Abstract
Studies of socially housed rodents have provided significant information regarding the consequences of exposure to stressors. Psychosocial stressors are known to alter the ingestion of ethanol and the activity of the dopaminergic neuronal system. Since both stressors and ethanol are known to affect the function of dopaminergic neurons, we employed amphetamine to assess the role of this neural system on the ingestion of ethanol by psychosocially stressed male rats. Male rats housed two per cage were designated as dominant or subdominant rats based on evaluations of agonistic behavior and body weight changes. The dyad-housed rats and a group of single-housed rats were sequentially assessed for ethanol intake after injections of saline or amphetamine (0.3, 0.9 or 2.7 mg/kg i.p.) both prior to dyad housing and subsequently again during dyad-housing. Prior to dyad housing ethanol intake of future subdominant rats was higher than that of future dominant rats. Dyad-housing significantly increased ethanol intake of dominant rats. Pre-dyad the highest dose of amphetamine potently depressed ethanol ingestion. Sensitivity to amphetamine’s depressant effect on ethanol intake was higher at the diad test in all subjects, most prominently in single-housed rats. In contrast to the single-housed rats, the dyad-housed rats displayed saccharin anhedonia. It can be concluded that dopaminergic system modulates, at least partially, the psychosocial stress-induced changes in ethanol intake. Furthermore, the level of ethanol ingestion at the pre-dyad test was predictive of future hierarchical status.
Keywords: Rank status, ethanol preference, saccharin intake, anhedonia, dominance, social isolation
1. INTRODUCTION
The stress response involves the coordinated physiological and behavioral response of an organism to a stressful challenge. While the stressful challenge (stressor) can be environmental or physiological, the adaptive coping mechanisms are both rapid in the case of acute stressors, and long term in the case of chronic stressors. Experimental evidence indicates that rodents display two general types of coping strategies when faced with a psychosocial stressor. These two extensively investigated strategies have been designated as “active” coping and “passive” coping (e.g., Benus et al., 1991; Koolhaas et al., 1999). Because of the distinct characteristics of “active” coping, generally associated with dominant (Dom) status, and of “passive” coping style, associated with non-Dom status (Benus et al., 1991; Koolhaas et al., 1999), it is likely that sensitivity to drugs may also differ with a subject’s rank status.
The influence of stress, on the ingestion of ethanol (EtOH), by humans and experimental animals, is now well recognized and is documented by several reviews (Björqvist, 2001; Jose et al., 2000; Pohorecky et al., 1990; Pohorecky et al, 1991; Uhart and Wand, 2008). The specific effects of psychosocial stress on the ingestion of EtOH have been evaluated employing a number of animal models. Studies on the effect of social isolation (single-housing, SiH) on the ingestion EtOH have provided inconsistent findings. While some stress researchers reported that isolation-stressed rodents ingested more EtOH compared to group-housed rodents (Ehlers et al., 2007; Juarez and Vazquez-Cortes, 2003; Parker and Radow, 1974; Roske et al., 1994), others reported a decline (Adams and Oldham, 1996) or no effect on EtOH intake (Doremus et al., 2005; Thorsell et al., 2005).
Stress researchers have also employed the resident-intruder model to evaluate the stressfulness of defeat on the ingestion of EtOH. These investigators reported a decline in EtOH intake in defeated rats (Funk et al., 2005; van Erp and Miczek, 2001) but not in mice (Croft et al., 2005; Keeney, Hogg, 1999). One of the most successful, and ethologically relevant, approaches to study the consequences of chronic social stress are models based on hierarchical stress. Such studies reported that EtOH intake was enhanced in group-housed non-Dom rats compared to the Dom rats (Blanchard et al, 1987; Blanchard et al., 1993; Ellison, 1987; Pohorecky, 2008; Pohorecky et al., 2010; Weisinger et al., 1989; Wolffgramm and Heyne, 1991). The triad-housing model developed in our laboratory allows for the rapid development of a stable and robust social hierarchy among co-housed male rats (Blakley and Pohorecky, 2006; Pohorecky et al., 1999; Pohorecky et al., 2004). This model involves the co-habitation of two or more male animals with continuous visual and olfactory contact. Employing this model we have reported that the change in intake of EtOH produced by distinct acute novel stressors depended on the rank/housing status of the subjects (Pohorecky et al., 2008). For example, enhancing anxiety levels by exposing rats to an elevated plus maze had a negative impact on the EtOH ingestion of triad-housed rats, but had no effect on EtOH intake of single-housed rats (Pohorecky et al., 2008). Additionally, studies with group-living non-human primates indicate that lower ranking animals ingested more EtOH compared to high-ranking animals (Crowley et al, 1990; Fahlke et al, 2002; Highley et al., 1996).
A significant body of evidence documents the effects of EtOH on the dopaminergic (DArgic) system in the brain. Acutely, EtOH has been shown to increase tonic dopamine (DA) release, while its effect on phasic release was less reliable (Robinson et al., 2009), and the firing rate of DA neurons in high EtOH preferring rats was higher compared to their non-EtOH preferring counterparts (Melis et al., 2009; Morzorati et al., 2010). Ingestion of EtOH also elevated the activity of DArgic neurons (Appel et al., 2003; Brodie et al., 1999; Morzorati et al., 2010), and DA levels in the nucleus accumbens (Di Chiara and Imperato, 1988). The DAgic system is believed to mediate, at least partially, both EtOH self-administration and the rewarding aspects of EtOH ingestion (Koob and Weiss, 1992; Melis et al., 2009).
Stressors also produce alterations in DArgic function (reviewed by Marinelli et al., 2006), and deficits in stress coping may reflect deficits in this system (Kapur and Mann, 1992). Significant evidence has also accrued on the effects of psychosocial stress on brain DA neurons. For instance, in monkeys the effect social stress on the DArgic systems was dependent on social rank. Morgan and associates (2002) have noted that cocaine functioned as a reinforcer for subordinate but not the dominant (Dom) monkeys, suggesting the differences in the DArgic systems to be rank dependent. In rodents defeat was associated with DArgic hyperactivity and withincreased phasic DAgic signaling of the mesolimbic pathway (Anstrom et al., 2009). Resident rats have been reported to have higher extraneuronal levels of DA compared to the intruder rats after a resident intruder test (Ferrari et al., 2003), while levels of DA D2 receptor binding were higher in Dom than in subordinate cynomolgus macaques (Morgan et al., 2002), and the binding capacity of DA transporters was found to be lower in repeatedly defeated tree shrews (Isovich et al., 2000).
Previous evidence indicated that the depressant effect of EtOH on behavior was dependent on the subjects rank status. For example, triad-housed subdominant rats were more sensitive than Dom rats the behavioral depressant effects of EtOH (Blakley and Pohorecky, 2006). The present studies address the hypothesis that rank status may also affect sensitivity to other drugs, specifically the psychostimulant drug amphetamine (Amp). Amphetamine was selected based on the evidence that implicates the DA neuronal system in both the ingestion of EtOH and in social stress. Drugs that release DA in the brain such as Amp (e.g.,Verheij and Cools, 2008 for a review), are known to increase the reward value of various stimuli (Di Chiara and Imperato, 1988;Tupala and Tiihonen, 2004) and would be expected to also alter the intake of EtOH. The additional aim of our study was to further evaluate the role of the DArgic system in the behavioral consequences of psychosocial stress. Lastly, to evaluate the specificity of Amp’s action on EtOH ingestion in psychosocially stressed rats, we also examined the intake of saccharin, a widely regarded rewarding substance for rodents.
2. METHODS
2.1. Subjects and Housing
The subjects were male adult Long Evans rats weighing approximately 450 g at the start of the experiment (Harlan, Indianapolis, Indiana). Purina chow and water were available ad libitum throughout the study. The animal room was on a reverse light/dark cycle (12 hours each, lights off at 12:30 ), and its ambient humidity and temperature (21±1° C) was strictly controlled. The housing cages were made of Plexiglas and had a wire mesh floor. One of the cage walls had either two (single cages) or four (dyad cages) 1-cm openings that accommodated the drinking spouts. The cages for the single-housed (SiH) rats were square (25 cm x 25 cm x 30 cm), and those for the dyad-housed rats were rectangular (26 cm wide x 82 cm long x 30 cm high). The dyad cages had a removable Plexiglas cage divider that partitioned the cage into two equal compartments. The bottom of the cage dividers consisted of a 6-cm high wire mesh screen that allowed rats to maintain sensory contact even when separated. These dividers were removed daily for a one-hour period that allowed the members of a dyad to interact and reinforce their social hierarchy. Rats were weighed daily during the periods when the intake of test solutions was being assessed, and at weekly intervals for the intervening periods. Our animal facility is certified by AAALAC, and the experimental protocols were approved by the Rutgers University review Committee for the use of Animal Subjects, and all principles of laboratory care were strictly adhered to.
Agonistic Behavior Rating
On the day of dyad formation subjects were placed into a dyad cage with the cage dividers in place for a 5-minute adaptation period that allowed them to explore their new environment. This initial adaptation period allowed us to video record the physical appearance of the two co-housed subjects to facilitate subsequent identification. The cage divider was then removed and the ensuing social interactions were recorded over the next 10-minutes of dyad housing. Agonistic behaviors were scored using a modified and expanded version (Pohorecky et. al., 1999; Pohorecky et. al., 2004) of the method originally described by Peterson and Pohorecky (1989). Twenty-three different behaviors were scored as previously described in greater detail (Pohorecky et al., 2004), and subsequently were grouped into four major categories: self-centered (rearing, self-grooming, genital grooming), affiliative (approach, sniff body, sniff genitals, groom other, mount other), defensive (defensive upright, defensive back chick, immobility, vocalization, flight/attempt to jump out of the cage) and aggressive (piloerection, aggressive push-under, pounce on, nip other, cage mark, offensive block or pacing, offensive back chick, lateral threat, on top, roll-tumble) (Pohorecky et al., 2004; 2006). The dyad subject that emitted 22 kHz ultrasonic calls was identified using a Mini Bat Detector (QMC Instruments Inc., London, UK), this rat generally remained immobile while vocalizing and its breathing rhythm appeared altered. Assignment of social status to the members of a dyad was subsequently based on the combined behavioral scores exhibited during the 10-min test, and the change in body weight determined 24 h after dyad formation, and could be further supported by the detection of the subject emitting ultrasonic and audible vocalizations (Pohorecky et al., 2004; 2006). Overall, the Dom rats displayed most of the offensive behaviors, while the subdominant rats (Sdom) displayed most of the defensive behaviors. Additionally, there was a group of single-housed (SiH) rats that remained single-caged for the duration of the study. When the display of agonistic interactions was insufficiently low to determine differences rank status, the dyad was either discarded (1 case), or a given dyad member was exchanged to allow a clearer distinction of rank status (1 case). Testing was continued until thirteen stable dyad pairs were established on day 1 of the study. At intervals during the study agonistic interactions were verified to assess the stability of rank assignments.
2.2. Drinking Protocols
Ethanol Intake
A 1-hour limited access drinking session began at approximately 13:30 PM, following the daily social interaction period, and after replacement of the cage dividers. Two drinking bottles were attached to each cage compartment; one contained tap water and the other a solution of EtOH. To control for position bias, the position of the two drinking tubes was alternated daily. The initial 3% EtOH concentration was increased every other day by 1% up to a 6% solution (v/v), which was then maintained for the duration of the study. The EtOH solutions were prepared from 95% of EtOH using tap water. Consumption of EtOH and water was determined by weighing the bottles, to the nearest 0.1 g, before and after each drinking session. Ethanol consumption was calculated as grams of EtOH consumed per kilogram of body weight. Ethanol preference was defined as the consumed volume of the EtOH solution divided by the volume of the total fluid consumed [ml of EtOH/(ml EtOH + ml water)]. Initial evaluation determined that there was no evidence of significant leakge from the drinking bottles.
Saccharin Intake
The protocol for saccharin intake mirrored that for EtOH intake, except that the 1-hour limited access for saccharin intake was evaluated over three consecutive test days. Beginning at 12:30 rats were allowed access to two drinking bottles, one contained a 0.1% saccharin solution in tap water (w/v), and the second contained tap water. The drinking tubes were refilled with fresh solutions daily, and their positions were also alternated daily dyad. Consumption of saccharin was averaged over the 4 test days and was calculated as grams per kilogram body weight.
2.3. Experimental Treatment
Saline and Amp injections (0.3 mg/kg, 0.9 mg/kg and 2.7 mg/kg) were administered IP 15 minutes prior to the EtOH intake test.
2.4. Experimental Design
The experimental protocol is outlined in Table 1. To adapt the rats to the conditions in our animal room, they were initially individually housed in hanging wire-mesh stainless steel cages. In order to adapt the rats to human contact, rats were handled daily during the last 5-days of the acclimatization period. After this 12-day period of acclimatization, the intake of saccharin was evaluated starting on day-13 of the pre-dyad period. Next rats were adapted to a daily 1-hour limited access to an EtOH solution beginning on day-19 and continuing up to day-46. The evaluation of the effect of Amp on EtOH intake was then conducted over a 13-day period (days 47–60) and was followed by a 28-day washout period during which the subjects continued with their daily routine of limited access to EtOH. On day-89 of the study all the subjects were weighed and transferred either to dyad or to individual cages, initiating the differential housing period. Rats were assigned to either dyad- or single-housed (SiH) based on their body weights (the two co-housed rats differed by less than 5% in body weight). Twenty-four hours after dyad formation all the subjects were again weighed. Only water was available from both drinking bottles during the initial 6 days of differential housing. Beginning on dyad day 95, and over the following three days, testing for saccharin intake was carried out, followed by 21 days of acclimatization to the 1-hour limited EtOH access test, and then by assessment of Amp’s effect on EtOH intake beginning on day 121.
Table 1.
Experimental Protocol for the dyad-housed rats
| Tests | Single- housing | Dyad- housing | Water only | Ethanol/Water choice | Timeline (days) |
|---|---|---|---|---|---|
| Acclimatization | x | x | 12 | ||
| Saccharin testing | x | x | 3 | ||
| Rest | x | x | 5 | ||
| Ethanol intake | x | x | 21 | ||
| Amphetamine test | x | x | 37 | ||
| Washout | x | x | 21 | ||
| - | - | - | - | - | |
| Saccharin testing | x | x | 3 | ||
| Rest | x | x | 5 | ||
| Ethanol intake | x | x | 21 | ||
| Amphetamine test | x | x | 37 |
The timeline for the testing schedule, and the availability of water and the choice of EtOH/water, for the single-housed rats was the same as for the dyad-housed rats except that these rats remained single-housed.
2.5. Statistical Data Analysis
All the data were analyzed using StatView version 5. A simple ANOVA was employed for the analysis of the data on the total behavioral social interactions scores displayed during the 10-min test and on the change in body weight, with the main factor being rank status/housing (Dom, Sdom, single). To assess the effect of Amp on EtOH intake and preference at the two test times, the ratio of change from pre-dyad to dyad was calculated as follows: (response at dyad test/[response at pre-dyad+dyad tests]). The calculated ratio data was then analyzed using a repeated measures ANOVA test with rank status/housing (Dom, Sdom, single) and drug treatment (saline, 0.3, 0.9 and 2.7 mg/kg of Amp) as the between subjects and the within subjects factors, respectively ; statistical significance was set at P =/< .05. Additionally, the intake data were analyzed using a single ANOVA test to compare the effect of rank/housing for each of the individual drug treatment, when appropriate, statistical significance between groups was assessed using the post-hoc Bonferroni’s test; with significance was set at P =/< .0167. All the data are presented as the means and standard errors of the means on 13 rats/rank of the dyad-housed rats and on 11 SiH rats (total n=37 rats).
3. RESULTS
3.1. Behavioral Ranking and Body Weight Changes
After being placed in a dyad cage each dyad member explored its respective compartment during the 5 min adaptation period. The divider separating the two rats was then removed and the behavior displayed by the dyad members was assessed during the first 10 minutes of co-housing. When the divider was removed, the rats began exploring their new environment and then engaged in an array of self-directed and interactive behaviors, beginning with mutual investigation that gradually evolved to brief play-like behavior or in displays of agonistic behaviors. Rank status was assigned to the two co-housed subjects based on the frequency of expression of these behaviors, and the observed changes in body weight noted during the initial twenty-four hours of dyad-housing. A subject’s hierarchical status was associated with a set of distinct behaviors. Specifically, for Dom rats aggressive behaviors represented 57.0±8.8% of the total behaviors expressed during the test, while self-centered, affiliative and defensive behaviors represented 23.0±3.0%, 15.5±2.8% and 4.5±1.3% respectively. By contrast, there was no significant difference in the frequencies of the behaviors displayed by the Sdom were comparable (22.5±3.5%, 28.9±4.2%, 25.3±5.9% and 23.3±4.6% for self-centered, affiliative, defensive and aggressive behaviors, respectively). The difference in self-centered behaviors were not influenced by rank status, however the Sdom rats engaged in significantly more affiliative and defensive behaviors than did the Dom rats (F1,24=9.513, P=.0051 and F1,24=12.440, P=.0017, respectively), on the other hand, the Dom rats displayed significantly more frequent offensive behaviors (F1,24=50.792, P<.0001).
On the day of dyad formation the three experimental groups did not differ in body weight (P>.05). Because the dyad-housed rats were separated by a cage-divider after their initial brief interaction, the decline in body weight consequent to the initial 24-hour dyad-housing was small (−3.13±0.91 – 3.70±0.72 and −0.70±0.44 for the Dom, Sdom and SiH rats, respectively) compared to the changes previously noted with triad-housed rats (Pohorecky et al.,1999; 2004). Similarly, on the last day of the study there were no group differences in body weight.
3.2. Ethanol Intake
The intake of EtOH was assessed over a 13-day testing period beginning 41-days prior to differential housing, and again over a 13-day period beginning on the 11th day of dyad-housing. Table 2 presents data on the intake of EtOH at the baseline test, when the subjects were injected with saline, for the pre-dyad test and subsequently when dyad-housed. A repeated measures ANOVA analysis indicated no significant main effect of rank/housing status or of test time on the intake of EtOH. Nevertheless, there was a statistically significant interaction effect of rank/housing status with test time on EtOH intake (F2,34=12.238, P<.0001). The significance of the interaction effect indicates that while overall rank/housing had no effect on the ingestion of EtOH intake, the effect of rank/housing may have differed at the two test times. Indeed, separate single ANOVA analysis of each test time indicated that rank/housing status had a main effect at the pre-dyad test as well as at the dyad tests (F2,34=5.177, P=.0109 and F2,34=3.696, P=.0353, respectively). At the pre-dyad test the future Dom rat ingested significantly less EtOH than the future Sdom rats (P=.0029). Conversely at the dyad test the EtOH intake of the Dom rats was higher compared to both the Sdom and SiH rats, but these differences missed statistical significance (P=.0284 and P=.0228, for the Sdom and SiH rats, respectively). Consequently, compared to the pre-dyad test EtOH intake of Dom rats increased significantly at the dyadtest (P=.0004), while the intakes of the Sdom and SiH rats tended to decline or were similar, respectively, at both test times. Neither rank/housing nor test time had an effect on EtOH preference (Table 1).
Table 2.
Ethanol intake and ethanol preference of saline-injected dyad-housed and single-housed rats
| Measure | Test time | Dominant | Subdominant | Single |
|---|---|---|---|---|
| Ethanol intake (g/kg) | Pre-diad | 0.587±0.049 | 0.790±0.055 D | 0.697±0.039 |
| Ethanol intake (g/kg) | Diad | 0.841±0.059* | 0.690±0.043 | 0.658±0.055 |
| Ethanol preference (%) | Pre-diad | 77.96±2.21 | 82.01±2.30 | 73.98±2.56 |
| Ethanol preference (%) | Diad | 78.20±1.53 | 82.81±1.32 | 78.44±1.38 |
Ethanol intake was assessed during a 1-hour daily session. Ethanol preference was calculated as a percent of the total fluid intake. Data represents the mean ± SEM for 13 rats per group.
Designates statistically significant difference from the dominant rats.
Designates statistically significant difference from the pre-diad test. Level significance was set at P<0.0167 as determined by the Bonferroni test
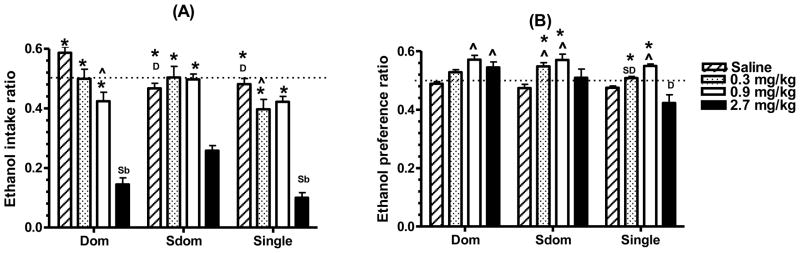
To compare the effectiveness of Amp on the intake of EtOH at the two test times the data are presented as the EtOH intake ratio. A ratio below 0.5 indicates that the effect of Amp was smaller at the dyad test compared to the pre-dyad test. Conversely, a ratio above 0.5 indicates that Amp’s effect was greater at the dyad compared to the pre-dyad test. A repeated measure ANOVA indicated that rank/housing status had a significant main effect on the ratio for EtOH intake (F2,102=5.057, P=.0120), as did the Amp treatment (F3,102=81.264, P<.0001) (Figure 1A). While overall, Amp had a dose-related depressant effect on EtOH intake, this dose-dependency was evident primarily in the Dom rats. In these subjects the 2.7 mg/kg dose of Amp drastically depressed the EtOH intake ratio compared to the other three treatments (P<.0001 for all three comparisons), and while the 0.9 mg/kg dose depressed the intake ratio compared to the saline (P=.0030), the decline after the 0.3 mg/kg dose compared to saline injection missed statistical significance (P=.0851). In Sdom rats the EtOH intake ratio was also drastically lower after the 2.7 mg/kg Amp dose (P<.0001 for all three comparisons), while after the two lower drug doses the ratio did not differ significantly compared to saline-treatment. Similarly in the SiH rats the 2.7 mg/kg dose of Amp drastically depressed the EtOH intake ratio (P<.0001 for all three comparisons); the two lower doses tended to depress the EtOH intake ratio but these effects missed statistical significance (P=.097 and P=.0597, for the 0.3 and 0.9 mg/kg doses respectively). Furthermore, single ANOVA analysis indicated that the EtOH intake ratio of the saline injected Sdom and SiH rats were significantly lower than those of the Dom rats (P<.0001 and P=.0006, respectively). After the 2.7 mg/kg dose of Amp the EtOH intake ratio of the Dom and SiH rats were lower compared to the Sdom rats (P=.0077 and P=.0006, respectively). Overall, the changes in the EtOH intake ratio for all the subjects indicated a greater Amp-induced decline in EtOH intake at the dyad compared to the pre-dyad test and furthermore, compared to Dom and SiH rats, the Sdom rats were less sensitive to Amp.
Figure 1.
Amphetamine treatment on ethanol intake and on ethanol preference of dyad- and single-housed rats. Rats were injected with saline or an amphetamine injection (0.3, 0.9 or 2.7 mg/kg IP) 30 min before a 1-hour drinking period during which they had a choice of a 6% ethanol solution and water. Testing was carried out before differential housing, while all rats were individually housed, and again when rats were housed either in dyads or SiH. (A) The ethanol intake ratio reflects the change in intake from the pre-dyad to the dyad test, and was calculated as explained in the Methods section. (B) Ethanol preference was calculated as the percent of the total fluid intake based on the ethanol intake data, and the ethanol preference ratio was calculated as explained in Methods section. Data represent the mean ± SEM for 13 dyad-housed rats and 11 SiH rats. Statistically significant differences from the saline and the 2.7 mg/kg groups are designated as (^) and (*), respectively. Significant group difference from the Dom rats, Sdom and the SiH group are designated by (D), (SD) and (Si), respectively. Significance level was set at P<.0167 as determined by the Bonferroni test.
At baseline, EtOH preference of the saline-injected subjects was not influenced by either rank/housing status or test time (Table 2). There was a tendency for EtOH preference of the Sdom rats to be higher than that of the Dom and SiH rats, but these differences were not statistically significant. The repeated measure ANOVA analysis indicated that there was a significant main effect of both rank/housing status and of Amp treatment on the ratio for EtOH preference (F2,102=4.873, P=.0138 and F3,102=18.523, P<.0001, respectively) (Figure 1B). Namely, Amp dose-relatedly enhanced the EtOH preference ratio. However this effect of Amp on the ratio was dependent on the subjects rank/housing status, as indicated by a significant interaction effect of rank/housing status with Amp treatment on (F6,102=3.144, P=.0072). Specifically, while the 0.3 mg/kg dose of Amp tended to increase the EtOH preference ratio compared to saline-injection in all three experimental groups, but the elevation was significant only for the Sdom rats (P=.0017). Compared to saline treatment, the ratio after the 0.9 mg/kg dose was higher in Dom, Sdom and SiH rats (P<.0001, P=.0027, and P=.0015, respectively), but the highest dose elevated the ratio only in the Dom rats (P=.0020). In fact, the significantly lower EtOH preference ratio after the 2.7 mg/kg Amp dose, compared to the other two drug doses, attests to the drug’s suppression of EtOH preference (Sdom rats:P<.0001, for both lower doses; SiH rats: P=.0004 and P<.0001, for the 0.3 mg/kg and 0.9 mg/kg doses, respectively). Lastly, a simple ANOVA analysis indicated that EtOH preference ratios for the SiH rats the after the 0.3 mg/kg were lower compared to the Sdom rats (P=.0098)), and after the 2.7 mg/kg Amp doses were lower compared to the Dom rats (P=.0026) Hence, except for the highest Amp dose, the ratio data indicates that the EtOH preference ratio was enhanced by Amp more at the dyad compared to the pre-dyad test.
3.3. Water Intake
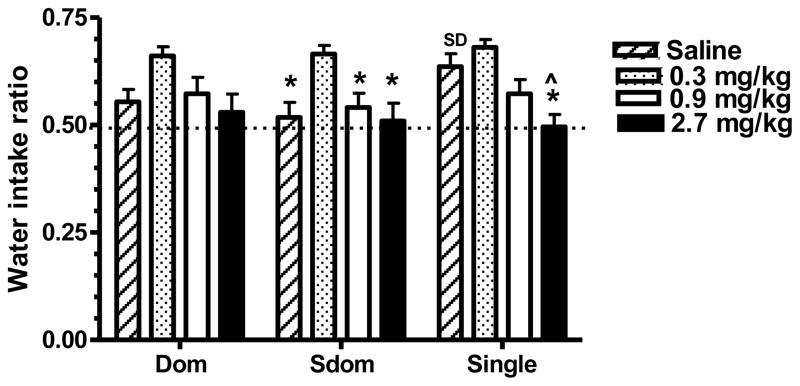
While the repeated measure ANOVA analysis indicated that Amp treatment affected the ratio for water intake (F3,102=12.433, P<.0001), rank/housing status and the interaction of rank/housing status with Amp treatment had no effect (Figure 2). Although the ratio for water intake of the Dom rats was highest after the 0.3 mg/kg Amp dose, this effect did not reach statistical significance. However in Sdom rats the ratio for water intake after the 0.3 mg/kg dose was significantly higher compared to saline and the two highest drug doses (P=.0016, P=.0074, P=.0012, for the saline, 0.9 mg/kg and 2.7 mg/kg doses respectively). For the SiH rats the water intake ratio of the 2.7 mg/kg Amp treated was significantly lower compared to treatments with either saline or the 0.3 mg/kg drug dose (P=.0012 and P<.0001, respectively). Lastly, separate single ANOVA analysis of individual rank/housing groups indicated that the ratio for water intake for the SiH saline-injected rats was higher compared to the corresponding Sdom rats (P=.0127), though the other rank/housing related differences were not statistically significant. Thus the lowest Amp dose significantly increased the water intake ratio and this effect was greater at the dyad test compared to the pre-dyad test.
Figure 2.
Amphetamine treatment on water intake of dyad- and single-housed rats. A saline or an amphetamine injection (0.3, 0.9 or 2.7 mg/kg IP) was administered 30 min before the 1-hour limited access test during which they had a choice of a 6% ethanol solution and water. Each test was carried out before differential housing while all rats were individually housed and again when rats were housed either in dyads or SiH. The water intake ratio was calculated as explained in the Methods section. Data represent the mean ± SEM for 13 dyad-housed rats and 11 SiH rats. Statistically significant differences from the saline and 0.3 mg/kg groups are designated as (^) and (*), respectively. (SD) Designates statistically significant differences from the Sdom group. Significance level was set at P<.0167 as determined by the Bonferroni test.
3.4. Saccharin Intake
To assess the specificity of the effect of Amp on EtOH drinking, we also evaluated the intake of a highly preferred saccharin solution. Saccharin intake was assessed on days 15 to 18 of the pre-dyad period and again on days 95–98 of the dyad period.
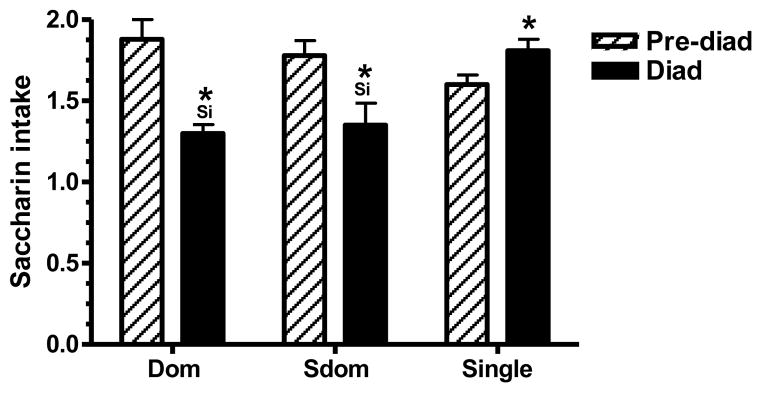
Rank/housing status had no effect on the intake of saccharin at either the pre-dyad or the dyad test (Figure 3). The repeated measures ANOVA analysis disclosed a significant effect of test time (F1,34=16.211, P=.0003), and a significant interaction effect for rank/housing status and test time (F2,34=12.513, P<.0001).. This significant interaction effect indicates that the change in saccharin intake with test time was highly dependent on the subject’s rank/housing status. Indeed, while the intake of saccharin of the dyad-housed rats declined at the dyad test (P=.0003 and P=.0063 for the Dom and Sdom rats, respectively), the saccharin intake of the SiH rats actually increased (P=.0408). When the two test times were analyzed separately, rank/housing status had a significant effect on saccharin intake at the dyad test (F2,34=8.001, P=.0014), but had no effect at the pre-dyad test. Overall, the SiH rats ingested more saccharin than the dyad-housed rats (P=.0008 and P=.0021 compared to the Dom and Sdom rats, respectively), but the intake of the Dom and Sdom rats did not differ.
Figure 3.
Amphetamine treatment on the intake of a saccharin solution by dyad and single-housed rats. Saccharin intake was evaluated during a 1-hour limited access test. Each test was carried out before differential housing while all rats were individually housed (Pre-dyad test), and again when rats were either dyad-housed or single-housed (Dyad). Data for saccharin intake (mg/kg) was calculate as the mean of four test days, and is presented as the mean ± SEM for 13 dyad-housed rats and 11 SiH rats. Statistically significant differences from the pre-dyad test are designated by (*). (Si) Designates significant differences from the single-housed group. Significance level was set at P<.0167 as determined by the Bonferroni test.
4. DISCUSSION
We have confirmed and expanded prior evidence that chronic psychosocial stress can significantly modify the ingestion of EtOH in rats. Specifically, chronically challenged Dom rats ingested more EtOH compared to their intake prior to the stressor. The data also indicate that future Dom rats differed from the other rats by their lower basal EtOH intake. Furthermore, sensitivity to Amp as assessed by its effect on EtOH ingestion, was also altered by psychosocial stress. While Amp generally depressed the voluntary ingestion of EtOH, this depression was greater in SiH rats compared to dyad-housed rats, particularly in the Dom rats. Concomitantly, Amp increased preference for EtOH, again most markedly in the dyad-housed rats. Overall, these findings tend to implicate the DArgic system in mediating the changes in EtOH ingestion produced by chronic psychosocial stress.
4.1. Effect of Psychosocial Stress
Psychosocial stress generated by dyad-housing had specific effects on the ingestion and on preference for EtOH. At the pre-dyad test the saline-injected future Dom rats ingested less EtOH than the future Sdom rats. Our findings indicate that the stress of dyad-housing increased EtOH intake specifically in the Dom rats since their intake increased significantly from the pre-dyad level, while the intake of Sdom and SiH rats was comparable at both tests. Previous research had indicated that compared to their Dom counterparts, the non-Dom group-housed animals ingested less (Blanchard et al., 1993; Van Erp et al., 2001), more (Caldwell and Riccio, 2010; Crowley et al., 1990; Ellison, 1987; Fahlke et al., 2002; Higley et al., 1991; Pohorecky, 2008; Pohorecky, 2010) or similar levels (Keeney and Hogg, 1999) of EtOH. The trend of higher sensitivity to Amp shown by the Dom compared to Sdom rats confirms the greater sensitivity to Amp noted in rats with high preference compared to low preference for EtOH (Fahlke et al., 1995). On the other hand, EtOH intake of the dyad-housed rats did not differ significantly from the SiH rats, confirming a previous report (Adams and Oldham, 1996), though others noted that SiH rats ingested more EtOH compared to group-housed rats (Ehlers et al., 2007; Parker and Radow, 1974). Conversely, psychosocial stress did not alter EtOH preference of saline treated subjects. It should be noted that EtOH intake and EtOH preference do not necessarily follow the same pattern, and may involve different mechanisms (e.g., Dhaher et al., 2009).
While the present data confirmed that psychosocial stress altered the ingestion of EtOH, these intake differences were not as notable as those in our previous studies with naive non-injected triad-housed rats. Differences in experimental variables may possibly account for the noted smaller impact of psychosocial stress on EtOH intake, as well as for some of the discrepancies among the above-mentioned studies. For example, since all the subjects in the present study were injected with saline, differences in sensitivity to this potentially stressful procedure may have minimized existing differences in EtOH ingestion (e.g., Drouet et al., 2010; Little et al., 1999). Handling of the test subjects may have also significantly altered the response to stressors (Andrews and File, 1993; Gallitano-Mendel et al., 2007). Moreover, temporal factors may have contributed to the inconsistencies in some of the above-mentioned studies. For example, in some of the mentioned studies with SiH and group-housed animals, the ingestion of EtOH was measured during exposure to the experimental stress period and in the context associated with the stressor. On the other hand, ingestion of EtOH in other studies was evaluated in the home cage after the resident-intruder stress. While in our study EtOH intake was determined during the first hour following the daily social interaction, a recent report indicated that enhancement of EtOH intake was optimal approximately two hours after a bout of defeat (Caldwell and Riccio, 2010), or even after a longer post-stress time delay (Croft et al., 2005). Lastly, it is highly unlikely that the Dom rat had a negative impact on the EtOH intake of the Sdom rat’s ingestive behaviors. First of all, the EtOH drinking spout was far removed from the cage divider so the drinking by the Sdom rat occurred at a distance from the Dom rat. Secondly, our observation of the behavior of the two rats during the 1-hour drinking period did not indicate any inhibitory effect of the Dom directed towards the Sdom rat. Therefore we believe that the differences in EtOH ingestion most likely reflect the stressfulness of the daily territorial challenge by the Sdom rat to the Dom rat.
An interesting shift in the distribution of test subjects was noted when they were categorized according to their level of EtOH-intake rather than rank (data not shown). Namely, at the pre-dyad test 8% of the Dom rats were ‘high’ EtOH drinkers (0.99±.05 g/kg) but this figure increased to 23% at the dyad test, and the percent of ‘low’ EtOH drinkers Doms (0.49±.04 g/kg) was higher at the pre-dyad compared to the dyad test. By contrast, the percent of ‘high’ EtOH Sdom drinkers declined at the dyad compared to the pre-dyad test, and the percentage of ‘low’ EtOH Sdom drinkers was the same at both test time, while the proportion of the ‘moderate’ EtOH drinkers increased slightly at the dyad test. This shift in Dom rats from ‘low’ to ‘high’ EtOH drinkers tends to support the notion that, in contrast to Sdom rats, the Dom rats could be ingesting EtOH to mitigate the stress of maintaining territorial dominance during the daily encounters with the Sdom rats.
In view of prior evidence that sensitivity to EtOH can be altered by stressors (Pohorecky, 1990,Pohorecky, 1991), it is relevant to also consider whether the stress of dyad-housing may have altered the interoceptive effects of the ingested EtOH. The higher ingestion of EtOH by Dom compared to Sdom rats may also have been influenced by a change in interoceptive properties of the drug. For example, psychosocial stress may alter the salience and/or the rewarding properties of drugs (Vekovischeva et al., 2004), and repeated exposure to elevated CORT levels, consequent to daily confrontations, may have altered sensitivity to the interoceptive effects of alcohol in rats (Besheer et al., 2011) and induced the Dom animals to consume more EtOH. Therefore, one may speculated that while at the pre-dyad test the future Dom rats tended to experienced less reward from ingesting EtOH’s compared to the future Sdom rats, on the other hand at the dyad test the Sdom rats may have found EtOH more aversive (Rezvani et al., 2010). However, whether psychosocial stress-induced alteration in the interospective effects of EtOH may have had an impact on its ingestion, remains to be determined.
Another relevant factor that might have contributed to the noted changes in EtOH intake was the ability of the dyad-housed rats to cope with the psychosocial stressor. Considerable evidence indicates that the impact of a stressor is highly dependent on a subject’s level of control, as critically evaluated in a recent review (Koolhaas et al., 2011). The removal of the cage barrier exposed the Dom rat’s territory to the daily challenge of its cage-sharing partner, resulting in higher CORT levels in the Dom (Pohorecky et al., 2011), which may indicate that the coping capacity of the Dom rats might have been impaired. Support for this notion stems from evidence that plasma CORT levels of group-housed rodents depend on social stability, and that the behavior of non-Dom rats may influence CORT levels of the Dom animal. For example, when stability of group-housed animals was impaired glucocorticoid levels of Dom subjects were higher compared to non-Dom subjects (Haemisch and Gartner, 1979; Setchell et al., 2010; Gesquiere et al., 2011). Furthermore, compared to ‘passively’ coping intruders, intruders that displayed ‘active’ coping behavior had a smaller adrenocortical response after their defeat (Peterson and Pohorecky, 1986; Walker et al., 2009). Corticosterone, the most commonly investigated stress-related hormone in rodents, is believed to have a facilitatory role on the ingestion of EtOH (Fahlke et al., 2000; Prasad and Prasad, 1995), though this issue remains controversial (Eriksson et al., 2001; O’Callaghan et al., 2005). Overall, it is likely that the elevated plasma CORT levels of the Dom rats may have contributed to their higher EtOH intake compared to the Sdom rats. Additionally, the ability to cope with a stressor may also impact the behavioral effects of EtOH. For example, in an acutely stressful situation coping behavior was reported to reduce EtOH’s ataxic effect. Drugan and associates (1996) noted that the ataxic effect of EtOH in rats was mitigated when they were subjected to escapable electric shocks, but not when the shocks were inescapable. Whether the noted group differences in EtOH intake may also reflect altered sensitivity to the central effects of the ingested alcohol remains to be established.
4.2. Effect of Amphetamine
Our data indicate that the SiH rats, were more sensitive to Amp’s depressant effect on EtOH intake at the dyad compared to the pre-dyad test. The greater Amp sensitivity of the SiH rats suggests that dyad-housing moderated its depressant effect on EtOH ingestion, confirming a previous report (Gaytan et al., 1996). The 0.9 mg/kg dose inhibited EtOH intake and enhanced EtOH preference in all the subjects, while the highest dose had a profound depressant effect on its intake, but EtOH preference was depressed only in the SiH rats. These dose-related differences in Amp’s effect on EtOH intake and preference, suggest that distinct underlying mechanisms mediated these two drug actions. A lack of correlation between EtOH preference and intake have been noted previously (Szumlinski et al., 2005; Tordoff et al., 2007), and may be related to the diverse roles of DA in “wanting” and “liking” a reward (Brennan et al., 2001). Since Amp was also found to reduce the ingestion of EtOH without changing its palatability in a taste reactivity test (Kaczmarek and Kiefer, 2000), it is unlikely that altered oral sensory perceptions were involved. Nevertheless, differences in the intake of EtOH may in part also reflect differences in its aversive properties (Rezvani et al., 2010).
Psychosocial stress is known to alter the activity of the neuronal DArgic system. Our data implicate the DArgic system in the just described differential stress-induced changes in EtOH ingestion. For example, studies indicate that defeat leads to DArgic hyperactivity (e.g., Berton et al., 2006; Ferrari et al., 2003), and the mesolimbic DA circuitry has also been implicated in innate resilience or susceptibility to social defeat stress. Namely, susceptibility and resilience to social defeat have been linked to the firing rate of ventral tegmental DArgic neurons (Anstrom et al., 2009; Krishnan et al., 2007; Razzoli et al., 2011). For example, in contrast to defeat-resilient mice, in defeat-susceptible mice chronic defeat engendered physiologic and behavioral changes, as well as the molecular adaptations in the mesolimbic DA neurons, were evident as long as four weeks after the last defeat experience (Krishnan et al., 2007). In fact, it has been suggested that an “increased phasic firing of DArgic neurons in the ventral tegmental area may be considered as a feature defining the lasting imprint of social defeat stress” (Razzoli et al., 2011). Further support for a role of the DAgic system in psychosocial stress effects comes from studies of other DAgic drugs such as cocaine (e.g., Covington and Miczek, 2001; Czoty et al., 2005). For example, subdominant non-human primates were found to be more sensitive to the relative reinforcing strength of cocaine (Czoty et.al., 2005). On the other hand the dopaminergic response to cocaine was found to be blunted in defeated female rats (Shimamoto et al., 2011).
Some evidence has also accrued on EtOH’s effect on the DAgic neuronal system in psychosocially stressed animals. For example, a small dose of intracerebrally microinjected EtOH increased extraneuronal levels DA in defeated Sdom rats, while it had no effect in Dom and in non-defeated Sdom rats (Yavich and Tiihoven, 2000). The availability of DA-D2 receptors was higher in dominant compared to non-dominant monkeys (Morgan et al., 2002). Interestingly, stimulation of DA-D2 receptors has been implicated in the enhancement of impulsivity produced by Amp (van Gaalen et al., 2009), and stimulation of these receptors has been also reported to predict trait impulsivity and the reinforcing properties of other drugs such as cocaine (Dalley et al., 2007). In fact, stress induced activation of DA in the frontal cortex was depended on the innate levels of sociability in rats, and possibly enhanced impulsivity (Tõnissaar et al., 2008), a finding that might be related to the rank related differences in the present study. However, because Amp reduced impulsivity dose-dependently yet had no effect on EtOH drinking (Oberlin et al., 2010), the role if any, of impulsivity in the present findings needs to be further investigated.
Amphetamine is known to induce dose-dependent behavioral stereotypy that consists of highly repetitive movements (e.g., sniffing, biting, head movements, licking) (Antoniou et al., 1998). This potential caveat may have interfered with drinking behavior. However, under our experimental conditions we did not observe any stereotypy even after the highest Amp dose, confirming the absence of stereotypic behaviors after a 2.5 mg/kg dose of Amp (Nordquist et al.,2008). Moreover,stereotypy-induced drinking impairment after the highest Amp dose would be expected to be similar for both EtOH and water drinking. However the high Amp dose only impaired the intake of EtOH and had no effect on water intake. It is possible that the extensive handling, testing and repeated injections may have altered the sensitivity to amphetamine-induced stereotyped behaviors.
Overall, the presented evidence suggests that in terms of EtOH ingestion, the Dom rats were more sensitive to Amp than were the Sdom rats. However, it is worth noting that Amp is also known to affect other non-DArgic neural systems in the brain, such as the noradrenergic and the serotonergic systems (Lanteri et al., 2008; McKittrick and Aberchromie, 2007). Consequently, the present interpretation of the role DA played in Amp’s action on EtOH ingestion is contingent on the evaluation of the involvement of other neuronal systems.
4.3. Social Stress and Anhedonia
To evaluate the specificity of the psychosocial stress-induced changes on EtOH ingestion, we also examined the intake of saccharin, another rewarding substance for rodents. While the dyad-housed rats clearly ingested less saccharin compared to their pre-dyad level, intake of the SiH rats at the dyad test was higher compared to their pre-dyad intake. These findings support previous reports of stress-induced anhedonia (Rygula et al., 2005; Stekalova et al., 2004; Tamashiro et al., 2007). Because stress-induced anhedonia tends to be influenced by such factors as repeated testing, handling of subjects and housing conditions (Hall et al., 1997; Domeney and Felden, 1998), the defeat-engendered anhedonia may have been partially mitigated in the dyad-housed rats (Grippo et al., 2007; Ruis et al., 1999).
4.4. Predictive Traits of Hierarchical Status
The present data indicate that for SiH rats the level of EtOH intake may serve as a predictor of future social rank. Specifically, future Dom rats ingested significantly less EtOH than future Sdoms. Also, while 31% of the future Sdom rats were “high“ EtOH drinkers, only 8% of the future Dom rats were in this drinking category. Since future Sdom rats tend to be innately more fearful and anxious than Dom rats (Pohorecky et al., 2011), the lower EtOH intake of by future Dom rats might be related to their lower level of innate emotionality, confirming the findings of Davis and associates (2009). Furthermore, the fact that future Dom animals were more susceptible to Amp’s effect on EtOH intake suggests that there were innate differences their DArgic systems. In fact, significant individual differences in the DArgic system are well known to exist in rodents (e.g., Verheij and Cools, 2008). Other behavioral and physiologic predictors of an animal’s hierarchical status, including such indices of anxiety, enhanced motivation for food reward, as well as in activity of the hypothalamo-pituitary-adrenal axis have been previously reported (Blanchard et al., 1998; 2001; Davis et al., 2009; Prasad and Prasad, 1995).
The present findings, in addition to some of the recent studies discussed above, have highlighted the crucial importance of trait characteristics in the determining the effect of stressors on the ingestion of EtOH. A variety of non-social stressors ranging from repeated footshock, forced swim, cold-immobilization, and the chronic variable stress paradigm, have been shown to enhance the voluntary intake of EtOH in rats and mice (Boyce-Rustay et al., 2008; Rockman et al., 1987; Sacharczuk et al., 2008; Yaroslavsky, Tejani-Butt, 2010; West et al., 2011). However, these stress-related increases in EtOH intake were evident primarily in select populations, such as rats bred for reduced struggling in a forced swim test (West et al., 2011), or pre-selected for low EtOH ingestion, but not medium or high EtOH-ingestion groups (Rockman et al., 1987), and in mice that showed low rather than high swim stress-induced analgesia (Sacharczuk et al., 2008). The challenge now is to unravel the potential commonality of the underlying neurobiologic changes engendered by these distinct stressors that modified the ingestion of EtOH.
In conclusion, the administration of Amp, a DA-releasing drug, demonstrated the involvement of the DArgic neuronal system in mediating the altered ingestion of EtOH in chronically psychosocially stressed rats. Compared to the SiH rats, both dyad-housed rats were less susceptible to the effects of Amp, suggesting that social housing may have dampened sensitivity to Amp. At the dyad test the Sdom rats appeared to be more resistant than the Dom rats to the lower doses of Amp. These findings suggest that chronic social stress can differentially alter the DArgic neural system, and that these changes may have significant behavioral and physiological consequences. Clearly, the relationship of social rank status and innate resistance or susceptibility to defeat is an issue that needs to be further explored. Moreover, more research is warranted to evaluate the distinct roles of rewarding and aversive properties of drugs in psychosocially stressed subjects. This research has relevance for the understanding of the role of social stress in the abuse of alcohol as well as other drugs.
Highlights.
Chronically stressed dominant rats ingested more ethanol than subdominant rats
Social stress altered differentially sensitivity to amphetamine
Amphetamine depressed ethanol ingestion more in single- than in dyad-housed rats
Dominant rats were more sensitive to amphetamine’s action than to the subdominant rats.
Dopaminergic system mediated social stress-induced changes in ethanol ingestion
Acknowledgments
This research was supported in part by funds from the National Institute of Alcoholism and Alcohol Abuse, Grant 1RO1AA10124 and the Center of Alcohol Studies. The excellent assistance of Rachel Grisham and Smeeta Sinha is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams N, Oldham TD. Seminatural housing increases subsequent ethanol intake in male Maudsley Reactive rats. J Stud Alcohol. 1996;57:349–51. doi: 10.15288/jsa.1996.57.349. [DOI] [PubMed] [Google Scholar]
- Andrews N, File SE. Handling history of rats modifies behavioural effects of drugs in the elevated plus-maze test of anxiety. Eur J Pharmacol. 1993;22(23):109–12. doi: 10.1016/0014-2999(93)90827-5. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161:3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou K, Kafetzopoulos E, Papadopoulou-Daifoti Z, Hyphantis T, Marselos M. D-amphetamine, cocaine and caffeine: a comparative study of acute effects on locomotor activity and behavioural patterns in rats. Neurosci Biobehav Rev. 1998;23:189–96. doi: 10.1016/s0149-7634(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Appel SB, Liu Z, McElvain MA, Brodie MS. Ethanol excitation of dopaminergic ventral tegmental area neurons is blocked by quinidine. J Pharmacol Exp Ther. 2003;306:437–46. doi: 10.1124/jpet.103.050963. [DOI] [PubMed] [Google Scholar]
- Baker SL, Kentner AC, Konkle AT, Santa-Maria Barbagallo L, Bielajew C. Behavioral and physiological effects of chronic mild stress in female rats. Physiol Behav. 2006;87:314–22. doi: 10.1016/j.physbeh.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Benus RF, Bohus B, Koolhaas JM, van Oortmerssen GA. Heritable variation for aggression as a reflection of individual coping strategies. Experientia. 1991;15(47):1008–19. doi: 10.1007/BF01923336. [DOI] [PubMed] [Google Scholar]
- Bertholomey ML, West CH, Jensen ML, Li TK, Stewart RB, Weiss JM, Lumeng L. Genetic propensities to increase ethanol intake in response to stress: studies with selectively bred swim test susceptible (SUS), alcohol-preferring (P), and non-preferring (NP) lines of rats. Psychopharmacology (Berl) 2011;218:157–67. doi: 10.1007/s00213-011-2381-6. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Grondin JJ, Cannady R, Hodge CW. The effects of repeated corticosterone exposure on the interoceptive effects of alcohol in rats. Psychopharmacology (Berl) 2011;218:xxx. doi: 10.1007/s00213-011-2533-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–42. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Blakley G, Pohorecky LA. Psychosocial stress alters ethanol's effect on open field behaviors. Pharmacol Biochem Behav. 2006;84:51–61. doi: 10.1016/j.pbb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Hori K, Tom P, Blanchard DC. Social structure and ethanol consumption in the laboratory rat. Pharmacol Biochem Behav. 1987;28:437–42. doi: 10.1016/0091-3057(87)90502-8. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Yudko EB, Blanchard DC. Alcohol, aggression and the stress of subordination. J Stud Alcohol Suppl. 1993;11:146–155. doi: 10.15288/jsas.1993.s11.146. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Hebert MA, Ferrari PF, Palanza P, Figueira R, Blanchard DC, Parmigiani S. Defensive behaviors in wild and laboratory (Swiss) mice: the mouse defense test battery. Physiol Behav. 1998;65:201–9. doi: 10.1016/s0031-9384(98)00012-2. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Yudko E, Dulloog L, Blanchard DC. Defense changes in stress nonresponsive subordinate males in a visible burrow system. Physiol Behav. 2001;72:635–42. doi: 10.1016/s0031-9384(00)00449-2. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Janos AL, Holmes A. Effects of chronic swim stress on EtOH-related behaviors in C57BL/6J, DBA/2J and BALB/cByJ mice. Behav Brain Res. 2008;186:133–7. doi: 10.1016/j.bbr.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan K, Roberts DC, Anisman H, Merali Z. Individual differences in sucrose consumption in the rat: motivational and neurochemical correlates of hedonia. Psychopharmacology (Berl) 2001;157:269–76. doi: 10.1007/s002130100805. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 199;23:1848–52. [PubMed] [Google Scholar]
- Caldwell EE, Riccio DC. Alcohol self-administration in rats: Modulation by temporal parameters related to repeated mild social defeat stress. Alcohol. 2010;44:265–74. doi: 10.1016/j.alcohol.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Andrews AE. Alcoholic-like drinking in simian social groups. Psychopharmacology (Berl) 1987;92:196–205. doi: 10.1007/BF00177915. [DOI] [PubMed] [Google Scholar]
- Croft AP, Brooks SP, Cole J, Little HJ. Social defeat increases alcohol preference of C57BL/10 strain mice; effect prevented by a CCKB antagonist. Psychopharmacology (Berl) 2005;183:163–70. doi: 10.1007/s00213-005-0165-6. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Williams EA, Jones RH. Initiating ethanol drinking in a simian social group in a naturalistic setting. Alcohol Clin Exp Res. 1990;14:444–55. doi: 10.1111/j.1530-0277.1990.tb00502.x. [DOI] [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. Assessment of the relative reinforcing strength of cocaine in socially housed monkeys using a choice procedure. J Pharmacol Exp Ther. 2005;312:96–102. doi: 10.1124/jpet.104.073411. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaher R, Finn DA, Oberbeck DL, Yoneyama N, Snelling CC, Wu W, Hitzemann RJ. Electrolytic lesions of the medial nucleus accumbens shell selectively decrease ethanol consumption without altering preference in a limited access procedure in C57BL/6J mice. Pharmacol Biochem Behav. 2009;92:335–42. doi: 10.1016/j.pbb.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Davis JF, Krause EG, Melhorn SJ, Sakai RR, Benoit SC. Dominant rats are natural risk takers and display increased motivation for food reward. Neuroscience. 2009;162:23–30. doi: 10.1016/j.neuroscience.2009.04.039. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeney A, Feldon J. The disruption of prepulse inhibition by social isolation in the Wistar rat: how robust is the effect? Pharmacol Biochem Behav. 1998;59:883–90. doi: 10.1016/s0091-3057(97)00534-0. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Drouet JB, Michel V, Peinnequin A, Alonso A, Fidier N, Maury R, Buguet A, Cespuglio R, Canini F. Metyrapone blunts stress-induced hyperthermia and increased locomotor activity independently of glucocorticoids and neurosteroids. Psychoneuroendocrinology. 2010;35:1299–310. doi: 10.1016/j.psyneuen.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Drugan RC, Coyle TS, Healy DJ, Chen S. Stress controllability influences the ataxic properties of both ethanol and midazolam in the rat. Behav Neurosci. 1996;110:360–7. doi: 10.1037//0735-7044.110.2.360. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Walker BM, Pian JP, Roth JL, Slawecki CJ. Increased alcohol drinking in isolate-housed alcohol-preferring rats. Behav Neurosci. 2007;121:111–9. doi: 10.1037/0735-7044.121.1.111. [DOI] [PubMed] [Google Scholar]
- Ellison G. Stress and alcohol intake: the socio-pharmacological approach. Physiol Behav. 1987;40:387–92. doi: 10.1016/0031-9384(87)90066-7. [DOI] [PubMed] [Google Scholar]
- Ely DL, Henry JP. Neuroendocrine response patterns in dominant and subordinate mice. Horm Behav. 1978;10:156–69. doi: 10.1016/0018-506x(78)90005-3. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hård E, Eriksson CJ, Engel JA, Hansen S. Amphetamine-induced hyperactivity: differences between rats with high or low preference for alcohol. Alcohol. 1995;12:363–7. doi: 10.1016/0741-8329(95)00019-n. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Lorenz JG, Long J, Champoux M, Suomi SJ, Higley JD. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcohol Clin Exp Res. 2000;24:644–50. [PubMed] [Google Scholar]
- Fahlke C, Garpenstrand H, Oreland L, Suomi SJ, Higley JD. Platelet monoamine oxidase activity in a nonhuman primate model of type 2 excessive alcohol consumption. Am J Psychiatry. 2002;159:2107. doi: 10.1176/appi.ajp.159.12.2107. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, van Erp AM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17:371–8. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- Funk D, Harding S, Juzytsch W, Lê AD. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2005;183:341–9. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Gallitano-Mendel A, Izumi Y, Tokuda K, Zorumski CF, Howell MP, Muglia LJ, Wozniak DF, Milbrandt J. The immediate early gene early growth response gene 3 mediates adaptation to stress and novelty. Neuroscience. 2007;148:633–43. doi: 10.1016/j.neuroscience.2007.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytan O, Swann AC, Dafny N. Effects of amphetamine at the beginning of the light cycle on multiple indices of motor activity in the rat. Eur J Pharmacol. 1996;300:1–8. doi: 10.1016/0014-2999(95)00843-8. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Learn NH, Simao MCM, Onyango PO, Alberts SC, Altmann J. Life at the top: ran and stress in wild male baboons. Science. 2011;333:357–60. doi: 10.1126/science.1207120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras MA, Cools AR. Analysis of the biphasic locomotor response to ethanol in high and low responders to novelty: a study in Nijmegen Wistar rats. Psychopharmacology (Berl) 1996;125:258–64. doi: 10.1007/BF02247337. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–80. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemisch A, Gärtner K. Effects of cage enrichment on territorial aggression and stress physiology in male laboratory mice. Acta Physiol Scand Suppl. 1997;640:73–6. [PubMed] [Google Scholar]
- Hall FS, Humby T, Wilkinson LS, Robbins TW. The effects of isolation-rearing on sucrose consumption in rats. Physiol Behav. 1997;62:291–7. doi: 10.1016/s0031-9384(97)00116-9. [DOI] [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc Natl Acad Sci U S A. 1991;88:7261–5. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A nonhuman primate model of type II excessive alcohol consumption? Part 1. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcohol Clin Exp Res. 1996;20:629–42. doi: 10.1111/j.1530-0277.1996.tb01665.x. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Liem BJ, Justice JB., Jr Sensitization and individual differences to IP amphetamine, cocaine, or caffeine following repeated intracranial amphetamine infusions. Pharmacol Biochem Behav. 1992;43(3):815–23. doi: 10.1016/0091-3057(92)90413-a. [DOI] [PubMed] [Google Scholar]
- Isovich E, Mijnster MJ, Flügge G, Fuchs E. Chronic psychosocial stress reduces the density of dopamine transporters. Eur J Neurosci. 2000;12:1071–8. doi: 10.1046/j.1460-9568.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- José BS, van Oers HA, van de Mheen HD, Garretsen HF, Mackenbach JP. Stressors and alcohol consumption. Alcohol Alcohol. 2000;35:307–12. doi: 10.1093/alcalc/35.3.307. [DOI] [PubMed] [Google Scholar]
- Juárez J, Vázquez-Cortés C. Alcohol intake in social housing and in isolation before puberty and its effects on voluntary alcohol consumption in adulthood. Dev Psychobiol. 2003;43:200–7. doi: 10.1002/dev.10133. [DOI] [PubMed] [Google Scholar]
- Kaczmarek HJ, Kiefer SW. Microinjections of dopaminergic agents in the nucleus accumbens affect ethanol consumption but not palatability. Pharmacol Biochem Behav. 2000;66:307–12. doi: 10.1016/s0091-3057(00)00182-9. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mann JJ. Role of the dopaminergic system in depression. Biol Psychiatry. 1992;32:1–17. doi: 10.1016/0006-3223(92)90137-o. [DOI] [PubMed] [Google Scholar]
- Keeney AJ, Hogg S. Behavioural consequences of repeated social defeat in the mouse: preliminary evaluation of a potential animal model of depression. Behav Pharmacol. 1999;10:753–64. doi: 10.1097/00008877-199912000-00007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Weiss F. Neuropharmacology of cocaine and ethanol dependence. Recent Dev Alcohol. 1992;10:201–33. doi: 10.1007/978-1-4899-1648-8_11. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vogt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MA, Blokhuis HJ. Coping styles in animals: current status in behavior and stress physiology. Neurosci Biobehav Rev. 1999;23:925–35. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wöhr M, Fuchs E. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35:1291–301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;19;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lanteri C, Salomon L, Torrens Y, Glowinski J, Tassin JP. Drugs of abuse specifically sensitize noradrenergic and serotonergic neurons via a non-dopaminergic mechanism. Neuropsychopharmacology. 2008;33:1724–34. doi: 10.1038/sj.npp.1301548. [DOI] [PubMed] [Google Scholar]
- Little HJ, O'Callaghan MJ, Butterworth AR, Wilson J, Cole J, Watson WP. Low alcohol preference among the "high alcohol preference" C57 strain of mice; preference increased by saline injections. Psychopharmacology (Berl) 1999;147:182–9. doi: 10.1007/s002130051159. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Rudick CN, Hu XT, White FJ. Excitability of dopamine neurons: modulation and physiological consequences. CNS Neurol Disord Drug Targets. 2006;5:79–97. doi: 10.2174/187152706784111542. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Abercrombie ED. Catecholamine mapping within nucleus accumbens: differences in basal and amphetamine-stimulated efflux of norepinephrine and dopamine in shell and core. J Neurochem. 2007;100:1247–56. doi: 10.1111/j.1471-4159.2006.04300.x. [DOI] [PubMed] [Google Scholar]
- Melis M, Diana M, Enrico P, Marinelli M, Brodie MS. Ethanol and acetaldehyde action on central dopamine systems: mechanisms, modulation, and relationship to stress. Alcohol. 2009;43:531–9. doi: 10.1016/j.alcohol.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–74. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Morzorati S, Marunde RL, Downey D. Limited access to ethanol increases the number of spontaneously active dopamine neurons in the posterior ventral tegmental area of nondependent P rats. Alcohol. 2010;44:257–64. doi: 10.1016/j.alcohol.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordquist RE, Vanderschuren LJ, Jonker AJ, Bergsma M, de Vries TJ, Pennartz CM, Voorn P. Expression of amphetamine sensitization is associated with recruitment of a reactive neuronal population in the nucleus accumbens core. Psychopharmacology (Berl) 2008;198:113–26. doi: 10.1007/s00213-008-1100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LF, Radow BL. Isolation stress and volitional ethanol consumption in the rat. Physiol Behav. 1974;12:1–3. doi: 10.1016/0031-9384(74)90060-2. [DOI] [PubMed] [Google Scholar]
- Peterson JT, Pohorecky LA. Effect of chronic ethanol administration on inter male aggression in rats. Aggress Behav. 1989;15:201–16. [Google Scholar]
- Pohorecky LA. The interaction of alcohol and stress: Research with experimental animals-An update. Alcohol Alcohol. 1990;25:263–276. doi: 10.1093/oxfordjournals.alcalc.a045000. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Stress and alcohol interaction: An update of human research. Acohol Clin Exp Res. 1991;15:438–459. doi: 10.1111/j.1530-0277.1991.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Housing and rank status of male Long-Evans rats modify ethanol's effect on open-field behaviors. Psychopharmacology (Berl) 2006;185:289–97. doi: 10.1007/s00213-005-0257-3. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Psychosocial stress and chronic ethanol ingestion in male rats: effects on elevated plus maze behavior and ultrasonic vocalizations. Physiol Behav. 2008;9(94):432–47. doi: 10.1016/j.physbeh.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Acute novel stressors modify ethanol intake of psychosocially stressed rats. Pharmacol Biochem Behav. 2010;95:390–400. doi: 10.1016/j.pbb.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Skiandos A, Zhang X, Rice KC, Benjamin D. Effect of chronic social stress on delta-opioid receptor function in the rat. J Pharmacol Exp Ther. 1999;290:196–206. [PubMed] [Google Scholar]
- Pohorecky LA, Blakley GG, Kubovcakova L, Krizanova O, Patterson-Buckendahl P, Kvetnansky R. Social hierarchy affects gene expression for catecholamine biosynthetic enzymes in rat adrenal glands. Neuroendocrinology. 2004;80:42–51. doi: 10.1159/000080664. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Sweeny A, Buckendahl P. Differential sensitivity to amphetamine's effect on open field behavior of psychosocially stressed male rats. Psychopharmacology (Berl) 2011;218:281–92. doi: 10.1007/s00213-011-2339-8. [DOI] [PubMed] [Google Scholar]
- Prasad C, Prasad A. A relationship between increased voluntary alcohol preference and basal hypercorticosteronemia associated with an attenuated rise in corticosterone output during stress. Alcohol. 1995;12:59–63. doi: 10.1016/0741-8329(94)00070-t. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Bristow RE, Heighton ME, Grahame NJ. Pharmacologic dissociation between impulsivity and alcohol drinking in high alcohol preferring mice. Alcohol Clin Exp Res. 2010;34:1363–75. doi: 10.1111/j.1530-0277.2010.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan MJ, Croft AP, Jacquot C, Little HJ. The hypothalamopituitary-adrenal axis and alcohol preference. Brain Res Bull. 2005;68:171–8. doi: 10.1016/j.brainresbull.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Razzoli M, Andreoli M, Michielin F, Quarta D, Sokal DM. Increased phasic activity of VTA dopamine neurons in mice 3 weeks after repeated social defeat. Behav Brain Res. 2011;218:253–7. doi: 10.1016/j.bbr.2010.11.050. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Sexton H, Levin ED. Persistent high alcohol consumption in alcohol-preferring (P) rats results from a lack of normal aversion to alcohol. Alcohol Alcohol. 2010;45:219–22. doi: 10.1093/alcalc/agq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman GE, Hall AM, Markert L, Glavin GB, Pare WP. Ethanol-stress interaction: immediate versus delayed effects of ethanol and handling on stress responses of ethanol-consuming rats. Alcohol. 1987;4:391–4. doi: 10.1016/0741-8329(87)90072-3. [DOI] [PubMed] [Google Scholar]
- Roske I, Baeger I, Frenzel R, Oehme P. Does a relationship exist between the quality of stress and the motivation to ingest alcohol? Alcohol. 1994;11:113–24. doi: 10.1016/0741-8329(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruis MA, te Brake JH, Buwalda B, De Boer SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JM. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24:285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–34. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sacharczuk M, Juszczak G, Sliwa AT, Tymosiak-Zielinska A, Lisowski P, Jaszczak K, Pluta R, Lipkowski A, Sadowski B, Swiergiel AH. Differences in ethanol drinking between mice selected for high and low swim stress-induced analgesia. Alcohol. 2008;42:487–92. doi: 10.1016/j.alcohol.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Setchell JM, Smith T, Wickings EJ, Knapp LA. Stress, social behaviour, and secondary sexual traits in a male primate. Horm Behav. 2010;58:720–8. doi: 10.1016/j.yhbeh.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–17. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugmann M, Cagle S, Welt K, During M, Worley PF, Middaugh LD, Kalivas PW. Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci. 2005;25:7054–61. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KL, Hegeman MA, Nguyen MM, Melhorn SJ, Ma LY, Woods SC, Sakai RR. Dynamic body weight and body composition changes in response to subordination stress. Physiol Behav. 2007;91:440–8. doi: 10.1016/j.physbeh.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Slawecki CJ, Khoury A, Mathe AA, Ehlers CL. Effect of social isolation on ethanol consumption and substance P/neurokinin expression in Wistar rats. Alcohol. 2005;36:91–7. doi: 10.1016/j.alcohol.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Tõnissaar M, Herm L, Eller M, Kõiv K, Rinken A, Harro J. Rats with high or low sociability are differently affected by chronic variable stress. Neuroscience. 2008;152:867–76. doi: 10.1016/j.neuroscience.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA, Reed DR. Forty mouse strain survey of water and sodium intake. Physiol Behav. 2007;91:620–31. doi: 10.1016/j.physbeh.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupala E, Hall H, Halonen P, Tiihonen J. Cortical dopamine D2 receptors in type 1 and 2 alcoholics measured with human whole hemisphere autoradiography. Synapse. 2004;54:129–37. doi: 10.1002/syn.20071. [DOI] [PubMed] [Google Scholar]
- Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addict Biol. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav. 2001a;73:301–11. doi: 10.1016/s0031-9384(01)00458-9. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Tachi N, Miczek KA. Short or continuous social stress: suppression of continuously available ethanol intake in subordinate rats. Behav Pharmacol. 2001b;12:335–42. doi: 10.1097/00008877-200109000-00004. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Unger L, Jongen-Rêlo AL, Schoemaker H, Gross G. Amphetamine decreases behavioral inhibition by stimulation of dopamine D2, but not D3, receptors. Behav Pharmacol. 2009;20:484–91. doi: 10.1097/FBP.0b013e3283305e3b. [DOI] [PubMed] [Google Scholar]
- Verheij MM, Cools AR. Twenty years of dopamine research: individual differences in the response of accumbal dopamine to environmental and pharmacological challenges. Eur J Pharmacol. 2008;585:228–44. doi: 10.1016/j.ejphar.2008.02.084. [DOI] [PubMed] [Google Scholar]
- Vekovischeva OY, Semenova SG, Verbitskaya EV, Zvartau EE. Effects of morphine and cocaine in mice with stable high aggressive and nonaggressive behavioral strategy. Pharmacol Biochem Behav. 2004;77:235–43. doi: 10.1016/j.pbb.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Walker FR, Masters LM, Dielenberg RA, Day TA. Coping with defeat: acute glucocorticoid and forebrain responses to social defeat vary with defeat episode behaviour. Neuroscience. 2009;162:244–53. doi: 10.1016/j.neuroscience.2009.04.041. [DOI] [PubMed] [Google Scholar]
- West CH, Boss-Williams KA, Weiss JM. Effects of fenfluramine, 8-OH-DPAT, and tryptophan-enriched diet on the high-ethanol intake by rats bred for susceptibility to stress. Alcohol. 2011 Sep 15; doi: 10.1016/j.alcohol.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Tejani-Butt SM. Voluntary alcohol consumption alters stress-induced changes in dopamine-2 receptor binding in Wistar-Kyoto rat brain. Pharmacol Biochem Behav. 2010;94:471–6. doi: 10.1016/j.pbb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavich L, Tiihonen J. Ethanol modulates evoked dopamine release in mouse nucleus accumbens: dependence on social stress and dose. Eur J Pharmacol. 2000;401:365–73. doi: 10.1016/s0014-2999(00)00456-8. [DOI] [PubMed] [Google Scholar]