Abstract
In this study, we examine whether corrections made during an ongoing movement are differentially affected by left hemisphere damage (LHD) and right hemisphere damage (RHD). Our hypothesis of motor lateralization proposes that control mechanisms specialized to the right hemisphere rely largely on online processes, while the left hemisphere primarily utilizes predictive mechanisms to specify optimal coordination patterns. We therefore predict that RHD, but not LHD, should impair online correction when task goals are unexpectedly changed. Fourteen stroke subjects (7 LHD, 7 RHD) and 14 healthy controls reached to 1 of the 3 targets that unexpectedly “jumped” during movement onset. RHD subjects showed a considerable delay in initiating the corrective response relative to controls and LHD subjects. However, both stroke groups made large final position errors on the target jump trials. Position deficits following LHD were associated with poor intersegmental coordination, while RHD subjects had difficulty terminating their movements appropriately. These findings confirm that RHD, but not LHD, produces a deficit in the timing of online corrections and also indicate that both stroke groups show position deficits that are related to the specialization of their damaged hemisphere. Further research is needed to identify specific neural circuits within each hemisphere critical for these processes.
Keywords: lateralization, motor control, reaching, stroke
Introduction
In his lectures on the anatomy of the human hand, Sir G.M. Humphry (1861, p. 200) questioned “why man [is] usually right-handed” and whether the superiority of the right hand for certain tasks, like “wielding the pen or the knife,” provided humans with greater motor skill. Implicit in this statement is the view that a larger repertoire of motor skills can result from the distribution of specialized functions to the right and left hands. New research is beginning to support this idea. To date, studies in right-handed adults have demonstrated that the left arm/hand is more skilled than the right arm/hand for proprioceptive matching and recall (Roy and MacKenzie 1978; Goble et al. 2006; Goble and Brown 2007), impeding dynamic perturbations (Bagesteiro and Sainburg 2003; Duff and Sainburg 2007; Schabowsky et al. 2007), and, under some conditions, achieving greater spatial accuracy (Guiard et al. 1983; Lenhard and Hoffmann 2007). In contrast, the right arm/hand in right-handers appears to better optimize dynamic parameters such as movement direction and trajectory shape (Sainburg and Kalakanis 2000; Bagesteiro and Sainburg 2002; Sainburg 2002). These interlimb differences in motor performance are thought to reflect hemispheric lateralization of motor control mechanisms within the central nervous system. It has been hypothesized that neural mechanisms for achieving and/or stabilizing limb position, which largely rely on feedback-based processes, are specialized in the right (nondominant) hemisphere in right-handed individuals. On the other hand, planning and coordinating limb trajectories through mechanisms that predict the effects of limb and task dynamics might be specialized in the left (dominant) hemisphere (see Sainburg 2005, 2010 for reviews). It is therefore likely that the preferred use of the right arm for tasks like throwing (Oldfield 1971) and the left arm for stabilizing functions emerges from these hemispheric specializations. This distribution of control mechanisms across the 2 hemispheres forms the basis of our dynamic-dominance hypothesis of motor lateralization.
Evidence supporting hemispheric lateralization of these complementary control processes has come from studies examining movement deficits in subjects with unilateral stroke. These studies have shown that left or right hemisphere lesions produce distinct behavioral deficits that reflect damage to control processes for which that hemisphere might be specialized (Kimura and Archibald 1974; Fisk and Goodale 1988; Haaland and Harrington 1989; Harrington and Haaland 1991; Haaland et al. 2004; Schaefer et al. 2007, 2009a, 2009b; Mutha et al. 2011). Most important is the finding that damage to a single hemisphere produces deficits in both the contralesional and the ipsilesional arms. In addition, deficits arising from damage to one hemisphere often do not occur when the opposite hemisphere is damaged. For example, damage to the left hemisphere produces deficits in intersegmental coordination, but coordination remains intact despite damage to the right hemisphere (Schaefer et al. 2009b). These findings indicate that both hemispheres contribute specific and necessary processes to movements of each arm.
Our hypothesis of lateralized trajectory and positional control mechanisms in the left and right hemisphere, respectively, is consistent with several studies that have proposed a distinction in mechanisms mediating these 2 features of movement (Gordon, Ghilardi, and Ghez 1994; Dizio and Lackner 1995; Kurtzer et al. 2005; Wang and Sainburg 2005; Ghez et al. 2007; Mutha and Sainburg 2007). Recently, Arce et al. (2009, 2010) demonstrated a potential functional advantage of such a distinction, by showing that healthy adults were able to flexibly use these separate control processes to adapt to different novel task conditions. It is possible that the lateralization of these control processes minimizes interference, thereby allowing the strategic use of these different processes during adaptation. This suggests that adaptation to novel conditions should be differentially affected following left hemisphere damage (LHD) or right hemisphere damage (RHD). In line with this idea, our recent studies on visuomotor adaptation (Schaefer et al. 2009a; Mutha et al. 2011) revealed that damage to the left, but not the right, hemisphere impaired the ability to improve movement direction over time, a deficit resulting from damage to mechanisms involved in movement planning. Surprisingly though, these subjects were able to make corrections as the movement progressed in order to bring their arm to the intended target, which we speculated was due to their intact right hemisphere. In contrast, subjects with RHD showed intact adaptation of movement direction but showed a reduced ability to accurately bring their arm to the target. While these findings demonstrated a vital role for the left hemisphere in movement planning, they also provided preliminary support for a critical role of the right hemisphere in online correction. This interpretation was tentative, however, given that movements were made to target locations that could be handled through predictive, or feedforward, mechanisms.
The purpose of the current study was to determine whether online error correction processes are differentially affected by LHD or RHD after stroke when reaching to target locations that change direction unexpectedly during movement. We predicted that 1) RHD should disrupt mechanisms that mediate rapid corrections to unexpected task changes during an ongoing movement; therefore, both the temporal efficiency and spatial efficacy of position corrections should be affected, while 2) LHD should not disrupt either aspect of these corrections. To test these predictions, in the current study, we use a double-step paradigm where the location of a visual target is unexpectedly perturbed to the right or left after the onset of a reaching movement and examine the nature of subsequent trajectory corrections. Differences in the quality of these corrective responses between groups of LHD and RHD subjects would indicate that the control processes for stable (position) and changing (trajectory) limb states are lateralized and can be flexibly recruited when the nervous system is intact. Such findings will provide a neural basis for the belief of Sir Humphry (1861) that “we acquire a greater degree of skillfulness and dexterity than we should do if both hands were equally employed” (p. 204).
Materials and Methods
Subjects
Fourteen right-handed subjects with chronic poststroke hemiparesis and 14 right-handed healthy controls (HCs; Table 1) were examined after obtaining approval from the Institutional Review Board of the New Mexico Veterans Affairs Healthcare System. Prior to participation, informed consent was obtained from each participant, according to the Declaration of Helsinki. All subjects were right-handed; handedness was determined by the Edinburgh inventory (Oldfield 1971). Subjects were screened and excluded based on history of 1) significant substance abuse and/or severe psychiatric diagnosis, 2) peripheral movement restrictions, such as neuropathy or orthopedic disorders, and 3) neurological diseases other than stroke for the stroke subjects and all neurological diagnoses for the control group. All stroke subjects in this study had hemiparesis in the contralesional arm, defined as a contralesional grip strength at least 1.5 standard deviations (SDs) below normal and at least 1.5 SDs less than ipsilesional grip strength using a hand dynamometer (Heaton et al. 2004). An additional measure of hemiparesis (upper extremity motor subscore of the Fugl-Meyer Motor Assessment) (Fugl-Meyer et al. 1975) and language comprehension (Kertesz 1982) were also used for descriptive purposes.
Table 1.
Summary of subject characteristics
| Subject | Sex | Age (years) | Education (years) | Poststroke (years)a | Lesion volume (cm3)b | Language abilityc | UE Fugl-Meyerd | Grip strength righte | Grip strength lefte | Lesion location |
| LHD1 | M | 44 | 14 | 7.0 | 147.2 | 46 | 22 | 0 | 51 | SMC, IC, BG, PC, IF, TC |
| LHD2 | M | 60 | 14 | 16.8 | 28.8 | 80 | 45 | 12 | 54 | IC, BG, PC, TC |
| LHD3 | M | 46 | 17 | 5.1 | 152.9 | 80 | 61 | 27 | 48 | SMC, IC, BG, PC, IF, TC |
| LHD4 | M | 61 | 14 | 17.3 | 125.2 | 66 | 27 | 12 | 44 | SMC, IC, BG, PC, IF, TC |
| LHD5 | M | 55 | 14 | 0.7 | 24.1 | 44 | 10 | 0 | 62 | BG |
| LHD6 | M | 76 | 12 | 12.0 | 182.9 | 80 | 33 | 9 | 46 | SMC, PC, TC |
| LHD7 | M | 53 | 18 | 4.2 | 59.1 | 53 | 5 | 0 | 46 | IC, BG, TC |
| LHD mean ± SD | 56.4 ± 10.8 | 14.7 ± 2.1 | 9.0 ± 6.4 | 102.9 ± 64.5 | 64.1 ± 16.4 | 29.0 ± 19.5 | 8.6 ± 9.9 | 50.1 ± 6.2 | ||
| LHC mean ± SD | 55.0 ± 7.7 | 14.4 ± 1.7 | 78.9 ± 3.0 | 50.7 ± 7.7 | 52.1 ± 6.9 | |||||
| RHD1 | M | 50 | 16 | 19.5 | 244.8 | 80 | 4 | 24 | 0 | SMC, IC, BG, PC, DLPF, IF, TC |
| RHD2 | M | 65 | 12 | 8.9 | 27.4 | 76 | 62 | 33 | 0 | SMC, DLPF |
| RHD3 | M | 52 | 12 | 10.5 | 274.7 | 80 | 49 | 34 | 5 | SMC, PC, DLPF, IF, TC |
| RHD4 | F | 58 | 16 | 9.0 | 137.3 | 80 | 6 | 33 | 0 | SMC, IC, BG, PC, DLPF, IF, TC |
| RHD5 | M | 63 | 18 | 3.8 | 118.7 | 80 | 5 | 38 | 0 | SMC, IC, BG, DLPF, IF, TC |
| RHD6 | M | 55 | 16 | 5.9 | 283 | 80 | 6 | 36 | 0 | SMC, IC, BG, PC, DLPF, IF, TC |
| RHD7 | F | 68 | 11 | 1.0 | 43.5 | 80 | 44 | 53 | 23 | TC |
| RHD mean ± SD | 58.7 ± 6.8 | 14.4 ± 2.7 | 8.4 ± 5.9 | 161.3 ± 107.1 | 79.4 ± 1.5 | 25.1 ± 25.4 | 35.9 ± 8.7 | 4.0 ± 8.6 | ||
| RHC mean ± SD | 59.9 ± 9.9 | 16.3 ± 1.7 | 78.9 ± 3.0 | 46.6 ± 7.8 | 48.6 ± 11.1 |
Note: UE: upper extremity; M: male; F: female; SMC: sensorimotor cortex (BA 4, 6, and/or 3, 1, 2); IC: internal capsule; BG: basal ganglia; PC: parietal cortex (BA 7 and/or 39, 40); DLPF: dorsolateral prefrontal cortex (BA 8, 9 and/or 46); IF: inferior frontal cortex (BA 44); TC: superior and middle temporal gyrus (BA 22, 21, and/or 37).
Years poststroke are calculated as time elapsed between incidence of stroke and day of data collection.
Lesion volume is computed from MRI or CT.
Language ability was assessed using the Sequential Command Subtest from the Western Aphasia Battery.
Maximum score on the UE motor subscore of the Fugl-Meyer Motor Assessment is 66.
Grip strength from dynamometer are expressed as standardized T scores.
Lesion Characteristics
Magnetic resonance images (MRIs) were obtained in 10 stroke subjects, while computed tomography (CT) scans were done for the remaining 4 subjects due to medical contraindications for MRI. A board-certified neurologist, who was blinded to the behavioral characteristics of the subjects, outlined the area of damage for each subject on 11 standardized horizontal sections derived from the DeArmond atlas (DeArmond et al. 1989) using T1-weighted MRI for anatomical detail and T2-weighted images to specify borders of the damaged tissue. These tracings were digitized to create overlap images and calculate lesion volume using a custom algorithm, the reliability of which has been previously verified (Knight et al. 1988).
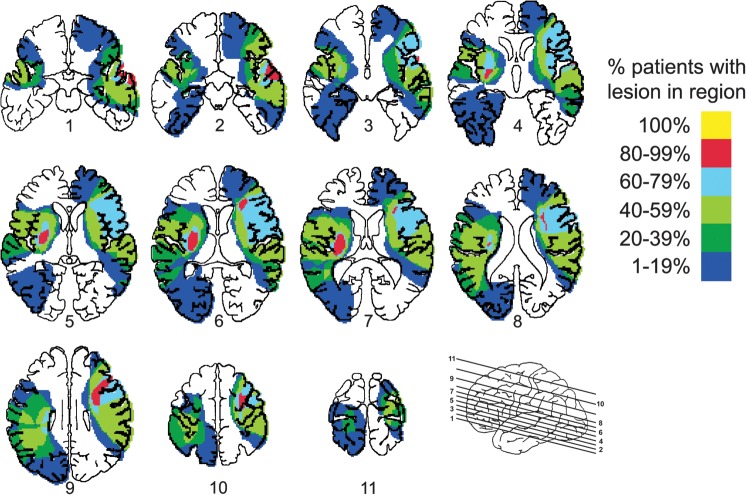
Lesion location for each LHD and RHD subject is described in Table 1, and overlap images are shown in Figure 1. In general, all subjects showed damage to widespread regions of cortex as well as subcortical regions. Maximum overlap in the LHD subjects occurred in basal ganglia, internal capsule, and insular cortex. A majority of these subjects also had damage to motor cortex (Brodmann Area [BA] 4), premotor cortex (BA 6), and somatosensory cortex (BA 3, 1, 2). Five LHD subjects had lesions to regions of the parietal cortex, particularly portions of the supramarginal gyrus (BA 40). RHD subjects had maximum overlap in prefrontal regions, particularly inferior frontal (BA 44) and dorsolateral prefrontal (BA 46) cortices. Several of these subjects also had damage in basal ganglia; internal capsule; insular cortex as well as motor (BA 4), premotor (BA 6), and somatosensory (BA 3, 1, 2) cortices. Inferior parietal regions (BA 40) were damaged in 4 of 7 RHD subjects. Regions of the superior, middle, and/or inferior temporal lobe (BA 22, 21, and/or 37) were also damaged in 6 subjects in each of the stroke groups. Thus, overall, lesions in both groups were quite large and covered widespread cortical and subcortical regions.
Figure 1.
Overlap of lesion location in the LHD and RHD groups. Colors of shaded regions denote percentage of LHD and RHD subjects with lesions in the corresponding region.
Experimental Setup
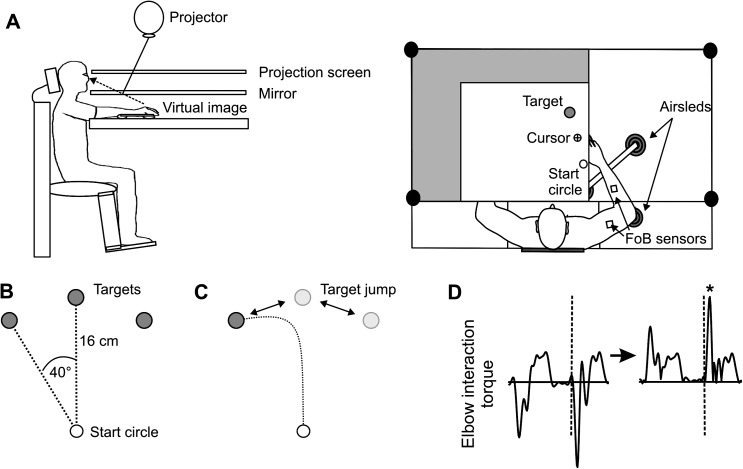
Figure 2A illustrates the experimental setup. Subjects sat facing a projection screen with their left or right arm supported just below shoulder height on an air-jet system to minimize the effects of friction and gravity. Under such conditions, subjects with hemiparesis are able to move across a larger workspace at higher speeds with better movement quality (Beer et al. 2004) and may be less susceptible to fatigue during participation (Rudroff et al. 2011). A start circle, a target, and a cursor that represented finger position were projected on a horizontal back-projection screen positioned above the arm, with a horizontal mirror positioned below this screen. The mirror reflected the visual display such that the projection appeared in the same horizontal plane as the fingertip. It is important to note that the virtual reality display was designed and calibrated to ensure that the projection was veridical (i.e., 1 cm leftward arm movements corresponded to 1 cm leftward cursor movements in the same plane). Subjects could not see their arm moving below the mirror; the displayed cursor was the only visual feedback available to the subjects.
Figure 2.
(A) Side and top view of experimental apparatus are shown. (B) Baseline and (C) target jump locations in the lateral, center, or medial direction relative to the starting position. All targets were presented in the ipsilateral hemispace relative to the arm. (D) Schematic of computation for corrective interaction torque (x-axis = time). Calculated interaction torque is on the left. Arrow indicates full-wave rectification; dashed line indicates correction time; asterisk indicates value of peak corrective interaction torque.
All joints distal to the elbow were immobilized using an adjustable brace. Position and orientation of the segments proximal and distal to the elbow joint were sampled at 103 Hz using a Flock of Birds (FoB; Ascension Technology) magnetic 6 degree-of-freedom movement recording system. A single sensor was attached to the upper arm segment via an adjustable plastic cuff, while another sensor was fixed to the air sled where the forearm was fitted. The sensors were positioned approximately at the center of each arm segment. The positions of the following 3 bony landmarks were digitized using a stylus that was rigidly attached to an FoB sensor: 1) index fingertip; 2) the lateral epicondyle of the humerus; 3) the acromion, directly posterior to the acromioclavicular joint. Custom software used the FoB sensor data to compute the 3D position of the index fingertip, and the recorded x–y coordinates of the fingertip were used to project a cursor onto the screen. Screen redrawing occurred fast enough to maintain the cursor centered on the fingertip throughout the sampled arm movements. The described experimental setup standardizes the experimental task (see below) and enables quantifiable comparisons of task performance (see Kinematic Data, Inverse Dynamics, and Performance Measures) across subjects.
Experimental Task
All subjects completed 1 session of 234 trials. Stroke subjects used the less affected ipsilesional arm, while the HCs were randomly assigned to perform the task with their left (LHC) or right (RHC) arm. For all trials, subjects were instructed to move from the start location to 1 of the 3 targets. All 3 targets were 2.5 cm in diameter and were projected in the same ipsilateral hemispace as the tested arm at a distance of 16 cm from the start position. The targets were oriented 40° clockwise, 0°, or 40° counterclockwise from the start position (Fig. 2B); thus, subjects were instructed to reach with their arm to a lateral (away from midline), center, and medial (toward midline) target. The cursor, which corresponded to the real-time position of the index fingertip, and the start circle were displayed on the screen prior to each trial. The target did not appear until after the subjects had held the cursor within the starting circle (for 200 ms) to trigger the audiovisual “go” signal; the target for that trial then appeared. Instructions were to move the finger (represented by the cursor) to “the center of the target and stop, using a single, uncorrected motion.” Visual feedback of the finger position (cursor) was provided only for positioning the finger in the start circle and was removed at the go signal. No visual feedback was given during the movement. Although explicit knowledge of performance or results was not given, subjects received a numerical score at the end of each trial to maintain motivation. This score was based on the location of the index finger relative to the target at movement end: Final position errors <1.25 cm from the center of the target (i.e., within the target circle) were awarded 10 points; 1.25–2.5 cm, 3 points; 2.5–3.75 cm, 1 point; >3.75 cm, no points. The purpose of awarding points to each trial was merely for motivation; these points were not analyzed as dependent variables. All trials, regardless of score, were recorded and saved. Following the display of the numerical score after each trial, the cursor was redisplayed for accurate positioning of the fingertip back at the start circle for the next trial. The 3 targets were presented in a pseudorandom order, and no single target was presented consecutively.
Within the testing session of 234 trials, there were 2 types of trials: 186 baseline trials and 48 “target jump” trials. During baseline trials, subjects moved as described above. For target jump trials, the target “jumped” to an adjacent location as movement was initiated (i.e., reaction time, see below) (Fig. 2C). Target jump trials were imposed every 4–6 trials, thereby making it highly likely that midmovement perturbations could occur during the testing session that were unpredictable in direction. Instructions for these trials did not change, and subjects were expected to still move to “the center of the target and stop.” The target jump locations were oriented 40° clockwise, 0°, or 40° counterclockwise from the start position, as in the baseline trials. The target location unpredictably, yet noticeably, shifted 10.9 cm from lateral to center, center to medial, center to lateral, or medial to center. Each type of target jump trial occurred 12 times in a session (4 target jump types × 12 trials each = 48 trials), but the pseudorandom order prevented the predictability of whether a jump would occur on a particular trial and the direction of the jump when it did occur. This ensured that the corrective response to the target jump was dependent on feedback-mediated processes.
Kinematic Data
The positions of the index finger, elbow point, and shoulder point were calculated from sensor position and orientation data from FoB (see above). Joint angles were calculated from these data. All kinematic data were low-pass filtered at 8 Hz (third order, dual pass Butterworth) and differentiated to yield velocity and acceleration values. Movement start was determined by identifying the time of peak velocity and searching backward in time for the first minimum in velocity (acceleration = 0) below 6% of peak tangential velocity. For baseline trials, movement end was similarly determined by searching forward in time from peak velocity to find the first minimum in velocity below 6% of peak tangential velocity, thereby excluding any small corrective submovements. For target jump trials, movement end was determined by searching forward in time from the second peak in velocity (corresponding to peak velocity of the corrective response) to find the first minimum in velocity below 6% of the first peak tangential velocity value.
Inverse Dynamics
Using inverse dynamics analysis, values for net torque, interaction torque, and muscle torque were calculated for each joint (elbow and shoulder) for all target jump trials. Full description of these calculations, as well as the equations detailing how each torque component was computed and analyzed, can be found in our earlier work (Bagesteiro and Sainburg 2002). The inertia and mass of the forearm support were 0.0247 kg·m2 and 0.58 kg, respectively. Limb segment inertia, center of mass, and mass were computed from regression equations using subjects’ body mass and measured limb segment lengths (Winter 1990). Positive torque values indicated joint flexion; negative torque values indicated joint extension.
Performance Measures
The following measures were calculated for each baseline trial: movement time, peak tangential velocity, and final position error. Movement time was defined as the elapsed time from movement start to movement end. Peak tangential velocity was defined as the absolute maximum tangential velocity occurring between movement start and end. Final position error was calculated as the absolute value of the distance from the index fingertip at movement end to the center of the target.
For each target jump trial, additional measures were calculated: correction time and corrective interaction torque. Correction time was determined by searching backward in time from the peak velocity of the corrective movement for the local minimum in tangential velocity. Mean correction time for each target jump direction (lateral to center, center to medial, center to lateral, or medial to center) was then expressed as a percentage of mean baseline movement time (lateral, center, or medial). For example, mean correction time for the lateral-to-center target jump trials was divided by the mean movement time for the lateral target baseline trials, multiplied by 100. This measure was used to quantify how much of the intended baseline trial was completed before the corrective movement began. This was done for each subject such that the timing of all subjects’ corrective responses was relative to their baseline performance (Shabbott and Sainburg 2008, 2009). To determine the corrective interaction torque, elbow interaction torque was full-wave rectified to convert the entire torque waveform to positive values (Fig. 2D). Corrective interaction torque was calculated as the maximum rectified elbow interaction torque during the corrective movement. This value represented the maximum amount of flexor or extensor interaction torque at the elbow in response to the unexpected change in target location.
Statistical Analysis
The means of all baseline performance measures were analyzed using 3-way mixed-model analysis of variance (ANOVA), with arm (left = L or right = R) and group (HC or hemisphere damage [HD]) as between-subject factors and target (lateral, center, and medial) as the within-subject factor. The means of all target jump performance measures were analyzed similarly with arm (L or R) and group (HC or HD) as between-subject factors and target (lateral to center, center to medial, center to lateral, or medial to center) as the within-subject factor. When warranted by significant main or interaction effects, post hoc analysis was performed using Tukey's test. Pearson product moment correlation coefficients, or r values (2-tailed significance), were also used across subjects or trials to determine whether there were linear relationships between specific variables. When used as a dependent variable, Pearson r values were normalized using Fisher transformation.
Results
Subject Characteristics
The corrective responses of the ipsilesional arm to unexpected changes in target location during reaching were studied in stroke subjects with contralesional hemiparesis after LHD or RHD and compared to the responses of the same arm of control subjects. Table 1 summarizes the characteristics of each HD subject, as well as overall for each group. Age (F3,24 = 0.42; P = 0.74) and education (F3,24 = 1.28; P = 0.30) were similar across all groups. Student’s t-test revealed that the LHD and RHD groups did not significantly differ in number of years poststroke (P = 0.85), lesion volume (P = 0.24), or degree of hemiparesis based on the upper extremity motor subscore of the Fugl-Meyer Motor Assessment (P = 0.75) or on contralesional grip strength (P = 0.37). Language comprehension was significantly lower in the LHD group than in the RHD group (P < 0.05) based on the Western Aphasia Battery, yet their motor performance indicated that it was adequate to perform the experimental task.
Baseline Performance
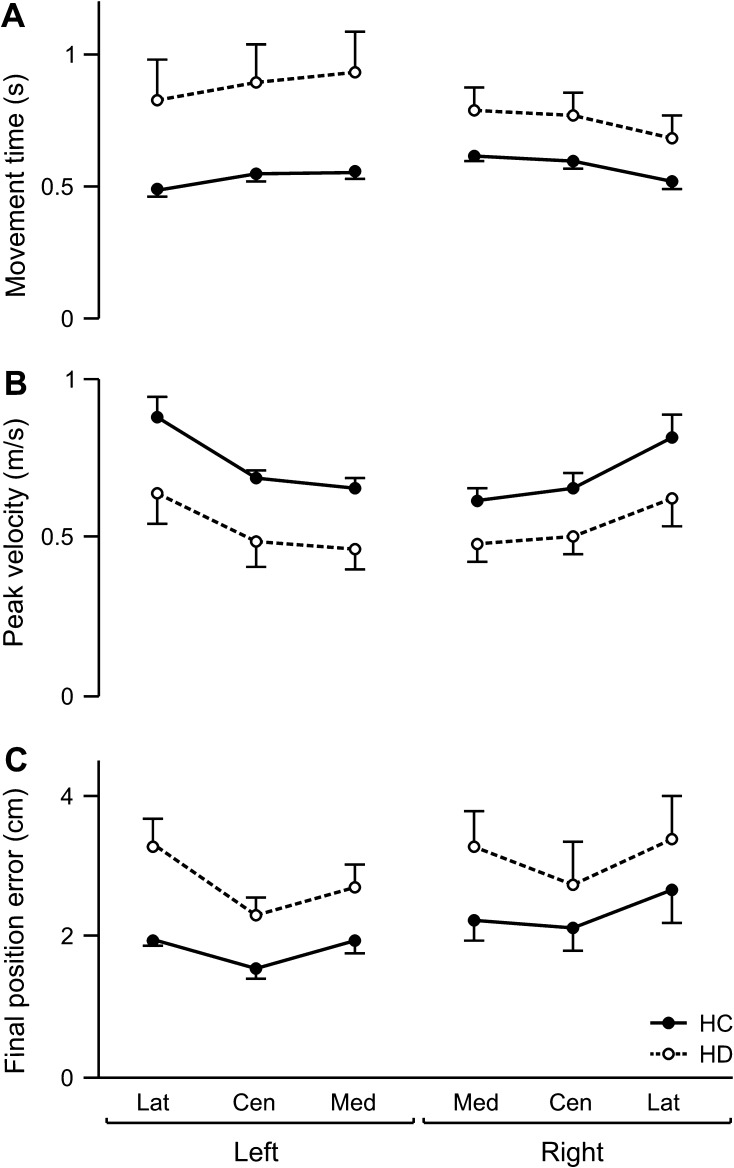
During baseline trials, subjects reached without visual feedback of their arm from a single start location to 3 targets. Measures of mean baseline performance are shown across targets in Figure 3. Repeated-measures ANOVA revealed no significant 2- or 3-way interactions, but a significant group effect on movement time (F1,24 = 8.93; P < 0.01), peak tangential velocity (F1,24 = 8.46; P < 0.01), and final position error (F1,24 = 5.49; P < 0.05) was observed. Subsequent analysis indicated that the stroke (HD) subjects took more time (Fig. 3A), moved more slowly (Fig. 3B), and were less accurate (Fig. 3C) than the HC groups. For all measures, there was a significant main effect of target (movement time: F2,48 = 64.51; P < 0.0001; peak velocity: F2,48 = 85.05; P < 0.0001; final position error: F2,48 = 14.27; P < 0.0001) yet no main effect of arm (movement time: F1,24 = 0.25; P = 0.62; peak velocity: F1,24 = 0.011; P = 0.75; final position error: F1,24 = 1.47; P = 0.24). Movements to the most medial target tended to be the slowest and also took longer than the other 2 target directions in the HC as well as the HD groups, consistent with other previous reports of planar reaching movement (Gordon, Ghilardi, Cooper, and Ghez 1994; Gordon, Ghilardi, and Ghez 1994; Schaefer et al. 2009b) in similar low-friction experimental conditions. This pattern was more pronounced in the HD group, who, regardless of arm and side of lesion, were slower and less accurate than the HC subjects across all targets during baseline performance. Such ipsilesional deficits in speed and accuracy may contribute to functional deficits during unconstrained naturalistic movements (Desrosiers et al. 1996; Schaefer et al. 2009b).
Figure 3.
Baseline performance. Mean (A) movement time, (B) peak tangential velocity, and (C) final position error for each target (Lat, lateral; Cen, center; and Med, medial location in ipsilesional hemispace) is displayed for the left and right arms of healthy control groups (LHC, RHC; solid line) and the ipsilesional arms of LHD and RHD groups (dashed line). Error bars indicate standard error.
Target Jump Performance
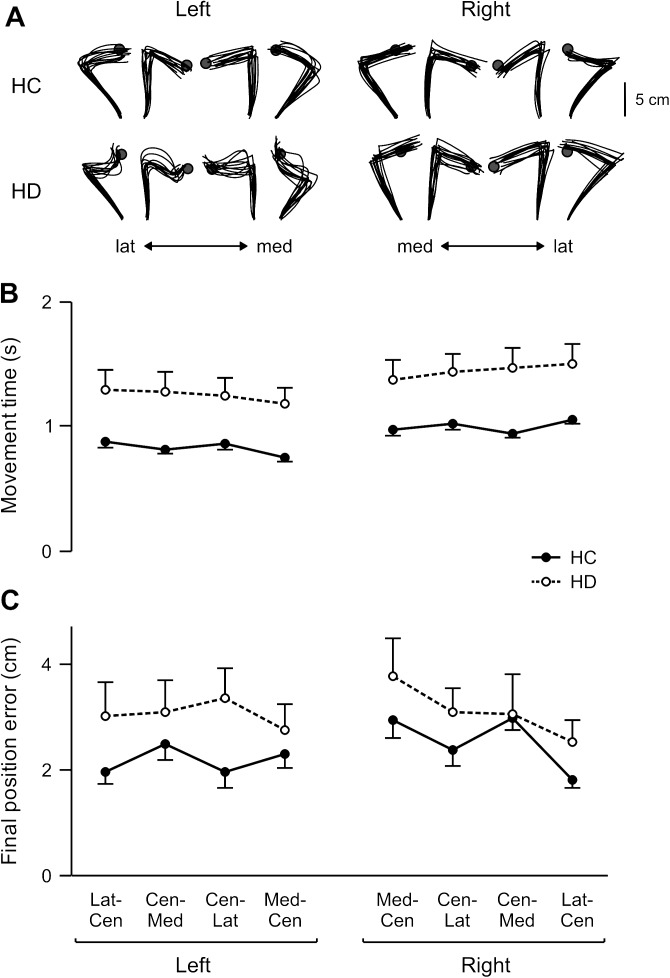
Figure 4A shows the handpaths of all target jump trials for representative control and HD subjects. Although the target changed location at reaction time, subjects continued to execute the originally planned reach and changed movement directions near the original target location. Thus, movement times for the target jump trials were nearly twice those of the baseline trials in all groups (mean target jump vs. baseline: LHC, 827 vs. 529 ms; RHC, 999 vs. 578 ms; LHD, 1247 vs. 883 ms; RHD, 1450 vs. 746 ms), as shown in Figure 4B. However, similar to baseline conditions, there was a significant main effect of group (F1,24 = 16.37; P < 0.001) and target (F3,72 = 5.29; P < 0.01) on movement time for the target jump trials, with no other significant effects. Post hoc tests indicated that movement time on the jump trials in the HD group was much longer than HC subjects and that target jump responses from the lateral to the center target took the longest to be completed. Further, both HD groups tended to be less accurate than the HC groups when the target jumped (Fig. 4C), with a marginal main effect of group on final position error in the target jump trials (F1,24 = 4.00; P = 0.056). Importantly, however, there was no significant effect of arm (F1,24 = 0.31; P = 0.58) or arm × group interaction (F1,24 = 0.17; P = 0.68) and therefore no indication of any difference in accuracy between the left and right arms of the HC groups, nor between the ipsilesional arm of the LHD and RHD groups. In fact, mean final position errors were quite comparable between the 2 HC groups (LHC: 2.2 ±0.1 cm, RHC: 2.5 ± 0.7 cm) as well as between the 2 HD groups (LHD: 3 ± 0.2 cm, RHD: 3.1 ± 0.3 cm).
Figure 4.
Target jump performance. (A) Top view of handpaths for all target jump trials for individual HC and HD subjects. Mean (B) movement time and (C) final position error for each target (Lat–Cen, lateral to center; Cen–Med, center to medial; Cen–Lat, center to lateral; Med–Cen, medial to center) is displayed for the left and right arms of healthy control groups (LHC, RHC; solid line) and the ipsilesional arms of LHD and RHD groups (dashed line). Error bars indicate standard error.
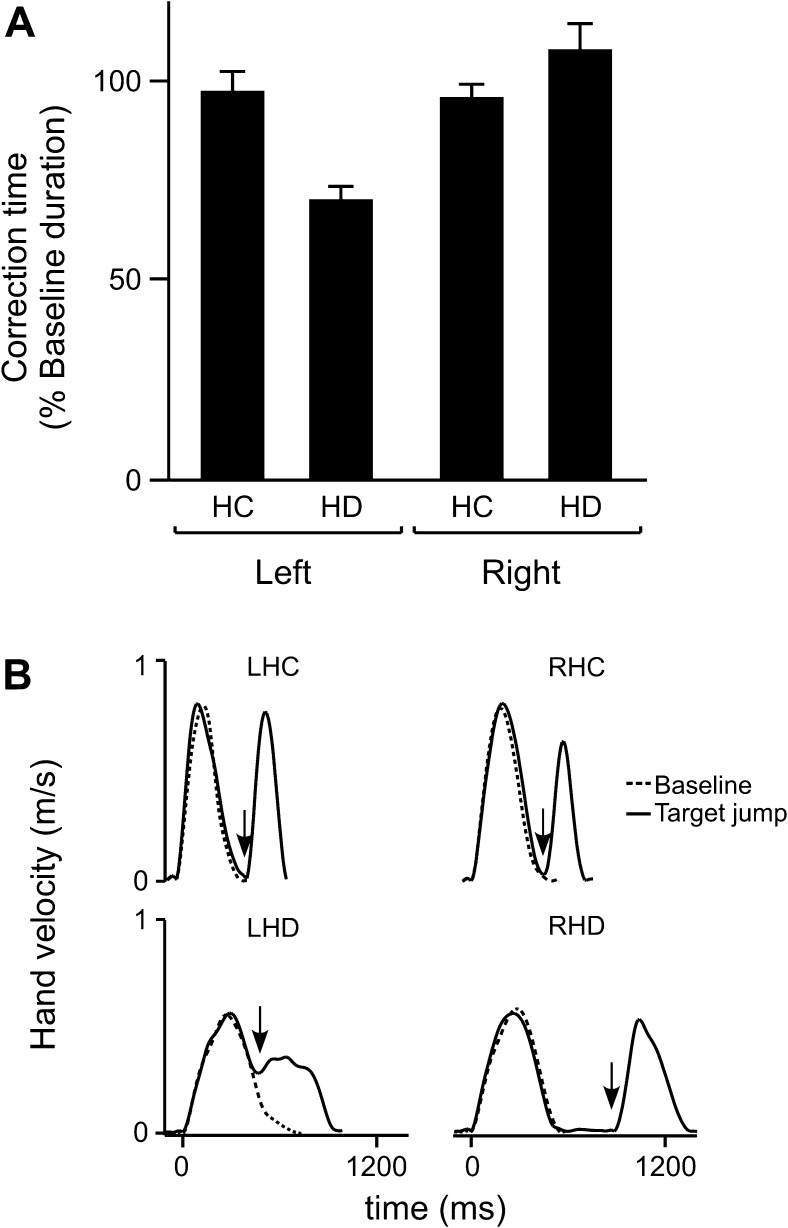
There were significant differences in the timing of each group’s corrective movements. Correction times were normalized for each subject with respect to mean baseline performance. Our ANOVA revealed a significant interaction between arm and group on correction time (F1,24 = 4.97; P < 0.05). This arm × group effect is illustrated in Figure 5A, such that correction times for both the LHC and RHC groups occurred at ∼100% of baseline movement time, whereas correction time for the LHD group occurred at ∼70% of baseline movement time. In contrast, RHD correction time occurred later, at ∼110% of baseline movement time. Post hoc analysis revealed that, relative to the originally planned baseline movement, correction times were significantly earlier for the LHD group and significantly later for the RHD group (P < 0.05). Velocity profiles of single baseline and target jump trials are shown in Figure 5B and clearly illustrate the delayed correction time in a representative RHD subject.
Figure 5.
Target jump performance. (A) Mean correction time (% baseline movement time) is displayed for the left and right arms of healthy control groups (LHC, RHC) and the ipsilesional arms of LHD and RHD groups. Error bars indicate standard error. (B) Tangential velocity profiles from individual baseline trials (thin line) to the center location (Cen) and target jump trials (thick line), when the target jumped from the center location to the medial location (Cen–Med), are shown for individual HC and HD subjects. Arrows indicate correction time for target jump trials.
Midmovement Dynamics Versus Final Position Errors
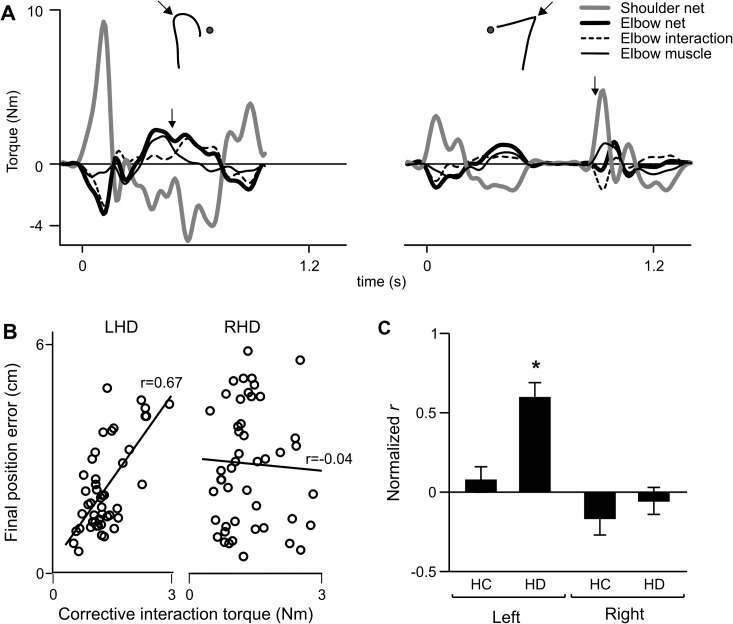
As shown in Figure 4A, the corrections of the LHD subject tended to be curved and variable. Depending on the location and direction of the target jump (medial or lateral), the handpaths curved toward or away from the body when moving to the new target. We had previously reported significant directional effects on handpath curvatures and final position errors of LHD subjects, which were attributed to direction-dependent variations in intersegmental dynamics that were not accurately accounted for by these subjects (Schaefer et al. 2009b). We therefore expected a similar deficit in intersegmental coordination in our LHD subjects when the required direction of a reach would suddenly change, as in the case of an unexpected target jump. We found this to be true. This effect is shown in Figure 6A, which displays profiles for shoulder net torque, elbow net torque, elbow interaction torque, and elbow muscle torque during the individual target jump trials of the LHD subject (left panel) and RHD subject (right panel) shown earlier in Figure 5B. Positive values indicate flexor torque. The corresponding handpaths are shown above the torque profiles, with arrows indicating correction time. For these representative trials, the target jumped from the center to medial location. The typical corrective response for these trials would involve flexion of the shoulder with little elbow acceleration. Instead of using shoulder motion to correct for the medial displacement of the target, the LHD subject initiated the correction with elbow action. Just prior to correction time (arrow on torque profile), a large burst of flexor elbow muscle torque caused flexor elbow acceleration (flexor elbow net torque). The effect of this flexor elbow muscle torque on the shoulder was to accelerate the shoulder into extension (extensor shoulder net torque), a strategy counter to that required for correcting to the medial target location. Just after the initiation of the correction, the extensor shoulder net torque resulted in a flexor interaction torque at the elbow, which became the primary driver of elbow motion and resulted in the “undershoot” of final position with respect to the new target location. Thus, dynamically, the LHD participant showed poor intersegmental coordination and relied largely on elbow motion during the corrective phase. In contrast, the response of the RHD subject to the same target jump was well coordinated, with the correction being initiated by shoulder action and elbow muscle and interaction torques compensating each other to produce minimal elbow acceleration. Again, the delayed correction time can be seen in Figure 6A, as the total movement was composed of 2 discrete reaches. The RHD subject also did not reach the displaced target location accurately, and the resulting undershoot of final position was of similar magnitude to that of the LHD subject. We therefore propose that the similar final position errors of the 2 subject groups are due to different mechanisms: RHD subjects made coordinated movements that were directed to erroneous locations, while the LHD subjects’ poor intersegmental coordination appeared to contribute to final position errors.
Figure 6.
(A) Torque profiles are shown for shoulder net torque (gray line), elbow net torque (thick black line), elbow muscle torque (thin black line), and elbow interaction torque (dashed line) during the individual target jump trials shown in Figure 5B for the LHD subject (left panel) and RHD subject (right panel). Corresponding handpaths are inset. Arrows indicate correction time. (B) Final position error of each target jump trial is plotted as a function of corrective interaction torque for an LHD and RHD subject, with corresponding Pearson r value. (C) Mean normalized Pearson r (Fisher z-score) of final position error versus corrective interaction torque is displayed for the left and right arms of healthy control groups (HC) and the ipsilesional arms of LHD and RHD groups (HD). Bars indicate standard error of mean. *Tukey's test: P < 0.05.
Both subjects shown in Figure 6A demonstrated similar magnitudes of elbow interaction torque early after correction time and had similar errors in final position. Figure 6B illustrates how final position errors were dependent on these interactions in each subject. In the LHD subject, the magnitude of final position error was substantially dependent on the magnitude of interaction torque acting at the elbow. These 2 variables were unrelated, however, in the RHD subject across the same range of torque and error values. To quantify this relationship within each group, final position error was plotted against corrective interaction torque of each target jump trial for each subject, yielding a Pearson r that was then normalized for across-subject comparison. Two-way ANOVA revealed a significant arm × group interaction effect on normalized r (F1,1 = 5.29; P < 0.05). Post hoc analysis revealed that the mean normalized r value for the LHD group (rnorm = 0.60) was significantly larger than all other groups (Fig. 6C), indicating that final position error during target jump trials was explained by interaction torque magnitude to a larger extent in the LHD group than in either the control group or the RHD group. This reflects a deficit in intersegmental coordination during the correction. Thus, even though both the LHD and RHD groups showed a deficit in accuracy of the corrective response, this deficit was associated with poor coordination only in the LHD group. The RHD group showed a deficit in timing (and accuracy) of the response but not its coordination.
Discussion
The purpose of this study was to determine whether online error correction processes are differentially affected by LHD or RHD due to stroke. Deficits in correcting movements to random target displacements were different between the 2 stroke groups, indicating that the roles of the left and right hemispheres are asymmetric. Corrective responses in the RHD group were initiated later and were less accurate compared to their control group. In contrast, corrective responses in the LHD group were initiated earlier, but were also less accurate compared to their control group. Errors in the RHD group emerged from well-coordinated corrective movements that were terminated in the wrong locations. In the LHD subjects, position errors resulted from deficits in coordination of intersegmental dynamics. These results demonstrate distinct effects of LHD and RHD on online corrections and indicate that both hemispheres play significant and complementary roles in the control of unilateral limb movement.
Left and Right Hemisphere Specialization for Different Aspects of Control
While several previous studies have examined responses to target displacements (Keele and Posner 1968; Soechting and Lacquaniti 1983; Goodale et al. 1986; Prablanc and Martin 1992; Desmurget et al. 1999; Sarlegna et al. 2003; Haaland et al. 2004; Mutha et al. 2008), only recently did Gritsenko et al. (2009) address the question of whether limb dynamics are accounted for during the correction. These authors compared actual responses following target jumps to the output of models in which the corrective signal was proportional to the kinematic error or also accounted for the dynamic requirements of making the correction. They found that the controller that accounted for limb dynamics best reproduced the behavioral results. We view this ability to account for intersegmental limb dynamics as arising from a left hemisphere specialization for predictive control. When using the ipsilesional left arm, the LHD subjects showed intact timing but poor intersegmental coordination while making the online correction. In fact, final position errors in these subjects were related to their coordination deficits. Our results thus agree with the conclusion of Gritsenko et al. (2009) and may provide a neural correlate for their findings. Our findings indicate that the ability to account for limb dynamics during an online correction depends on processes within the left hemisphere.
Together with our previous results, our current findings demonstrate that damage to left hemisphere regions produces significant deficits in planning well-coordinated movements. During unperturbed movements, these deficits are evident as inefficient dynamic profiles, large direction errors, and curved hand trajectories (Schaefer et al. 2009b; Mutha et al. 2010). In cases of perturbations, as in the current study, they appear to be manifest as poor intersegmental coordination during the corrective action as well. Although we have not yet evaluated the effects of focal lesions on the coordination of online corrections, our recent work has indicated that parietal regions in the left hemisphere are critical for planning coordinated movements, given that damage to left, but not right, parietal regions impaired the ability to adapt initial movement direction and plan a straight movement to the intended target (Mutha et al. 2011). Further, our recent findings in subjects with ideomotor limb apraxia, which commonly occurs following left parietal lesions, showed impaired interjoint coordination and large variations in movement direction during simple visually targeted reaching actions, lending further support to the idea of parietal-mediated motor planning (Mutha et al. 2010). Some studies have shown, however, little to no significant relationship between limb apraxia and deficits of ipsilesional reaching and grasping (Hermsdorfer et al. 1999, 2003; Schaefer et al. 2007), suggesting that reaching studies in limb apraxic subjects may have to be interpreted with some caution with respect to neuroanatomical correlates. Nonetheless, a large body of evidence from other work suggests that parietal regions appear to be a key contributor to the left hemisphere specialization for predictive control, as has also been recently proposed by Oliveira et al. (2010). Whether the same regions also contribute to planning of coordinated corrective responses requires further investigation.
Desmurget et al. (1999) showed that transcranial magnetic stimulation over parietal cortex in the left hemisphere impacted upon the accuracy of responses to target displacements. They attributed this deficit to a disruption in the ability to estimate the current location of the hand in order to generate the corrective response. Our results demonstrate that final position errors in the LHD group arise as a consequence of poor dynamic coordination; knowledge of the current hand location is necessary in order to accurately compute movement dynamics (Desmurget et al. 1998; Sainburg et al. 2003; Sober and Sabes 2003; Sarlegna et al. 2009). It is thus possible that parietal damage in our LHD subjects impaired the ability to generate estimates of hand location, and thereby resulted in poor specification of limb dynamics for the corrective action, causing the positional error. It must be pointed out, however, that estimation of limb location might not be exclusively dependent on parietal regions. Our other studies in which movement corrections were required, but task goals remained stable, showed accurate final positions in LHD subjects, several of whom had parietal damage (Schaefer et al. 2009a; Mutha et al. 2011). As mentioned earlier, future studies with subjects with focal parietal damage might help to resolve the specific role of parietal regions in the movement correction process.
It is also highly likely that the movement correction process varies as a function of task goals. During experimental reaching tasks, the same stimulus has in fact been shown to elicit a different response depending on the statistical properties of the environment (Fine and Thoroughman 2007), suggesting that the design of task protocols can alter how the nervous system processes errors. Although our current findings for baseline movements appear to be at odds with our previous findings in LHD and RHD subjects as they used their ipsilesional arm to reach across multiple directions (Schaefer et al. 2009b), these differences in task performance may be attributable to the uncertainty of final position introduced by our current perturbation protocol. We have previously shown that LHD subjects can be as, if not more, accurate than their control group when using the ipsilesional left arm (Schaefer et al. 2007, 2009b), while the RHD group has been substantially less accurate despite using their ipsilesional yet dominant right arm when reaching to predictable target locations. In the current study, when comparing baseline performance between each HD group and their respective control groups, LHD subjects had larger errors that were only slightly better than the errors made by RHD subjects. We suggest that this reduced accuracy in baseline movements may be attributable in part to the unpredictable nature of the current paradigm because target jumps were imposed every 4–6 trials and in either direction relative to the original target, thereby increasing the temporal and spatial uncertainty of final position. It is plausible that under these “noisier” task conditions, the neural strategies used to plan each reach are suboptimal (van Beers et al. 2002). Under these conditions, neither HD group could therefore achieve the level of final position accuracy that they did under more predictable conditions in which target location was unperturbed (Schaefer et al. 2009b). Our previous work in both healthy adults and individuals after stroke has demonstrated a specialized role of the left hemisphere for movement planning, which suggests that the performance of LHD subjects could be more susceptible to increased task uncertainty than that of RHD subjects. The accuracy of predictive mechanisms, which is likely already impaired by HD, may be further affected by the unpredictable experimental environment. This interpretation could, in part, account for the reduced accuracy in the LHD group and is consistent with previous work showing that the nature and quality of feedforward mechanisms vary with the likelihood of experiencing task-related errors (Fine and Thoroughman 2007). However, variations in other factors such as the task environment, the availability of arm support, and the type of feedback provided could also contribute to the differences between the current findings during baseline movements and the results from other studies (Fisk and Goodale 1988; Haaland and Harrington 1989; Schaefer et al. 2009b).
It should also be stressed that our previous research has often, but not always, shown final position advantages for the nondominant arm in healthy adults and LHD subjects (Sainburg and Kalakanis 2000; Bagesteiro and Sainburg 2002; Sainburg 2002; Schaefer et al. 2007), emphasizing the critical role that task requirements and movement goals play in controlling reaching behavior. The processing of contextual factors, like behavioral risk and reward, has been attributed largely to frontoparietal and striatal areas (Tobler et al. 2009; Turner and Desmurget 2010), which all play considerable roles in the preparation and execution of voluntary movement (Alexander and Crutcher 1990). Although many of the LHD and RHD subjects in this study had lesions in these regions (see Table 1), future studies involving more focal brain damage are needed to directly assess their relative contributions in establishing a movement’s context.
We nevertheless propose that the nondominant right hemisphere has become specialized for feedback-mediated error correction mechanisms, which can result in positional accuracy advantages. We designed the current study to specifically compare the effects of LHD and RHD on error correction mechanisms. Although the LHD group showed little advantage in the spatial accuracy of movement under baseline conditions, these subjects showed a very strong advantage in the timing of feedback-mediated corrections to target jumps. This earlier onset of movement correction relative to controls may appear to be contradictory to clinical neuropsychological reasoning as brain damage is thought to slow down neural processing. The relative advantage seen in this study within the LHD group, along with other specific relative advantages in aspects of motor behavior (i.e., better accuracy after LHD and better coordination after RHD; see Schaefer et al. 2009b), are collectively consistent with the idea of hemispheric competition. This concept is supported by data from hemispherectomied and split-brain monkeys and humans (see Gazzaniga 2000, 2005 for review), which have demonstrated that some aspects of neural processing necessary for successful motor performance are performed better with one isolated hemisphere than with 2 intact hemispheres (Nakamura and Gazzaniga 1978), presumably due to lack of interference or “competition” from the other hemisphere. Overall performance of complex real-world movement, however, tends to be worse than in intact subjects because it relies on processes from each hemisphere. Recent work in split-brain subjects has, in fact, suggested that integration of specialized information from each hemisphere is critical for intact error processing during visuomotor learning (Hochman et al. 2011) and supports the principle that lateralization affords a larger functional repertoire by recruiting both hemispheres for specific processes.
For example, we found that RHD subjects took longer to initiate movement corrections than HCs, as well as the LHD subjects. Thus, the effect of stroke alone did not explain the longer latency for movement modification; rather the delay in the time of correction was an effect of right hemisphere stroke only. In RHD subjects, movements in the target jump condition were segmented (see Figs 4A and 5B), such that they completed the first reach to the expected location and initiated a second reach to the new location after a prolonged delay. This disruption in rapid online control is consistent with studies that have suggested a prominent role for regions of the right hemisphere in the executive control of actions. These studies have suggested that frontal regions of the right hemisphere, along with their subcortical connections, are important for inhibiting predominant responses and substituting them with responses appropriate for the current goal of the task (Aron and Poldrack 2006; Mars et al. 2007; Swann et al. 2009; Neubert et al. 2010). This leads to the prediction that damage to these right hemisphere regions should impair this ability to rapidly update ongoing actions in cases where task goals change unexpectedly. Our current results in the RHD group fit well with this prediction. When target location was unexpectedly changed, our RHD subjects showed a strong tendency to complete the initial (baseline) movement and then initiated a second movement to the displaced target location after a long delay. Thus, the ability to inhibit the predominant baseline response and rapidly update the ongoing action was impaired following RHD. It has been suggested that frontal regions, especially inferior frontal and dorsolateral prefrontal cortex, in the right hemisphere are critical for this behavior. Indeed, all but one of our RHD subjects had dorsolateral prefrontal involvement and all but 2 RHD subjects had inferior frontal lesions, which likely contributed to their deficit. However, because of damage to other regions in the right hemisphere, as well as the lack of significant damage in similar frontal regions in the LHD group (see Table 1), more research comparing subjects with focal damage is necessary to confirm a specialized role of right frontal regions in updating an ongoing action.
Besides the delay between the first and second components of the movements in the RHD subjects, these subjects also showed large final position errors. Further, in these subjects, unlike the LHD group, we noted that these errors were not associated with poor dynamic coordination. In fact, intersegmental coordination in these subjects remained intact, likely due to spared left hemisphere regions. However, the accuracy of their corrections was still affected. These findings suggest that right hemisphere lesions resulted in damage to mechanisms involved in achieving a stable final position despite intact motor planning. Whether the same mechanisms mediate predominant response inhibition, action updating, and achieving a desired final position following initiation of the corrective response remains unclear. Nevertheless, the finding that final position accuracy was adversely impacted following RHD is in line with our previous studies. Consistent with other studies (Hermsdorfer et al. 2003; Darling et al. 2008), we have shown poor final position control in RHD subjects during simple reaching movements (Schaefer et al. 2009b) and now extend it to conditions where movement goals can unexpectedly change.
Hemispheric Specialization of Movement Control Affords Motor Skill
While contralesional arm deficits that occur after stroke have been emphasized most frequently (Bourbonnais et al. 1989; Dewald et al. 1995; Levin 1996; Beer et al. 2000; Dewald and Beer 2001; Fang et al. 2007), our current results, along with other studies (Winstein and Pohl 1995; Haaland et al. 2004; Yarosh et al. 2004; Quaney et al. 2005, 2010), have shown that significant hemisphere-specific deficits exist even in the ipsilesional “unaffected” arm following unilateral stroke. Though the hemisphere-specific nature of stroke-related ipsilesional motor deficits has been characterized by controlled and hypothesis-driven experimentation, the real-world effects of these deficits have been realized as poorer functional task performance (Desrosiers et al. 1996; Wetter et al. 2005; Nowak et al. 2007; Chestnut and Haaland 2008; Schaefer et al. 2009b). Despite documented deficits in its performance, the ipsilesional limb is still often favored relative to the contralesional limb when spontaneously performing activities of daily living (Nakayama et al. 1994; Sterr et al. 2002; Taub et al. 2006; Uswatte et al. 2006). This increased reliance on the ipsilesional limb is also illustrated by higher levels of monitored motor activity compared to the contralesional limb (Uswatte et al. 2005, 2006; Lang et al. 2007; Rinehart et al. 2009; Thrane et al. 2011). Animal studies have demonstrated, however, that ipsilesional deficits following motor cortex damage can improve over time and through training. For example, the ipsilesional limb can move faster and with more skill when reaching to grasp small food items following high doses of daily repetitions of functionally important upper extremity task practice over several weeks (Bury and Jones 2002; Luke et al. 2004; Kaeser et al. 2010). Spontaneous improvements in ipsilesional hand motor skill over a 12-month period have recently been documented in nonhuman primates with focal lesions to primary motor cortex (Darling et al. 2011). While these findings in animal models are promising, they are different from human clinical findings that show that ipsilesional deficits persist for many years, following stroke (Desrosiers et al. 1996; Schaefer et al. 2009b). It is plausible that the more persistent ipsilesional deficits in humans are related to the larger degree of cortical and subcortical damage that typically results from a naturally occurring stroke. The findings from animal models of motor cortex lesions provide strong evidence and promise that the ipsilesional limb can show functional recovery, but future work is necessary in translating studies in animals into clinical interventions in humans.
Studies of unilateral brain lesions have shown that the intact hemisphere can undergo some reorganization after stroke and that the nature of these changes may depend on the lesion’s location and size (Allred and Jones 2004; Luke et al. 2004; Hsu and Jones 2006). These changes may even continue into the chronic stage (Cramer et al. 1997; Elbert and Rockstroh 2004; Luft et al. 2004; Schaechter et al. 2006). In the current study, the broad ranges of upper extremity Fugl-Meyer scores and time poststroke in both HD groups (see Table 1) limit our ability to test direct relationship between kinematic/kinetic variables and stroke severity/duration. Our data suggest, however, that an intact left or right hemisphere is unable to fully compensate for the functional loss due to damage to the other hemisphere following unilateral stroke, consistent with other work (Nonnekes et al. 2010). In other words, after a stroke in the right hemisphere, the right arm has greater position-based deficits than the right arm of a healthy adult, despite both individuals having intact left hemispheres. Similarly, LHD produces significant coordination deficits in the ipsilesional left arm compared to HCs using the same arm. These are strong predictions of our dynamic-dominance model, which proposes that each hemisphere contributes unique mechanisms for controlling movements of a single limb. These mechanisms likely result in a limb-specific set of motor commands appropriate for a given task based on the evaluation of certain costs and rewards (Trommershauser et al. 2006; Shadmehr and Krakauer 2008; Arce et al. 2009). The specialization of the left and right hemispheres for controlling different but complementary aspects of movement in parallel can thus produce effective performance for a given task goal. Thus, by testing our model of hemispheric specialization in people with unilateral hemispheric damage after stroke, we provide quantitative neurological evidence of Sir Humphry’s hypothesis that lateralized neural organization gives rise to a greater degree of skillfulness.
Funding
This work was supported by grants from the National Institutes of Health (R01 HD39311, R01HD059783 to R.L.S.), the Veterans Affairs Office of Research and Development (Clinical Science Research and Development and Rehabilitation Research and Development grant B4125R to K.Y.H.), and the National Institute on Aging Interdisciplinary Training in Gerontology grant (T32AG00048 to S.Y.S.). This project was also funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds. The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Acknowledgments
Thanks to 1) Jennifer Hogan, Rena Singleton, Lee Stapp, and Melissa Daniels for data collection and Jenna Keller for subject recruitment; 2) Dr Robert Knight for MRI tracings and neuroanatomical consultation; 3) Dr Brad Cushnyr for clinical neuroradiological consultation; and 4) Drs John Adair and Sally Harris, as well as HealthSouth and Lovelace Rehabilitation Hospitals, for subject referral. Conflict of Interest : None declared.
References
- Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol. 1990;64:133–150. doi: 10.1152/jn.1990.64.1.133. [DOI] [PubMed] [Google Scholar]
- Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage in female rats: forelimb behavioral effects and dendritic structural plasticity in the contralateral homotopic cortex. Exp Neurol. 2004;190:433–445. doi: 10.1016/j.expneurol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Arce F, Novick I, Shahar M, Link Y, Ghez C, Vaadia E. Differences in context and feedback result in different trajectories and adaptation strategies in reaching. PLoS One. 2009;4:e4214. doi: 10.1371/journal.pone.0004214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce F, Novick I, Vaadia E. Discordant tasks and motor adjustments affect interactions between adaptations to altered kinematics and dynamics. Front Hum Neurosci. 2010;3:65. doi: 10.3389/neuro.09.065.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagesteiro LB, Sainburg RL. Handedness: dominant arm advantages in control of limb dynamics. J Neurophysiol. 2002;88:2408–2421. doi: 10.1152/jn.00901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagesteiro LB, Sainburg RL. Nondominant arm advantages in load compensation during rapid elbow joint movements. J Neurophysiol. 2003;90:1503–1513. doi: 10.1152/jn.00189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer RF, Dewald JP, Dawson ML, Rymer WZ. Target-dependent differences between free and constrained arm movements in chronic hemiparesis. Exp Brain Res. 2004;156:458–470. doi: 10.1007/s00221-003-1807-8. [DOI] [PubMed] [Google Scholar]
- Beer RF, Dewald JP, Rymer WZ. Deficits in the coordination of multijoint arm movements in patients with hemiparesis: evidence for disturbed control of limb dynamics. Exp Brain Res. 2000;131:305–319. doi: 10.1007/s002219900275. [DOI] [PubMed] [Google Scholar]
- Bourbonnais D, Vanden Noven S, Carey KM, Rymer WZ. Abnormal spatial patterns of elbow muscle activation in hemiparetic human subjects. Brain. 1989;112:85–102. doi: 10.1093/brain/112.1.85. [DOI] [PubMed] [Google Scholar]
- Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. J Neurosci. 2002;22:8597–8606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chestnut C, Haaland KY. Functional significance of ipsilesional motor deficits after unilateral stroke. Arch Phys Med Rehabil. 2008;89:62–68. doi: 10.1016/j.apmr.2007.08.125. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, Kennedy DN, Finklestein SP, Rosen BR. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- Darling WG, Bartelt R, Pizzimenti MA, Rizzo M. Spatial perception errors do not predict pointing errors by individuals with brain lesions. J Clin Exp Neuropsychol. 2008;30:102–119. doi: 10.1080/13803390701249036. [DOI] [PubMed] [Google Scholar]
- Darling WG, Pizzimenti MA, Hynes SM, Rotella DL, Headley G, Ge J, Stilwell-Morecraft KS, McNeal DW, Solon-Cline KM, Morecraft RJ. Volumetric effects of motor cortex injury on recovery of ipsilesional dexterous movements. Exp Neurol. 2011;231:56–71. doi: 10.1016/j.expneurol.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeArmond SJ, Fusco MM, Dewey MM. Structure of the human brain: a photographic atlas. New York: Oxford University Press; 1989. [Google Scholar]
- Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat Neurosci. 1999;2:563–567. doi: 10.1038/9219. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Pelisson D, Rossetti Y, Prablanc C. From eye to hand: planning goal-directed movements. Neurosci Biobehav Rev. 1998;22:761–788. doi: 10.1016/s0149-7634(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Desrosiers J, Bourbonnais D, Bravo G, Roy PM, Guay M. Performance of the ‘unaffected’ upper extremity of elderly stroke patients. Stroke. 1996;27:1564–1570. doi: 10.1161/01.str.27.9.1564. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 2001;24:273–283. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118:495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Dizio P, Lackner JR. Motor adaptation to Coriolis force perturbations of reaching movements: endpoint but not trajectory adaptation transfers to the nonexposed arm. J Neurophysiol. 1995;74:1787–1792. doi: 10.1152/jn.1995.74.4.1787. [DOI] [PubMed] [Google Scholar]
- Duff SV, Sainburg RL. Lateralization of motor adaptation reveals independence in control of trajectory and steady-state position. Exp Brain Res. 2007;179:551–561. doi: 10.1007/s00221-006-0811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert T, Rockstroh B. Reorganization of human cerebral cortex: the range of changes following use and injury. Neuroscientist. 2004;10:129–141. doi: 10.1177/1073858403262111. [DOI] [PubMed] [Google Scholar]
- Fang Y, Yue GH, Hrovat K, Sahgal V, Daly JJ. Abnormal cognitive planning and movement smoothness control for a complex shoulder/elbow motor task in stroke survivors. J Neurol Sci. 2007;256:21–29. doi: 10.1016/j.jns.2007.01.078. [DOI] [PubMed] [Google Scholar]
- Fine MS, Thoroughman KA. Trial-by-trial transformation of error into sensorimotor adaptation changes with environmental dynamics. J Neurophysiol. 2007;98:1392–1404. doi: 10.1152/jn.00196.2007. [DOI] [PubMed] [Google Scholar]
- Fisk JD, Goodale MA. The effects of unilateral brain damage on visually guided reaching: hemispheric differences in the nature of the deficit. Exp Brain Res. 1988;72:425–435. doi: 10.1007/BF00250264. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123(Pt 7):1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Forty-five years of split-brain research and still going strong. Nat Rev Neurosci. 2005;6:653–659. doi: 10.1038/nrn1723. [DOI] [PubMed] [Google Scholar]
- Ghez C, Scheidt R, Heijink H. Different learned coordinate frames for planning trajectories and final positions in reaching. J Neurophysiol. 2007;98:3614–3626. doi: 10.1152/jn.00652.2007. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Brown SH. Task-dependent asymmetries in the utilization of proprioceptive feedback for goal-directed movement. Exp Brain Res. 2007;180:693–704. doi: 10.1007/s00221-007-0890-7. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Lewis CA, Brown SH. Upper limb asymmetries in the utilization of proprioceptive feedback. Exp Brain Res. 2006;168:307–311. doi: 10.1007/s00221-005-0280-y. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Pelisson D, Prablanc C. Large adjustments in visually guided reaching do not depend on vision of the hand or perception of target displacement. Nature. 1986;320:748–750. doi: 10.1038/320748a0. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghilardi MF, Cooper SE, Ghez C. Accuracy of planar reaching movements. II. Systematic extent errors resulting from inertial anisotropy. Exp Brain Res. 1994;99:112–130. doi: 10.1007/BF00241416. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghilardi MF, Ghez C. Accuracy of planar reaching movements. I. Independence of direction and extent variability. Exp Brain Res. 1994;99:97–111. doi: 10.1007/BF00241415. [DOI] [PubMed] [Google Scholar]
- Gritsenko V, Yakovenko S, Kalaska JF. Integration of predictive feedforward and sensory feedback signals for online control of visually guided movement. J Neurophysiol. 2009;102:914–930. doi: 10.1152/jn.91324.2008. [DOI] [PubMed] [Google Scholar]
- Guiard Y, Diaz G, Beaubaton D. Left-hand advantage in right-handers for spatial constant error: preliminary evidence in a unimanual ballistic aimed movement. Neuropsychologia. 1983;21:111–115. doi: 10.1016/0028-3932(83)90106-9. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL. Hemispheric control of the initial and corrective components of aiming movements. Neuropsychologia. 1989;27:961–969. doi: 10.1016/0028-3932(89)90071-7. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Prestopnik JL, Knight RT, Lee RR. Hemispheric asymmetries for kinematic and positional aspects of reaching. Brain. 2004;127:1145–1158. doi: 10.1093/brain/awh133. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY. Hemispheric specialization for motor sequencing: abnormalities in levels of programming. Neuropsychologia. 1991;29:147–163. doi: 10.1016/0028-3932(91)90017-3. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan battery: demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz (FL): Psychological Assessment Resources, Inc; 2004. [Google Scholar]
- Hermsdorfer J, Blankenfeld H, Goldenberg G. The dependence of ipsilesional aiming deficits on task demands, lesioned hemisphere, and apraxia. Neuropsychologia. 2003;41:1628–1643. doi: 10.1016/s0028-3932(03)00097-6. [DOI] [PubMed] [Google Scholar]
- Hermsdorfer J, Laimgruber K, Kerkhoff G, Mai N, Goldenberg G. Effects of unilateral brain damage on grip selection, coordination, and kinematics of ipsilesional prehension. Exp Brain Res. 1999;128:41–51. doi: 10.1007/s002210050815. [DOI] [PubMed] [Google Scholar]
- Hochman EY, Eviatar Z, Barnea A, Zaaroor M, Zaidel E. Hemispheric integration is critical for intact error processing. Neuropsychologia. 2011;49:1816–1823.. doi: 10.1016/j.neuropsychologia.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Hsu JE, Jones TA. Contralesional neural plasticity and functional changes in the less-affected forelimb after large and small cortical infarcts in rats. Exp Neurol. 2006;201:479–494. doi: 10.1016/j.expneurol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Humphry GM. The human foot and the human hand. London: MacMillan and Co; 1861. [Google Scholar]
- Kaeser M, Wyss AF, Bashir S, Hamadjida A, Liu Y, Bloch J, Brunet JF, Belhaj-Saif A, Rouiller EM. Effects of unilateral motor cortex lesion on ipsilesional hand's reach and grasp performance in monkeys: relationship with recovery in the contralesional hand. J Neurophysiol. 2010;103:1630–1645. doi: 10.1152/jn.00459.2009. [DOI] [PubMed] [Google Scholar]
- Keele SW, Posner MI. Processing of visual feedback in rapid movements. J Exp Psychol. 1968;77:155–158. doi: 10.1037/h0025754. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western aphasia battery. New York: The Psychological Corporation; 1982. [Google Scholar]
- Kimura D, Archibald Y. Motor functions of the left hemisphere. Brain. 1974;97:337–350. doi: 10.1093/brain/97.1.337. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL, Clayworth C. The effects of lesions of superior temporal gyrus and inferior parietal lobe on temporal and vertex components of the human AEP. Electroencephalogr Clin Neurophysiol. 1988;70:499–509. doi: 10.1016/0013-4694(88)90148-4. [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Herter TM, Scott SH. Random change in cortical load representation suggests distinct control of posture and movement. Nat Neurosci. 2005;8:498–504. doi: 10.1038/nn1420. [DOI] [PubMed] [Google Scholar]
- Lang CE, Wagner JM, Edwards DF, Dromerick AW. Upper extremity use in people with hemiparesis in the first few weeks after stroke. J Neurol Phys Ther. 2007;31:56–63. doi: 10.1097/NPT.0b013e31806748bd. [DOI] [PubMed] [Google Scholar]
- Lenhard A, Hoffmann J. Constant error in aiming movements without visual feedback is higher in the preferred hand. Laterality. 2007;12:227–238. doi: 10.1080/13576500701203891. [DOI] [PubMed] [Google Scholar]
- Levin MF. Interjoint coordination during pointing movements is disrupted in spastic hemiparesis. Brain. 1996;119:281–293. doi: 10.1093/brain/119.1.281. [DOI] [PubMed] [Google Scholar]
- Luft AR, Waller S, Forrester L, Smith GV, Whitall J, Macko RF, Schulz JB, Hanley DF. Lesion location alters brain activation in chronically impaired stroke survivors. Neuroimage. 2004;21:924–935. doi: 10.1016/j.neuroimage.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Luke LM, Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage induces contralesional synaptogenesis and enhances skilled reaching with the ipsilateral forelimb in adult male rats. Synapse. 2004;54:187–199. doi: 10.1002/syn.20080. [DOI] [PubMed] [Google Scholar]
- Mars RB, Piekema C, Coles MG, Hulstijn W, Toni I. On the programming and reprogramming of actions. Cereb Cortex. 2007;17:2972–2979. doi: 10.1093/cercor/bhm022. [DOI] [PubMed] [Google Scholar]
- Mutha PK, Boulinguez P, Sainburg RL. Visual modulation of proprioceptive reflexes during movement. Brain Res. 2008;1246:54–69. doi: 10.1016/j.brainres.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL. Control of velocity and position in single joint movements. Hum Mov Sci. 2007;26:808–823. doi: 10.1016/j.humov.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL, Haaland KY. Coordination deficits in ideomotor apraxia during visually targeted reaching reflect impaired visuomotor transformations. Neuropsychologia. 2010;48:3855–3867. doi: 10.1016/j.neuropsychologia.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL, Haaland KY. Left parietal regions are critical for adaptive visuomotor control. J Neurosci. 2011;31:6972–6981. doi: 10.1523/JNEUROSCI.6432-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura RK, Gazzaniga MS. Hemispherectomy vs commissurotomy in the monkey: one hemisphere can be better than two. Exp Neurol. 1978;59:202–208. doi: 10.1016/0014-4886(78)90150-4. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Compensation in recovery of upper extremity function after stroke: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75:852–857. doi: 10.1016/0003-9993(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Buch ER, Olivier E, Rushworth MF. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc Natl Acad Sci U S A. 2010;107:13240–13245. doi: 10.1073/pnas.1000674107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonnekes JH, Talelli P, de Niet M, Reynolds RF, Weerdesteyn V, Day BL. Deficits underlying impaired visually triggered step adjustments in mildly affected stroke patients. Neurorehabil Neural Repair. 2010;24:393–400. doi: 10.1177/1545968309348317. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Dafotakis M, Kust J, Karbe H, Fink GR. Dexterity is impaired at both hands following unilateral subcortical middle cerebral artery stroke. Eur J Neurosci. 2007;25:3173–3184. doi: 10.1111/j.1460-9568.2007.05551.x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oliveira FT, Diedrichsen J, Verstynen T, Duque J, Ivry RB. Transcranial magnetic stimulation of posterior parietal cortex affects decisions of hand choice. Proc Natl Acad Sci U S A. 2010;107:17751–17756. doi: 10.1073/pnas.1006223107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prablanc C, Martin O. Automatic control during hand reaching at undetected two-dimensional target displacements. J Neurophysiol. 1992;67:455–469. doi: 10.1152/jn.1992.67.2.455. [DOI] [PubMed] [Google Scholar]
- Quaney BM, He J, Timberlake G, Dodd K, Carr C. Visuomotor training improves stroke-related ipsilesional upper extremity impairments. Neurorehabil Neural Repair. 2010;24:52–61. doi: 10.1177/1545968309341646. [DOI] [PubMed] [Google Scholar]
- Quaney BM, Perera S, Maletsky R, Luchies CW, Nudo RJ. Impaired grip force modulation in the ipsilesional hand after unilateral middle cerebral artery stroke. Neurorehabil Neural Repair. 2005;19:338–349. doi: 10.1177/1545968305282269. [DOI] [PubMed] [Google Scholar]
- Rinehart JK, Singleton RD, Adair JC, Sadek JR, Haaland KY. Arm use after left or right hemiparesis is influenced by hand preference. Stroke. 2009;40:545–550. doi: 10.1161/STROKEAHA.108.528497. [DOI] [PubMed] [Google Scholar]
- Roy EA, MacKenzie C. Handedness effects in kinesthetic spatial location judgements. Cortex. 1978;14:250–258. doi: 10.1016/s0010-9452(78)80051-3. [DOI] [PubMed] [Google Scholar]
- Rudroff T, Justice JN, Holmes MR, Matthews SD, Enoka RM. Muscle activity and time to task failure differ with load compliance and target force for elbow flexor muscles. J Appl Physiol. 2011;110:125–136. doi: 10.1152/japplphysiol.00605.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res. 2002;142:241–258. doi: 10.1007/s00221-001-0913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL. Handedness: differential specializations for control of trajectory and position. Exerc Sport Sci Rev. 2005;33:206–213. doi: 10.1097/00003677-200510000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL. Lateralization of goal-directed movement. In: Elliott D, Khan M, editors. Vision and goal-directed movement: neurobehavioral perspectives. Champaign (IL): Human Kinetics; 2010. pp. 219–238. [Google Scholar]
- Sainburg RL, Kalakanis D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J Neurophysiol. 2000;83:2661–2675. doi: 10.1152/jn.2000.83.5.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Lateiner JE, Latash ML, Bagesteiro LB. Effects of altering initial position on movement direction and extent. J Neurophysiol. 2003;89:401–415. doi: 10.1152/jn.00243.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlegna F, Blouin J, Bresciani JP, Bourdin C, Vercher JL, Gauthier GM. Target and hand position information in the online control of goal-directed arm movements. Exp Brain Res. 2003;151:524–535. doi: 10.1007/s00221-003-1504-7. [DOI] [PubMed] [Google Scholar]
- Sarlegna FR, Przybyla A, Sainburg RL. The influence of target sensory modality on motor planning may reflect errors in sensori-motor transformations. Neuroscience. 2009;164:597–610. doi: 10.1016/j.neuroscience.2009.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabowsky CN, Hidler JM, Lum PS. Greater reliance on impedance control in the nondominant arm compared with the dominant arm when adapting to a novel dynamic environment. Exp Brain Res. 2007;182:567–577. doi: 10.1007/s00221-007-1017-x. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, Moore CI, Connell BD, Rosen BR, Dijkhuizen RM. Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain. 2006;129:2722–2733. doi: 10.1093/brain/awl214. [DOI] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Ipsilesional motor deficits following stroke reflect hemispheric specializations for movement control. Brain. 2007;130:2146–2158. doi: 10.1093/brain/awm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Dissociation of initial trajectory and final position errors during visuomotor adaptation following unilateral stroke. Brain Res. 2009a;1298:78–91. doi: 10.1016/j.brainres.2009.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KY, Sainburg RL. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia. 2009b;47:2953–2966. doi: 10.1016/j.neuropsychologia.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabbott BA, Sainburg RL. Differentiating between two models of motor lateralization. J Neurophysiol. 2008;100:565–575. doi: 10.1152/jn.90349.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabbott BA, Sainburg RL. On-line corrections for visuomotor errors. Exp Brain Res. 2009;195:59–72. doi: 10.1007/s00221-009-1749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sober SJ, Sabes PN. Multisensory integration during motor planning. J Neurosci. 2003;23:6982–6992. doi: 10.1523/JNEUROSCI.23-18-06982.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soechting JF, Lacquaniti F. Modification of trajectory of a pointing movement in response to a change in target location. J Neurophysiol. 1983;49:548–564. doi: 10.1152/jn.1983.49.2.548. [DOI] [PubMed] [Google Scholar]
- Sterr A, Freivogel S, Schmalohr D. Neurobehavioral aspects of recovery: assessment of the learned nonuse phenomenon in hemiparetic adolescents. Arch Phys Med Rehabil. 2002;83:1726–1731. doi: 10.1053/apmr.2002.35660. [DOI] [PubMed] [Google Scholar]
- Swann N, Tandon N, Canolty R, Ellmore TM, MCEvoy LK, Dreyer S, SiSano M, Aron AR. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci. 2009;29:12675–12685. doi: 10.1523/JNEUROSCI.3359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E, Uswatte G, Mark VW, Morris DM. The learned nonuse phenomenon: implications for rehabilitation. Eura Medicophys. 2006;42:241–256. [PubMed] [Google Scholar]
- Thrane G, Emaus N, Askim T, Anke A. Arm use in patients with subacute stroke monitored by accelerometry: association with motor impairment and influence on self-dependence. J Rehabil Med. 2011;43:299–304. doi: 10.2340/16501977-0676. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Christopoulos GI, O'Doherty JP, Dolan RJ, Schultz W. Risk-dependent reward value signal in human prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106:7185–7190. doi: 10.1073/pnas.0809599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommershauser J, Landy MS, Maloney LT. Humans rapidly estimate expected gain in movement planning. Psychol Sci. 2006;17:981–988. doi: 10.1111/j.1467-9280.2006.01816.x. [DOI] [PubMed] [Google Scholar]
- Turner RS, Desmurget M. Basal ganglia contributions to motor control: a vigorous tutor. Curr Opin Neurobiol. 2010;20:704–716. doi: 10.1016/j.conb.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uswatte G, Foo WL, Olmstead H, Lopez K, Holand A, Simms LB. Ambulatory monitoring of arm movement using accelerometry: an objective measure of upper-extremity rehabilitation in persons with chronic stroke. Arch Phys Med Rehabil. 2005;86:1498–1501. doi: 10.1016/j.apmr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Uswatte G, Taub E, Morris D, Light K, Thompson PA. The Motor Activity Log-28: assessing daily use of the hemiparetic arm after stroke. Neurology. 2006;67:1189–1194. doi: 10.1212/01.wnl.0000238164.90657.c2. [DOI] [PubMed] [Google Scholar]
- van Beers RJ, Baraduc P, Wolpert DM. Role of uncertainty in motor control. Philos Trans R Soc Lond B Biol Sci. 2002;357:1137–1145. doi: 10.1098/rstb.2002.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Adaptation to visuomotor rotations remaps movement vectors, not final positions. J Neurosci. 2005;25:4024–4030. doi: 10.1523/JNEUROSCI.5000-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter S, Poole JL, Haaland KY. Functional implications of ipsilesional motor deficits after unilateral stroke. Arch Phys Med Rehabil. 2005;86:776–781. doi: 10.1016/j.apmr.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Pohl PS. Effects of unilateral brain damage on the control of goal-directed hand movements. Exp Brain Res. 1995;105:163–174. doi: 10.1007/BF00242191. [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and motor control of human movement. New York: John Wiley and Sons; 1990. [Google Scholar]
- Yarosh CA, Hoffman DS, Strick PL. Deficits in movements of the wrist ipsilateral to a stroke in hemiparetic subjects. J Neurophysiol. 2004;92:3276–3285. doi: 10.1152/jn.00549.2004. [DOI] [PubMed] [Google Scholar]