I. Introduction
Cross sections of the neuroepithelium reveal a striking feature of neural progenitor cells: interphase nuclei are observed throughout the epithelium, but mitotic cells are found only at the apical surface. Nuclei are distributed this way because they migrate within the epithelial cells, undergoing division at the apical surface and moving basally during interphase. This behavior has been termed interkinetic nuclear migration (INM.) Although the nuclear behavior itself has been documented recently by time-lapse imaging, many cellular events during INM are not clear. Furthermore, the underlying molecular mechanisms remain unknown or controversial. This is because several obstacles preclude a full understanding of INM and its functions: 1) A large number of genes and molecular processes have been reported to affect INM, however many may not have a direct effect on nuclear migration. For example, loss of cell polarity disrupts INM, but may primarily cause loss of epithelial structure, with nuclear migration affected as a consequence. 2) Another issue is that INM occurs in many different species in a wide variety of epithelia that give rise to diverse cell types. Results obtained about INM in one context are often assumed to be general features of INM, without cross-tissue or cross-species comparisons. 3) Additionally, most investigations of INM study fixed sections, but INM is a dynamic process. Proper analysis of it requires time-lapse observations of living tissue, which has only been possible recently. The goal of this review is to summarize the tissues in which INM happens, analyze the available evidence examining the mechanisms of INM in the neuroepithelium, and suggest possible functions of INM.
A. The history of INM
INM is not a recently discovered process. Cajal and His in the 19th century suggested that the neuroepithelium is comprised of two different cell populations, mitotic cells at the ventricular surface and quiescent neuroblasts further from the ventricle. Schaper was the first to suggest that there is only one population of neuroblasts, which move to the ventricle for division (Schaper, 1897a; b). In 1935, FC Sauer observed that there is no clear distinction between the “spongiblasts” and mitotic germinal cells. He coined the term “interkinetic nuclear migration,” and made a case for its existence, based on calculations of nuclear size (as an indirect measure of DNA content) and distance from the apical surface (Sauer, 1935). Watterson demonstrated that inhibition of mitosis at metaphase using colchicine results in several layers of mitotic cells that increase in number with the length of time colchicine is applied (Watterson et al., 1956). This suggests that the elongated cells are not quiescent, but are actively cycling to produce the mitotic cells at the apical surface. FC Sauer’s wife, ME Sauer, and others were able to prove that nuclei move from basal positions in the epithelium to divide at the apical surface using pulses of H3-thymidine. Short pulses label only S-phase cells at basal positions, whereas longer pulses label mitotic cells at the apical surface, thus strongly suggesting that the apical mitotic cells are the same population as the basal S-phase cells (Sauer, 1959; Fujita, 1962). BrdU combined with H3-thymidine allowed double labeling of successive S phases, suggesting that nuclei move in a basal direction during G1 phase, while apical nuclear migration happens during G2 phase (Hayes and Nowakowski, 2000).
INM was eventually observed directly with time-lapse microscopy (Noctor et al., 2001; Miyata et al., 2001), and when combined with BrdU labeling suggests apical nuclear migration commences soon after entering G2 phase (Tamai et al., 2007). Cell-cycle indicators in living cells have also been used to demonstrate that basal nuclear movement happens in G1 whereas apical nuclear movement occurs in G2 phase (Kosodo et al., 2011; Sugiyama et al., 2009).
B. The structure of the neuroepithelium
Since FC Sauer’s initial observations on pig and chick neural tubes, research on INM has focused on the apical progenitors of the neuroepithelium, because the physical separation of mitotic nuclei from interphase nuclei is the most prominent due to the unique cytoarchitecture of the neuroepithelium. Apical progenitor cells collectively refer to neuroepithelial cells early in embryogenesis and to the radial glial cells as neurogenesis proceeds, both of which undergo INM. Apical progenitors are thin and very elongated compared to the cells that comprise many epithelia. Consequently, nuclear migration during INM in the neural tube is more dramatic than in many other epithelia. In the radial glia of the cerebral cortex, nuclei may translocate up to 100 μm (Miyata, 2007).
Apical progenitor cells are structurally and molecularly polarized. For example, the adherens junctions connecting the cells to each other are found just lateral to the apical surface (Baum and Georgiou, 2011; Chenn et al., 1998). These are comprised of N-cadherin in the neuroepithelium. Beta catenin, Cdc42, and Par3 are associated with the adherens junctions along with a belt of actin (Konno et al., 2008; Afonso and Henrique, 2006; Baum and Georgiou, 2011). Centrosomes are also localized near the apical surface (Chenn et al., 1998). The centrosomes nucleate the cilium, which extends into the central lumen, and microtubules, which extend basally from the centrosome to the nucleus (Tsai et al., 2010; Chenn et al., 1998; Kosodo et al., 2011; Miyata, 2007; Hinds and Ruffett, 1971).
C. INM happens in a diverse range of tissues
Although INM is most obvious in the vertebrate neuroepithelium and radial glial cells, it is not confined to vertebrate organisms, nor is it only observed in neural tissue. INM is observed in Drosophila wing disc epithelia and Nematostella ectodermal layers (Meyer et al., 2011). In vertebrate embryos, the lens (Zwaan et al., 1969), liver, lung buds, pancreatic bud (Bort et al., 2006), small intestine (Grosse et al., 2011), epithelial somites (Langman and Nelson, 1968), and mesonephric tubules also undergo INM (Sauer, 1936). Similarly INM has been observed in the adult intestine crypts (Jinguji and Ishikawa, 1992; Sauer, 1936), and hair cells of the inner ear in adult chickens (Tsue et al., 1994). Because the tissues that undergo INM are diverse, the mechanisms and functions are likely to be different as well. For example, the transcription factor Hex is necessary for the transition of the liver from a columnar epithelium to a pseudostratified epithelium. However, loss of Hex affects only the liver epithelium; it does not affect the neuroepithelium (Bort et al., 2006). Most of these tissues have not been observed with time-lapse microscopy and so significant differences in INM may exist between them.
II. Molecular basis of INM
The mechanisms that regulate INM in apical progenitors are poorly understood. For example, nuclei migrate apically during G2 phase and basally during G1 phase, but whether these movements occur via microtubules or actin is controversial. It has been suggested that mitosis initiates at the apical surface because the centrosome is positioned there, but initiation of mitosis has not yet been observed in live tissue through time-lapse microscopy.
A. Apical nuclear movement
Nuclei migrate toward the apical surface during G2 phase. There has been disagreement about the specific mechanisms that regulate apical nuclear migration. Microtubules and the minus-end-directed microtubule motor protein, dynein, are believed to be required for apical nuclear migration in the cerebral cortex (Tsai et al., 2005, 2007) because a considerable number of dynein or microtubule-associated proteins, when perturbed, disrupt INM there: 1) Lis1 is a regulator of dynein. RNAi of Lis1 in the mouse cerebral cortex prevents apical nuclear migration and inhibits mitosis, suggesting that dynein moves nuclei apically along microtubules (Tsai et al., 2005). Also, a reduction in Lis1 causes ectopic mitosis in apical progenitors of the cerebral cortex (Gambello et al., 2003). 2) NudC, another regulator of dynein, is also required for apical nuclear movement in the rat neocortex (Cappello et al., 2011). 3) Cep120, a centrosomal protein, and 4) transforming acidic coiled-coil proteins (TACCs) associated with the centrosomes are necessary for the integrity of the microtubule cytoskeleton. These proteins are also essential for INM in the mouse neocortex (Xie et al., 2007). 5) RNAi of centrosomal proteins Hook3 or PCM1 results in a reduction of pericentriolar satellites, reduces microtubule anchorage at the centrosomes, and impairs INM in the mouse neocortex (Ge et al., 2010). 6) TPX2 appears to organize the microtubule array in G2 phase and is also essential for normal nuclear movement during INM (Kosodo et al., 2011). 7) Decreased levels of dynactin, an activator of dynein, result in increased average nuclear distance from the apical surface during interphase and slower apical nuclear migration in zebrafish retinal progenitors (Del Bene et al., 2008). 8) Inhibition of casein kinase 2 in the rat retina causes a loss of microtubule integrity and slows INM (Carneiro et al., 2008). 9) The KASH protein Syne-2 and the SUN proteins, SUN 1 and SUN 2, are important for INM in the mouse retina (Yu et al., 2011). KASH and SUN proteins bridge the nuclear envelope and are involved in many nuclear migration events (reviewed Starr, 2009). Syne 2 interacts with kinesin and dynein in mouse brain and retinal lysates, and colocalizes in immunohistochemistry with dynein and kinesin in mouse retinal progenitor cells and mouse cerebral cortex apical progenitors (Zhang et al., 2009; Yu et al., 2011). Taken together, these studies are consistent with a model of apical nuclear migration where nuclei migrate along microtubules via dynein toward the apical centrosome.
Evidence also suggests that actin and myosin are involved in apical nuclear migration during INM. Cytochalasin B, an actin depolymerizing agent, has long been known to disrupt INM and cause ectopic mitotic figures in the neural tube. The interpretation has been that the drug inhibited apical nuclear migration and that mitosis occurred wherever the nuclei stopped. This was never shown with time-lapse imaging, however (Murciano et al., 2002; Karfunkel, 1972). In the zebrafish retina, myosin inhibition with blebbistatin also stops apical nuclear migration, suggesting that actomyosin drives the process. Consistent with a critical role for actomyosin in INM, Norden and colleagues reported that colcemide-induced depolymerization of microtubules, and loss of dynactin function with a dominant-negative p150 construct does not affect apical nuclear movement in zebrafish retina. (Norden et al., 2009).
Actin-driven and microtubule-driven mechanisms are not necessarily mutually exclusive. For example, both actomyosin and microtubules are required for cell division and maintenance of epithelial structure (Hildebrand, 2005). Results from our lab unexpectedly revealed that apical movement during INM is a two-step process. Nuclei move apically during G2 phase, but do not reach the lumen of the chick neural tube during interphase. Instead, nuclei enter M phase marked by nuclear envelope breakdown (NEBD) on average 16 μm away from the apical surface. The mitotic cell then rounds up the rest of the way to the apical surface. We find that nuclear movement during G2 phase is dependent on microtubules, whereas apical rounding during mitosis is dependent on actin. Disruption of the microtubule cytoskeleton with colcemide prohibits apical nuclear movement during G2 phase, but does not prevent mitosis, nor does it stop cells from moving to the apical surface during mitosis. Conversely, cytochalasin B does not stop intact nuclei from moving apically during INM, but instead causes ectopic divisions by preventing mitotic cells from rounding up to the apical surface. Thus, the seemingly contradictory results could be explained by two parts of the process being controlled by microtubules and actomyosin.
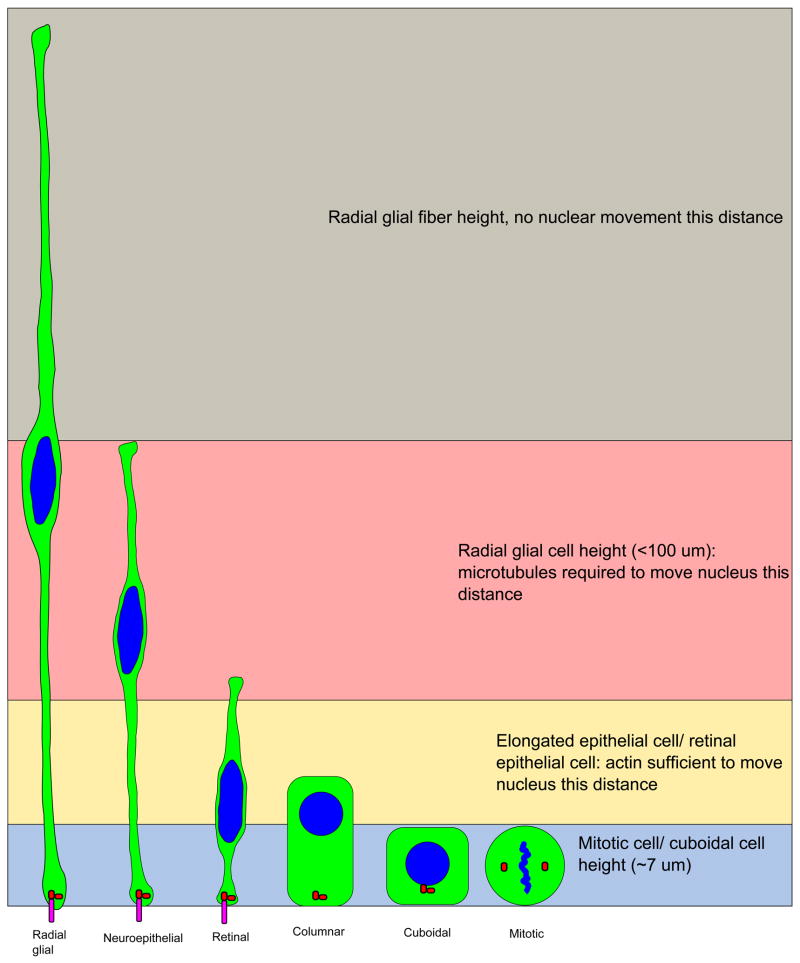
It is also possible that the disagreement over mechanism comes from the relative size of the tissues being studied and markers being used. By some estimates, the zebrafish retina is 3 to 4 nuclei thick in the apical-to-basal direction, whereas the mammalian cortex is closer to 10 nuclei in width (Taverna and Huttner, 2010; Miyata, 2007). Different mechanisms may be required to move the nuclei the greater distances observed in thicker regions of the neuroepithelium. FC Sauer noted a difference in nuclear movement between the neural tube and the thinner garter snake epithelium of the gut. In the neural tube, nuclear size (as a marker for stage in the cell cycle) and position were consistent with nuclei moving gradually during G1 and S-phase, as the nucleus gets larger. In the gut epithelium, size and position are not as well correlated, suggesting that mostly stochastic movements occur during interphase (Sauer, 1936). Similarly, Norden and colleagues report that basal nuclear movement in the shorter zebrafish retina and hindbrain is also less gradual and more punctuated than published observations in mammalian cerebral cortex, taking place only early in G1 phase (Norden et al., 2009; Tsai et al., 2005; Leung et al., 2011). We propose that microtubules and microtubule motor proteins may be required for the longer, more gradual nuclear movements observed during G1 and G2 in the elongated apical progenitor cells of the cerebral cortex. Microtubules may be insufficient to move the nucleus all the way to the apical surface, however. Myosin and actin may be sufficient to move the nucleus in a more stochastic manner over a more limited range observed in shorter epithelial cells, and may be required for ensuring mitotic cells are completely rounded up to the apical surface (Figure 1).
Figure 1. Different lengths of epithelial cells may dictate the mechanism of apical nuclear movement for mitosis.
Microtubule-dependent and actin-dependent forces may contribute to apical movement during division, but the length of the cell may determine which mechanism contributes the most. Highly elongated cells, such as radial glia, may require the microtubule cytoskeleton. Shorter epithelia undergo shorter nuclear movements to and from the apical surface. In this case, actin-based forces that round up the cell may suffice, with little need for the microtubule cytoskeleton. Cytoplasm of the cells is shown in green, nuclei and DNA are shown in blue. Centrosomes are red and cilia are magenta.
B. Initiation of mitosis in the neuroepithelium
How mitosis initiates in the neuroepithelium is unclear. Apically positioned centrosomes are presumed to trigger mitosis only after nuclei have reached the apical surface (Schenk et al., 2009; Frade, 2002; Miyata, 2007; Tamai et al., 2007; Taverna and Huttner, 2010; Smart, 1972; Hinds and Ruffett, 1971). The centrosome is critical for entry into mitosis. Mitosis is triggered by a cascade of proteins, culminating in the activation of aurora A kinase, which in turn activates the cyclin B/cdk1 complex. This cascade is localized to the centrosome (Hirota et al., 2003; Jackman et al., 2003). Centrosomes are important for nuclear envelope breakdown (NEBD) as well (Basto and Pines, 2007; Margalit et al., 2005). However, it has long been noted that early prophase cells were found away from apical surface, suggesting that mitosis may not initiate at the apical surface (Hinds and Ruffett, 1971; Sauer, 1936). Reduction of Lis1, loss of microtubule stability, and inhibition of actin produce ectopic mitotic cells in the neuroepithelium (Gambello et al., 2003; Xie et al., 2007; Murciano et al., 2002), suggesting that either mitosis does not initiate at the centrosomes or that centrosomes do not stay at the apical surface. We have observed that the latter is true. We have previously reported that in the dorsal neural tube, centrosomes leave the apical surface prior to mitosis (Ahlstrom and Erickson, 2009). More recently, we have discovered that late in G2 phase, centrosomes of the lateral neural tube and cerebral cortex leave the apical surface and contact the nucleus to initiate mitosis, which we discuss in the next section.
C. Centrosome positioning and initiation of mitosis
Centrosome positioning is critical for normal INM since disruption of centrosome position perturbs INM. For example, loss-of-function mutations of atypical protein kinase C λ (aPKC λ) cause disorganization of cell polarity and displace centrosomes from the apical surface in the neuroepithelium. Subsequently, mitosis occurs non-apically (Imai et al., 2006). It has also been observed that loss of Pax6 is associated with abnormal INM. In this circumstance, the centrosomes move away from the apical surface early in the cell cycle, and are frequently found in ectopic locations. (Tamai et al., 2007).
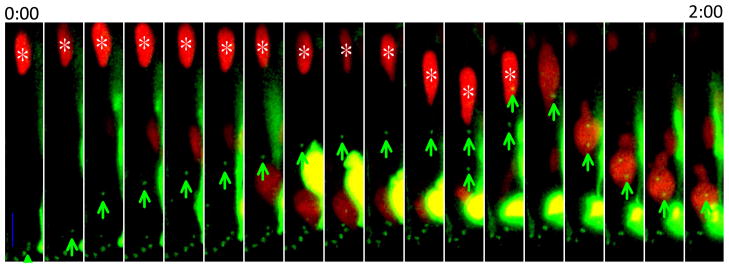
How centrosomes are localized to the apical surface is not certain, however. Centrosomes are physically connected to the cilium. Therefore it is likely that the centrosome is anchored at the apical surface via a primary cilium (Dubreuil et al., 2007). Consistent with this idea, when Pax6 is reduced in the neuroepithelium, cells often lack a primary cilium and under these circumstances centrosomes move away from the apical surface. We have observed in the chicken neural tube that cilia are disassembled late in G2 phase. Concomitant with this event, centrosomes depart from the apical surface before the incoming nucleus reaches the apical surface (Figure 2). When the centrosomes arrive at the nucleus, nuclear envelope breakdown proceeds. In 66% of mitoses observed in the chicken neural tube, nuclear envelope breakdown began more than 7μm from the apical surface.
Figure 2. Centrosomes leave the apical surface to initiate mitosis.
The intact nucleus is marked with NLS-tdTomato and an asterisk; centrosomes are marked with GFP-centrin and green arrows. The average distance from site of nuclear envelope breakdown to final position during mitosis is 16.5 μm (N = 15). All mitotic cells are observed to undergo apical rounding during mitosis (N = 62). NLS-tdTomato and GFP-centrin were introduced to neural tube cells by in-ovo electroporation at Hamburger/Hamilton stage 12 to 16. Neural tubes were sectioned and imaged less than 24 hours later on an Olympus confocal microscope. Images were taken every 7 minutes. Scale bar = 10 μm
D. Basal movement
As with apical nuclear movement, there is also disagreement about the mechanism of basally-directed nuclear movement during INM. Schenk and colleagues suggest that myosin II and actin control basal nuclear movement in the mouse cortex, whereas Tsai and colleagues propose that nuclei are driven along microtubules via kinesin 3 (Schenk et al., 2009; Tsai et al., 2010). Yet a third possibility initially suggested by FC Sauer, but more recently supported experimentally by Kosodo and colleagues, is that basal nuclear movement during INM is not an active, cell-autonomous process (Kosodo et al., 2011; Sauer, 1935). When magnetic beads are implanted into the neuroepithelium, they are observed to move basally. However, if apical nuclear migration is blocked by inhibiting S-phase with hydroxyurea, beads fail to move basally, suggesting that basal displacement is passively driven by active apical nuclear movement (Kosodo et al., 2011).
As with apical nuclear movement, these mechanisms need not be mutually exclusive. Myosin II was not shown to be required cell autonomously for basal nuclear movement because tissue sections were incubated in blebbistatin, a myosin inhibitor, which affected all cells (Schenk et al., 2009). Cells in mitosis moving to the apical surface may require myosin II to round up forcefully and consequently displace their neighbors basally. Based on the fact that kinesin 3 and myosin both seem essential for basal nuclear migration, we suggest that kinesin 3 may be required in G1 cells as a ratchet that prevents back-sliding and therefore allows effective movement in one direction only, rather than as an active motor. This would ensure that pressures from rounding neighboring mitotic cells would move nuclei toward the plus ends of microtubules, and away from the apical surface, instead of pushing the nucleus basally and apically randomly. Alternatively, intrinsic microtubule-based nuclear movement in G1 driven by kinesin may be required for persistent basal nuclear movement after neighboring cells have already pushed G1 nuclei away from the apical surface.
E. Relationship of cell cycle and nuclear movement
Cell cycle is correlated with nuclear movement. Mitotic nuclei are found at the apical surface for reasons previously discussed. S-phase cells are found at more basal positions. Time-lapse imaging combined with S-phase labeling suggest that G1 nuclei are migrating basally, whereas G2 nuclei are migrating apically (Hayes and Nowakowski, 2000; Tamai et al., 2007). Cell cycle progression appears to be essential for nuclear migration. Inhibiting completion of mitosis with 5-azacytidine prevents basal nuclear movement, as evidenced by an increase of mitotic figures at the apical surface (Ueno et al., 2006).
There is more controversy about whether the completion of S-phase is necessary for apical nuclear migration. In support of this, overexpression of p18Ink4c arrests cells in G1 phase, and results in an increase of cells in the basal region of the neuroepithelium (Kosodo et al., 2011). Also, morphine is known to slow down the cell cycle of radial glial cells and delays apical nuclear migration (Sargeant et al., 2008). Finally, Ueno and colleagues found that cyclophosphamide-induced arrest in S-phase prevents apical nuclear migration, and Leung and colleagues demonstrated that arresting retinal cells with aphidicolin and hydroxyurea inhibits apical nuclear migration (Ueno et al., 2006; Kosodo et al., 2011; Leung et al., 2011). Curiously, Murciano and colleagues reported that inhibition of S-phase with hydroxyurea does not inhibit apical nuclear migration in the chicken diencephalon (Murciano et al., 2002). The discrepancy between their results and others detailed above is not explained.
How cell cycle might regulate nuclear movement is not known. A possible link between the S-to-G2 phase transition and apical nuclear movement is TPX2, which is contained in the nucleus prior to G2 phase, and relocates to the apical process during G2 phase. TPX2 is a microtubule-binding protein that reorganizes the microtubule cytoskeleton, and is essential for apical nuclear movement (Kosodo et al., 2011). Potentially, INM is controlled by a cell-cycle-regulated release of TPX2.
III. Possible functions of INM
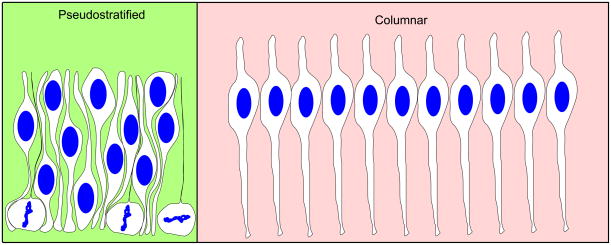
The simplest function of INM may be to allow epithelia to pack more progenitor cells within a limited surface area by displacing most of the thick nuclei basally (Miyata, 2007; Frade, 2002; Smart, 1972). Elongated objects with a bulge, like the nuclear bulge in neuroepithelial cells that must be joined by a thin end in a limited area, can be confined to a smaller area if the position of the bulge is varied (Figure 3). INM serves to vary the distances of the nuclei from the apical surface, and thereby allows more progenitor cells to attach to a limited apical surface area than would be possible with columnar epithelia.
Figure 3. Pseudostratified conformations allow for more cells to be contained with limited surface area.
Neural epithelial cells are connected to a limited surface area. Since the nuclei take up more room than the apical endfeet, pseudostratification allows the same number of cells to occupy less space.
Identifying other functions of INM has proven more difficult than establishing the mechanisms involved. One reason is that, as with testing mechanisms of nuclear movement, it is difficult to affect only INM. Most published experimental perturbations that disrupt INM also involve loss of epithelial structure and defects in mitosis. In these cases, the proposed functions of INM may instead be related to functions of the epithelial structure itself or may be affected more by timely mitosis. Nonetheless, several common themes from disrupting INM have emerged and suggest potential functions.
A. Epithelial remodeling
One of the first suggested functions of INM is to regulate the shape of the neural plate for neurulation (Hsin-Yi, 1976; Messier and Auclair, 1974; Langman et al., 1966). Hinge points are areas of the epithelium where patches of cells have assumed a wedge shape, thereby bending the epithelium and contributing to neural tube closure (Eom et al., 2011). It has been suggested that the wedge-shaped cells in hinge points are shaped by the position of the nuclei. If the cell cycle is lengthened in G1 or S phase, more nuclei would be found at basal positions, leading to a more contracted apical surface and a wider basal region (Smith and Schoenwolf, 1997; Smith et al., 1994; Smith and Schoenwolf, 1987).
INM is found in other epithelia undergoing morphogenetic movements beside the formation of hinge points. The lens placode and neural retina both invaginate, and the lung buds, liver bud, and pancreatic buds evaginate from the endoderm (Bort et al., 2006; Zwaan et al., 1969). All these morphological events are associated with concave apical surfaces. Stem cells stimulated to differentiate into optic vesicles undergo INM in culture and spontaneously invaginate to form optic-cup-like structures, suggesting that INM and epithelial shaping are intrinsic properties of the epithelium. Proper formation of the cup structure is inhibited when cell proliferation is blocked using aphidicolin, suggesting that cell division at the apical surface may be important for generating forces to shape the epithelium (Eiraku et al., 2011). The relationship between INM and epithelia undergoing remodeling should be examined more closely since it is not known how cell division during INM controls cell-shape change.
B. Maintenance of Epithelial Structure and Polarity
Apical division may also be required to maintain the structure and/or the polarity of the epithelium. The adhesions between the cells are found at the apical end of the cell. Regulators of cell polarity, such as Par3, Par6, and aPKC, are also concentrated at the apical surface, and are required for the maintenance of the adherens junctions (Baum and Georgiou, 2011). Loss of these proteins results in the disruption of the epithelial structure (Imai et al., 2006; Cappello et al., 2006). Apical division in the neocortex allows for both daughter cells to inherit the adherens junctions and therefore remain attached in the VZ (Konno et al., 2008). Similarly, mitotic cells of the intestine crypt round up to the apical surface and undergo cytokinesis while maintaining the apical tight junctions and microvilli bordering the lumen of the intestine (Jinguji and Ishikawa, 1992). Apical cytokinesis may therefore be required to allow both daughter cells to inherit polarity signals and cell-cell adhesions in order to maintain the integrity of the epithelium during cell division.
C. Regulation of neurogenesis and proliferation
In the retina, cells that detach from the apical surface become post-mitotic neurons (Austin et al., 1995; Zolessi et al., 2006). In the brain, cells leaving the apical surface continue to proliferate as transit amplifying basal progenitors, but have limited potential for self-renewal (Noctor et al., 2004; Miyata et al., 2004; Haubensak et al., 2004; reviewed Gotz and Huttner, 2005). Different types of post-mitotic cells are produced over time (Shen et al., 2006; reviewed Livesey and Cepko, 2001). To produce the correct number and type of neurons, neurogenesis must be balanced against proliferation of progenitor cells. Neurogenesis is regulated by a variety of mechanisms, but it has been suggested that INM may determine which progenitor cells produce a neuron (reviewed Gotz and Huttner, 2005; Baye and Link, 2008).
Neurogenesis and INM are tightly correlated. In the embryo, INM begins before neurogenesis and continues as neurons are born. Neurons of the fetal brain, retina, and spinal cord can be traced to apical progenitors of the neuroepithelium that undergo INM (Miyata et al., 2004; Noctor et al., 2004). The premigratory neural crest cells in the dorsal trunk neural tube also undergo INM, later giving rise to neurons and glial cells of the peripheral nervous system (Ahlstrom and Erickson, 2009; Dupin et al., 2007). INM is also associated with neurogenic regions of the adult organism. For example, the adult canary brain and turtle spinal cord undergo INM during neurogenesis, whereas non-neurogenic regions do not (Alvarez-Buylla et al., 1998; Russo et al., 2008). INM is observed in cultured stem cells stimulated to produce neurons as well, suggesting it is an intrinsic feature of neurogenic epithelia (Eiraku et al., 2011; Lazzari et al., 2006).
The link between INM and neurogenesis is likely not to be coincidental. Indeed, most perturbations affecting INM in the neuroepithelium also affect neurogenesis. Also, nuclei that move further from the apical surface are associated with increased chances of producing a post-mitotic neuron (Baye and Link, 2007; Murciano et al., 2002; Del Bene et al., 2008). Baye and Link show that the converse is not true, however; inhibiting neurogenesis does not alter depth of nuclear migration, suggesting that nuclear migration affects neurogenesis and not vice versa (Baye and Link, 2007).
There are several ways in which INM may regulate neurogenesis. It is possible that the effect of nuclear migration on neurogenesis is controlled by Notch signaling (Del Bene et al., 2008; Murciano et al., 2002; Frade, 2002; Taverna and Huttner, 2010; Latasa et al., 2009; Buchman and Tsai, 2008). When the Notch receptor on a neural progenitor binds to Delta ligand on an adjacent cell, neurogenesis is inhibited (reviewed Latasa et al., 2009). Two studies have suggested that the Notch and Delta form apical-basal gradients in the neuroepithelium, with low levels of Notch basally (Murciano et al., 2002; Del Bene et al., 2008). It has been proposed that those nuclei that migrate further basally encounter less signal to inhibit neurogenesis, whereas nuclei staying more apically are more likely to continue proliferating.
IV. Conclusion
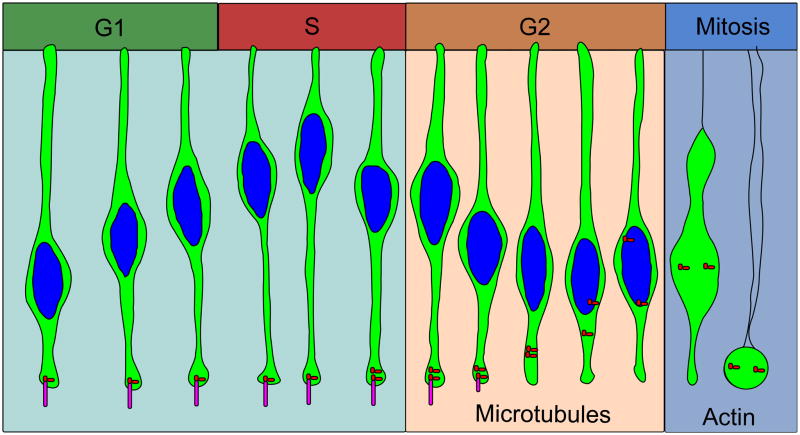
We propose a model of INM for the neural epithelium in which nuclei are passively displaced from the apical surface during G1 by neighboring cells that are rounding up for mitosis. Centrosomes nucleate a microtubule array extending toward the basal side of the epithelium. Nuclei pushed away from the apical surface by neighboring mitotic cells then move basally by actin transport along this microtubule array toward the plus ends as a result of kinesin 3. Sometime before entry into G2 phase, nuclei reach their peak basal position. At the S-phase to G2-phase transition, dynein is activated at, or localized to, the nucleus and begins pulling nuclei along microtubules toward the minus-end located at the centrosomes, which are in turn held at the apical surface by the cilium. Late in G2 phase, the cilium is lost, and the centrosome is pulled to the nucleus by dynein. Nuclear envelope breakdown begins when the centrosome reaches the nucleus. The cell is then rounded up to the apical surface via actomyosin prior to cytokinesis (Figure 4). Shorter epithelial cells may undergo little microtubule-based nuclear migration, and only use mitotic rounding regulated by actin. How densely the cells are packed may affect how nuclei migrate during the cell cycle, which may affect Notch lateral inhibition and thereby balance proliferation and differentiation.
Figure 4. Proposed model of INM.
Nuclei are moved by actin contraction and along microtubules. Short nuclear movements may be accomplished by actin alone. Longer nuclear movements may require microtubules. Nuclei move basally during G1 phase, reaching a peak distance during S-phase. Nuclei begin moving apically along microtubules using the dynein motor protein during G2 phase. Tpx2 initiates apical nuclear migration at G2 phase. After cilia are lost, centrosomes can move to the nucleus during late G2 to initiate nuclear envelope breakdown and apical rounding, dependent on actin. Cytoplasm of the cells are shown in green, and nuclei are shown in blue. Centrosomes are red dots and cilia are magenta.
One of the biggest questions about INM remains how similar or different the process is between tissue types. Time-lapse microscopy has revealed much about INM in the neural epithelium, but to our knowledge, has not been used to observe INM in other tissues, such as the epithelial somite, or the intestinal crypt. Significant questions remain about actin and/or myosin-regulated mechanisms for apical movement prior to mitosis. Most cells assume a round morphology during mitosis as a result of the actin cytoskeleton. It will be important to determine if neuroepithelial cells round up to the apical surface using mechanisms that are common to other mitotic events, or if they use mechanisms specific to elongated cells. Finally, how INM may balance proliferation and differentiation, and whether it has this role in other epithelia, should be investigated.
Acknowledgments
We thank Dr. D. Starr for reading a draft of this manuscript. This research was supported by the NIH (grant number RO1 GM53258).
References
- Afonso C, Henrique D. PAR3 acts as a molecular organizer to define the apical domain of chick neuroepithelial cells. J Cell Sci. 2006;119:4293–304. doi: 10.1242/jcs.03170. [DOI] [PubMed] [Google Scholar]
- Ahlstrom JD, Erickson CA. The neural crest epithelial-mesenchymal transition in 4D: a ‘tail’ of multiple non-obligatory cellular mechanisms. Development. 2009;136:1801–1812. doi: 10.1242/dev.034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garci3a-Verdugo JM, Mateo AS, Merchant-Larios H. Primary Neural Precursors and Intermitotic Nuclear Migration in the Ventricular Zone of Adult Canaries. The Journal of Neuroscience. 1998;18:1020–1037. doi: 10.1523/JNEUROSCI.18-03-01020.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin CP, Feldman DE, Ida JA, Jr, Cepko CL. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development. 1995;121:3637–3650. doi: 10.1242/dev.121.11.3637. [DOI] [PubMed] [Google Scholar]
- Basto R, Pines J. The Centrosome Opens the Way to Mitosis. Developmental Cell. 2007;12:475–477. doi: 10.1016/j.devcel.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. The Journal of Cell Biology. 2011;192:907–917. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baye LM, Link BA. Nuclear migration during retinal development. Brain Res. 2008;1192:29–36. doi: 10.1016/j.brainres.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene F, Wehman AM, Link BA, Baier H. Regulation of neurogenesis by interkinetic nuclear migration through an apical-basal notch gradient. Cell. 2008;134:1055–65. doi: 10.1016/j.cell.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bort R, Signore M, Tremblay K, Martinez Barbera JP, Zaret KS. Hex homeobox gene controls the transition of the endoderm to a pseudostratified, cell emergent epithelium for liver bud development. Dev Biol. 2006;290:44–56. doi: 10.1016/j.ydbio.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Buchman JJ, Tsai LH. Putting a notch in our understanding of nuclear migration. Cell. 2008;134:912–4. doi: 10.1016/j.cell.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Cabernard C, Doe CQ. Stem Cell Self-Renewal: Centrosomes on the Move. Current Biology. 2007;17:R465–R467. doi: 10.1016/j.cub.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Cappello S, Attardo A, Wu X, Iwasato T, Itohara S, Wilsch-Brauninger M, Eilken HM, Rieger MA, Schroeder TT, Huttner WB, Brakebusch C, Gotz M. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci. 2006;9:1099–107. doi: 10.1038/nn1744. [DOI] [PubMed] [Google Scholar]
- Cappello S, Monzo P, Vallee RB. NudC is required for interkinetic nuclear migration and neuronal migration during neocortical development. Dev Biol. 2011;357:326–335. doi: 10.1016/j.ydbio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro ACD, Fragel-Madeira L, Silva-Neto MA, Linden R. A role for CK2 upon interkinetic nuclear migration in the cell cycle of retinal progenitor cells. Dev Neurobiol. 2008;68:620–631. doi: 10.1002/dneu.20613. [DOI] [PubMed] [Google Scholar]
- Chenn A, Zhang YA, Chang BT, McConnell SK. Intrinsic Polarity of Mammalian Neuroepithelial Cells. Molecular and Cellular Neuroscience. 1998;11:183–193. doi: 10.1006/mcne.1998.0680. [DOI] [PubMed] [Google Scholar]
- Dubreuil V, Marzesco AM, Corbeil D, Huttner WB, Wilsch-Bräuninger M. Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. The Journal of Cell Biology. 2007;176:483–495. doi: 10.1083/jcb.200608137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin E, Calloni G, Real C, Gonçalves-Trentin A, Le Douarin NM. Neural crest progenitors and stem cells. Comptes Rendus Biologies. 2007;330:521–529. doi: 10.1016/j.crvi.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- Eom DS, Amarnath S, Fogel JL, Agarwala S. Bone morphogenetic proteins regulate neural tube closure by interacting with the apicobasal polarity pathway. Development. 2011;138:3179–3188. doi: 10.1242/dev.058602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade JM. Interkinetic nuclear movement in the vertebrate neuroepithelium: encounters with an old acquaintance. Prog Brain Res. 2002;136:67–71. doi: 10.1016/s0079-6123(02)36007-2. [DOI] [PubMed] [Google Scholar]
- Fujita S. Kinetics of cellular proliferation. Experimental Cell Research. 1962;28:52–60. doi: 10.1016/0014-4827(62)90311-7. [DOI] [PubMed] [Google Scholar]
- Gambello MJ, Darling DL, Yingling J, Tanaka T, Gleeson JG, Wynshaw-Boris A. Multiple dose-dependent effects of Lis1 on cerebral cortical development. J Neurosci. 2003;23:1719–29. doi: 10.1523/JNEUROSCI.23-05-01719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Frank CL, Calderon de Anda F, Tsai L-H. Hook3 Interacts with PCM1 to Regulate Pericentriolar Material Assembly and the Timing of Neurogenesis. Neuron. 2010;65:191–203. doi: 10.1016/j.neuron.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–88. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Grosse AS, Pressprich MF, Curley LB, Hamilton KL, Margolis B, Hildebrand JD, Gumucio DL. Cell dynamics in fetal intestinal epithelium: implications for intestinal growth and morphogenesis. Development. 2011;138:4423–4432. doi: 10.1242/dev.065789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci USA. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes NL, Nowakowski RS. Exploiting the dynamics of S-phase tracers in developing brain: interkinetic nuclear migration for cells entering versus leaving the S-phase. Dev Neurosci. 2000;22:44–55. doi: 10.1159/000017426. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD. Shroom regulates epithelial cell shape via the apical positioning of an actomyosin network. J Cell Sci. 2005;118:5191–203. doi: 10.1242/jcs.02626. [DOI] [PubMed] [Google Scholar]
- Hinds JW, Ruffett TL. Cell proliferation in the neural tube: an electron microscopic and golgi analysis in the mouse cerebral vesicle. Z Zellforsch Mikrosk Anat. 1971;115:226–64. doi: 10.1007/BF00391127. [DOI] [PubMed] [Google Scholar]
- Hirota T, Kunitoku N, Sasayama T, Marumoto T, Zhang D, Nitta M, Hatakeyama K, Saya H. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 2003;114:585–98. doi: 10.1016/s0092-8674(03)00642-1. [DOI] [PubMed] [Google Scholar]
- Hsin-Yi L. Inhibition of neurulation and interkinetic nuclear migration by concanavalin a in explanted early chick embryos. Developmental Biology. 1976;48:392–399. doi: 10.1016/0012-1606(76)90100-7. [DOI] [PubMed] [Google Scholar]
- Imai F, Hirai S, Akimoto K, Koyama H, Miyata T, Ogawa M, Noguchi S, Sasaoka T, Noda T, Ohno S. Inactivation of aPKClambda results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development. 2006;133:1735–44. doi: 10.1242/dev.02330. [DOI] [PubMed] [Google Scholar]
- Jackman M, Lindon C, Nigg EA, Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- Jinguji Y, Ishikawa H. Electron microscopic observations on the maintenance of the tight junction during cell division in the epithelium of the mouse small intestine. Cell Struct Funct. 1992;17:27–37. doi: 10.1247/csf.17.27. [DOI] [PubMed] [Google Scholar]
- Karfunkel P. The activity of microtubules and microfilaments in neurulation in the chick. J Exp Zool. 1972;181:289–301. doi: 10.1002/jez.1401810302. [DOI] [PubMed] [Google Scholar]
- Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, Miyata T, Matsuzaki F. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- Kosodo Y, Suetsugu T, Suda M, Mimori-Kiyosue Y, Toida K, Baba SA, Kimura A, Matsuzaki F. Regulation of interkinetic nuclear migration by cell cycle-coupled active and passive mechanisms in the developing brain. EMBO J. 2011;30:1690–1704. doi: 10.1038/emboj.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langman J, Nelson GR. A radioautographic study of the development of the somite in the chick embryo. J Embryol Exp Morphol. 1968;19:217–226. [PubMed] [Google Scholar]
- Langman J, Guerrant RL, Freeman BG. Behavior of neuro-epithelial cells during closure of the neural tube. The Journal of Comparative Neurology. 1966;127:399–411. doi: 10.1002/cne.901270308. [DOI] [PubMed] [Google Scholar]
- Latasa MJ, Cisneros E, Frade JM. Cell cycle control of Notch signaling and the functional regionalization of the neuroepithelium during vertebrate neurogenesis. The International Journal of Developmental Biology. 2009;53:895–908. doi: 10.1387/ijdb.082721ml. [DOI] [PubMed] [Google Scholar]
- Lazzari G, Colleoni S, Giannelli SG, Brunetti D, Colombo E, Lagutina I, Galli C, Broccoli V. Direct derivation of neural rosettes from cloned bovine blastocysts: a model of early neurulation events and neural crest specification in vitro. Stem Cells. 2006;24:2514–21. doi: 10.1634/stemcells.2006-0149. [DOI] [PubMed] [Google Scholar]
- Leung L, Klopper AV, Grill SW, Harris WA, Norden C. Apical migration of nuclei during G2 is a prerequisite for all nuclear motion in zebrafish neuroepithelia. Development. 2011;138:5003–5013. doi: 10.1242/dev.071522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- Margalit A, Vlcek S, Gruenbaum Y, Foisner R. Breaking and making of the nuclear envelope. J Cell Biochem. 2005;95:454–65. doi: 10.1002/jcb.20433. [DOI] [PubMed] [Google Scholar]
- Messier PE, Auclair C. Effect of cytochalasin B on interkinetic nuclear migration in the chick embryo. Developmental Biology. 1974;36:218–223. doi: 10.1016/0012-1606(74)90206-1. [DOI] [PubMed] [Google Scholar]
- Meyer EJ, Ikmi A, Gibson MC. Interkinetic Nuclear Migration Is a Broadly Conserved Feature of Cell Division in Pseudostratified Epithelia. Current Biology. 2011;21:485–491. doi: 10.1016/j.cub.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Miyata T. Development of three-dimensional architecture of the neuroepithelium: Role of pseudostratification and cellular “community”. Development, Growth & Differentiation. 2007;50:S105–S112. doi: 10.1111/j.1440-169X.2007.00980.x. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric Inheritance of Radial Glial Fibers by Cortical Neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131:3133–3145. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- Murciano A, Zamora J, López-Sánchez J, Frade JM. Interkinetic Nuclear Movement May Provide Spatial Clues to the Regulation of Neurogenesis. Molecular and Cellular Neuroscience. 2002;21:285–300. doi: 10.1006/mcne.2002.1174. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Norden C, Young S, Link BA, Harris WA. Actomyosin Is the Main Driver of Interkinetic Nuclear Migration in the Retina. Cell. 2009;138:1195–1208. doi: 10.1016/j.cell.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo RE, Reali C, Radmilovich M, Fernandez A, Trujillo-Cenoz O. Connexin 43 delimits functional domains of neurogenic precursors in the spinal cord. J Neurosci. 2008;28:3298–309. doi: 10.1523/JNEUROSCI.5736-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargeant TJ, Day DJ, Miller JH, Steel RW. Acute in utero morphine exposure slows G2/M phase transition in radial glial and basal progenitor cells in the dorsal telencephalon of the E15.5 embryonic mouse. Eur J Neurosci. 2008;28:1060–7. doi: 10.1111/j.1460-9568.2008.06412.x. [DOI] [PubMed] [Google Scholar]
- Sauer FC. Mitosis in the neural tube. The Journal of Comparative Neurology. 1935;62:377–405. [Google Scholar]
- Sauer FC. The interkinetic migration of embryonic epithelial nuclei. J Morphol. 1936;60:1–11. [Google Scholar]
- Sauer MEW. Radiographic study of interkinetic nuclear migration in the neural tube. Proc Soc Exp Biol Med. 1959;101:557–600. doi: 10.3181/00379727-101-25014. [DOI] [PubMed] [Google Scholar]
- Schaper A. Die frühesten Differenzirungsvorgänge im Centralnervensystem. Archiv für Entwickelungsmechanik der organismen. 1897a;5:81–132. [Google Scholar]
- Schaper A. The earliest differentiation in the central nervous system of Vertebrates. Science. 1897b;5:430–431. [Google Scholar]
- Schenk J, Wilsch-Bräuninger M, Calegari F, Huttner WB. Myosin II is required for interkinetic nuclear migration of neural progenitors. Proceedings of the National Academy of Sciences. 2009;106:16487–16492. doi: 10.1073/pnas.0908928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- Smart IH. Proliferative characteristics of the ependymal layer during the early development of the spinal cord in the mouse. J Anat. 1972;111:365–380. [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Schoenwolf GC. Cell cycle and neuroepithelial cell shape during bending of the chick neural plate. Anat Rec. 1987;218:196–206. doi: 10.1002/ar.1092180215. [DOI] [PubMed] [Google Scholar]
- Smith JL, Schoenwolf GC. Neurulation: coming to closure. Trends Neurosci. 1997;20:510–7. doi: 10.1016/s0166-2236(97)01121-1. [DOI] [PubMed] [Google Scholar]
- Smith JL, Schoenwolf GC, Quan J. Quantitative analyses of neuroepithelial cell shapes during bending of the mouse neural plate. J Comp Neurol. 1994;342:144–151. doi: 10.1002/cne.903420113. [DOI] [PubMed] [Google Scholar]
- Starr DA. A nuclear-envelope bridge positions nuclei and moves chromosomes. J Cell Sci. 2009;122:577–586. doi: 10.1242/jcs.037622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama M, Sakaue-Sawano A, Iimura T, Fukami K, Kitaguchi T, Kawakami K, Okamoto H, Higashijima S-ichi, Miyawaki A. Illuminating cell-cycle progression in the developing zebrafish embryo. Proceedings of the National Academy of Sciences. 2009;106:20812–20817. doi: 10.1073/pnas.0906464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai H, Shinohara H, Miyata T, Saito K, Nishizawa Y, Nomura T, Osumi N. Pax6 transcription factor is required for the interkinetic nuclear movement of neuroepithelial cells. Genes Cells. 2007;12:983–96. doi: 10.1111/j.1365-2443.2007.01113.x. [DOI] [PubMed] [Google Scholar]
- Taverna E, Huttner WB. Neural progenitor nuclei IN motion. Neuron. 2010;67:906–914. doi: 10.1016/j.neuron.2010.08.027. [DOI] [PubMed] [Google Scholar]
- Tsai JW, Bremner KH, Vallee RB. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat Neurosci. 2007;10:970–9. doi: 10.1038/nn1934. [DOI] [PubMed] [Google Scholar]
- Tsai JW, Chen Y, Kriegstein AR, Vallee RB. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J Cell Biol. 2005;170:935–45. doi: 10.1083/jcb.200505166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JW, Lian WN, Kemal S, Kriegstein AR, Vallee RB. Kinesin 3 and cytoplasmic dynein mediate interkinetic nuclear migration in neural stem cells. Nat Neurosci. 2010;13:1463–1471. doi: 10.1038/nn.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsue TT, Watling DL, Weisleder P, Coltrera MD, Rubel EW. Identification of hair cell progenitors and intermitotic migration of their nuclei in the normal and regenerating avian inner ear. J Neurosci. 1994;14:140–152. doi: 10.1523/JNEUROSCI.14-01-00140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Katayama K, Yamauchi H, Nakayama H, Doi K. Cell cycle progression is required for nuclear migration of neural progenitor cells. Brain Res. 2006;1088:57–67. doi: 10.1016/j.brainres.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, Shi SH. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature. 2009;461:947–955. doi: 10.1038/nature08435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson RL, Venezaiano P, Bartha A. American Association of Anatomists. Sixty-ninth annual session; Marquette University School of Medicine, Milwaukee, Wisconsin, April 4, 5, 6, 1956. Officers, abstracts, demonstrations (pp. 249–388) The Anatomical Record. 1956;124:379. doi: 10.1002/ar.1091240209. [DOI] [PubMed] [Google Scholar]
- Wilsch-Bräuninger M, Peters J, Paridaen JTML, Huttner WB. Basolateral rather than apical primary cilia on neuroepithelial cells committed to delamination. Development. 2012;139:95–105. doi: 10.1242/dev.069294. [DOI] [PubMed] [Google Scholar]
- Xie Z, Moy LY, Sanada K, Zhou Y, Buchman JJ, Tsai LH. Cep120 and TACCs Control Interkinetic Nuclear Migration and the Neural Progenitor Pool. Neuron. 2007;56:79–93. doi: 10.1016/j.neuron.2007.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Lei K, Zhou M, Craft CM, Xu G, Xu T, Zhuang Y, Xu R, Han M. KASH protein Syne-2/Nesprin-2 and SUN proteins SUN1/2 mediate nuclear migration during mammalian retinal development. Hum Mol Genet. 2011;20:1061–1073. doi: 10.1093/hmg/ddq549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, Xu R, Han M. SUN1/2 and Syne/Nesprin-1/2 Complexes Connect Centrosome to the Nucleus during Neurogenesis and Neuronal Migration in Mice. Neuron. 2009;64:173–187. doi: 10.1016/j.neuron.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolessi FR, Poggi L, Wilkinson CJ, Chien CB, Harris WA. Polarization and orientation of retinal ganglion cells in vivo. Neural Develop. 2006;1:2. doi: 10.1186/1749-8104-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaan J, Bryan PR, Jr, Pearce TL. Interkinetic nuclear migration during the early stages of lens formation in the chicken embryo. J Embryol Exp Morphol. 1969;21:71–83. [PubMed] [Google Scholar]