potentials, as well as activity-dependent H2O2 generation in these cells. Interestingly, when 30 brief current injection pulses are used instead of local stimulation to evoke action potentials in MSNs, no increase in DCF fluorescence is seen (Avshalumov and others 2008). One explanation is that glutamate-receptor activation is an important upstream step in the generation of modulatory H2O2 in the striatum.
Regulation of SNc DA neuron activity by H2O2 and KATP channels
Basal H2O2 levels modulate the firing rate of SNc DA neurons via KATP channels
Does tonically generated H2O2 in SNc DA neurons (Fig. 2A) influence cell activity? Yes, it does: depletion of intracellular H2O2 by including catalase in the recording pipette used for whole-cell recording in these neurons causes a ~40% increase in spontaneous firing rate in all DA neurons tested (Avshalumov and others 2005). The mechanism by which H2O2 regulates cellular activity involves H2O2-dependent opening of KATP channels (Avshalumov and others 2005). Blocking KATP channels in SNc DA neurons not only causes a similar increase in spontaneous firing rate to that seen with catalase, but also occludes the usual increase with catalase (Avshalumov and others 2005).
KATP channels are octameric proteins composed of four inward rectifier K+ channel subunits that form a central pore, typically Kir6.2 in neurons and Kir6.1 in glia (Karschin and others 1997; Aschroft and Gribble 1998), and four surrounding sulfonylurea-binding subunits, either SUR1 or SUR2 (Babenko and others 1998; Aguilar-Bryan and others 1998). Channels based on SUR1 or SUR2 subunits can be distinguished by their differential sensitivity to KATP-channel openers (Inagaki and others 1996; Babenko and others 2000). Previous physiological studies demonstrated that exogenous H2O2 can cause membrane hyperpolarization by activating a K+ conductance in a variety of cell types, including pancreatic β-cells (Krippeit-Drews and others 1999) and CA1 hippocampal neurons (Seutin and others 1995). Studies of the nigrostriatal pathway provided the first evidence that endogenous H2O2 can activate KATP channels (Avshalumov and others 2003, 2005; Avshalumov and Rice 2003).
The effect of catalase on DA neuron firing rate and the occlusion of this by a non-selective KATP channel blocker, glibenclamide, indicate that basal H2O2 contributes to regulation of DA neuron excitability by maintaining a KATP-channel tone (Avshalumov and others 2005). It should be noted that the backfill solution in those whole-cell recording studies contained 3 mM ATP, at which concentration, KATP channels in DA neurons should be closed (Häusser and others 1991). Thus, it is unlikely that the resting KATP channel tone in DA neurons is caused by low ATP. Indeed, previous studies using inside-out membrane patches from cardiac cells have shown a direct, concentration-dependent effect of H2O2 on KATP channel opening by decreasing channel sensitivity to ATP (Ichinari and others 1996). Whether regulation by H2O2 in intact cells is direct or indirect in DA neurons remains to be determined.
H2O2 elevation can inhibit SNc DA neuron firing via KATP channels
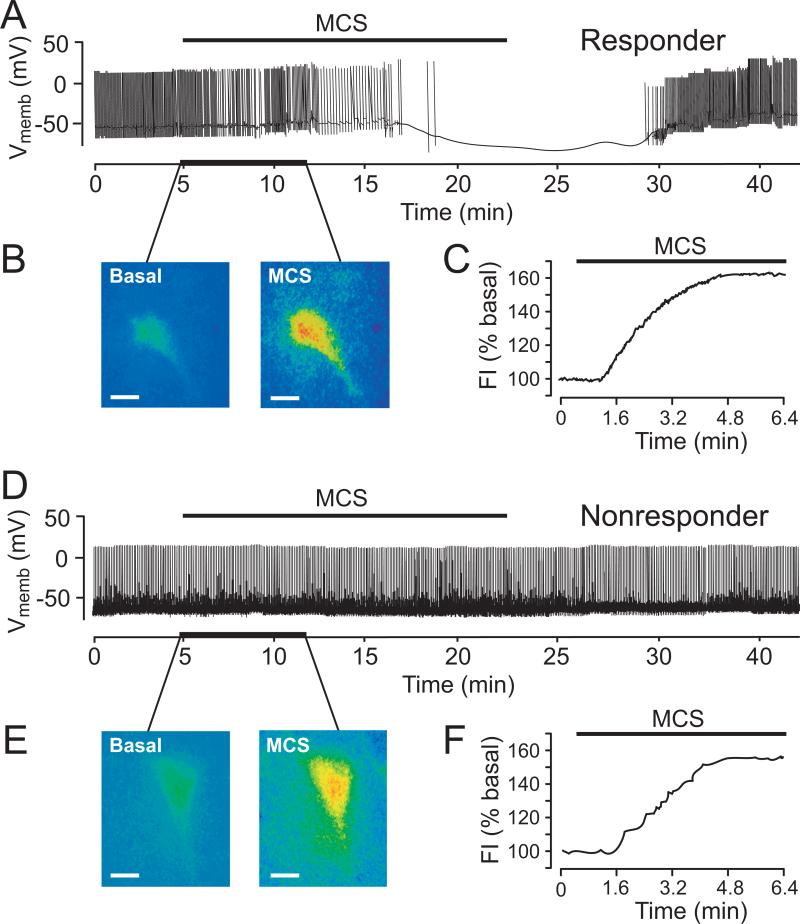
Does elevation of cellular H2O2 above basal levels have additional effects on SNc DA neuron activity? The answer depends on the level of H2O2 elevation. Whole-cell recording studies with simultaneous fluorescence imaging of DCF to visualize intracellular H2O2 in DA neurons showed that moderate increases in H2O2 (≤ 25% increase in fluorescence intensity) during partial inhibition of GSH peroxidase by MCS (0.1-0.3 mM) has no effect on DA neuron firing rate. However, with greater GSH inhibition (1 mM MCS) (Fig. 3A) or application of exogenous H2O2 (1.5 mM), 50% of recorded cells, ‘responders’, show KATP-channel dependent hyperpolarization within minutes of H2O2 elevation (Fig. 3B,C) (Avshalumov and others 2005). The other 50% of recorded cells show no change in membrane properties with MCS (Fig. 3D), although the increase in DCF FI with MCS in ‘nonresponders’ was indistinguishable from that in responders (Fig. 3E,F). This differential responsiveness is similar to that of metabolically ‘sensitive’ and ‘insensitive’ DA neurons described by Liss and others (1999), who showed that greater metabolic sensitivity is conveyed by SUR1- versus SUR2-based KATP channels.
Figure 3. Effects of GSH peroxidase inhibition on membrane properties and intracellular H2O2 levels in SNc DA neurons in guinea-pig midbrain slices.
A) Inhibition of GSH peroxidase by MCS (1 mM) caused hyperpolarization and cessation of spontaneous activity in one population of DA neurons (responders; n = 20/38). B) Simultaneously recorded DCF fluorescence in a responder (A) before (basal) and during MCS exposure; (C) time course of H2O2 elevation (fluorescence intensity, FI) in this cell. D) MCS did not affect spontaneous activity in a second population of DA neurons (non-responders; n = 18/38). E) Simultaneously recorded DCF fluorescence images before and during MCS in the non-responder in (D); F) time course of H2O2 elevation in this cell (scale bars in DCF images = 20 μm) (modified from Avshalumov and others 2005; copyright Journal of Neuroscience, used with permission).
Differential responsiveness to H2O2 elevation also proved to be a consequence of channel subtype. Using KATP channel openers selective for either SUR1- or SUR2-based channels showed that H2O2-responders hyperpolarize with SUR1-selective diazoxide, but not SUR1-selective cromakalim, with the opposite pattern seen in non-responders (Avshalumov and others 2005). Thus, SUR1 expression conveys enhanced sensitivity to H2O2 elevation. Interestingly, when endogenous H2O2 levels are increased by inhibiting catalase, the predominant peroxidase in SNc (Hung and Lee 1998), with 3-aminotriazole (ATZ), all DA neurons respond with glibenclamide-reversible hyperpolarization. Fluorescence imaging with DCF showed a more rapid and slightly greater increase in DFC FI with ATZ than with MCS (Avshalumov and others 2005), suggesting that SUR2- as well as SUR1-based KATP channels can be activated by sufficiently high and/or rapid H2O2 transients. Overall, these data show that H2O2 has auto-regulatory role in SNc DA neurons, in which an activity-dependent H2O2 generation suppresses neuronal activity via KATP channels, adding a new factor in the reciprocal relationship between metabolism and excitability in these cells.
Modulation of DA release in dorsal striatum by glutamate and GABA requires AMPAR-dependent H2O2 generation and KATP channels
Regulation of striatal DA release by glutamate and GABA requires H2O2
Endogenous H2O2 also provides regulation of the nigrostratial DA pathway at the level of DA axons in the dorsal striatum. As discussed, locally evoked DA release in the striatum can be suppressed by exogenous H2O2 or by elevation of endogenous H2O2 during peroxidase inhibition (Chen and others 2001, 2002; Avshalumov and others 2003, 2008) (Fig. 1B). However is it important to appreciate that dynamically generated H2O2 modulates striatal DA release, without amplification by peroxidase inhibition. This was first seen in experiments to examine regulation of striatal DA release by endogenous glutamate and GABA in striatal slices. Local electrical stimulation elicits the release of these transmitters, as well as release of DA. The effect of concurrently released glutamate and GABA on [DA]o evoked by pulse train stimulation (e.g., 10 Hz, 30 pulses) was assessed using selective receptor antagonists. An initially surprising result in these experiments was that blockade of AMPARs by GYKI-52466 causes up to a 100% increase in evoked [DA]o, indicating that AMPAR activation normally leads to inhibition of DA release (Avshalumov and others 2003) (Fig. 4A). The apparent absence of ionotropic glutamate receptors on DA terminals (Bernard and Bolam 1998; Chen and others 1998) suggested that any glutamatergic influence must be indirect, possibly through AMPAR-dependent regulation of GABAergic microcircuitry. However, neither GABAA receptor (GABAAR) nor GABAB receptor (GABABR) blockade led to an increase in evoked [DA]o. In fact, blockade of GABAARs by picrotoxin causes a nearly 50% decrease in peak evoked [DA]o (Fig. 4B), and blockade of GABABRs by saclofen is without effect (Avshalumov and others 2003). These data indicate that GABA, acting through GABAAR, normally enhances DA release. The influence of GABA on DA release, like that of glutamate, must be indirect, since DA axons in dorsal striatum apparently do not express GABAARs (Fujiyama and others 2000).
Figure 4. Regulation of DA release in dorsal striatum by glutamate and GABA requires H2O2.
A) AMPAR blockade by GYKI-52466 (GYKI; 50 μM) causes a ~100% increase in evoked [DA]o in striatum (p < 0.001, n = 6). B) GABAA receptor blockade by picrotoxin (PTX; 100 μM) causes a ~50% decrease in evoked [DA]o (p < 0.001, n = 6). B) The effect of AMPAR blockade is prevented by catalase (Cat), an H2O2-metabolizing enzyme. D) Catalase abolishes the effect of picrotoxin. Responses in the presence of heat-inactivated catalase were the same as control. Data are means ± SEM, shown as percentage of same-site control (modified from Avshalumov and others 2003; copyright Journal of Neuroscience, used with permission).
Previous observations that elevation of endogenous H2O2 can inhibit DA release (Chen and others 2001, 2002) and that glutamate-receptor activation can enhance mitochondrial H2O2 generation (Dugan and others 1995; Reynolds and Hastings 1995; Bindokas and others 1996; Carriedo and others 2000) led to the hypothesis that AMPAR-dependent suppression of DA release might be mediated by H2O2. This proved to be true. The dramatic effect of AMPAR blockade on evoked DA release is abolished in the presence of exogenous catalase (Fig. 4C) or GSH peroxidase (Avshalumov and others 2003). The effect of GABAAR blockade by picrotoxin on evoked [DA]o is also prevented by catalase (Fig. 4D), demonstrating that GABAergic regulation of striatal DA release requires H2O2 generation, as well. The additional finding that picrotoxin has no effect when AMPARs are blocked suggests that regulation of striatal DA release by glutamate and GABA depends on a common source of modulatory H2O2 (Avshalumov and others 2003).
This AMPAR-dependent process appears to be the primary source of modulatory H2O2 in the striatum, because the usual inhibitory effect of GSH peroxidase inhibition by MCS on DA release is absent when AMPARs are blocked by GYKI-52466 (Avshalumov and others 2003). In contrast to the tonic regulation of SNc DA neuron activity by basal H2O2 levels, MCS also has no effect on DA release evoked by a single stimulus pulse (Avshalumov and others 2003), implying that there is little tonic regulation of the excitability of DA axons by H2O2 in striatal slices. However, suppression of evoked [DA]o is seen within a few hundred milliseconds of the onset of pulse-train stimulation, indicating that dynamically generated H2O2 downstream from AMPAR activation can rapidly suppress DA release during concurrent or subsequent stimulation of DA axons.
Activation of KATP channels underlies H2O2-dependent inhibition of striatal DA release
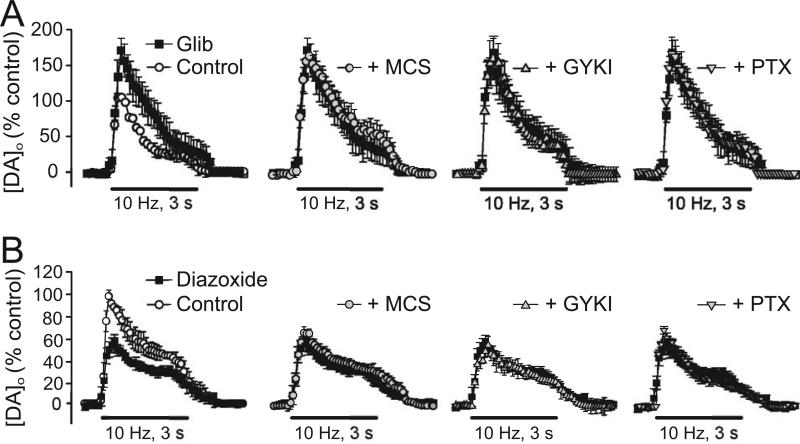
As in SNc DA neurons, elevation of H2O2 in the striatum leads to the opening of KATP channels, presumably located on DA axons, to inhibit striatal DA release (Avshalumov and Rice 2003; Avshalumov and others 2003, 2005). Antagonism of KATP-channels using sulfonylurea agents, either tolbutamide (Avshalumov and others 2003) or glibenclamide (Fig. 5A) (Avshalumov and Rice 2003), causes a significant increase in [DA]o evoked by pulse-train stimulation, indicating that inhibitory KATP channels are normally activated during local stimulation and mediate dynamic suppression of DA release. Furthermore, when KATP channels are blocked, the usual effects of MCS, GYKI-52466, and picrotoxin are prevented (Fig. 5A), demonstrating that KATP channels are required for modulation of DA release by H2O2, glutamate, and GABA.
Figure 5. Inhibition of striatal DA release by endogenous H2O2 is mediated by KATP channels.
A) KATP-channel blockade by glibenclamide (Glib; 3 μM) increased evoked [DA]o in guinea-pig striatal slices (p < 0.01, n = 5) and prevented the usual modulation of DA release by MCS (1 mM), GYKI-52466 (GYKI; 50 μM), and picrotoxin (PTX; 100 μM) (n = 5 for each). B) Diazoxide (30 μM), a SUR1-selective KATP-channel opener, decreased evoked [DA]o (p < 0.01, diazoxide vs. control; n = 5) and also abolished the effects of MCS, GYKI-52466, and PTX (n = 5). Data are means ± SEM (modified from Avshalumov and Rice 2003; copyright 2003 National Academy of Sciences, USA, used with permission).
Are SUR1-based KATP channels again involved? Interestingly, activation of KATP channel opening by either diazoxide or cromakalim causes ~40% decrease in evoked DA release (Fig. 5B) (Avshalumov and Rice 2003). However, in the presence of diazoxide, the effects of MCS, GYKI-52466, and picrotoxin on DA release are lost (Fig. 5B), whereas these changes persist after KATP channel opening by SUR2-selective cromakalim, implying that glutamate-H2O2-dependent modulation of striatal DA release involves SUR1-based KATP channels (Avshalumov and Rice 2003). Thus, differential SUR1 versus SUR2 expression appears to be maintained throughout the nigrostriatal pathway, although the functional significance of this remains unexplored.
Modulatory H2O2 in dorsal striatum is generated in MSNs
To address the cellular source of AMPAR-dependent H2O2 signaling requires a general understanding of striatal circuitry and receptor localization. The overall circuitry of the basal ganglia is relatively well established (Kemp and Powell 1971; Albin and others 1989; Smith and Bolam 1990). Motor regions of the dorsal striatum receive excitatory input from cortex and thalamus and provide the major inhibitory output of the basal ganglia to subcortical regions (Albin and others 1989; Smith and Bolam 1990). The principal striatal efferent cells are GABAergic MSNs (Kemp and Powell 1971), which receive synaptic glutamate input to their dendrites (Smith and Bolam 1990; Bernard and Bolam 1998; Chen and others 1998). These neurons also receive synaptic DA input from SNc DA neurons in the midbrain (Albin and others 1989; Smith and Bolam 1990). The absence of ionotropic glutamate receptors (Bernard and Bolam 1998; Chen and others 1998) and GABAARs (Fujiyama and others 2000) on DA axons suggests that glutamate-dependent H2O2 must be generated in non-DA cells. Prevention of the modulatory effects of endogenous glutamate and GABA by exogenously applied peroxidase enzymes, which are likely to remain in the extracellular compartment, is also consistent with a requirement for extracellular diffusion of H2O2 to inhibit DA release from presynaptic (or non-synaptic) sites.
Current evidence implicates MSNs as the primary cellular source of modulatory H2O2 in dorsal striatum (Avshalumov and others 2008). Not only are these the most abundant striatal neurons (90-95% of the striatal neuron population; Kemp and Powell 1971), but the pattern of sensitivity of DA release to glutamate and GABA antagonists (Avshalumov and others 2003) also mirrors the electrophysiological responsiveness of these cells (Jiang and North 1991; Kita 1996). As already discussed, local stimulation causes a ~30% increase over basal DCF fluorescence in a majority of MSNs (Fig. 2B); this response doubles when GSH peroxidase is inhibited by MCS, confirming amplification of H2O2 signaling with MCS (Avshalumov and others 2008) (Fig. 6A,C). Conversely, AMPAR blockade by GYKI-52466 eliminates both stimulus-induced action potentials in MSNs and activity-dependent H2O2 generation (Fig. 6B,C). These patterns of modulation of H2O2 levels in MSNs are consistent with the patterns of DA release regulation seen with MCS and GYKI-52466.
Figure 6. Patterns of H2O2 regulation in striatal MSNs mirror those predicted from modulation of evoked striatal DA release.
A) Inhibition of GSH peroxidase by MCS (1 mM) amplifies stimulus-evoked increases in DCF FI (30 pulses, 10 Hz) (upper panel), with no effect on action potential generation in recorded MSNs (lower panel). In MCS, 7 of 7 MSNs showed a significant increase in DCF FI (p < 0.001). B) The usual stimulus-induced increase in DCF FI in MSNs (Fig. 2B) was prevented by an AMPAR antagonist, GYKI-52466 (50-100 μM) (upper panel), an AMPAR antagonist, as were stimulus-evoked action potentials monitored during simultaneous current-clamp recording (lower panel) (n = 7; p > 0.05 vs. basal) (in (A) and (B), scale bar = 20 μm). C. Average stimulus-induced changes in DCF FI in H2O2 source MSNs under control conditions (Con; n = 7), in the presence of MCS (n = 7), or in the presence of GYKI (n = 7) (**p < 0.01 vs. basal; ***p < 0.001 vs. basal). The increase in DCF FI in MCS was nearly 2-fold greater than under control conditions, whereas AMPAR blockade with GYKI markedly attenuated the usual control response (###p < 0.001 vs. control) (modified from Avshalumov and others 2008; copyright American Physiological Society, used with permission).
The first report of DA release inhibition by endogenous H2O2 concluded with the hypothesis that DA axons might be the primary source of activity-dependent H2O2 (Chen and others 2001), given the abundance of axonal mitochondria within a few hundred nanometers of DA synapses (Nirenberg and others 1997). In this scenario, H2O2 would be an autoinhibitor of DA release, in the same way that activity-dependent H2O2 is an autoregulator of SNc DA neuron activity (Avshalumov and others 2005). However, subsequent results do not support a role for DA axons in self-generation of modulatory H2O2. Most obviously, the requirement for AMPAR activation (Avshalumov and others 2003) suggests that DA axons in dorsal striatum cannot be the primary source of modulatory H2O2 because they lack AMPARs. Moreover, when AMPARs are blocked, the usual inhibition of evoked [DA]o seen during GSH peroxidase by MCS is absent, implying that there is no H2O2 signal to amplify (Avshalumov and others 2003).
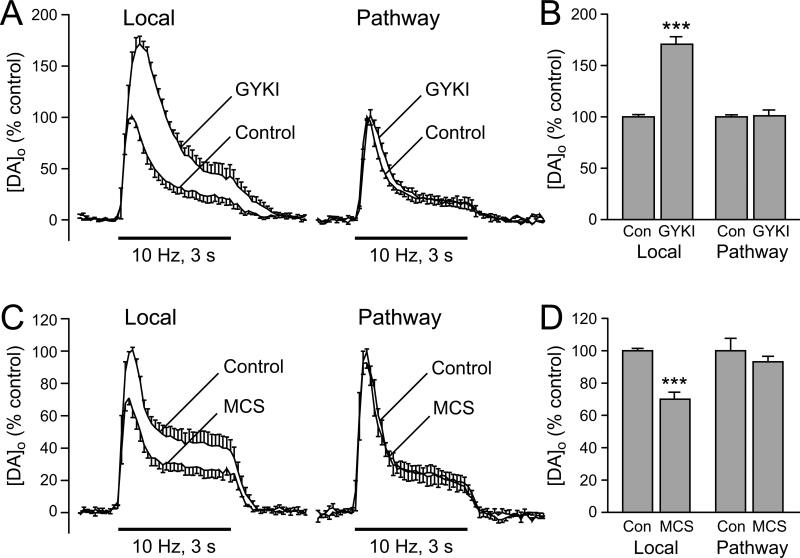
Direct evidence for lack of DA axon involvement has come from experiments using parasagittal slices that allow distal pathway stimulation of DA axons without concurrent activation of local corticostriatal or thalamostriatal input (Avshalumov and others 2008). In those experiments, DA release was recorded from a single site during alternating local and pathway stimulation. The absence of concurrent glutamate release was indicated by a lack of effect of GYKI-52466 on pathway-evoked [DA]o, but the usual enhancement during local stimulation (Fig. 7A,B). Confirming little, if any, direct AMPAR-independent contribution from DA axons to the generation of modulatory H2O2, GSH peroxidase inhibition also has no effect on pathway-evoked DA release (Fig. 7C.D) (Avshalumov and others 2008).
Figure 7. Modulatory H2O2 is not generated in striatal DA axons.
A) Average extracellular DA concentration ([DA]o) versus time profiles evoked at a single site by alternating local and pathway stimulation (30 pulses, 10 Hz) in the absence and presence of an AMPAR antagonist, GYKI-52466 (GYKI; 50-100 μM) (n = 6). B. Summary of the effect of GYKI on peak [DA]o evoked by local and pathway stimulation; control peak evoked [DA]o for either local or DA pathway stimulation is 100%. Blockade of AMPARs caused a significant increase in [DA]o evoked by local stimulation but had no effect on pathway-evoked DA release (n = 6; ***p < 0.001 vs. local control). C. Average [DA]o versus time profiles evoked at a single site by alternating local and pathway stimulation in the absence and presence of the GSH peroxidase inhibitor mercaptosuccinate (MCS; 1 mM) (n = 6). D. Summary of the effect of MCS on peak [DA]o at a given site evoked by local versus DA pathway stimulation; control peak evoked [DA]o for either local or pathway stimulation was 100%. MCS caused a significant decrease in [DA]o evoked by local stimulation but had no effect on pathway evoked [DA]o (n = 6; ***p < 0.001 vs. local control) (modified from Avshalumov and others 2008: copyright American Physiological Society, used with permission).
These data also imply that there are no indirect contributions to dynamically generated, modulatory H2O2 from metabolism or autoxidation of released DA. The lack of AMPAR-dependent modulation of DA release by pathway stimulation also argues against a contribution from glutamate co-released from DA axons (Avshalumov and others 2008). Indeed, although glutamate co-release from DA axons was recently confirmed in the shell of the nucleus accumbens (ventral striatum) using optogenetic methods (Tecuapetla and others 2010; Stuber and others 2010), it was shown to be absent in dorsal striatum (Stuber and others 2010).
Mitochondria are the source of AMPAR-dependent H2O2 signaling in dorsal striatum
What cellular processes generate modulatory H2O2? There are three primary sources of H2O2 in the CNS. The most ubiquitous is mitochondrial respiration, as already discussed. A second source of particular relevance in monoaminergic regions is MAO, which catalyzes deamination of DA and other biogenic amines via a two-electron reduction of O2 to H2O2, with one molecule of H2O2 produced for each molecule of DA metabolized (Sandri and others 1990; Cohen and others 1997). The isoforms of MAO are type A (MAO-A) and type B (MAO-B), with MAO-A primarily in neurons and MAO-B primarily in glia (Azarro and others 1985; Levitt and others 1985). The third source of H2O2 is NADPH oxidase, a family of membrane-associated, multi-subunit enzymes that catalyze the one-electron reduction of O2 to form •O2- and subsequently H2O2 (Babior 1984; Lambeth 2004; Infanger and others 2006; Rhee 2006; Bedard and Krause 2007).
The source of H2O2 generation for rapid neuronal signaling proved to be mitochondrial respiration (Bao and others 2009). This was shown using a combination of rotenone, a complex I inhibitor, and succinate, a mitochondrial complex II substrate, which was found to limit H2O2 production, but maintain tissue ATP content (Bao and others 2009). In the presence of this mitochondrial ‘cocktail’, the usual effects of AMPAR blockade and GSH peroxidase inhibition on evoked [DA]o are completely lost. These data implicate mitochondrial complex I as the primary source of modulatory H2O2 in the striatum. Moreover, neither a combination of MAO inhibitors nor an NADPH oxidase inhibitor altered the effect of H2O2 amplification by MCS on evoked [DA]o. Thus, dynamic, glutamate-dependent modulation of striatal DA release requires H2O2 that originates from mitochondria, rather than MAO or NADPH oxidase. Of course, this does not exclude a role for MAO-dependent DA metabolism or NADPH oxidase as sources of H2O2 and/or •O2- that participate in other aspects of neuronal regulation on a longer time scale (Cohen 1994; Zekry and others 2003; Infanger and others 2006; Kishida and Klann, 2007; Miller and others 2007b; Brennan and others 2009).
Triad of DA, glutamate, and GABA synapses bound functionally by diffusible H2O2
Together, the data summarized in the previous sections indicate that H2O2 must be generated in the mitochondria of non-DA cells or processes, then diffuse from there to inhibit axonal DA release via KATP channel activation. Although the ability of H2O2 to act as a diffusible messenger has been recently questioned (Mishina and others 2010), these data demonstrate that rapid cellular elevation of H2O2 allows this ROS to escape the intracellular antioxidant network and affect adjacent targets. Notably, glutamatergic synapses are closely apposed to DA synapses on the dendritic spines of striatal MSNs (Smith and Bolam 1990; Bernard and Bolam 1998; Chen and others 1998), and are thus ideally positioned to modulate DA release via postsynaptically generated H2O2 in these neurons (Fig. 8). Dendrites of MSNs also express GABAARs at sites near spines (Fujiyama and others 2000). This facilitates GABAergic inhibition that can oppose AMPAR-mediated excitation and consequent H2O2 generation from mitochondria, which are abundant in dendrites, but absent from spines (Smith and Bolam 1990; Chen and others 1998). Following AMPAR activation, mitochondrial respiration would be expected to increase to meet the energy demand required for clearance of intracellular Na+ and Ca2+ entry via Ca2+-permeable AMPARs (Carter and Sabatini 2004), as well as via voltage-dependent Na+ and Ca2+ channels (Stefani and others 1998).
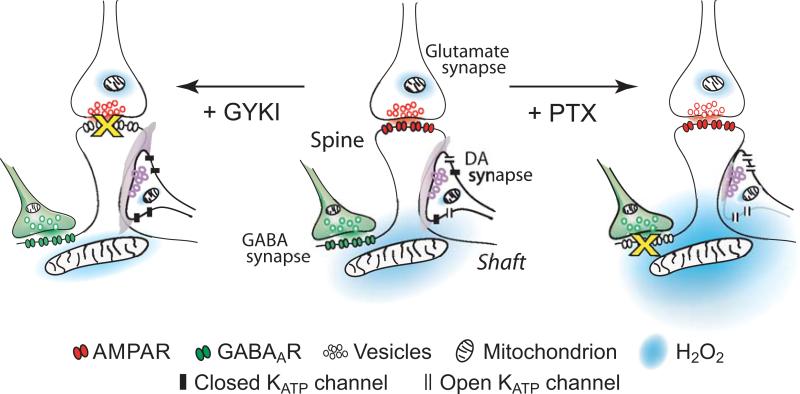
Figure 8. Triad of striatal DA, glutamate and GABA synapses on a medium spiny neuron dendrite bound together functionally by diffusible H2O2.
Generation of modulatory H2O2 when GABA as well as glutamate is released (center); GABAA-receptor (GABAAR) blockade by picrotoxin (+PTX, right), and with AMPA-receptor (AMPAR) blockade by GYKI-52466 (+GYKI, left) (circuitry and locations of receptors and mitochondria are from Smith and Bolam, 1990; Bernard and Bolam, 1998; Chen and others 1998; Fujiyama and others 2000) (modified from Avshalumov and others 2008; copyright American Physiological Society, used with permission).
The resulting model of striatal DA release regulation by glutamate and GABA involves a triad of DA, glutamate, and GABA synapses, separated by a few micrometers on the dendrites of MSNs (Smith and Bolam 1990; Bernard and Bolam 1998; Chen and others 1998; Fujiyama and others 2000). In this model, mitochondrial H2O2 generated in dendrites of MSNs after AMPAR activation diffuses to adjacent DA synapses where it inhibits DA release via opening of SUR1-based KATP channels (Fig. 8, center). By decreasing dendritic excitability, GABA attenuates AMPAR-dependent H2O2 production. When GABAARs are blocked (+ PTX), or GABA input is absent, H2O2 production increases, leading to greater suppression of DA release (Fig. 8, right). On the other hand, when AMPARs are blocked (+ GYKI), or glutamatergic input is absent, H2O2 generation is minimal, DA release is no longer inhibited by H2O2-dependent KATP channel opening, and GABAAR-dependent regulation is lost (Fig. 8, left). Like a brake applied when a car is parked, GABA has no direct influence on DA release when MSNs are not activated by glutamate.
False H2O2 signaling during mitochondrial dysfunction
The finding that mitochondria are the source of H2O2 for dynamic neuronal signaling via KATP-channel activation suggests a potential pathological role for H2O2 under conditions of mitochondrial dysfunction. In isolated mitochondria, partial mitochondrial inhibition by nanomolar concentrations of rotenone alone leads to an increase in H2O2 production (e.g., Votyakova and Reynolds 2001). Consistent with the expected consequences of unregulated H2O2 production, exposure of striatal slices to 30-100 nM rotenone causes a concentration-dependent inhibition of [DA]o evoked by either single- or multiple-pulse stimulation that can be prevented by catalase or by KATP-channel blockade (Bao and others 2005). Tissue ATP content was not changed under these conditions, indicating that “false” H2O2 signaling, not ATP depletion, was the cause of KATP-channel-dependent DA release suppression (Bao and others 2005). The observation that rotenone-induced suppression of DA release is seen with single-pulse stimulation indicates that enhanced H2O2 generation during mitochondrial inhibition is independent of concurrent glutamate release and AMPAR activation, which are the usual triggers for “true” H2O2 signaling in striatum (Bao and others 2005). These findings with rotenone have potentially important implications for Parkinson's disease because mitochondrial dysfunction has been implicated as a casual factor in nigrostriatal degeneration (Schapira and others 1990; Greenamyre and others 2001). The finding that striatal DA release can be compromised by ‘false’ H2O2 signaling during partial mitochondrial dysfunction suggests a mechanism for functional DA denervation that could contribute to motor deficits before structural degeneration of the nigrostriatal pathway.
Modulation of non-DA neurons by H2O2, including excitation via TRP channels
One final point about dynamic neuromodulation by H2O2 is that it is not limited to the nigrostriatal DA system. Other studies have shown that H2O2 influences characteristics of LTP in the hippocampus (Auerbach and Segal 1997; Kamsler and Segal 2003, 2004), which has implications for memory formation. Indeed, instead of the usual expectation that H2O2 is hazardous for brain health, there is evidence that elevated H2O2 production improves spatial memory in aged mice (Kamsler and others 2007). Additionally, diffusible H2O2 mediates neuron-glia signaling in the hippocampus, in which neuronal activation leads to H2O2-dependent phosphorylation of myelin basic protein in oligodendrocytes (Atkins and Sweatt 1999). Recent evidence also suggests that H2O2 may be a neuromodulator in cardiovascular regulation by the nucleus tractus solitarius (Cardoso and others 2009), with bradycardia and hypotension seen after local elevation of H2O2 by exogenous application or following catalase inhibition. These cardiovascular effects appears to involve activation of glutamatergic neurons, but the mechanism has not yet been addressed (Cordoso and others 2009).
One mechanism by which H2O2 can excite neurons is through activation of TRP channels. A specific TRP channel subtype implicated is TRPM2, originally named TRPC7 or LTRPC-2 (Clapham and others 2005), which exhibit activation in the presence of H2O2 and other oxidants (Smith and others 2003; Perraud and others 2003; McNulty and Fonfria 2005). How H2O2 activates TRPM2 channels is somewhat better understood than its actions at KATP channels. Although there is evidence for direct activation of TRPM2 channels (Wehage and others 2002), recent data argue against this (Toth and Csanady 2010). Instead, activation may be mediated by H2O2-dependent elevation of ADP ribose or a synergistic action of H2O2 and ADP ribose (Perraud and others 2005; Hecquet and Malik 2009).
Consistent with a role for TRP channels as targets of H2O2, H2O2 elevation increases the excitability of striatal GABAergic MSNs through activation of a TRP channel, presumed to be TRPM2 (Smith and others 2003; Bao and others 2005). Evidence for H2O2-dependent activation of TRP channels was also recently obtained GABAergic neurons of the substantia pars reticulata (SNr) in guinea-pig midbrain slices (Lee and Rice 2008). Elevation of endogenous H2O2 increases the spontaneous activity of these neurons; equally important, H2O2 depletion leads to a decrease in firing rate and regularity of SNr neurons (Lee and Rice 2008). This H2O2-dependent excitation of SNr GABAergic neurons is diametrically opposed to the inhibitory effect of both tonic and elevated H2O2 in SNc DA neurons mediated by KATP channels (Avshalumov and others 2005). Importantly, much of the inhibitory input to SNc DA neurons is from axon collaterals of SNr GABAergic neurons (Lee and Tepper 2009). Thus, under conditions of H2O2 elevation, enhanced GABAergic inhibition of SNc DA neurons with H2O2 elevation could contribute to the previously reported inhibition of somatodendritic DA release in the SNc seen with exogenous H2O2 or GSH peroxidase inhibition (Chen and others 2002).
Summary and conclusions
The role of H2O2 and other ROS as signaling molecules is increasingly appreciated. Signaling by H2O2 on time scales of minutes to hours is exemplified by its role in mediating downstream effects of growth factors that activate neuronal NADPH oxidase to generate H2O2 (e.g., Miller and others 2007b), which then regulates the activities of intracellular kinases, phosphatases, and other enzymes. The dynamic H2O2 signaling described in this review differs from such slow regulation in four key aspects: 1) the time scale is subsecond to seconds; 2) the source is mitochondria; 3) the targets of H2O2 are ion channels; and 4) dynamically generated H2O2 can act as a diffusible, as well as intracellular signaling agent. In the substantia nigra, H2O2 acts as an autoregulatory signal that modulates the activity of SNc DA and SNr GABAergic neurons. In dorsal striatum, H2O2 acts as a diffusible modulator that reverses the predicted effects of excitatory glutamate and inhibitory GABA on striatal DA release.
Somewhat surprisingly, SNc DA neurons, SNr GABAergic neurons, and striatal MSNs all express H2O2-sensitive KATP and TRP channels (Häusser and others 1991; Schwanstecher and Panten 1993; Roeper and Ashcroft 1995; Stanford and Lacey 1995; Smith and others 2003; Bao and others 2005; Lee and Tepper 2007; Freestone and others 2009). Nonetheless, population-specific responses to H2O2 are seen. This indicates that whether H2O2 will inhibit or excite a given neuron depends on the relative responsiveness of inhibitory KATP channels versus excitatory TRP channels, and possibly other emerging redox-sensitive ion channels (e.g., Tang and others 2004). Other contributing factors include the extent of H2O2 generation and regulation in a given cell. Thus, the relative expression of H2O2-sensitive ion channels coupled with cell-specific H2O2 regulation will define the specificity of dynamic neuromodulation by H2O2.
Acknowledgements
The author is grateful to Drs. Jyoti C. Patel and Christian R. Lee for insightful discussions during the preparation of this review, as well as their generous contribution of time spent reading and editing the final version.
Funding sources
The author is grateful for support from NIH/NINDS grant R01 NS036362, NIH/NINDS grant F32 NS063656 (to Dr. Christian R. Lee), the Ricciardi Research Fund, and the National Parkinson Foundation.
References
- Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxidants and Redox Signaling. 2005;7:1140–1149. doi: 10.1089/ars.2005.7.1140. [DOI] [PubMed] [Google Scholar]
- Adimora NJ, Jones DP, Kemp ML. A model of redox kinetics implicates the thiol proteome in cellular hydrogen peroxide responses. Antioxidants and Redox Signaling. 2010;13:731–743. doi: 10.1089/ars.2009.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Bryan L, Clement JPT, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of KATP channels. Physiological Reviews. 1998;78:227–245. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in Neurosciences. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Antunes F, Cadenas E. Estimation of H2O2 gradients across biomembranes. FEBS Lett. 2000;475:121–26. doi: 10.1016/s0014-5793(00)01638-0. [DOI] [PubMed] [Google Scholar]
- Antunes F, Cadenas E. Cellular titration of apoptosis with steady state concentrations of H2O2: submicromolar levels of H2O2 induce apoptosis through Fenton chemistry independent of the cellular thiol state. Free Radic Biol Med. 2001;30:1008–18. doi: 10.1016/s0891-5849(01)00493-2. [DOI] [PubMed] [Google Scholar]
- Arnaiz SL, Coronel MF, Boveris A. Nitric oxide, superoxide, and hydrogen peroxide production in brain mitochondria after haloperidol treatment. Nitric Oxide. 1999;3:235–43. doi: 10.1006/niox.1999.0229. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Gribble FM. Correlating structure and function in ATP-sensitive K+ channels. Trends in Neurosciences. 1998;21:288–294. doi: 10.1016/s0166-2236(98)01225-9. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Sweatt JD. Reactive oxygen species mediate activity-dependent neuron-glia signaling in output fibers of the hippocampus. Journal of Neuroscience 1999. 1999;19:7241–7248. doi: 10.1523/JNEUROSCI.19-17-07241.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach JM, Segal M. Peroxide modulation of slow onset potentiation in rat hippocampus. Journal of Neuroscience. 1997;17:8695–8701. doi: 10.1523/JNEUROSCI.17-22-08695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Bao L, Patel JC, Rice ME. H2O2 signaling in the nigrostriatal dopamine pathway via ATP-sensitive potassium channels: issues and answers. Antioxidants and Redox Signaling. 2007;9:219–231. doi: 10.1089/ars.2007.9.219. [DOI] [PubMed] [Google Scholar]
- Avshalumov MV, Chen BT, Kóos T, Tepper JM, Rice ME. Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels. Journal of Neuroscience. 2005;25:4222–4231. doi: 10.1523/JNEUROSCI.4701-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Chen BT, Marshall SP, Peña DM, Rice ME. Glutamate-dependent inhibition of dopamine release in striatum is mediated by a new diffusible messenger, H2O2. Journal of Neuroscience. 2003;23:2744–2750. doi: 10.1523/JNEUROSCI.23-07-02744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Chen BT, Rice ME. Mechanisms underlying H2O2-mediated inhibition of synaptic transmission in rat hippocampal slices. Brain Research. 2000;882:86–94. doi: 10.1016/s0006-8993(00)02835-3. [DOI] [PubMed] [Google Scholar]
- Avshalumov MV, MacGregor DG, Sehgal LM, Rice ME. The glial antioxidant network and neuronal ascorbate: permissive yet protective for H2O2 signaling? Neuron Glia Biology. 2004;1:65–376. doi: 10.1017/S1740925X05000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Patel JC, Rice ME. AMPA receptor-dependent H2O2 generation in striatal medium spiny neurons, but not dopamine axons: one source of a retrograde signal that can inhibit dopamine release. Journal of Neurophysiology. 2008;100:1590–1601. doi: 10.1152/jn.90548.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Rice ME. NMDA-receptor activation mediates hydrogen peroxide-induced pathophysiology in rat hippocampal slices. J Neurophysiol. 2002;87:2896–2903. doi: 10.1152/jn.2002.87.6.2896. [DOI] [PubMed] [Google Scholar]
- Avshalumov MV, Rice ME. Activation of ATP-sensitive K+ (KATP) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release. Proceedings of the National Academy of Sciences (U.S.A.) 2003;100:11729–11734. doi: 10.1073/pnas.1834314100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzaro AJ, King J, Kotzuk J, Schoepp DD, Frost J, Schochet S. Guinea pig striatum as a model of human dopamine deamination: the role of monoamine oxidase isozyme ratio, localization, and affinity for substrate in synaptic dopamine metabolism. Journal of Neurochemistry. 1985;45:949–956. doi: 10.1111/j.1471-4159.1985.tb04086.x. [DOI] [PubMed] [Google Scholar]
- Babenko AP, Aguilar-Bryan L, Bryan J. A view of SUR/KIR6.X, KATP channels. Annual Review of Physiology. 1998;60:667–687. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- Babenko AP, Gonzalez G, Bryan J. Pharmaco-topology of sulfonylurea receptors. Separate domains of the regulatory subunits of KATP channel isoforms are required for selective interaction with K+ channel openers. Journal of Biological Chemistry. 2000;275:717–720. doi: 10.1074/jbc.275.2.717. [DOI] [PubMed] [Google Scholar]
- Babior BM. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984;64:959–966. [PubMed] [Google Scholar]
- Bao L, Avshalumov MV, Patel JC, Lee CR, Miller EW, Chang CJ, Rice ME. Mitochondria are the source of hydrogen peroxide for dynamic brain-cell signaling. Journal of Neuroscience. 2009;29:9002–9010. doi: 10.1523/JNEUROSCI.1706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Avshalumov MV, Rice ME. Partial mitochondrial inhibition causes striatal dopamine release suppression and medium spiny neuron depolarization via H2O2 elevation, not ATP depletion. Journal of Neuroscience. 2005;25:10029–10040. doi: 10.1523/JNEUROSCI.2652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological Reviews. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bernard V, Bolam JP. Subcellular and subsynaptic distribution of the NR1 subunit of the NMDA receptor in the neostriatum and globus pallidus of the rat: colocalization at synapses with the GluR2/3 subunit of the AMPA receptor. European Journal of Neuroscience. 1998;10:3721–3738. doi: 10.1046/j.1460-9568.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochimica Biophysica Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Bienert GP, Møller AL, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–92. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- Bindokas VP, Jordan J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. Journal of Neuroscience. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide: general properties and the effect of hyperbaric oxygen. Biochemical Journal. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nature Neuroscience. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso LM, Colombari DS, Menani JV, Toney GM, Chianca DA, Jr, Colombari E. Cardiovascular responses to hydrogen peroxide into the nucleus tractus solitarius. American Journal of Physiology, Regulatory Integrative Comparative Physiology. 2009;297:R462–R469. doi: 10.1152/ajpregu.90796.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriedo SG, Sensi SL, Yin HZ, Weiss JH. AMPA exposures induce mitochondrial Ca2+ overload and ROS generation in spinal motor neurons in vitro. Journal of Neuroscience. 2000;20:240–250. doi: 10.1523/JNEUROSCI.20-01-00240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron. 2004;44:483–493. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Chen BT, Avshalumov MV, Rice ME. H2O2 is a novel, endogenous modulator of synaptic dopamine release. Journal of Neurophysiology. 2001;85:2468–2476. doi: 10.1152/jn.2001.85.6.2468. [DOI] [PubMed] [Google Scholar]
- Chen BT, Avshalumov MV, Rice ME. Modulation of somatodendritic dopamine release by endogenous H2O2: susceptibility in substantia nigra but resistance in the ventral tegmental area. Journal of Neurophysiology. 2002;87:1155–1158. doi: 10.1152/jn.00629.2001. [DOI] [PubMed] [Google Scholar]
- Chen Q, Veenman L, Knopp K, Yan Z, Medina L, Song WJ, Surmeier DJ, Reiner A. Evidence for the preferential localization of glutamate receptor-1 subunits of AMPA receptors to the dendritic spines of medium spiny neurons in rat striatum. Neuroscience. 1998;83:749–761. doi: 10.1016/s0306-4522(97)00452-1. [DOI] [PubMed] [Google Scholar]
- Chung KK, Lipski J. Dopaminergic neurons of the substantia nigra express functional TRPM2 channels. Proceedings of the International Australasian Winter Conference on Brain Research. 2007;25 abstract 10.2. [Google Scholar]
- Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacological Reviews. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- Coffman JA, Coluccio A, Planchart A, Robertson AJ. Oral-aboral axis specification in the sea urchin embryo III. Role of mitochondrial redox signaling via H2O2. Developmental Biology. 2009;330:123–130. doi: 10.1016/j.ydbio.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. Enzymatic/nonenzymatic sources of oxyradicals and regulation of antioxidant defenses. Annals of the New York Academy of Sciences. 1994;738:8–14. doi: 10.1111/j.1749-6632.1994.tb21784.x. [DOI] [PubMed] [Google Scholar]
- D'Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nature Reviews Molecular Cell Biology. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994;14:5147–59. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desagher S, Glowinski J, Premont J. Astrocytes protect neurons from hydrogen peroxide toxicity. Journal of Neuroscience. 1996;16:2553–2562. doi: 10.1523/JNEUROSCI.16-08-02553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Pawlowski PG, Hirrlinger J. Peroxide detoxification by brain cells. Journal of Neuroscience Research. 2005;79:157–165. doi: 10.1002/jnr.20280. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, Goldberg MP, Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-d-aspartate. Journal of Neuroscience. 1995;15:6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freestone PS, Chung KK, Guatteo E, Mercuri NB, Nicholson LF, Lipski J. Acute action of rotenone on nigral dopaminergic neurons -- involvement of reactive oxygen species and disruption of Ca2+ homeostasis. European Journal of Neuroscience. 2009;30:1849–1859. doi: 10.1111/j.1460-9568.2009.06990.x. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Fritschy JM, Stephenson FA, Bolam JP. Synaptic localization of GABAA receptor subunits in the striatum of the rat. Journal of Comparative Neurology. 2000;416:158–172. [PubMed] [Google Scholar]
- Funke F, Gerich FJ, Müller M. Dynamic, semi-quantitative imaging of intracellular ROS levels and redox status in rat hippocampal neurons. Neuroimage. 2011;54:2590–602. doi: 10.1016/j.neuroimage.2010.11.031. [DOI] [PubMed] [Google Scholar]
- Gerich FJ, Funke F, Hildebrandt B, Fasshauer M, Müller M. H2O2-mediated modulation of cytosolic signaling and organelle function in rat hippocampus. Pflugers Archiv. 2009;458:937–952. doi: 10.1007/s00424-009-0672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenamyre JT, Sherer TB, Betarbet R, Panov AV. Complex I and Parkinson's disease. IUBMB Life. 2001;52:135–141. doi: 10.1080/15216540152845939. [DOI] [PubMed] [Google Scholar]
- Groeger G, Quiney C, Cotter TG. Hydrogen peroxide as a cell-survival signaling molecule. Antioxidants and Redox Signaling. 2009;11:2655–2671. doi: 10.1089/ars.2009.2728. [DOI] [PubMed] [Google Scholar]
- Häusser MA, de Weille JR, Lazdunski M. Activation by cromakalim of pre- and post-synaptic ATP-sensitive K+ channels in substantia nigra. Biochemical and Biophysical Research Communications. 1991;174:909–914. doi: 10.1016/0006-291x(91)91504-6. [DOI] [PubMed] [Google Scholar]
- Hecquet CM, Malik AB. Role of H2O2-activated TRPM2 calcium channel in oxidant-induced endothelial injury. Thrombosis and Haemostasis. 2009;101:619–625. [PMC free article] [PubMed] [Google Scholar]
- Hofmann B, Hecht HJ, Flohe L. Peroxiredoxins. Biol Chem. 2002;383:347–64. doi: 10.1515/BC.2002.040. [DOI] [PubMed] [Google Scholar]
- Jin H, Heller DA, Kalbacova M, Kim JH, Zhang J, Boghossian AA, Maheshri N, Strano MS. Detection of single-molecule H2O2 signalling from epidermal growth factor receptor using fluorescent single-walled carbon nanotubes. Nature Nanotechnology. 2010;5:302–209. doi: 10.1038/nnano.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung HC, Lee EH. MPTP produces differential oxidative stress and antioxidative responses in the nigrostriatal and mesolimbic dopaminergic pathways. Free Radical Biology and Medicine. 1998;24:76–848. doi: 10.1016/s0891-5849(97)00206-2. [DOI] [PubMed] [Google Scholar]
- Ichinari K, Kakei M, Matsuoka T, Nakashima H, Tanaka H. Direct activation of the ATP-sensitive potassium channel by oxygen free radicals in guinea-pig ventricular cells: its potentiation by MgADP. Journal of Molecular and Cellular Cardiology. 1996;28:1867–1877. doi: 10.1006/jmcc.1996.0179. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxidants and Redox Signaling. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, North RA. Membrane properties and synaptic responses of rat striatal neurones in vitro. Journal of Physiology (London) 1991;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamsler A, Avital A, Greenberger V, Segal M. Aged SOD overexpressing mice exhibit enhanced spatial memory while lacking hippocampal neurogenesis. Antioxidants and Redox Signaling. 2007;9:181–189. doi: 10.1089/ars.2007.9.181. [DOI] [PubMed] [Google Scholar]
- Kamsler A, Segal M. Hydrogen peroxide as a diffusible signal molecule in synaptic plasticity. Molecular Neurobiology. 2004;29:167–178. doi: 10.1385/MN:29:2:167. [DOI] [PubMed] [Google Scholar]
- Kamsler A, Segal M. Hydrogen peroxide modulation of synaptic plasticity. Journal of Neuroscience. 2003;23:269–276. doi: 10.1523/JNEUROSCI.23-01-00269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin C, Ecke C, Ashcroft FM, Karschin A. Overlapping distribution of KATP channel-forming Kir6.2 subunit and the sulfonylurea receptor SUR1 in rodent brain. FEBS Letters. 1997;401:59–64. doi: 10.1016/s0014-5793(96)01438-x. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The structure of the caudate nucleus of the cat: light and electron microscopy. Philosophical Transactions of the Royal Society London B: Biological Sciences. 1971;262:383–401. doi: 10.1098/rstb.1971.0102. [DOI] [PubMed] [Google Scholar]
- Kennedy RT, Jones SR, Wightman RM. Simultaneous measurement of oxygen and dopamine: coupling of oxygen consumption and neurotransmission. Neuroscience. 1992;47:603–612. doi: 10.1016/0306-4522(92)90169-3. [DOI] [PubMed] [Google Scholar]
- Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxidants and Redox Signaling. 2007;9:233–244. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JP, Vizi ES. Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends Neurosci. 2001;24:211–15. doi: 10.1016/s0166-2236(00)01745-8. [DOI] [PubMed] [Google Scholar]
- Kita H. Glutamatergic and GABAergic postsynaptic responses of striatal spiny neurons to intrastriatal and cortical stimulation recorded in slice preparations. Neuroscience. 1996;70:925–940. doi: 10.1016/0306-4522(95)00410-6. [DOI] [PubMed] [Google Scholar]
- Klann E, Thiels E. Modulation of protein kinases and protein phosphatases by reactive oxygen species: implications for hippocampal synaptic plasticity. Progress in Neuropsychopharmacology and Biological Psychiatry. 1999;23:359–376. doi: 10.1016/s0278-5846(99)00002-0. [DOI] [PubMed] [Google Scholar]
- Krippeit-Drews P, Kramer C, Welker S, Lang F, Ammon HP, Drews G. Interference of H2O2 with stimulus-secretion coupling in mouse pancreatic beta-cells. Journal of Physiology (London) 1999;514:471–481. doi: 10.1111/j.1469-7793.1999.471ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nature Reviews Immunology. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Lee CR, Tepper JM. A calcium-activated nonselective cation conductance underlies the plateau potential in rat substantia nigra GABAergic neurons. Journal of Neuroscience. 2007;27:6531–6541. doi: 10.1523/JNEUROSCI.1678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Tepper JM. Basal ganglia control of substantia nigra dopaminergic neurons. Journal of Neural Transmission Supplement. 2009;73:71–90. doi: 10.1007/978-3-211-92660-4_6. [DOI] [PubMed] [Google Scholar]
- Lee CR, Witkovsky P, Rice ME. Regulation of substantia nigra pars reticulata GABAergic neuron activity by H2O2 via flufenamic acid-sensitive channels and KATP channels. Front Syst Neurosci. 2011;5:14. doi: 10.3389/fnsys.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P, Maxwell GD, Pintar JE. Specific cellular expression of monoamine oxidase B during early stages of quail embryogenesis. Developmental Biology. 1985;110:346–361. doi: 10.1016/0012-1606(85)90094-6. [DOI] [PubMed] [Google Scholar]
- Li X, Liu Y, Zhu A, Luo Y, Deng Z, Tian Y. Real-time electrochemical monitoring of cellular H2O2 integrated with in situ selective cultivation of living cells based on dual functional protein microarrays at Au-TiO2 surfaces. Analytical Chemistry. 2010;82:6512–6518. doi: 10.1021/ac100807c. [DOI] [PubMed] [Google Scholar]
- Liss B, Bruns R. Roeper J. Alternative sulfonylurea receptor expression defines metabolic sensitivity to of K-ATP channels in dopaminergic midbrain neurons. EMBO Journal. 1999;18:833–846. doi: 10.1093/emboj/18.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss B, Haeckel O, Wildmann J, Miki T, Seino S, Roeper J. K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nature Neuroscience. 2005;8:1742–1751. doi: 10.1038/nn1570. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. Journal of Neurochemistry. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- Maker HS, Weiss C, Silides DJ, Cohen G. Coupling of dopamine oxidation (monoamine oxidase activity) to glutathione oxidation via the generation of hydrogen peroxide in rat brain homogenates. Journal of Neurochemistry. 1981;36:589–593. doi: 10.1111/j.1471-4159.1981.tb01631.x. [DOI] [PubMed] [Google Scholar]
- Makino N, Sasaki K, Hashida K, Sakakura Y. A metabolic model describing the H2O2 elimination by mammalian cells including H2O2 permeation through cytoplasmic and peroxisomal membranes: comparison with experimental data. Biochim Biophys Acta. 2004;1673:149–59. doi: 10.1016/j.bbagen.2004.04.011. [DOI] [PubMed] [Google Scholar]
- McNulty S, Fonfria E. The role of TRPM channels in cell death. Pflugers Archiv. 2005;451:235–242. doi: 10.1007/s00424-005-1440-4. [DOI] [PubMed] [Google Scholar]
- Meyer AJ, Dick TP. Fluorescent protein-based redox probes. Antioxidants and Redox Signaling. 2010;13:621–650. doi: 10.1089/ars.2009.2948. [DOI] [PubMed] [Google Scholar]
- Miller EW, Bian SX, Chang CJ. A fluorescent sensor for imaging reversible redox cycles in living cells. Journal of the American Chemical Society. 2007a;129:3458–3459. doi: 10.1021/ja0668973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EW, Tulyathan O, Isacoff EY, Chang CJ. Molecular imaging of hydrogen peroxide produced for cell signaling. Nature Chemical Biology. 2007b;3:263–267. doi: 10.1038/nchembio871. [DOI] [PubMed] [Google Scholar]
- Mishina NM. Does cellular hydrogen peroxide diffuse or act locally? Antioxidants and Redox Signaling. 2010 doi: 10.1089/ars.2010.3539. others. (in press; doi: 10.1089/ars2010.3539) [DOI] [PubMed] [Google Scholar]
- Nemoto S, Takeda K, Yu ZX, Ferrans VJ, Finkel T. Role for mitochondrial oxidants as regulators of cellular metabolism. Molecular and Cellular Biology. 2000;20:7311–7318. doi: 10.1128/mcb.20.19.7311-7318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Liu Y, Edwards RH, Pickel VM. Vesicular monoamine transporter-2: immunogold localization in striatal axons and terminals. Synapse. 1997;26:194–198. doi: 10.1002/(SICI)1098-2396(199706)26:2<194::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Nishida M, Maruyama Y, Tanaka R, Kontani K, Nagao T, Kurose H. Gαi and Gαo are target proteins of reactive oxygen species. Nature. 2000;408:492–495. doi: 10.1038/35044120. [DOI] [PubMed] [Google Scholar]
- Oyama Y, Hayashi A, Ueha T, Maekawa K. Characterization of 2',7'-dichlorofluorescin fluorescence in dissociated mammalian brain neurons: estimation on intracellular content of hydrogen peroxide. Brain Research. 1994;635:113–117. doi: 10.1016/0006-8993(94)91429-x. [DOI] [PubMed] [Google Scholar]
- Pellmar T. Electrophysiological correlates of peroxide damage in guinea pig hippocampus in vitro. Brain Research. 1986;364:377–381. doi: 10.1016/0006-8993(86)90851-6. [DOI] [PubMed] [Google Scholar]
- Pellmar TC. Peroxide alters neuronal excitability in the CA1 region of guinea-pig hippocampus in vitro. Neuroscience. 1987;23:447–456. doi: 10.1016/0306-4522(87)90068-6. [DOI] [PubMed] [Google Scholar]
- Perraud AL, Schmitz C, Scharenberg AM. TRPM2 Ca2+ permeable cation channels: from gene to biological function. Cell Calcium. 2003;33:519–531. doi: 10.1016/s0143-4160(03)00057-5. [DOI] [PubMed] [Google Scholar]
- Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, Knowles HM, Ferraris D, Li W, Zhang J, Stoddard BL, Scharenberg AM. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem. 2005;280:6138–48. doi: 10.1074/jbc.M411446200. [DOI] [PubMed] [Google Scholar]
- Peuchen S, Bolanos JP, Heales SJ, Almeida A, Duchen MR, Clark JB. Interrelationships between astrocyte function, oxidative stress and antioxidant status within the central nervous system. Progress in Neurobiology. 1997;52:261–281. doi: 10.1016/s0301-0082(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Ramasarma T. Generation of H2O2 in biomembranes. Biochemica Biophysica Acta. 1983;694:69–93. doi: 10.1016/0304-4157(82)90014-4. [DOI] [PubMed] [Google Scholar]
- Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. Journal of Neuroscience. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Kang SW, Chang TS, Jeong W, Kim K. Peroxiredoxins: a novel family of peroxidases. IUBMB Life. 2001;52:35–41. doi: 10.1080/15216540252774748. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17:183–89. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–16. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213–1223. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- Rigoulet M, Yoboue ED, Devin A. Mitochondrial ROS generation and its regulation Mechanisms involved in H2O2 signaling. Antioxidants and Redox Signaling. 2010 doi: 10.1089/ars.2010.3363. (in press) [DOI] [PubMed] [Google Scholar]
- Roeper J, Ashcroft FM. Metabolic inhibition and low internal ATP activate K-ATP channels in rat dopaminergic substantia nigra neurones. Pflugers Archiv. 1995;430:44–54. doi: 10.1007/BF00373838. [DOI] [PubMed] [Google Scholar]
- Samanta S, Perkinton MS, Morgan M, Williams RJ. Hydrogen peroxide enhances signal-responsive arachidonic acid release from neurons: role of mitogen-activated protein kinase. Journal of Neurochemistry. 1998;70:2082–2090. doi: 10.1046/j.1471-4159.1998.70052082.x. [DOI] [PubMed] [Google Scholar]
- Sandri G, Panfili E, Ernster L. Hydrogen peroxide production by monoamine oxidase in isolated rat-brain mitochondria: its effect on glutathione levels and Ca2+ efflux. Biochim Biophys Acta. 1990;1035:300–5. doi: 10.1016/0304-4165(90)90092-b. [DOI] [PubMed] [Google Scholar]
- Sanford AL, Morton SW, Whitehouse KL, Oara HM, Lugo-Morales LZ, Roberts JG, Sombers LA. Voltammetric detection of hydrogen peroxide at carbon fiber microelectrodes. Analytical Chemistry. 2010;82:5205–5210. doi: 10.1021/ac100536s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. Journal of Neurochemistry. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Schwanstecher C, Panten U. Tolbutamide- and diazoxide-sensitive K+ channel in neurons of substantia nigra pars reticulata. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:113–17. doi: 10.1007/BF00168546. [DOI] [PubMed] [Google Scholar]
- Seutin V, Scuvee-Moreau J, Masotte L, Dresse A. Hydrogen peroxide hyperpolarizes rat CA1 pyramidal neurons by inducing an increase in potassium conductance. Brain Research. 1995;683:275–278. doi: 10.1016/0006-8993(95)00436-t. [DOI] [PubMed] [Google Scholar]
- Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurons. Trends in Neurosciences. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- Smith MA, Herson PS, Lee K, Pinnock RD, Ashford ML. Hydrogen-peroxide-induced toxicity of rat striatal neurones involves activation of a non-selective cation channel. J Physiol. 2003;547:417–425. doi: 10.1113/jphysiol.2002.034561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikun D, Albers AE, Nam CI, Iavarone AT, Chang CJ. Organelle-targetable fluorescent probes for imaging hydrogen peroxide in living cells via SNAP-tag protein labeling. Journal of the American Chemical Society. 2010;132:4455–4465. doi: 10.1021/ja100117u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford IM, Lacey MG. Electrophysiological investigation of adenosine trisphosphate-sensitive potassium channels in the rat substantia nigra pars reticulata. Neuroscience. 1996;74:499–509. doi: 10.1016/0306-4522(96)00151-0. [DOI] [PubMed] [Google Scholar]
- Stefani A, Chen Q, Flores-Hernandez J, Jiao Y, Reiner A, Surmeier DJ. Physiological and molecular properties of AMPA/Kainate receptors expressed by striatal medium spiny neurons. Developmental Neuroscience. 1998;20:242–252. doi: 10.1159/000017318. [DOI] [PubMed] [Google Scholar]
- Stone JR. An assessment of proposed mechanisms for sensing hydrogen peroxide in mammalian systems. Arch Biochem Biophys. 2004;422:119–24. doi: 10.1016/j.abb.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxidants and Redox Signaling. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. Journal of Neuroscience. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stults FH, Forstrom JW, Chiu DTY, Tappel AL. Rat liver glutathione peroxidase: purification and study of multiple forms. Archives of Biochemistry and Biophysics. 1977;183:490–497. doi: 10.1016/0003-9861(77)90384-8. [DOI] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- Tang XD, Garcia ML, Heinemann SH, Hoshi T. Reactive oxygen species impair Slo1 BK channel function by altering cysteine-mediated calcium sensing. Nat Struct Mol Biol. 2004;11:171–8. doi: 10.1038/nsmb725. [DOI] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koós T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. Journal of Neuroscience. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth B, Csanady L. Identification of direct and indirect effectors of the transient receptor potential melastatin 2 (TRPM2) cation channel. Journal of Biological Chemistry. 2010 doi: 10.1074/jbc.M109.066464. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votyakova TV, Reynolds IJ. ΔΨm-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. Journal of Neurochemistry. 2001;9:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- Wehage E, Eisfeld J, Heiner I, Jüngling E, Zitt C, Lückhoff A. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. A splice variant reveals a mode of activation independent of ADP-ribose. Journal of Biological Chemistry. 2002;277:23150–23156. doi: 10.1074/jbc.M112096200. [DOI] [PubMed] [Google Scholar]
- Zekry D, Epperson TK, Krause KH. A role for NOX NADPH oxidases in Alzheimer's disease and other types of dementia? IUBMB Life. 2003;55:307–313. doi: 10.1080/1521654031000153049. [DOI] [PubMed] [Google Scholar]