Abstract
Accumulating evidence suggests a role for the medial temporal lobe (MTL) in working memory (WM). However, little is known concerning its functional interactions with other cortical regions in the distributed neural network subserving WM. To reveal these, we availed of subjects with MTL damage and characterized changes in effective connectivity while subjects engaged in WM task. Specifically, we compared dynamic causal models, extracted from magnetoencephalographic recordings during verbal WM encoding, in temporal lobe epilepsy patients (with left hippocampal sclerosis) and controls. Bayesian model comparison indicated that the best model (across subjects) evidenced bilateral, forward, and backward connections, coupling inferior temporal cortex (ITC), inferior frontal cortex (IFC), and MTL. MTL damage weakened backward connections from left MTL to left ITC, a decrease accompanied by strengthening of (bidirectional) connections between IFC and MTL in the contralesional hemisphere. These findings provide novel evidence concerning functional interactions between nodes of this fundamental cognitive network and sheds light on how these interactions are modified as a result of focal damage to MTL. The findings highlight that a reduced (top-down) influence of the MTL on ipsilateral language regions is accompanied by enhanced reciprocal coupling in the undamaged hemisphere providing a first demonstration of “connectional diaschisis.”
Keywords: dynamic causal modeling, effective connectivity, magnetoencephalography, temporal lobe epilepsy, working memory
Introduction
Extensive evidence indicates that medial temporal lobe (MTL) is not exclusively involved in long-term memory (LTM). Human neuroimaging studies have reported activation of MTL during working memory (WM) tasks that engage informational encoding (Campo et al. 2005; Karlsgodt et al. 2005; Mainy et al. 2007), maintenance of information (Ranganath and D'Esposito 2001; Axmacher et al. 2007), and retrieval (Cabeza et al. 2002; Schon et al. 2009). Also supporting this view, neuropsychological and neuroimaging studies have revealed impaired performance and abnormalities in MTL activity during WM tasks in patients with MTL damage with various causes (Owen et al. 1996; Krauss et al. 1997; Abrahams et al. 1999; Grady et al. 2001; Lancelot et al. 2003; Lee et al. 2006; Olson et al. 2006; Piekema et al. 2007; Ezzyat and Olson 2008; Wagner et al. 2009). However, based on the assumption that cognitive processes engage distributed neural networks, if we want to gain a clearer understanding of the functional role of MTL in WM it cannot be considered as an independent processor. It is, therefore, necessary to characterize that role from the perspective of the functional systems (Bullmore and Sporns 2009). Accordingly, the goal of the current study was to investigate the conjoint function of MTL and other functionally related brain regions involved in verbal WM as a large-scale network (Bressler and Menon 2010). We obtained whole-head magnetoencephalographic (MEG) recordings during a verbal WM task, which was designed to ensure that participants encoded words semantically (Campo et al. 2005, 2009), as prior neuroimaging investigations have demonstrated that depth processing modulates MTL activity (Kapur et al. 1994; Lepage et al. 2000).
Although few previous studies have used connectivity analyses to investigate the interactions between MTL and other key structures in the neural network involved in visual and verbal WM (Petersson et al. 2006; Nee and Jonides 2008; Rissman et al. 2008), our study diverges from those in 2 main aspects. First, we studied temporal lobe epilepsy (TLE) patients with left hippocampal sclerosis (HS) (Trenerry et al. 1993; Thom et al. 2005) in order to evaluate the impact of unilateral MTL pathology on functional organization and connectivity among brain regions engaged in verbal WM encoding. This is considered as a useful approach that allows the characterization of changes in the functional organization of interconnected brain regions following focal brain damage (Guye et al. 2008). Second, we used dynamic causal modeling (DCM) (Friston et al. 2003; David et al. 2006; Daunizeau et al. forthcoming) to characterize the effective connectivity in the WM network, in subjects with and without MTL damage (Seghier et al. 2010). Effective connectivity denotes “directed or causal relationships between elements” (Bullmore and Sporns 2009) and in the present context refers to the change that the activity in one brain region causes in the activity of another, and how this is modulated by experimental factors (Stephan and Friston 2007). Effective connectivity can be estimated with Bayesian model inversion by perturbing the system and measuring its response (Friston and Price 2001; Garrido, Kilner, Kiebel, and Friston 2007)—this is DCM. DCM represents a fundamental variation from alternative methods to estimate connectivity because it employs a generative model of measured brain responses that takes into account their nonlinear and dynamic nature. As opposed to functional connectivity measures that explore nondirectional statistical dependencies between brain regions, DCM explicitly estimates the causal influence of one area over another.
On the basis of previous findings showing changes in functional connectivity (correlations) during declarative memory in TLE patients (Addis et al. 2007; Wagner et al. 2007; Bettus et al. 2009; Frings et al. 2009; Voets et al. 2009), we hypothesized that TLE patients with left HS would show decreased connectivity between left MTL and ipsilateral brain regions (prefrontal and temporal cortices) and an increased connectivity in contralateral homologous structures (Bettus et al. 2009). More specifically, we expected changes in the connectivity of MTL with inferior temporal language cortex and IFC/ventrolateral prefrontal cortex (VLPFC) (Fiebach et al. 2006; Nee and Jonides 2008; Rissman et al. 2008; Ojemann et al. 2009; Saling 2009; Hashimoto et al. 2010). Neurons in ITC respond selectively to task relevant features of stimuli in visual WM (Fuster 1990), are active during verbal WM when semantic processing is required (Fiebach et al. 2006, 2007), and have been shown to be affected in patients with semantic WM deficits (Hoffman et al. 2009). Interestingly, an interaction of ITC with rhinal cortex is considered to be part of a semantic associative memory subsystem (Saling et al. 1993; Saling 2009). Furthermore, previous studies have highlighted the relevance of hippocampus–ITC connectivity in strengthening mnemonic traces during visual WM (Axmacher et al. 2008; Rissman et al. 2008), while VLPFC-MTL interactions have been associated with semantic memory processing during WM in a prior study (Nee and Jonides 2008) and have been proposed to be fundamental for memory formation (Ranganath et al. 2005).
Materials and Methods
Participants
Eleven patients (6 males) with refractory MTL epilepsy were consecutively recruited following presurgical evaluation at the “Hospital Ruber Internacional” and participated in the study. They ranged in age from 24 to 43 years (mean = 32.91; standard deviation [SD] = 6.89). Diagnosis was established according to clinical EEG and magnetic resonance imaging (MRI) data. All patients underwent neurological examination, continuous video-EEG monitoring, and high-resolution 1.5-T brain MRI. Patients were included in the study when clinical data and MRI and EEG findings were suggestive of unilateral mesial TLE related to left HS. All patients had: 1) seizures with typical temporal lobe semiology that were not controlled with antiepileptic drugs (AEDs) and 2) moderate to severe decreased volume (and abnormally increased T2 and FLAIR signal) of the left hippocampus on brain MRI. No lesions were observed in other structures beyond left MTL. Bedside video-EEG monitoring showed interictal epileptiform activity ipsilateral to the side of HS and in 5 cases complex partial seizures with an ictal onset in left anterior temporal electrodes. No seizure occurred within 24 h prior to the experiment. At the time of study, patients were on AED treatment, including levetiracetam, lamotrigine, oxcarbazepine, carbamazepine, valproate, topiramate, zonisamide, clonazepam, lorazepam, either in monotherapy or multitherapy.
As a control group, we recruited 11 healthy volunteers (6 males), ranging in age from 26 to 34 years (mean = 31.09; SD = 2.63). Participants were interviewed and entered in the study if they met the following inclusion criteria: 1) absence of a previous history of neuropathological conditions or psychopathological diseases and 2) no antecedent of drug or alcohol abuse. There was no significant difference between groups in terms of age (t20 = 0.82, P > 0.20).
Demographic and clinical information about patients and controls is provided in Table 1.
Table 1.
Demographic and clinical information of patients and controls
| TLE (n = 11) | Controls (n = 11) | |
| Age | 32.91 (6.89) | 31.09 (2.63) |
| Years of education | 15.18 (2.40) | 16.91 (1.04) |
| Duration of epilepsy (years) | 18.23 (11.70) | |
| Age at epilepsy onset (years) | 14.68 (10.76) | |
| Seizure frequency (per month) | 2.54 (0.93) | |
| AED (number) | 1.73 (0.47) |
Participants were right handed according to the Edinburgh Handedness Inventory (Oldfield 1971), and Spanish was their primary language. All participants signed a consent form detailing the procedures of the study in accordance with the Declaration of Helsinki (1991).
Stimuli and Tasks
We used the same verbal WM task as in our previous studies (Campo et al. 2005, 2009). In each trial, subjects first saw a stimulus array comprising 4 words, located centrally in the display. The to-be-remembered array remained on the screen for 3000 ms. After a 2500 ms delay interval, participants were presented with 3 consecutive probe displays comprising a semantic category name for 500 ms. They were required to make a push-button response to indicate whether any of the words belonged to the semantic category represented by one of probe words. Thus, correct performance required subjects to maintain the target words in memory and make a semantic categorization; ensuring a deep processing of probe words. There was an interval between probes of 500–700 ms. Match and no-match trials occurred with equal probability.
Concrete words were used, 4–7 letters in length (5.62 ± 1.57) and of moderate frequency (Algarabel 1996). A total of 120 trials were presented. The stimuli were projected through a LCD video projector (SONY VPL-X600E), situated outside the shielded room, onto a series of in-room mirrors, the last of which was suspended approximately 50 cm above the subject’s face and subtended a visual angle of 1–3o horizontally and 0.5o vertically.
Data Acquisition and Analysis
All MEG recordings were obtained using a whole-head neuromagnetometer comprising an array of 148 magnetometers (4-D 2500, San Diego) housed in a magnetically shielded room. Neuromagnetic signals were digitized continuously at 678 Hz and were band-pass filtered between 0.1 and 100 Hz. MEG data were submitted to an interactive noise reduction procedure to reduce environmental noise (4-D 2500, San Diego). Data were analyzed using SPM8 (Wellcome Trust Centre for Neuroimaging, London; http://www.fil.ion.ucl.ac.uk/spm/). The continuous time series for each participant was subjected to a Butterworth band-pass filter at 3–30 Hz. We analyzed epoched encoding period activity for each trial, for each participant. Trials including eye blinks or other myogenic or mechanical artifacts were removed using the thresholding artifact rejection algorithm implemented in SPM8 (trials containing signal strength exceeding 3000 fT were excluded). After artifact rejection, epochs were baseline corrected from −100 to 0 ms and then averaged.
Source Localization
Multiple sparse priors (as implemented in SPM8) were used to estimate the cortical origin of the neuronal response during the encoding period (Friston et al. 2008). This model specifies 512 sparse patches of activation and then iteratively reduces them until an optimal number and location of active patches are found using a (variational) Bayesian scheme. The hyperparameters of these multiple sparse priors are optimized using a greedy search. A tessellated cortical mesh template surface in canonical (Montreal Neurological Institute [MNI]) served as a brain model to estimate the current source distribution (Mattout et al. 2007). This dipole mesh was used to calculate the forward solution using a spherical head model. The inverse solution was calculated over a time window from 0 to 1000 ms during the encoding epoch. These reconstructions were analyzed using a general linear model (Kilner and Friston 2010), as described in Furl et al. (2010).
Effective Connectivity Analysis: DCM
Determining effective connectivity requires a causal model of the interactions among the constituents of the neural network subject to study (Stephan and Friston 2007). DCM considers the brain as “a deterministic nonlinear dynamical system that is subject to inputs and produces outputs” (David 2007).
DCM is a hypothesis-driven method that relies on the specification of a plausible biophysical and physiological model of interacting brain regions (Stephan and Friston 2007). The model is specified by its regions connections and by whether these connections are unidirectional (forward or backward) or bidirectional (both forward and backward). Forward and backward connections are defined according to the connectivity rules outlined in Felleman and Van Essen (1991) and specified in DCM to convey bottom-up and top-down effects, respectively. This model is then supplemented with a forward model of how neuronal or synaptic activity is transformed into a measured response (Kiebel et al. 2006). This enables the parameters of the neuronal model (i.e., effective connectivity) and spatial model (i.e., dipole orientations) to be estimated from observed data using a Bayesian scheme. Estimating the parameters of a DCM model relies on estimating the hidden states and parameters of the modeled system, which corresponds to the sources that comprise the model (David et al. 2006). DCM for MEG uses a neural mass model to explain source activity (David and Friston 2003) and has been described in detail elsewhere (David et al. 2006).
DCM Specification: Hypotheses Tested
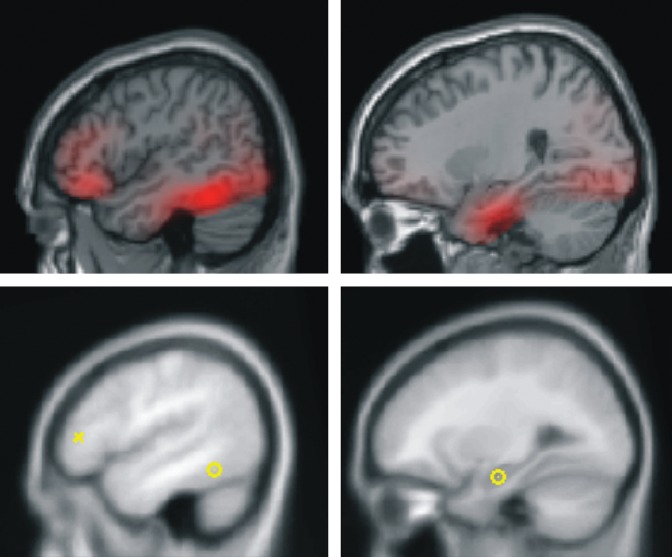
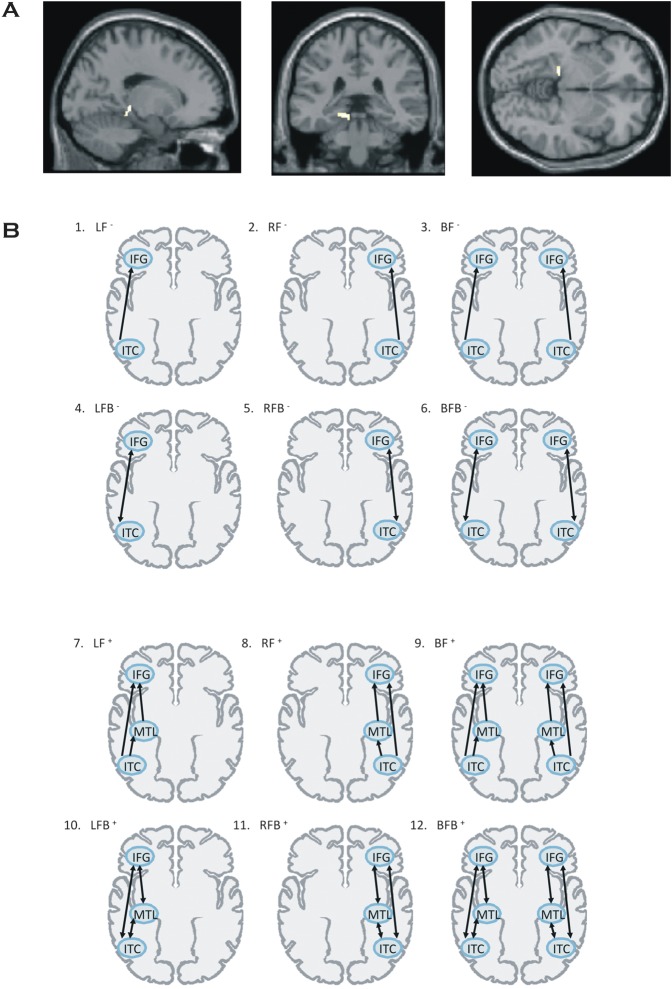
Network architecture was specified on the basis of the inverse solutions (source localizations; see Fig. 1) for single subjects using multiple sparse priors (Friston et al. 2008) and was constrained by recent studies of functional connectivity on verbal WM (Fiebach et al. 2006; Nee and Jonides 2008). Accordingly, we considered for our models 6 regions that corresponded to ITC, MTL, and VLPFC/IFC bilaterally. These sources were modeled as equivalent current dipoles, which were superimposed on an MRI of a standard brain in MNI space (Fig. 1), whose prior mean locations coordinates (x, y, z) are: bilateral ITC: −43, −54, −15 (left); 43, −54, −15 (right); bilateral MTL: −27, −15, −20 (left); 27, −15, −20 (right); and bilateral IFC/VLPFC: −54, 35, 6 (left); 54, 35, 6 (right). Twelve models were specified and inverted separately for each subject (Fig. 2b). In all models, left and right ITC were chosen as visual input nodes for semantic processing of words (Bitan et al. 2005; Heim et al. 2009). The models were specified starting with simple architectures and adding hierarchical levels (i.e., sources and extrinsic connections). The simplest models only included the ITC and IFC/VLPFC sources, while more complex models included MTL sources. The sources were left unilateral, right unilateral, or bilateral. Models also differed in terms of their connections; forward only or both forward and backward. Accordingly, model lF − included left unilateral and forward connections, while model bFB − included bilateral sources with forward and backward connections. Models with MTL sources were created by simply adding MTL sources; that is, model lF+ included left unilateral and forward connections and the MTL. See Figure 2b, for details. The MTL models allowed an evaluation of the involvement of MTL in verbal WM and the functional relevance of the connections of this region within the network.
Figure 1.
Source localization for a representative subject using multiple sparse priors (upper panel). Sources of activity, modeled as dipoles (estimated posterior moments and locations) superimposed in an MRI of a standard brain in MNI space (lower panel).
Figure 2.
(A) Significant differences between groups in left MTL derived from conventional SPM8 analysis rendered on an averaged normalized brain. (B) Outline of the 12 DCM models for the effective connectivity analysis shown on axial brain schematics (see text for coordinates of all regions). The brain regions comprising the network architecture for each model are represented by circles. Arrows between the regions indicate the directionality of the connections (i.e., forward or forward and backward). IFG, inferior frontal gyrus; ITC, inferior temporal cortex; MTL, medial temporal lobe.
Model Comparison
One of the advantages of DCM is that it can be used to compare competing hypotheses about functional architectures (David 2007; Garrido, Kilner, Kiebel, Stephan, et al. 2007; Friston 2009; Garrido, Kilner, Kiebel, Stephan, et al. 2009). This is accomplished by specifying a model (hypothesis), in terms of anatomical connections between brain regions. Using Bayesian model selection, DCM tests a group of competing models and provides evidence in favor of one model, relative to others (Penny et al. 2004). The model log-evidence or the marginal log-likelihood of each model is compared against the remaining models. The model with the highest evidence (i.e., the model with the best balance of accuracy and complexity) is then considered the best or optimal model. A difference of 3 or more in favor of one model as compared with others is required (Penny et al. 2004). We performed a fixed-effect analysis for comparing model log-evidence at the group level (i.e., patient group and control group), which is accomplished by summing the log-evidence of each participant for each model, finding the highest valued model and comparing it with the summed log evidence of the next highest model (Garrido, Kilner, Kiebel, and Friston 2007; Garrido, Kilner, Kiebel, Stephan, et al. 2009; Reyt et al. forthcoming). We also performed a random-effect analysis for comparing model evidence, an approach that admits different models for different subjects and that is relevant when investigating “cognitive tasks that can be performed with different strategies” (Stephan et al. 2009; Penny et al. 2010; Reyt et al. forthcoming). The 12 models were also compared at a single subject level (Penny et al. 2004; Garrido, Kilner, Kiebel, and Friston 2009). After selecting the optimal model, its subject-specific parameters (restricted to posterior probabilities of 90% or more) were analyzed using paired t-tests, to test for group differences in the usual way (Noppeney et al. 2006; Werner and Noppeney 2010). Following previous studies (Mechelli et al. 2007; Benetti et al. 2009), we controlled for Type-I error derived from multiple comparisons using a statistical threshold of P < 0.025.
Results
Behavioral Performance
We assessed performance in the verbal WM task was in terms of correct hits for each stimulus set. We observed a mean accuracy level of 75.55% (SD = 8.79) in the control group and mean accuracy of 58.93% (SD = 12.53) in the patient group. Control subjects performed significantly better than patients in terms of accuracy (t20 = 3.60; P < 0.001). No significant differences were found for reaction time (RT) measure between groups (t20 = 0.76, P > 0.20). Average RTs were 776.73 for patients (SD = 83.37) and 753.36 for controls (SD = 57.86).
Group Differences in Spatiotemporal Activation
Differences between groups during verbal WM encoding were associated with greater left MTL activation in the control group between 200 and 800 ms (t20 = 3.17, P < 0.003, uncorrected). Further analyses were conducted on more specific time windows of 200 ms duration. These analyses revealed a greater activation in left MTL for the control group between 200 and 400 ms (t20 = 3.59; P < 0.001) (Fig. 2a), between 400 and 600 ms (t20 = 3.28, P < 0.002, uncorrected), and between 600 and 800 ms (t20 = 2.89, P < 0.005, uncorrected). We detected no differential activity for the reverse contrast (patients > controls).
Bayesian Model Selection
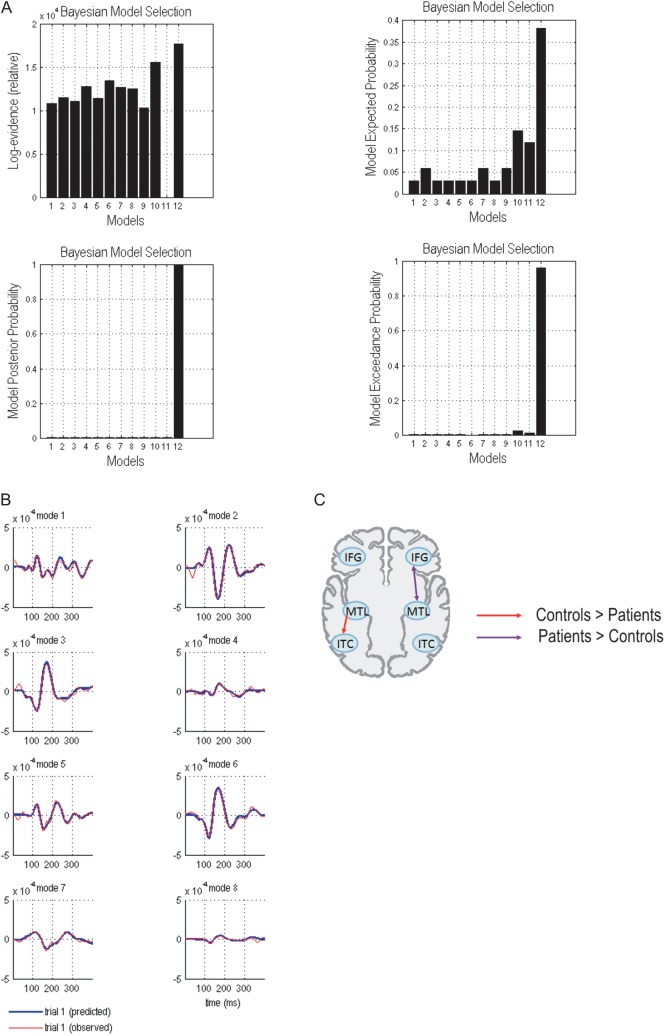
To determine changes that left MTL damage can produce in the functional organization of interconnected brain regions during WM encoding, we evaluated the model evidence for 12 (DCM) models described in the Materials and Methods section (see Fig. 2b). This established the best functional architecture over all subjects, which we then used to test for group differences in connection strengths. A fixed-effects analysis (Garrido, Kilner, Kiebel, Stephan, et al. 2007; Garrido, Kilner, Kiebel, Stephan, et al. 2009) revealed that model bFB+ (i.e., bilateral forward and backward connections) supervened (Bayes factor relative to the second best model [model lFB+] = 452.07). These results constitute “very strong” evidence in favor of model bFB+ (Penny et al. 2004). A random-effect analysis (allowing for random effects on models) yielded similar results (exceedance probability for model bFB+ = 0.965). A representation of the fixed effect and random effect approaches is shown in Figure 3a. Model comparison was also performed for each participant individually. This confirmed that, for the majority of the patients (8 of 11) and controls (6 of 11), model bFB+ was superior to all other models (see Table 2).
Figure 3.
(A) Group level Bayesian selection of the 12 tested models. Left: fixed effect analysis (FFX) showing log-evidence and model posterior probability. Right: random fixed effects (RFX) showing model expected probability and model exceedance probability. Results indicate the best model is one with bilateral forward and backward connections comprising IFG, ITC, and MTL. (Bayes factor relative to the second best model [model lFB+] = 452.07; exceedance probability for model bFB+ = 0.965). 1. LF −; 2. RF −; 3. BF −; 4. LFB −; 5. RFB −; 6. BFB −; 7. LF +; 8. RF +; 9. BF +; 10. LFB +; 11. RFB +; 12. BFB +. L, left; R, right; B, bilateral; F, forward; FB, forward and backward; − model architecture not including MTL; + model architecture including MTL. (B) Predicted (blue) and observed (red) responses in measurement space for the best model. (C) Group differences in effective connectivity assessed using subject-specific (maximum a posteriori) parameter estimates.
Table 2.
Individual Bayes factor for model comparison
| bFB+–lFB+ | bFB+–rF+ | rFB+–rF+ | lFB+–rFB+ | bFB+–rFB+ | bF+–lF+ | rFB+–bFB+ | lFB+–bFB+ | |
| P#1 | 128.85 | |||||||
| P#2 | 152.33 | |||||||
| P#3 | 17.53 | |||||||
| P#4 | 88.94 | |||||||
| P#5 | 20.55 | |||||||
| P#6 | 44.17 | |||||||
| P#7 | 78.68 | |||||||
| P#8 | 95.20 | |||||||
| P#9 | 301.59 | |||||||
| P#10 | 153.24 | |||||||
| P#11 | 72.62 | |||||||
| C#1 | 140.45 | |||||||
| C#2 | 76.02 | |||||||
| C#3 | 21.78 | |||||||
| C#4 | 673.19 | |||||||
| C#5 | 77.69 | |||||||
| C#6 | 169.92 | |||||||
| C#7 | 6.65 | |||||||
| C#8 | 140.03 | |||||||
| C#9 | 98.74 | |||||||
| C#10 | 100.78 | |||||||
| C#11 | 60.15 |
Note: P, patient; C, control; l, left; r, right; b, bilateral; F, forward; FB, forward and backward; − model architecture not including MTL; + model architecture including MTL.
Once the best model had been determined, group differences in effective connectivity were assessed using subject-specific (maximum a posteriori) parameter estimates (Fig. 3c). A two-sample t-test revealed that the extrinsic backward connection from left MTL to left ITC was stronger in controls (mean = 1.40; SD = 0.64) than patients (mean = 0.74; SD = 0.33) (t20 = 2.99, P < 0.01). In the right hemisphere, contralateral to the lesion, backward connection between VLPFC/IFC and MTL was greater for patients (mean = 0.97; SD = 0.34) as compared with controls (mean = 0.51; SD = 0.17) (t20 = 3.98, P < 0.001). A significant greater forward connection from right MTL to right VLPFC/IFC in favor of patients (mean = 1.31; SD = 0.44) (for controls, mean = 0.72; SD = 0.37) was also observed (t20 = 3.36, P < 0.005). We failed to detect differences in the remaining connections (all P > 0.15).
To assess the functional significance of group differences in effective connectivity, connectivity strengths were correlated with task performance. A linear regression analysis showed that the backward connections from right VLPFC/IFC to right MTL were inversely related to task performance (R2 = −0.649, P < 0.002). We also observed a trend for a positive correlation between backward connections from left IFG to left MTL and task performance in the control group (R2 = 0.519, P = 0.10). When an outlier (a control subject with very high performance but low connectivity strength) was eliminated from this analysis, this correlation reached significance (R2 = 0.641, P < 0.05).
A factor that may have influenced these results is the difference in task performance between epilepsy patients and controls. We used a median-split approach to identify a group of patients and controls that were matched on performance because neural activity from patients cannot be unambiguously interpreted unless the 2 groups are matched on this variable (Brown and Eyler 2006). Consequently, a subgroup of patients (n = 7) and a subgroup of controls (n = 7) with similar task performance were selected. These groups did not significantly differ in terms of age (t12 = 1.12; P > 0.20), level of education (t12 = 0.32; P > 0.50), nor in task performance (t12 = 1.04; P > 0.30). Bayesian model comparison showed that model bFB+ (i.e., bilateral forward and backward connections) supervened (fixed effects Bayes factor relative to the second best model [model lFB+] = 391.33). These results constitute very strong evidence in favor of model bFB+ (Penny et al. 2004). Furthermore, we found the same differences in connectivity. That is, extrinsic backward connections from left MTL to left ITC were reduced in patients (mean = 0.81; SD = 0.38) as compared with controls (mean = 1.50; SD = 0.65) (t12 = 2.37, P < 0.02). In the nonlesional hemisphere, a significantly greater forward connection from right MTL to right VLPFC/IFC was seen in patients (mean = 1.34; SD = 0.42) (vs. controls mean = 0.71; SD = 0.39; t13 = 2.85, P < 0.01). Backward connections between VLPFC/IFC and MTL were greater in patients (mean = 0.80; SD = 0.28) than in controls (mean = 0.57; SD = 0.13; t12 = 2.05, P = 0.031).
Discussion
The main focus of the current study was the functional organization expressed in terms of effective connectivity among MTL and other functionally related brain regions subserving verbal WM encoding. This is the first study of effective connectivity in relation to the impact of MTL damage on mnemonic function and we note that all previous work has examined functional connectivity that may or may not be mediated by directed neuronal connections (i.e., effective connectivity). This is because functional connectivity simply establishes a statically dependency between sources and does not address how these dependencies are mediated. Studying effective connectivity allows us to understand the effect of MTL damage precisely since we can examine the causal influences in the network and ask what connections to or from the MTL are affected. The findings of this study corroborate a framework that the MTL is a part of an extended neural network engaged in verbal WM. Moreover, the data provide new evidence about its functional interactions during a WM task and sheds light on how these interactions are modified as a result of localized damage to MTL. Interestingly, we found a bilateral network model with forward and backward connections including MTL, ITC, and IFC/VLPFC (see Materials and Methods section) was the best model across participants. Note that because the prior source locations were based on source reconstructions of the channel data, they are optimized for the particular subjects we studied. The bilateral nature of the model is in agreement with functional imaging studies showing that left and right MTL contribute to verbal memory processes, especially with semantic encoding (Lepage et al. 2000; Davachi and Wagner 2002). However, a left MTL preponderance with “semantically meaningful verbal inputs during stimulus processing and encoding” (Giovagnoli et al. 2005) is supported by model lFB+, which was the best for 3 controls and the second best model in 5 of 8 of the remaining controls
Model comparison allowed us to compare a number of competing hypotheses about the involvement of MTL in verbal WM and the functional relevance of the connections of this region within the network. By comparing models with and without MTL, we found compelling (very strong) evidence for fundamental involvement of MTL during verbal WM (David et al. 2006). Importantly, there was not a single subject for whom a model without MTL had the highest evidence. These results, along with performance deficits, support the notion that the contribution of MTL to WM extends beyond novel or complex stimuli and includes familiar verbal stimuli.
When considering the pattern of interactions among the network regions, we found both an attenuation in effective connectivity in the lesional hemisphere and an enhancement in the contralesional hemisphere in the group of patients as compared with controls. The ipsilateral backward connection from left MTL to left ITC was significantly weakened in the patient group, compared with the control group. Structural connectivity between MTL regions and ITC has been demonstrated in vivo using diffusion tensor imaging in healthy subjects (Powell et al. 2004). Hence, changes in coupling between these regions could be mediated by alterations in white matter connections due to MTL pathology in TLE (Yogarajah et al. 2008; Voets et al. 2009). Alternatively, although not mutually exclusive, a weakened interaction between temporal neocortex and MTL could be caused by damage to rhinal or parahippocampal cortex, commonly reduced in TLE, which has been proposed to regulate this interaction (Squire 1991; Saling 2009). Hence, reduced function and use-dependent changes in gray and/or white matter could lead to regression of connectivity (Fuster 1995). In fact, important reductions of functional connectivity of the lesional MTL has been observed in the ipsilateral hemisphere (Pereira et al. 2010). Furthermore, the duration of epilepsy has been related to a decreased coupling between MTL and ipsilesional temporal cortex in patients with left TLE (Wagner et al. 2007). On the purely functional side, the interaction between left MTL and left ITC is compatible with feedback from MTL to cortex during memory formation (Ranganath et al. 2005; Rissman et al. 2008) and has been reported in previous studies of functional connectivity (Rajah et al. 1999; Grady et al. 2003; Gagnepain et al. 2011), although in the context of a LTM task. Greater functional connectivity between the hippocampus and temporal cortex has been suggested to be indicative of higher functional network integrity in a presurgical group of TLE patients (Wagner et al. 2007). Enhancement of the interplay between MTL and ITC has been also identified during visual WM tasks (Axmacher et al. 2008; Rissman et al. 2008). Interestingly, Axmacher et al. (2008) described this interaction as an increased top-down control (i.e., backward influence) of ITC by the MTL. Likewise, findings from studies with non-human primates (Higuchi and Miyashita 1996; Woloszyn and Sheinberg 2009) have suggested a crucial role of backward signals from MTL to ITC in memory processing during visual WM. This influence can be framed in terms of the projections from MTL to representational posterior brain regions as a key mediator of memory processes (Fuster 1995; Ranganath and D'Esposito 2005). Therefore, the organization of MTL-ITC connectivity during verbal WM can be depicted as the interaction between a region engaged in the semantic processing of verbal information, the ITC (Fiebach et al. 2006, 2007; Nee and Jonides 2008), and a group of structures involved in the temporary retention of incoming information, the MTL (Saling 2009).
The IFC/VLPFC has been shown to manifest increased its connectivity with MTL and ITC during verbal and visual WM tasks (Grady et al. 2001; Simons and Spiers 2003; Fiebach et al. 2007; Nee and Jonides 2008). It has also been shown that the pattern of fronto–limbic interactions in the hemisphere ipsilateral to the lesioned MTL are impaired during memory tasks in patients with TLE (Addis et al. 2007; Voets et al. 2009). Surprisingly, our connectivity analyses failed to identify any differences in the pattern of connectivity of left IFC/VLPFC, either with left ITC or left MTL between groups. Despite this lack of significant differences, we observed a trend to a significant positive correlation between backward connections from left IFC/VLPFC to left MTL in the control group. A significant correlation emerged when we one control with very high performance but low connectivity value was excluded from the analysis. Increased connectivity between VLPFC and MTL during encoding of words has been previously found to correlate with better performance in healthy young subjects (Grady et al. 2003). It is possible that this interaction constitutes a common mechanism supporting encoding process both in WM and LTM (Fuster 1995), as has been recently suggested for retrieval processes (Öztekin et al. 2010).
In contrast, compared with controls, the coupling between IFC/VLPFC and the MTL in the contralesional hemisphere was enhanced in patients. This strengthening of connections was bidirectional. This change in the pattern of connectivity between these regions is interesting, considering that brain activation analyses did not reveal evidence for substantial differences in activation in the contralesional MTL between groups and reflect a dissociation between regional activation and connectivity measures (Grady et al. 2001; Ranganath et al. 2005; Benetti et al. 2009). In this sense, our findings provide evidence for a “connectional diaschisis.” Diaschisis (from Greek, meaning “shocked throughout”) usually refers to loss of neuronal activity, due to lost afferents from a lesion area. It has been generalized to cover “dynamic diaschisis” (Price et al. 2001), which refers to a selective changes in neuronal responses, due to lost afferents. We suggest that the phenomenon we report here reflects a connectional diaschisis—a selective change in coupling due to lost afferents; in this case, from the contralateral (lesioned) nodes of the network.
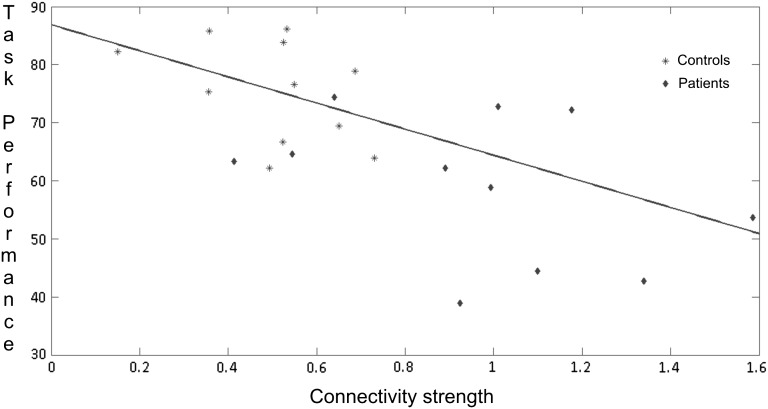
Enhanced recruitment of right prefrontal cortex has been previously reported in left TLE patients while processing verbal material (Maccotta et al. 2007) as well as increased basal functional connectivity involving contralesional MTL in patients with intractable epilepsy of MTL origin (Bettus et al. 2009). Interpretation of these effects as reflecting compensatory mechanisms is controversial (Maccotta et al. 2007; Vlooswijk et al. 2008; Saling 2009). Increased levels of activity or changes in connectivity dynamics in response to a pathological state can be interpreted in different ways, taking into account their relation with task execution (Maccotta et al. 2007). A linear regression analysis showed that the backward connections from right VLPFC/IFC to right MTL was inversely related to task performance (see Fig. 4). Crucially, the relationship between coupling and behavior is seen over both patients and controls, suggesting that the remote effects of lesions on connectivity are functionally (behaviorally) relevant and may reflect compensatory or adaptive changes that are similar to differences among normal subjects. More generally, this correlation lends the coupling estimates predictive validity, in relation to function or task performance. Interestingly, when we controlled for behavioral differences between patients and normal subjects (by examining a subset of subjects), there was still evidence for significant differences in coupling. This suggests that both compensatory and pathophysiological mechanisms underlie the increased connection strength in patients. In this sense, enhanced contralesional fronto–limbic coupling can be regarded “as a marker of network disruption in the presence of mesial temporal pathology” (Saling 2009), which is in agreement with previous work (Dupont et al. 2000; Maccotta et al. 2007; Powell et al. 2007; Vlooswijk et al. 2008). The pathological nature of this pattern of connectivity is reinforced by recent findings showing an increased functional connectivity between hippocampus and diffuse areas of prefrontal cortex which was negatively correlated with performance on a memory task in amnestic mild cognitive impairment patients showing hippocampal atrophy (Bai et al. 2009).
Figure 4.
Correlation of task performance and effective connectivity measures between right VLPFC/IFC and right MTL in patients and controls.
As both groups differed in task performance, correct inferences about connectivity measures require comparisons between a subgroup of patients and controls that were matched on performance. We found that the differences in connectivity measures observed at the group level were maintained at the subgroup level. In view of these results, differences in connectivity between MTLE patients and controls cannot be attributed completely to performance differences. This could suggest that group differences are mediated by the disruption of the network supporting WM encoding due to MTL damage (Mueller et al. 2011). That is, MTL lesion not only affects local neural processing but also interactions with other brain regions that constitute a network supporting a specific cognitive process. Nonetheless, further studies including patients with epileptogenic lesions in other locations (i.e., right MTLE, extratemporal epilepsy) will be needed to evaluate this interpretation.
The potential impact of AEDs on cognitive functioning, brain activation, and connectivity measures cannot be discounted as contributing to the differences reported here. AEDs have been reported to have both positive and negative effects on cognition, in patients, and in healthy controls (Prevey et al. 1996; Thompson et al. 2000; Aldenkamp et al. 2002; Meador et al. 2007; Seo et al. 2007; Park and Kwon 2008) and vary in the type and degree of their associated side effects, depending upon several factors such as the type and dosage of AED used (Meador 2006; Schilbach et al. 2007; Baxendale et al. 2010; Canevini et al. 2010; Hermann et al. 2010). Additionally, it is difficult to dissociate AEDs effects in epileptic patients from the effect of epilepsy itself and associated psychosocial variables (Gualtieri and Johnson 2006; Bocquillon et al. 2009). Although, we can discount an effect of AEDS that is mediated through performance differences (see above), the role of AEDs on brain activation differences cannot be excluded. The effects of AEDs in brain activation are difficult to disentangle since previous studies have shown a decrease in electrophysiological measures of amplitude (Tuunainen et al. 1995) and power (Zaveri et al. 2010) or hemodynamic signals (Chen et al. 2009) either during AED discontinuation or associated with AEDs (Sun et al. 2007). However, it is important to emphasize that activation differences between patients and controls in the current study were restricted to the lesional temporal lobe, a finding that matches with a previous study showing a decrease in power in signals recorded from epileptogenic mesial temporal lobe structures as compared with nonepileptogenic regions (Bettus et al. 2008). Finally, the impact of AEDs on connectivity measures must be also considered. Studies addressing the effects of AEDs on functional connectivity in epilepsy patients are scarce. Chen et al. (2009) have shown an attenuation of frontal-hippocampal connections after AED withdrawal. Similarly, Fingelkurts et al. (2004) observed a widespread increase in functional connectivity after administering Lorazepam to a group of healthy volunteers. Contrary to these findings, van Dellen et al. (2009) found a significant lower phase-lag index in epilepsy patients on multiple AED therapy, compared with those on monotherapy, although no effect of AEDs were found on network configuration measures. It is important to highlight that these results were restricted to the lesional temporal lobe and contradict those from a study showing an increase functional connectivity within the MTL when comparing epilepsy patients and healthy controls at rest (Liao et al. 2010). In relation to our findings, is unlikely that AED effects could account for both an increase and a decrease in specific connections observed in the current study, especially those observed in the nonlesional hemisphere.
The current study has some limitations: One is the relatively small size of the patient group. Therefore, the findings should be considered preliminary and need to be replicated in further patient cohorts. The fact that we obtained significant results with such a small sample size suggests that the sizes of the effects reported above are large; however, our homogenous patient group was selected carefully, and our findings may or may not generalize to other groups. As we have mentioned before, another limitation is that only patients with left MTLE were included. Studies of patients with right MTLE, patients with temporal neocortical lesions and extratemporal epilepsy, should afford a more reliable test of our hypothesis.
In summary, our findings revealed that, 1) MTL is part of a network of functionally related regions subserving verbal WM encoding, 2) this network is best defined as a bilateral cortico-limbic system encompassing IFC/VLPFC, MTL, and ITC, with forward and backward connections, 3) changes caused by damage within left MTL in the aforementioned network are characterized by weakened connections from left MTL to left ITC and by the strengthening of forward/backward connections between IFC/VLPFC and MTL in the contralesional hemisphere, and 4) the pattern of connectivity identified in the patient group may not be an effective compensation for MTL damage but reflect a greater engagement of the remaining components of a damaged network subserving verbal WM (i.e., constituting a connectional diaschisis) and could be considered an indication that the network supporting a specific process (i.e., encoding) has been perturbed by pathophysiology (Mueller et al. 2011).
Funding
Ramon y Cajal Fellowship from the Spanish Ministry of Science and Innovation (RYC-2010-05748 to P.C.); Wellcome Trust (to M.G., R.M., R.D., K.F.).
Acknowledgments
Conflict of Interest : None declared.
References
- Abrahams S, Morris RG, Polkey CE, Jarosz JM, Cox TC, Graves M, Pickering A. Hippocampal involvement in spatial and working memory: a structural MRI analysis of patients with unilateral mesial temporal lobe sclerosis. Brain Cogn. 1999;41:39–65. doi: 10.1006/brcg.1999.1095. [DOI] [PubMed] [Google Scholar]
- Addis DR, Moscovitch M, McAndrews MP. Consequences of hippocampal damage across the autobiographical memory network in left temporal lobe epilepsy. Brain. 2007;130:2327–2342. doi: 10.1093/brain/awm166. [DOI] [PubMed] [Google Scholar]
- Aldenkamp AP, Arends J, Bootsma HP, Diepman L, Hulsman J, Lambrechts D, Leenen L, Majoie M, Schellekens A, de Vocht J. Randomized double-blind parallel-group study comparing cognitive effects of a low-dose lamotrigine with valproate and placebo in healthy volunteers. Epilepsia. 2002;43:19–26. doi: 10.1046/j.1528-1157.2002.29201.x. [DOI] [PubMed] [Google Scholar]
- Algarabel S. Indices de interés psicolinguístico de 1917 palabras castellanas. Cognitiva. 1996;8:43–88. [Google Scholar]
- Axmacher N, Mormann F, Fernandez G, Cohen MX, Elger CE, Fell J. Sustained neural activity patterns during working memory in the human medial temporal lobe. J Neurosci. 2007;27:7807–7816. doi: 10.1523/JNEUROSCI.0962-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Schmitz DP, Wagner T, Elger CE, Fell J. Interactions between medial temporal lobe, prefrontal cortex, and inferior temporal regions during visual working memory: a combined intracranial EEG and functional magnetic resonance imaging study. J Neurosci. 2008;28:7304–7312. doi: 10.1523/JNEUROSCI.1778-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Zhang Z, Watson DR, Yu H, Shi Y, Yuan Y, Zang Y, Zhu C, Qian Y. Abnormal functional connectivity of hippocampus during episodic memory retrieval processing network in amnestic mild cognitive impairment. Biol Psychiatry. 2009;65:951–958. doi: 10.1016/j.biopsych.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Baxendale S, Heaney D, Thompson PJ, Duncan JS. Cognitive consequences of childhood-onset temporal lobe epilepsy across the adult lifespan. Neurology. 2010;75:705–711. doi: 10.1212/WNL.0b013e3181eee3f0. [DOI] [PubMed] [Google Scholar]
- Benetti S, Mechelli A, Picchioni M, Broome M, Williams S, McGuire P. Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain. 2009;132:2426–2436. doi: 10.1093/brain/awp098. [DOI] [PubMed] [Google Scholar]
- Bettus G, Guedj E, Joyeux F, Confort-Gouny S, Soulier E, Laguitton V, Cozzone PJ, Chauvel P, Ranjeva JP, Bartolomei F, et al. Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp. 2009;30:1580–1591. doi: 10.1002/hbm.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettus G, Wendling F, Guye M, Valton L, Regis J, Chauvel P, Bartolomei F. Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res. 2008;81:58–68. doi: 10.1016/j.eplepsyres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Bitan T, Booth JR, Choy J, Burman DD, Gitelman DR, Mesulam MM. Shifts of effective connectivity within a language network during rhyming and spelling. J Neurosci. 2005;25:5397–5403. doi: 10.1523/JNEUROSCI.0864-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocquillon P, Dujardin K, Betrouni N, Phalempin V, Houdayer E, Bourriez JL, Derambure P, Szurhaj W. Attention impairment in temporal lobe epilepsy: a neurophysiological approach via analysis of the P300 wave. Hum Brain Mapp. 2009;30:2267–2277. doi: 10.1002/hbm.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Brown GG, Eyler LT. Methodological and conceptual issues in functional magnetic resonance imaging: applications to schizophrenia research. Annu Rev Clin Psychol. 2006;2:51, 81. doi: 10.1146/annurev.clinpsy.2.022305.095241. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16:317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Campo P, Maestu F, Garcia-Morales I, Gil-Nagel A, Strange B, Morales M, Ortiz T. Modulation of medial temporal lobe activity in epilepsy patients with hippocampal sclerosis during verbal working memory. J Int Neuropsychol Soc. 2009;15:536–546. doi: 10.1017/S135561770909078X. [DOI] [PubMed] [Google Scholar]
- Campo P, Maestu F, Ortiz T, Capilla A, Fernandez S, Fernandez A. Is medial temporal lobe activation specific for encoding long-term memories? Neuroimage. 2005;25:34–42. doi: 10.1016/j.neuroimage.2004.07.074. [DOI] [PubMed] [Google Scholar]
- Canevini MP, De Sarro G, Galimberti CA, Gatti G, Licchetta L, Malerba A, Muscas G, La Neve A, Striano P, Perucca E. Relationship between adverse effects of antiepileptic drugs, number of coprescribed drugs, and drug load in a large cohort of consecutive patients with drug-refractory epilepsy. Epilepsia. 2010;51:797–804. doi: 10.1111/j.1528-1167.2010.02520.x. [DOI] [PubMed] [Google Scholar]
- Chen Q, Wu X, Zhou B, Lui S, Tang HH, Gong QY, Shang H, Yan B, Zhou D. Functional connectivity changes in frontal-hippocampus circuity after withdrawal of topiramate in epilepsy patients. 2009 28th International Epilepsy Congress; 28th June–2nd July; Budapest. Available from: www.epilepsybudapest2009.org. [Google Scholar]
- Daunizeau J, David O, Stephan KE. Dynamic causal modelling: a critical review of the biophysical and statistical foundations. Neuroimage. Forthcoming doi: 10.1016/j.neuroimage.2009.11.062. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- David O. Dynamic causal models and autopoietic systems. Biol Res. 2007;40:487–502. [PMC free article] [PubMed] [Google Scholar]
- David O, Friston KJ. A neural mass model for MEG/EEG: coupling and neuronal dynamics. Neuroimage. 2003;20:1743–1755. doi: 10.1016/j.neuroimage.2003.07.015. [DOI] [PubMed] [Google Scholar]
- David O, Kiebel SJ, Harrison LM, Mattout J, Kilner JM, Friston KJ. Dynamic causal modeling of evoked responses in EEG and MEG. Neuroimage. 2006;30:1255–1272. doi: 10.1016/j.neuroimage.2005.10.045. [DOI] [PubMed] [Google Scholar]
- Dupont S, Van de Moortele PF, Samson S, Hasboun D, Poline JB, Adam C, Lehericy S, Le Bihan D, Samson Y, Baulac M. Episodic memory in left temporal lobe epilepsy: a functional MRI study. Brain. 2000;123(Pt 8):1722–1732. doi: 10.1093/brain/123.8.1722. [DOI] [PubMed] [Google Scholar]
- Ezzyat Y, Olson IR. The medial temporal lobe and visual working memory: comparisons across tasks, delays, and visual similarity. Cogn Affect Behav Neurosci. 2008;8:32–40. doi: 10.3758/cabn.8.1.32. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Smith EE, Swinney D. Lateral inferotemporal cortex maintains conceptual-semantic representations in verbal working memory. J Cogn Neurosci. 2007;19:2035–2049. doi: 10.1162/jocn.2007.19.12.2035. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Rissman J, D'Esposito M. Modulation of inferotemporal cortex activation during verbal working memory maintenance. Neuron. 2006;51:251–261. doi: 10.1016/j.neuron.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingelkurts AA, Kivisaari R, Pekkonen E, Ilmoniemi RJ, Kahkonen S. Enhancement of GABA-related signalling is associated with increase of functional connectivity in human cortex. Hum Brain Mapp. 2004;22:27–39. doi: 10.1002/hbm.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings L, Schulze-Bonhage A, Spreer J, Wagner K. Remote effects of hippocampal damage on default network connectivity in the human brain. J Neurol. 2009;256:2021–2029. doi: 10.1007/s00415-009-5233-0. [DOI] [PubMed] [Google Scholar]
- Friston K. Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS Biol. 2009;7:220–225. doi: 10.1371/journal.pbio.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Harrison L, Daunizeau J, Kiebel S, Phillips C, Trujillo-Barreto N, Henson R, Flandin G, Mattout J. Multiple sparse priors for the M/EEG inverse problem. Neuroimage. 2008;39:1104–1120. doi: 10.1016/j.neuroimage.2007.09.048. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Price CJ. Dynamic representations and generative models of brain function. Brain Res Bull. 2001;54:275–285. doi: 10.1016/s0361-9230(00)00436-6. [DOI] [PubMed] [Google Scholar]
- Furl N, van Rijsbergen NJ, Kiebel SJ, Friston KJ, Treves A, Dolan RJ. Modulation of perception and brain activity by predictable trajectories of facial expressions. Cereb Cortex. 2010;20:694–703. doi: 10.1093/cercor/bhp140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. Inferotemporal units in selective visual attention and short-term memory. J Neurophysiol. 1990;64:681–697. doi: 10.1152/jn.1990.64.3.681. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Memory in the cerebral cortex. Cambridge: MIT Press; 1995. [Google Scholar]
- Gagnepain P, Henson R, Chételat G, Desgranges B, Lebreton K, Eustache F. Is neocortical-hippocampal connectivity a better predictor of subsequent recollection than local increases in hippocampal activity? New insights on the role of priming. J Cogn Neurosci. 2011;23:391–403. doi: 10.1162/jocn.2010.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido MI, Kilner JM, Kiebel SJ, Friston KJ. Evoked brain responses are generated by feedback loops. Proc Natl Acad Sci U S A. 2007;104:20961–20966. doi: 10.1073/pnas.0706274105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido MI, Kilner JM, Kiebel SJ, Friston KJ. Dynamic causal modeling of the response to frequency deviants. J Neurophysiol. 2009;101:2620–2631. doi: 10.1152/jn.90291.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido MI, Kilner JM, Kiebel SJ, Stephan KE, Baldeweg T, Friston KJ. Repetition suppression and plasticity in the human brain. Neuroimage. 2009;48:269–279. doi: 10.1016/j.neuroimage.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido MI, Kilner JM, Kiebel SJ, Stephan KE, Friston KJ. Dynamic causal modelling of evoked potentials: a reproducibility study. Neuroimage. 2007;36:571–580. doi: 10.1016/j.neuroimage.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovagnoli AR, Erbetta A, Villani F, Avanzini G. Semantic memory in partial epilepsy: verbal and non-verbal deficits and neuroanatomical relationships. Neuropsychologia. 2005;43:1482–1492. doi: 10.1016/j.neuropsychologia.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Grady CL, Furey ML, Pietrini P, Horwitz B, Rapoport SI. Altered brain functional connectivity and impaired short-term memory in Alzheimer's disease. Brain. 2001;124:739–756. doi: 10.1093/brain/124.4.739. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13:572–586. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- Gualtieri CT, Johnson LG. Comparative neurocognitive effects of 5 psychotropic anticonvulsants and lithium. MedGenMed. 2006;8:46. [PMC free article] [PubMed] [Google Scholar]
- Guye M, Bartolomei F, Ranjeva JP. Imaging structural and functional connectivity: towards a unified definition of human brain organization? Curr Opin Neurol. 2008;21:393–403. doi: 10.1097/WCO.0b013e3283065cfb. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Lee K, Preus A, McCarley RW, Wible CG. An fMRI study of functional abnormalities in the verbal working memory system and the relationship to clinical symptoms in chronic schizophrenia. Cereb Cortex. 2010;20:46–60. doi: 10.1093/cercor/bhp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, Ischebeck AK, Friederici AD, Stephan KE, Amunts K. Effective connectivity of the left BA 44, BA 45, and inferior temporal gyrus during lexical and phonological decisions identified with DCM. Hum Brain Mapp. 2009;30:392–402. doi: 10.1002/hbm.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann B, Meador KJ, Gaillard WD, Cramer JA. Cognition across the lifespan: antiepileptic drugs, epilepsy, or both? Epilepsy Behav. 2010;17:1–5. doi: 10.1016/j.yebeh.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Miyashita Y. Formation of mnemonic neuronal responses to visual paired associates in inferotemporal cortex is impaired by perirhinal and entorhinal lesions. Proc Natl Acad Sci U S A. 1996;93:739–743. doi: 10.1073/pnas.93.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P, Jefferies E, Ehsan S, Hopper S, Ralph MA. Selective short-term memory deficits arise from impaired domain-general semantic control mechanisms. J Exp Psychol Learn Mem Cogn. 2009;35:137–156. doi: 10.1037/a0013985. [DOI] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Tulving E, Wilson AA, Houle S, Brown GM. Neuroanatomical correlates of encoding in episodic memory: levels of processing effect. Proc Natl Acad Sci U S A. 1994;91:2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Shirinyan D, van Erp TG, Cohen MS, Cannon TD. Hippocampal activations during encoding and retrieval in a verbal working memory paradigm. Neuroimage. 2005;25:1224–1231. doi: 10.1016/j.neuroimage.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Kiebel SJ, David O, Friston KJ. Dynamic causal modelling of evoked responses in EEG/MEG with lead field parameterization. Neuroimage. 2006;30:1273–1284. doi: 10.1016/j.neuroimage.2005.12.055. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ. Topological inference for EEG and MEG data. Ann Appl Stat. 2010;14:1272–1290. [Google Scholar]
- Krauss GL, Summerfield M, Brandt J, Breiter S, Ruchkin D. Mesial temporal spikes interfere with working memory. Neurology. 1997;49:975–980. doi: 10.1212/wnl.49.4.975. [DOI] [PubMed] [Google Scholar]
- Lancelot C, Samson S, Ahad P, Baulac M. Effect of unilateral temporal lobe resection on short-term memory for auditory object and sound location. Ann N Y Acad Sci. 2003;999:377–380. doi: 10.1196/annals.1284.046. [DOI] [PubMed] [Google Scholar]
- Lee AC, Buckley MJ, Gaffan D, Emery T, Hodges JR, Graham KS. Differentiating the roles of the hippocampus and perirhinal cortex in processes beyond long-term declarative memory: a double dissociation in dementia. J Neurosci. 2006;26:5198–5203. doi: 10.1523/JNEUROSCI.3157-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M, Habib R, Cormier H, Houle S, McIntosh AR. Neural correlates of semantic associative encoding in episodic memory. Brain Res Cogn Brain Res. 2000;9:271–280. doi: 10.1016/s0926-6410(00)00005-7. [DOI] [PubMed] [Google Scholar]
- Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, Luo C, Lu G, Chen H. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS One. 2010;5:e8525. doi: 10.1371/journal.pone.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccotta L, Buckner RL, Gilliam FG, Ojemann GA. Changing frontal contributions to memory before and after medial temporal lobectomy. Cereb Cortex. 2007;17:443–456. doi: 10.1093/cercor/bhj161. [DOI] [PubMed] [Google Scholar]
- Mainy N, Kahane P, Minotti L, Hoffmann D, Bertrand O, Lachaux JP. Neural correlates of consolidation in working memory. Hum Brain Mapp. 2007;28:183–193. doi: 10.1002/hbm.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattout J, Henson RN, Friston KJ. Canonical source reconstruction for MEG. Comput Intell Neurosci. 2007:67613. doi: 10.1155/2007/67613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador KJ. Cognitive and memory effects of the new antiepileptic drugs. Epilepsy Res. 2006;68:63–67. doi: 10.1016/j.eplepsyres.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Gevins A, Loring DW, McEvoy LK, Ray PG, Smith ME, Motamedi GK, Evans BM, Baum C. Neuropsychological and neurophysiologic effects of carbamazepine and levetiracetam. Neurology. 2007;69:2076–2084. doi: 10.1212/01.wnl.0000281104.55418.60. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Allen P, Amaro E, Jr., Fu CH, Williams SC, Brammer MJ, Johns LC, McGuire PK. Misattribution of speech and impaired connectivity in patients with auditory verbal hallucinations. Hum Brain Mapp. 2007;28:1213–1222. doi: 10.1002/hbm.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Laxer KD, Scanlon C, Garcia P, McMullen WJ, Loring DW, Meador KJ, Weiner MW. Different structural correlates for verbal memory impairment in temporal lobe epilepsy with and without mesial temporal lobe sclerosis. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Jonides J. Neural correlates of access to short-term memory. Proc Natl Acad Sci U S A. 2008;105:14228–14233. doi: 10.1073/pnas.0802081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppeney U, Price CJ, Penny WD, Friston KJ. Two distinct neural mechanisms for category-selective responses. Cereb Cortex. 2006;16:437–445. doi: 10.1093/cercor/bhi123. [DOI] [PubMed] [Google Scholar]
- Ojemann GA, Schoenfield-McNeill J, Corina D. The roles of human lateral temporal cortical neuronal activity in recent verbal memory encoding. Cereb Cortex. 2009;19:197–205. doi: 10.1093/cercor/bhn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olson IR, Moore KS, Stark M, Chatterjee A. Visual working memory is impaired when the medial temporal lobe is damaged. J Cogn Neurosci. 2006;18:1087–1097. doi: 10.1162/jocn.2006.18.7.1087. [DOI] [PubMed] [Google Scholar]
- Owen AM, Morris RG, Sahakian BJ, Polkey CE, Robbins TW. Double dissociations of memory and executive functions in working memory tasks following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Brain. 1996;119(Pt 5):1597–1615. doi: 10.1093/brain/119.5.1597. [DOI] [PubMed] [Google Scholar]
- Öztekin I, Davachi L, McElree B. Are representations in working memory distinct from representations in long-term memory? Neural evidence in support of a single store. Psychol Sci. 2010;21:1123–1133. doi: 10.1177/0956797610376651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SP, Kwon SH. Cognitive effects of antiepileptic drugs. J Clin Neurol. 2008;4:99–106. doi: 10.3988/jcn.2008.4.3.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Daunizeau J, Rosa MJ, Friston KJ, Schofield TM, Leff AP. Comparing families of dynamic causal models. PLoS Comput Biol. 2010;6:e1000709. doi: 10.1371/journal.pcbi.1000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. Comparing dynamic causal models. Neuroimage. 2004;22:1157–1172. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Pereira FR, Alessio A, Sercheli MS, Pedro T, Bilevicius E, Rondina JM, Ozelo HF, Castellano G, Covolan RJ, Damasceno BP, et al. Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC Neurosci. 2010;11:66. doi: 10.1186/1471-2202-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson KM, Gisselgard J, Gretzer M, Ingvar M. Interaction between a verbal working memory network and the medial temporal lobe. Neuroimage. 2006;33:1207–1217. doi: 10.1016/j.neuroimage.2006.07.042. [DOI] [PubMed] [Google Scholar]
- Piekema C, Fernandez G, Postma A, Hendriks MP, Wester AJ, Kessels RP. Spatial and non-spatial contextual working memory in patients with diencephalic or hippocampal dysfunction. Brain Res. 2007;1172:103–109. doi: 10.1016/j.brainres.2007.07.066. [DOI] [PubMed] [Google Scholar]
- Powell HW, Guye M, Parker GJ, Symms MR, Boulby P, Koepp MJ, Barker GJ, Duncan JS. Noninvasive in vivo demonstration of the connections of the human parahippocampal gyrus. Neuroimage. 2004;22:740–747. doi: 10.1016/j.neuroimage.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Powell HW, Richardson MP, Symms MR, Boulby PA, Thompson PJ, Duncan JS, Koepp MJ. Reorganization of verbal and nonverbal memory in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsia. 2007;48:1512–1525. doi: 10.1111/j.1528-1167.2007.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevey ML, Delaney RC, Cramer JA, Cattanach L, Collins JF, Mattson RH. Effect of valproate on cognitive functioning. Comparison with carbamazepine. The Department of Veterans Affairs Epilepsy Cooperative Study 264 Group. Arch Neurol. 1996;53:1008–1016. doi: 10.1001/archneur.1996.00550100086018. [DOI] [PubMed] [Google Scholar]
- Price CJ, Warburton EA, Moore CJ, Frackowiak RS, Friston KJ. Dynamic diaschisis: anatomically remote and context-sensitive human brain lesions. J Cogn Neurosci. 2001;13:419–429. doi: 10.1162/08989290152001853. [DOI] [PubMed] [Google Scholar]
- Rajah MN, McIntosh AR, Grady CL. Frontotemporal interactions in face encoding and recognition. Brain Res Cogn Brain Res. 1999;8:259–269. doi: 10.1016/s0926-6410(99)00030-0. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D'Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31:865–873. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D'Esposito M. Directing the mind's eye: prefrontal, inferior and medial temporal mechanisms for visual working memory. Curr Opin Neurobiol. 2005;15:175–182. doi: 10.1016/j.conb.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Heller A, Cohen MX, Brozinsky CJ, Rissman J. Functional connectivity with the hippocampus during successful memory formation. Hippocampus. 2005;15:997–1005. doi: 10.1002/hipo.20141. [DOI] [PubMed] [Google Scholar]
- Reyt S, Picq C, Sinniger V, Clarencon D, Bonaz B, David O. Dynamic causal modelling and physiological confounds: a functional MRI study of vagus nerve stimulation. Neuroimage. 2010;52:1456–1464. doi: 10.1016/j.neuroimage.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Dynamic adjustments in prefrontal, hippocampal, and inferior temporal interactions with increasing visual working memory load. Cereb Cortex. 2008;18:1618–1629. doi: 10.1093/cercor/bhm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saling MM. Verbal memory in mesial temporal lobe epilepsy: beyond material specificity. Brain. 2009;132:570–582. doi: 10.1093/brain/awp012. [DOI] [PubMed] [Google Scholar]
- Saling MM, Berkovic SF, O'Shea MF, Kalnins RM, Darby DG, Bladin PF. Lateralization of verbal memory and unilateral hippocampal sclerosis: evidence of task-specific effects. J Clin Exp Neuropsychol. 1993;15:608–618. doi: 10.1080/01688639308402582. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Koubeissi MZ, David N, Vogeley K, Ritzl EK. Being with virtual others: studying social cognition in temporal lobe epilepsy. Epilepsy Behav. 2007;11:316–323. doi: 10.1016/j.yebeh.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Schon K, Quiroz YT, Hasselmo ME, Stern CE. Greater working memory load results in greater medial temporal activity at retrieval. Cereb Cortex. 2009;19:2561–2571. doi: 10.1093/cercor/bhp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Zeidman P, Neufeld NH, Leff AP, Price CJ. Identifying abnormal connectivity in patients using dynamic causal modeling of fMRI responses. Front Syst Neurosci. 2010;4:142. doi: 10.3389/fnsys.2010.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JG, Lee DI, Hwang YH, Lee HW, Jung DK, Suh CK, Kwon SH, Park SP. Comparison of cognitive effects of lamotrigine and oxcarbazepine in epilepsy patients. J Clin Neurol. 2007;3:31–37. doi: 10.3988/jcn.2007.3.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Squire LR. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ. Models of effective connectivity in neural systems. In: Jirsa VK, McIntosh AR, editors. Handbook of brain connectivity. Berlin (Germany): Springer-Verlag; 2007. pp. 303–326. [Google Scholar]
- Stephan KE, Penny WD, Daunizeau J, Moran RJ, Friston KJ. Bayesian model selection for group studies. Neuroimage. 2009;46:1004–1017. doi: 10.1016/j.neuroimage.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Wang W, Wu X, Wang Y. Antiepileptic drugs and the significance of event-related potentials. J Clin Neurophysiol. 2007;24:271–276. doi: 10.1097/WNP.0b013e31803bb334. [DOI] [PubMed] [Google Scholar]
- Thom M, Zhou J, Martinian L, Sisodiya S. Quantitative post-mortem study of the hippocampus in chronic epilepsy: seizures do not inevitably cause neuronal loss. Brain. 2005;128:1344–1357. doi: 10.1093/brain/awh475. [DOI] [PubMed] [Google Scholar]
- Thompson PJ, Baxendale SA, Duncan JS, Sander JW. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry. 2000;69:636–641. doi: 10.1136/jnnp.69.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenerry MR, Jack CR, Jr, Ivnik RJ, Sharbrough FW, Cascino GD, Hirschorn KA, Marsh WR, Kelly PJ, Meyer FB. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology. 1993;43:1800–1805. doi: 10.1212/wnl.43.9.1800. [DOI] [PubMed] [Google Scholar]
- Tuunainen A, Nousiainen U, Pilke A, Mervaala E, Riekkinen P. Lateralization of event-related potentials during discontinuation of antiepileptic medication. Epilepsia. 1995;36:262–269. doi: 10.1111/j.1528-1157.1995.tb00994.x. [DOI] [PubMed] [Google Scholar]
- van Dellen E, Douw L, Baayen JC, Heimans JJ, Ponten SC, Vandertop WP, Velis DN, Stam CJ, Reijneveld JC. Long-term effects of temporal lobe epilepsy on local neural networks: a graph theoretical analysis of corticography recordings. PLoS One. 2009;4:e8081. doi: 10.1371/journal.pone.0008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlooswijk MC, Jansen JF, Reijs RP, de Krom MC, Kooi ME, Majoie HJ, Hofman PA, Backes WH, Aldenkamp AP. Cognitive fMRI and neuropsychological assessment in patients with secondarily generalized seizures. Clin Neurol Neurosurg. 2008;110:441–450. doi: 10.1016/j.clineuro.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Voets NL, Adcock JE, Stacey R, Hart Y, Carpenter K, Matthews PM, Beckmann CF. Functional and structural changes in the memory network associated with left temporal lobe epilepsy. Hum Brain Mapp. 2009;30:4070–4081. doi: 10.1002/hbm.20830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Sziklas V, Garver KE, Jones-Gotman M. Material-specific lateralization of working memory in the medial temporal lobe. Neuropsychologia. 2009;47:112–122. doi: 10.1016/j.neuropsychologia.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Wagner K, Frings L, Halsband U, Everts R, Buller A, Spreer J, Zentner J, Schulze-Bonhage A. Hippocampal functional connectivity reflects verbal episodic memory network integrity. Neuroreport. 2007;18:1719–1723. doi: 10.1097/WNR.0b013e3282f0d3c5. [DOI] [PubMed] [Google Scholar]
- Werner S, Noppeney U. Distinct functional contributions of primary sensory and association areas to audiovisual integration in object categorization. J Neurosci. 2010;30:2662–2675. doi: 10.1523/JNEUROSCI.5091-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloszyn L, Sheinberg DL. Neural dynamics in inferior temporal cortex during a visual working memory task. J Neurosci. 2009;29:5494–5507. doi: 10.1523/JNEUROSCI.5785-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogarajah M, Powell HW, Parker GJ, Alexander DC, Thompson PJ, Symms MR, Boulby P, Wheeler-Kingshott CA, Barker GJ, Koepp MJ, et al. Tractography of the parahippocampal gyrus and material specific memory impairment in unilateral temporal lobe epilepsy. Neuroimage. 2008;40:1755–1764. doi: 10.1016/j.neuroimage.2007.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri HP, Pincus SM, Goncharova, Novotny EJ, Duckrow RB, Spencer DD, Blumenfeld H, Spencer SS. Background intracranial EEG spectral changes with anti-epileptic drug taper. Clin Neurophysiol. 2010;121:311–317. doi: 10.1016/j.clinph.2009.11.081. [DOI] [PubMed] [Google Scholar]